Proton Craniospinal Irradiation for Patients with Solid Tumor Leptomeningeal Disease: Real-World Feasibility, Toxicity, and Outcome Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
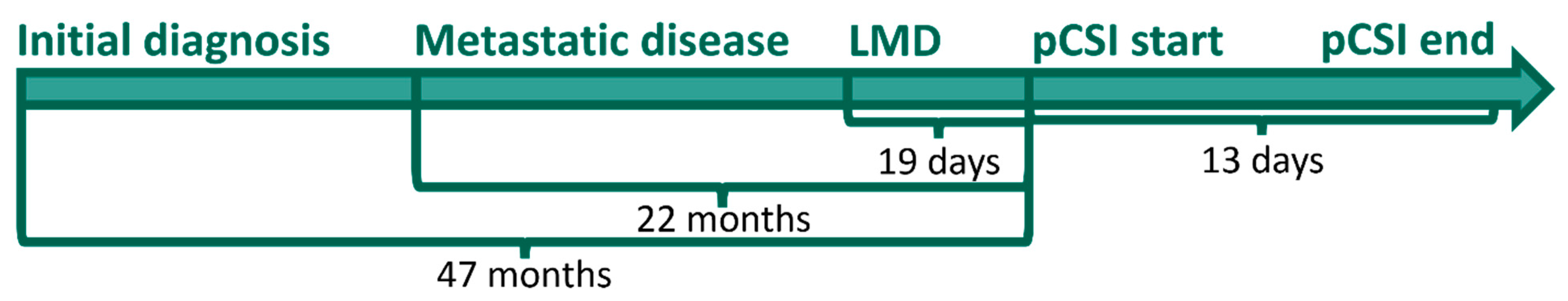
2.1. Patient Cohort
2.2. Treatment Planning and Delivery
2.3. Statistical Considerations
3. Results
3.1. Feasibility and Toxicity Measures
3.2. Progression-Free Survival and Overall Survival
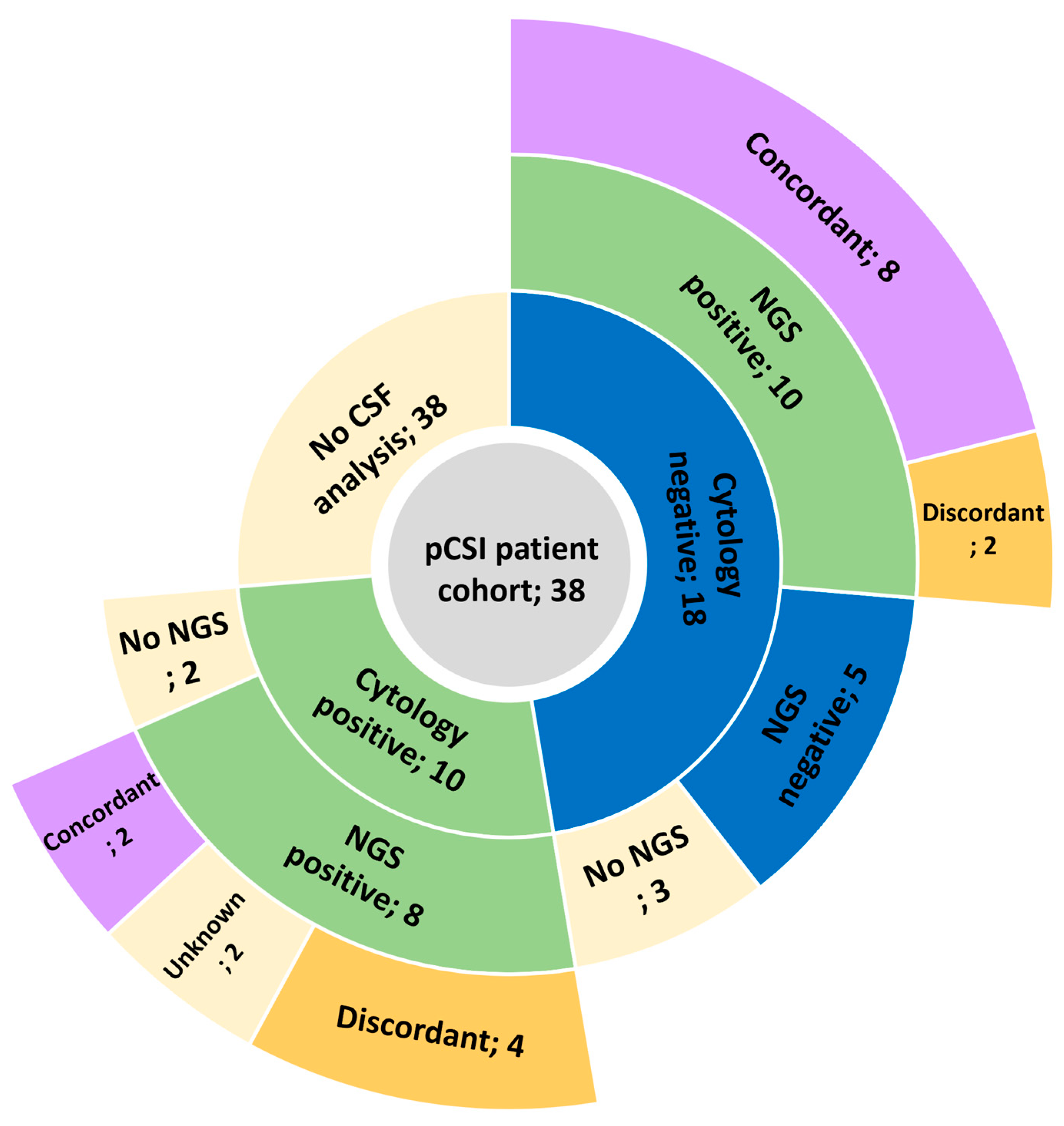
3.3. CSF Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clarke, J.L.; Perez, H.R.; Jacks, L.M.; Panageas, K.S.; DeAngelis, L.M. Leptomeningeal metastases in the MRI era. Neurology 2010, 74, 1449–1454. [Google Scholar] [CrossRef]
- Emoto, S.; Ishigami, H.; Yamaguchi, H.; Yamashita, H.; Kaisaki, S.; Kitayama, J. Frequent development of leptomeningeal carcinomatosis in patients with peritoneal dissemination of gastric cancer. Gastric Cancer 2011, 14, 390–395. [Google Scholar] [CrossRef]
- Lai, R.; Dang, C.T.; Malkin, M.G.; Abrey, L.E. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer 2004, 101, 810–816. [Google Scholar] [CrossRef]
- Omuro, A.M.P.; Kris, M.G.; Miller, V.A.; Franceschi, E.; Shah, N.; Milton, D.T.; Abrey, L.E. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer 2005, 103, 2344–2348. [Google Scholar] [CrossRef]
- Boogerd, W.; Hart, A.A.M.; van der Sande, J.J.; Engelsman, E. Meningeal carcinomatosis in breast cancer. Prognostic factors and influence of treatment. Cancer 1991, 67, 1685–1695. [Google Scholar] [CrossRef]
- Buszek, S.M.; Chung, C. Radiation Therapy for Leptomeningeal Disease. In Radiopharmaceuticals in the Management of Leptomeningeal Metastasis; Springer: Berlin/Heidelberg, Germany, 2022; pp. 125–141. [Google Scholar]
- Buszek, S.M.; Chung, C. Radiotherapy in Leptomeningeal Disease: A Systematic Review of Randomized and Non-randomized Trials. Front. Oncol. 2019, 9, 1224. [Google Scholar] [CrossRef]
- Schiopu, S.; Habl, G.; Haefner, M.; Katayama, S.; Herfarth, K.; Debus, J.; Sterzing, F. Helical tomotherapy in patients with leptomeningeal metastases. Cancer Manag. Res. 2018, 11, 401–409. [Google Scholar] [CrossRef]
- Devecka, M.; Duma, M.N.; Wilkens, J.J.; Kampfer, S.; Borm, K.J.; Münch, S.; Straube, C.; Combs, S.E. Craniospinal irradiation(CSI) in patients with leptomeningeal metastases: Risk-benefit-profile and development of a prognostic score for decision making in the palliative setting. BMC Cancer 2020, 20, 501. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Nguyen, E.K.; Soliman, H. An overview of leptomeningeal disease. Ann. Palliat. Med. 2021, 10, 909–922. [Google Scholar] [CrossRef]
- Yang, T.J.; Wijetunga, N.A.; Yamada, J.; Wolden, S.; Mehallow, M.; Goldman, D.A.; Zhang, Z.; Young, R.J.; Kris, M.G.; Yu, H.A.; et al. Clinical trial of proton craniospinal irradiation for leptomeningeal metastases. Neuro-Oncology 2021, 23, 134–143. [Google Scholar] [CrossRef]
- Mathis, N.J.; Wijetunga, N.A.; Imber, B.S.; Pike, L.R.G.; Yang, J.T. Recent Advances and Applications of Radiation Therapy for Brain Metastases. Curr. Oncol. Rep. 2022, 24, 335–342. [Google Scholar] [CrossRef]
- Yang, J.T.; Wijetunga, N.A.; Pentsova, E.; Wolden, S.; Young, R.J.; Correa, D.; Zhang, Z.; Zheng, J.; Steckler, A.; Bucwinska, W.; et al. Randomized Phase II Trial of Proton Craniospinal Irradiation Versus Photon Involved-Field Radiotherapy for Patients With Solid Tumor Leptomeningeal Metastasis. J. Clin. Oncol. 2022, 40, 3858–3867. [Google Scholar] [CrossRef]
- Le Rhun, E.; Weller, M.; van den Bent, M.; Brandsma, D.; Furtner, J.; Rudà, R.; Schadendorf, D.; Seoane, J.; Tonn, J.C.; Wesseling, P.; et al. Leptomeningeal metastasis from solid tumours: EANO–ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. ESMO Open 2023, 8, 101624. [Google Scholar] [CrossRef]
- Wilcox, J.A.; Chukwueke, U.N.; Ahn, M.-J.; Aizer, A.A.; Bale, T.A.; Brandsma, D.; Brastianos, P.K.; Chang, S.; Daras, M.; Forsyth, P.; et al. Leptomeningeal metastases from solid tumors: A Society for Neuro-Oncology and American Society of Clinical Oncology consensus review on clinical management and future directions. Neuro-Oncology 2024, 26, 1781–1804. [Google Scholar] [CrossRef]
- Lam, K.; Nasr, L.F.; Andersen, C.R.; Marqueen, K.E.; Li, J.; Wang, C.; Beckham, T.H.; Majd, N.; Aaroe, A.E.; Loghin, M.; et al. Early Outcomes from Proton Craniospinal Irradiation for Leptomeningeal Metastasis from Solid Tumors. Adv. Radiat. Oncol. 2024, 10, 101697. [Google Scholar] [CrossRef]
- Dornisch, A.; Tringale, K.R.; Murphy, J.D. Cost-Effectiveness of Proton Craniospinal Irradiation (pCSI) in Patients with Solid Tumor Leptomeningeal Metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, S191. [Google Scholar] [CrossRef]
- Nasr, L.F.; Li, J.; Swanson, T.A.; Ghia, A.J.; Wang, C.; Yeboa, D.N.; Grosshans, D.R.; McAleer, M.F.; Beckham, T.; McGovern, S.L. Early Outcomes from Proton Craniospinal Irradiation (pCSI) for Leptomeningeal Disease from Solid Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, e139–e140. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Ehret, F.; Yuan, A.M.; Zieminski, S.; Leland, P.A.; Khandekar, M.J.; Oh, K.S.; Shih, H.A. RADT-44. EARLY CLINICAL EXPERIENCE WITH PROTON CRANIOSPINAL IRRADIATION FOR THE TREATMENT OF LEPTOMENINGEAL DISEASE. Neuro-Oncology 2024, 26, viii82. [Google Scholar] [CrossRef]
- El Shafie, R.A.; Böhm, K.; Weber, D.; Lang, K.; Schlaich, F.; Adeberg, S.; Paul, A.; Haefner, M.F.; Katayama, S.; Sterzing, F.; et al. Outcome and prognostic factors following palliative craniospinal irradiation for leptomeningeal carcinomatosis. Cancer Manag. Res. 2019, 11, 789–801. [Google Scholar] [CrossRef]
- Takeda, K.; Umezawa, R.; Yamamoto, T.; Takahashi, N.; Jingu, K. Craniospinal irradiation for leptomeningeal metastasis of solid tumors: Survival analysis and prognostic factors. J. Radiat. Res. 2024, 65, 667–675. [Google Scholar] [CrossRef]
- Keit, E.; Peterson, J.; Johnstone, P.A.S.; Robinson, T.J.; Figura, N.B. Modern Photon-Based Craniospinal Irradiation in Leptomeningeal Disease. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, e242. [Google Scholar] [CrossRef]
- Prabhu, R.S.; Turner, B.E.; Asher, A.L.; Marcrom, S.R.; Fiveash, J.B.; Foreman, P.M.; Press, R.H.; Patel, K.R.; Curran, W.J.; Breen, W.G.; et al. A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neuro-Oncology 2019, 21, 1049–1059. [Google Scholar] [CrossRef]
- Bartsch, R.; Jerzak, K.J.; Larrouquere, L.; Müller, V.; Le Rhun, E. Pharmacotherapy for leptomeningeal disease in breast cancer. Cancer Treat. Rev. 2024, 122, 102653. [Google Scholar] [CrossRef]
- Yang, J.C.H.; Kim, S.-W.; Kim, D.-W.; Lee, J.-S.; Cho, B.C.; Ahn, J.-S.; Lee, D.H.; Kim, T.M.; Goldman, J.W.; Natale, R.B.; et al. Osimertinib in Patients with Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study. J. Clin. Oncol. 2020, 38, 538–547. [Google Scholar] [CrossRef]
- Alder, L.; Trapani, D.; Bradbury, C.; Van Swearingen, A.E.D.; Tolaney, S.M.; Khasraw, M.; Anders, C.K.; Lascola, C.D.; Hsu, L.; Lin, N.U.; et al. Durable responses in patients with HER2+ breast cancer and leptomeningeal metastases treated with trastuzumab deruxtecan. npj Breast Cancer 2023, 9, 19. [Google Scholar] [CrossRef]
- Sener, U.; Webb, M.; Breen, W.G.; Neth, B.J.; Laack, N.N.; Routman, D.; Brown, P.D.; Mahajan, A.; Frechette, K.; Dudek, A.Z.; et al. Proton Craniospinal Irradiation with Immunotherapy in Two Patients with Leptomeningeal Disease from Melanoma. J. Immunother. Precis. Oncol. 2024, 7, 1–6. [Google Scholar] [CrossRef]
- Webb, M.J.; Breen, W.G.; Laack, N.N.; Leventakos, K.; Campian, J.L.; Sener, U. Proton craniospinal irradiation with bevacizumab and pembrolizumab for leptomeningeal disease: A case report. CNS Oncol. 2023, 12, CNS101. [Google Scholar] [CrossRef]
- Wijetunga, N.A.; Goglia, A.G.; Weinhold, N.; Berger, M.F.; Cislo, M.; Higginson, D.S.; Chabot, K.; Osman, A.M.; Schaff, L.; Pentsova, E.; et al. Dynamic Mutational Landscape of Cerebrospinal Fluid Circulating Tumor DNA and Predictors of Survival after Proton Craniospinal Irradiation for Leptomeningeal Metastases. Clin. Cancer Res. 2023, 29, 775–783. [Google Scholar] [CrossRef]
- Ahmed, K.; Kumthekar, P.; Kim, Y.; DeJesus, M.; Pina, Y.; Oliver, D.; Evernden, B.; Arrington, J.; Vogelbaum, M.; Rosa, M.; et al. Octs-07 Trial in Progress: Phase Ii Study of Radiation Therapy Followed by Intrathecal Trastuzumab/Pertuzumab in the Management of Her2+ Breast Leptomeningeal Disease. Neuro-Oncol. Adv. 2024, 6, i28. [Google Scholar] [CrossRef]


| Variable | Frequency (n) | Rate (%) |
|---|---|---|
| Age, years: median (range) | 58 (23–82) | |
| Sex | ||
| Male | 6 | 16 |
| Female | 32 | 84 |
| KPS | ||
| 90–100 | 20 | 53 |
| 70–80 | 18 | 47 |
| Primary tumor histology | ||
| Breast | 23 | 61 |
| HER2+ | 9 | 24 |
| NSCLC | 10 | 26 |
| EGFR+ | 4 | 11 |
| ALK+ | 2 | 5 |
| SCLC | 2 | 5 |
| Other * | 3 | 8 |
| Extracranial disease status | ||
| Active | 18 | 47 |
| Stable/Controlled | 9 | 24 |
| None | 11 | 29 |
| LMD detection method | ||
| MRI | 38 | 100 |
| CSF | 20 | 53 |
| Involved compartment | ||
| Brain | 13 | 34 |
| Spine | 5 | 13 |
| Both | 20 | 53 |
| Parenchymal brain metastases | ||
| Yes | 32 | 84 |
| No | 6 | 16 |
| Toxicity/Grade | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Non-Hematologic: Frequency, n (Rate, %) | ||||
| Alopecia | 4 (11) | 11 (29) | 0 (0) | 0 (0) |
| Anorexia | 4 (11) | 3 (8) | 0 (0) | 0 (0) |
| Conjunctivitis | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Constipation | 2 (5) | 0 (0) | 0 (0) | 0 (0) |
| Dermatitis | 2 (5) | 0 (0) | 0 (0) | 0 (0) |
| Dizziness | 3 (8) | 0 (0) | 0 (0) | 0 (0) |
| Dry eye | 2 (5) | 0 (0) | 0 (0) | 0 (0) |
| Fatigue | 11 (29) | 6 (16) | 0 (0) | 0 (0) |
| Headache | 7 (18) | 1 (3) | 0 (0) | 0 (0) |
| Memory impairment | 2 (5) | 0 (0) | 0 (0) | 0 (0) |
| Nausea | 7 (18) | 2 (5) | 0 (0) | 0 (0) |
| Seizure | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 4 (11) | 1 (3) | 0 (0) | 0 (0) |
| Total | 20 (53) | 15 (39) | 0 (0) | 0 (0) |
| Hematologic: Frequency, n (Rate, %) | ||||
| Anemia | 0 (0) | 2 (5) | 0 (0) | 0 (0) |
| Lymphopenia | 0 (0) | 4 (11) | 16 (42) | 1 (3) |
| Neutropenia | 0 (0) | 1 (3) | 0 (0) | 1 (3) |
| Thrombocytopenia | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Total | 1 (3) | 6 (16) | 16 (42) | 2 (5) |
| Variable | Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|
| Level | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| PFS | |||||
| Age | Continuous | 1.03 (0.99–1.07) | 0.13 | 1.05 (1.01–1.10) | 0.021 |
| KPS | 90–100 | REF | REF | ||
| 70–80 | 3.08 (1.19–7.94) | 0.02 | 3.14 (1.18–8.37) | 0.022 | |
| Primary tumor | Lung | REF | REF | ||
| Breast | 2.35 (0.68–8.20) | 1.79 | 3.50 (0.90–13.60) | 0.071 | |
| Other | 5.34 (1.15–24.77) | 0.03 | 4.19 (0.85–20.68) | 0.079 | |
| OS | |||||
| Age | Continuous | 1.03 (0.99–1.07) | 0.10 | 1.04 (0.99–1.09) | 0.079 |
| KPS | 90–100 | REF | REF | ||
| 70–80 | 2.16 (0.82–5.72) | 0.12 | 2.08 (0.68–6.35) | 0.199 | |
| Primary tumor | Lung | REF | REF | ||
| Breast | 1.47 (0.40–5.34) | 0.56 | 1.18 (0.27–5.06) | 0.824 | |
| Other | 5.76 (1.22–27.09) | 0.03 | 5.47 (1.00–30.09) | 0.051 | |
| CSF Analysis | Non-positive | REF | REF | ||
| Positive | 2.65 (0.93–7.56) | 0.07 | 3.40 (1.03–11.17) | 0.044 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gal, O.; La Rosa, A.; Hall, M.D.; Press, R.H.; Fellows, Z.; Wroe, A.J.; Gutierrez, A.N.; Odia, Y.; Mehta, M.P.; Kotecha, R. Proton Craniospinal Irradiation for Patients with Solid Tumor Leptomeningeal Disease: Real-World Feasibility, Toxicity, and Outcome Analysis. Cancers 2025, 17, 1046. https://doi.org/10.3390/cancers17061046
Gal O, La Rosa A, Hall MD, Press RH, Fellows Z, Wroe AJ, Gutierrez AN, Odia Y, Mehta MP, Kotecha R. Proton Craniospinal Irradiation for Patients with Solid Tumor Leptomeningeal Disease: Real-World Feasibility, Toxicity, and Outcome Analysis. Cancers. 2025; 17(6):1046. https://doi.org/10.3390/cancers17061046
Chicago/Turabian StyleGal, Omer, Alonso La Rosa, Matthew D. Hall, Robert H. Press, Zachary Fellows, Andrew J. Wroe, Alonso N. Gutierrez, Yazmin Odia, Minesh P. Mehta, and Rupesh Kotecha. 2025. "Proton Craniospinal Irradiation for Patients with Solid Tumor Leptomeningeal Disease: Real-World Feasibility, Toxicity, and Outcome Analysis" Cancers 17, no. 6: 1046. https://doi.org/10.3390/cancers17061046
APA StyleGal, O., La Rosa, A., Hall, M. D., Press, R. H., Fellows, Z., Wroe, A. J., Gutierrez, A. N., Odia, Y., Mehta, M. P., & Kotecha, R. (2025). Proton Craniospinal Irradiation for Patients with Solid Tumor Leptomeningeal Disease: Real-World Feasibility, Toxicity, and Outcome Analysis. Cancers, 17(6), 1046. https://doi.org/10.3390/cancers17061046







