Simple Summary
Acute myeloid leukemia (AML) is characterized by genetic mutations that influence disease course and treatment response. Mutations in the nucleophosmin (NPM1) gene are among the most common genetic abnormalities in AML and are generally associated with favorable outcomes. However, our comprehensive analysis of 2811 patients revealed that this prognostic benefit is strongly age-dependent. While NPM1-mutated AML patients younger than 65 years demonstrated significantly improved survival compared to their wild-type counterparts, this advantage disappeared in older patients. Our investigation identified several factors contributing to this discrepancy: older patients exhibited enrichment of adverse co-mutations, more frequently presented with abnormal chromosomal patterns, and received less intensive therapeutic interventions. Additionally, we found differences in the clonal dominance patterns of NPM1 mutations between age groups. These findings underscore the necessity for age-adapted risk stratification in NPM1-mutated AML, which could prevent potential overestimation of prognosis in older patients and inform more appropriate therapeutic decision making, ultimately improving clinical outcomes.
Abstract
Background: While NPM1-mutated AML in the absence of FLT3-ITD generally carries a favorable prognosis, large registry studies suggest the positive prognostic benefit may not extend to patients > 65 years of age. We examined this preferential, age-dependent prognostic impact through a real-world analysis of 2811 adult AML patients. Results: The median overall survival (OS) was significantly better in NPM1MT compared to NPM1WT patients [20.86 vs. 17 mo., p = 0.003]. When stratified by age, NPM1MT patients had higher OS than NPM1WT patients in the 55–65-year age group (28.62 vs. 16.3 mo., p ≤ 0.0001). This OS benefit was heterogenous and prevailed most strikingly in the 55–60 (68.3 vs. 15.6 mo., p = 0.002), and up to the 60–65-year group (mOS not estimable vs. 20 mo., p = 0.007), but not beyond 65 y. Notably, the ≤65 cohort was more enriched with dominant NPM1 (21% vs. 15%, p ≤ 0.001), while the >65 cohort was enriched with abnormal karyotype (20% in >65 years vs. 16% in ≤65 years, p = 0.001), and co-occurring SRSF2 and ASXL1 mutations (18.7% vs. 7.5%, p ≤ 0.0001 and 13.5% vs. 4.1%, p ≤ 0.0001 resp.). Conclusions: We demonstrate that in a real-world setting, the prognostic benefit of NPM1 does not extend beyond age 65, underscoring the need for age-adapted risk stratification models. This granular approach could prevent the potential overestimation of prognosis in older patients with NPM1MT AML and inform therapeutic decision making.
1. Introduction
Somatic mutations in Nucleophosmin 1 or NPM1 gene (NPM1MT) are earlier driver hits in leukemogenesis and one of the most commonly identified genetic abnormalities in de novo acute myeloid leukemia (AML; ~30% of cases) [1] The distinct molecular and clinical features of NPM1MT AML led to its recognition as a separate entity in the 2017 World Health Organization (WHO) classification of myeloid neoplasms [2].
The prognostic significance of NPM1, particularly in conjunction with co-occurring mutations such as FLT3-ITD and myelodysplasia-related gene signature (AML-MR), has become increasingly central to risk stratification, as reflected in the 2022 European LeukemiaNet (ELN) guidelines [3,4,5]. Historically, NPM1MT has been associated with favorable outcomes in AML patients [6,7]. Early observations from Falini et al. based on 591 AML patients showed that 291 NPM1MT patients achieved a higher complete response (CR) rate and harbored less chemotherapy-refractory disease after induction therapy compared to NPM1 wild-type (NPM1WT) [8]. Particularly, their work also highlighted that the favorable prognostic impact of NPM1MT seemed limited to younger patients with median ages between 41.9 and 51.8. This age-related effect coincides with the observation that NPM1MT occurs less frequently in older patients, who instead show enrichment for AML-MR. Subsequent analyses revealed stark survival differences between NPM1MT patients above and below age 65 (median OS: 10.5 years vs. 1.7 years) [9].
Clinical trial-based registry studies have also suggested age as a strong prognostic indicator in NPM1MT AML. More recently, it was discovered that the favorable prognostic impact of NPM1MT was particularly pronounced in patients aged 55 to 65 years but diminished significantly in patients aged > 65 [10]. However, with age increasingly influencing treatment selection, the real-world implications of NPM1MT age-dependent prognostic benefit remain poorly characterized outside of clinical trials [11]. Although it is conceived that chemotherapy without hematopoietic stem cell transplant is adequate for treating NPM1MT AML, these non-uniform outcomes necessitate an exploratory analysis in a real-world cohort study. Accordingly, our work presents a comprehensive age-stratified analysis of a large cohort of NPM1MT AML patients predominantly managed outside of conventional clinical trials to validate the differential prognosis and address the potential reasons underlying variable outcomes in this genetically defined subset of AML.
2. Methods
2.1. Cohort Selection
For this study, we used a large, well-annotated cohort of patients treated at Karmanos Cancer Institute (KCI) between 2006 and 2021 and expanded it using a publicly available dataset from cBioPortal and a meta-analytic registry (CCF) of AML patients from previously reported series [12]. More extensive details on the inclusion criteria for patients in the real-world meta-analytic registry are available elsewhere [12]. Patients with de novo AML were classified as primary (pAML), and patients with Myelodysplasia-Related Changes (MRC) or evolving from an antecedent myeloid neoplasm were classified as secondary AML (sAML). Due to a very small number of therapy-associated AML patients included in our analysis, this subgroup was also reclassified as secondary AML. Patients aged ≤65 and >65 years were classified as younger and older cohorts, respectively, for the purpose of this study. Patients were further subdivided into more granular age groups (<55, 55–60, 60–65, 65–75, and >75) in order to capture the age-related impact of NPM1 mutation on overall survival. These cut-offs represent clinically meaningful inflection points in aging where disease biology, preexisting comorbidities, and treatment tolerability tend to shift overall survival and may otherwise be missed with broader age groupings. We analyzed the baseline clinical and molecular characteristics and performed survival analyses with respect to multiple genetic variables, focusing on the differences across the age groups. The clonal hierarchy of NPM1MT in relation to other mutations was defined with a cut-off VAF difference of ≥5%. When the difference in VAF between the co-occurring mutations was less than 5%, the call was deemed codominant, as previously described [13].
Treatment was characterized as either intensive or non-intensive. Intensive therapies included high-dose cytarabine regimens with anthracyclines (such as the standard 7 + 3 regimen) or purine analogs (such as fludarabine, cladribine, or clofarabine). Non-intensive therapy primarily consisted of hypomethylating agents (azacitidine or decitabine) or low-dose cytarabine. Only a minority of patients received venetoclax, likely since it had not yet become the standard of care at the time of diagnosis for many of these patients. Additionally, The BEAT-AML Master trial dataset was used to validate the available data from KCI and CCF to study the impact of the treatment intensity [14].
2.2. Genetic Studies
For the genetic data generated from KCI, multiplex PCR of 568 amplicons was performed using the Illumina TruSight Myeloid Panel (Illumina, San Diego, CA, USA)to target frequently mutated regions of 49 myeloid genes. Next-generation sequencing was performed on Illumina’s MiSeqDx with 150 bp paired-end reads and a mean depth of coverage of 1000×. Sequencing data were analyzed with Illumina’s Local Run Manager software using the published human genome build UCSC hg19 as the reference sequence. Agilent’s Alissa Interpret 5.4 software (Agilent, Santa Clara, CA, USA) was used to evaluate sequence variations in individuals. All the reported sequence variants in patients with variant allele fractions less than 10% or 10–20% with <500× read depth were confirmed by Sanger sequencing. FLT3-ITD mutations and CALR exon 9 insertions or deletions were tested by multiplex fragment analysis. CEBPA sequencing was performed by Sanger sequencing for amplicons with less than 100x coverage by next-generation sequencing. Mutation nomenclature was based on the recommendations of the Human Genome Variation Society (HGVS). Variants were classified according to the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists standards and guidelines for interpreting and reporting sequence variants in cancer [15].
2.3. Statistical Analysis
Survival outcomes were assessed using the Kaplan–Meier methodology. Overall survival (OS) was defined as the time from initial diagnosis to death from any cause, with censoring at the date of last follow-up for surviving patients. Differences in survival curves were evaluated using the log-rank test. The reverse Kaplan–Meier method was employed to calculate the median follow-up time. For comparisons between groups, categorical variables were analyzed using the chi-squared test, while the Mann–Whitney test was used to compare medians of continuous variables. Statistical significance was set at p < 0.05 using two-tailed tests for all the analyses. All the statistical analyses were performed using the R statistical software (version 4.30), with Kaplan–Meier curves generated using GraphPad Prism 9.5.0.
3. Results
The patient clinical characteristics are listed in Table 1. We performed rigorous quality control on our entire cohort of 2811 patients and the remaining 2309 were further subdivided into NPM1WT (n = 1820) and NPM1MT (n = 489) (Supplementary Figure S1). The patient subgroup stratification is also detailed in the Supplemental Methods. Among the clinically relevant differences between the two groups, the NPM1WT patients had a higher median age at diagnosis than NPM1MT (p = 0.0006). The NPM1WT group was more enriched with secondary AML (p = 0.0001), abnormal karyotype (p < 0.0001), and non-FLT3-ITD patients (p < 0.0001). Conversely, NPM1MT had a hi gher proportion of primary AML (p = 0.0001), normal karyotype (p < 0.0001), and FLT3-ITDPOS (p < 0.0001) patients. NPM1MT was also associated with a higher bone marrow blast percentage (p < 0.0001) and peripheral white blood cell count (p < 0.0001) at diagnosis.

Table 1.
Patient characteristics.
We then explored survival outcomes based on various genetic variables and molecular profiles of our cohort.
3.1. Cohort Overview and OS Based on Co-Occurring Mutational Count
In our entire cohort, the NPM1MT patients had a significantly higher median overall survival (OS) than NPM1WT (20.86 mo. vs. 17 mo., p = 0.003) (Figure 1A). The median follow-up time for our cohort was 45.6 months, and the 4-year OS reached 33.1% for the NPM1WT patients (30.5–36.0%, 95% CI) and 43.5% for NPM1MT (38.9–48.6%, 95% CI). When the NPM1MT patients were stratified further based on ENL2022 favorable-risk criteria (NPM1MT without FLT3-ITD), the NPM1MT patients had a better OS compared to the wild-type population (29 mo. vs. 16 mo., p ≤ 0.0001) (Figure 1B).
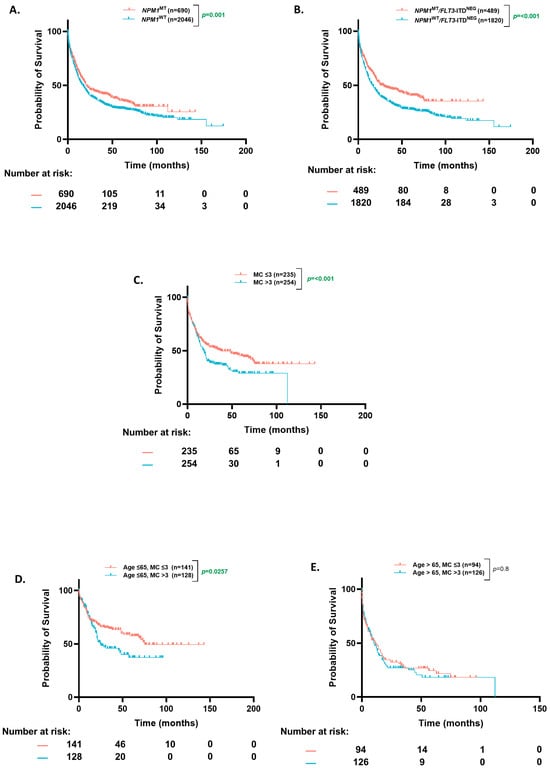
Figure 1.
Overall survival based on NPM1 status and mutational burden in patients ≤65 and >65 years. (A) Kaplan–Meier estimates of overall survival (OS) in all patients according to NPM1 mutation status. (B) Kaplan–Meier estimates of OS in NPM1MT patients based on FLT3-ITDNEG status (C) Kaplan–Meier estimates of OS based on co-occurring mutation count in NPM1MT patients. (D,E) Kaplan–Meier estimates of OS in NPM1MT patients according to co-occurring mutation count [age ≤ 65 (D) and age > 65 (E)]. MC = mutation count.
A number of co-occurring mutations can predict clinical behavior in myeloid neoplasms [16]. Based on prior studies, we analyzed differences in OS of the NPM1MT AML patients with respect to the number of co-occurring mutations (≤3 or >3). There was a higher proportion of patients with more than three mutations in the older age group (44% vs. 33%, p = 0.01). The patients with more than three than less than or equal to three co-occurring mutations had a worse OS (18 mo. vs. 38 mo. p < 0.0001) (Figure 1C). When stratified by an age cut-off of 65, the difference in OS based on mutation count was statistically significant in the younger (75 mo. vs. 25 mo., p = 0.026) (Figure 1D) but not the older patients (10.3 mo. vs. 11.3 mo., p = 0.8) (Figure 1E). However, most of the benefit in all comers was derived from the patients younger than 65.
3.2. Age-Stratified OS (Based on NPM1 Status)
In order to understand the heterogeneity of the prognostic benefit provided by NPM1, we stratified the cohort (n = 2309) into clinically relevant age groups and observed significant differences. The NPM1MT patients in the 55–65-year age group had a higher OS when compared to the NPM1WT group (75.3 mo. vs. 19 mo., p ≤ 0.001). This benefit was preserved even if further sub-grouped into 55–60-year (68.3 mo. vs. 16 mo., p = 0.02) and 60–65-year (not estimable (NE) vs. 20 mo., p = 0.0007) age cohorts. In 65–75 and above-75-year groups, there was no difference in overall survival between the NPM1MT versus NPM1WT patients (65–75 years. age group, 13 vs. 14 mo., p = 0.19; >75 years. age group, 3.4 vs. 5.8 mo., p = 0.33). Patients younger than 55 years performed well regardless of the NPM1MT status, and the NPM1MT patients had a numerical survival advantage over NPM1WT (NE vs. 81 mo., p = 0.51) (Figure 2).
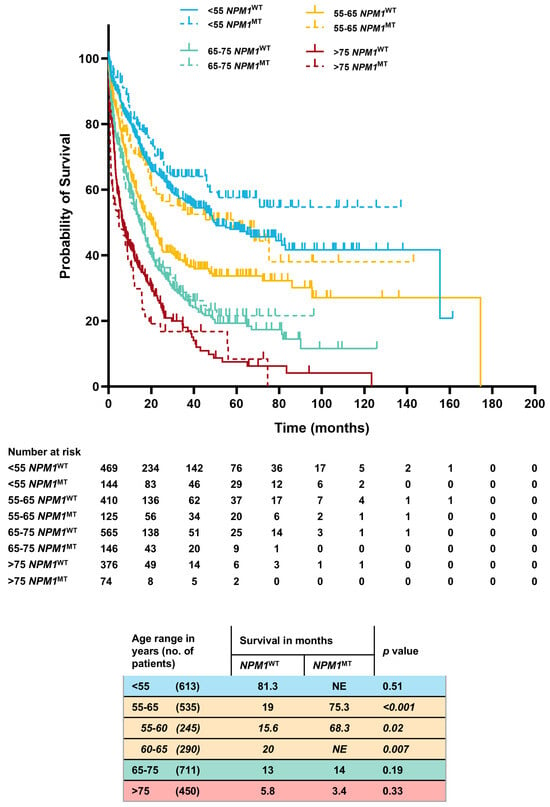
Figure 2.
Comprehensive Kaplan–Meier estimates of OS stratified by age, NPM1 mutation status, and FLT3-ITDNEG genotype.
3.3. Molecular Profile of NPM1MT Patients
Among the co-occurring mutations with NPM1MT, the association of DNMT3A (46% vs. 39%, p = 0.27), IDH2 (25% vs. 22%, p = 0.61), FLT3-ITD (31% vs. 19%, p = 0.16), IDH1 (19% vs. 15%, p = 0.25), PTPN11 (5% vs. 8%, p = 0.18), and CEBPA (5% vs. 6%, p = 0.63) were not different in the ≤65 vs. >65-year groups. TET2 (13% vs. 27%, p = 0.0001), SRSF2 (5% vs. 15%, p = 0.0002), and ASXL1 (1% vs. 7%, p = 0.0006) were more associated with NPM1MT in the >65 years age group, whereas NRAS (13% vs. 7%, p = 0.03) and WT1 (10% vs. 4%, p = 0.01) were more commonly seen in the ≤65 years cohort (Table 2, and Supplementary Figure S2). The strength of associations between these mutations was slightly different between the younger and older age groups (Figure 3A,B). We sought to investigate the impact of prognostically significant co-mutations with NPM1 (DNMT3A, FLT3-ITD, and WT1) on overall survival in the younger (aged ≤ 65) and older (aged > 65) patients (Supplementary Figure S3). These three co-mutations have been previously recognized as defining a key archetype of NPM1MT AMLs [16,17,18]. When NPM1 was co-mutated with DNMT3A, OS was the most favorable among the triplet, and the younger patients had a significantly higher OS compared to the older ones (68 vs. 11 mo.; p ≤ 0.0001). Furthermore, in the younger patients, WT1 co-mutation resulted in the next highest OS compared to the elderly (46 vs. 9 mo.; p ≤ 0.0001). In contrast, among the elderly patients, FLT3-ITD co-mutated with NPM1 had the highest OS (Supplementary Figure S3; 21 mo. vs. 10 mo. for DNMT3A vs. 9 mo. for WT1; p ≤ 0.0001).

Table 2.
Distribution of mutations.
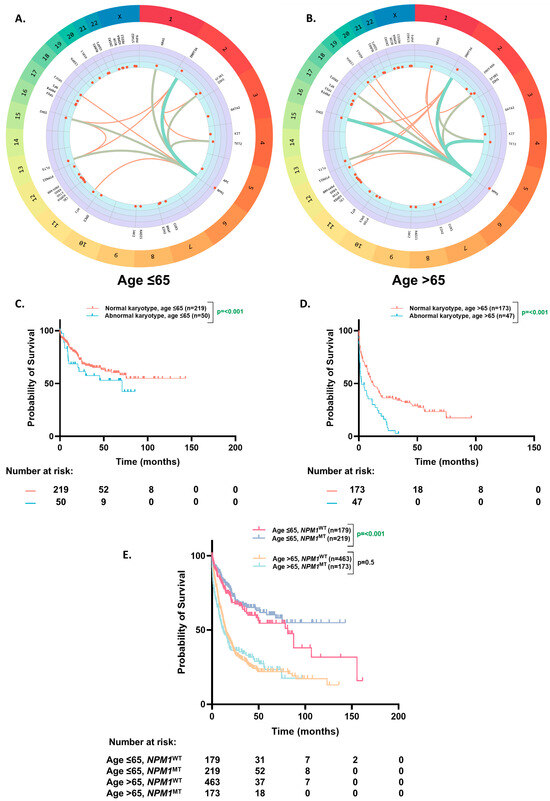
Figure 3.
Co-occurring mutational profile and overall survival based on karyotype. (A,B). Circos plot illustrating the co-occurrence of somatic mutations in the NPM1MT patients aged ≤ 65 (A) and aged > 65 (B). (C,D). Kaplan–Meier estimates of OS according to karyotype in the NPM1MT patients aged ≤ 65 (C) and aged > 65 (D). (E). Kaplan–Meier estimates of OS based on the patients aged ≤ 65 and >65 and NPM1 mutation status with a normal karyotype.
3.4. OS Based on Karyotype
A relatively higher proportion of the older patients had an abnormal karyotype than the younger patients (20% vs. 16%, p ≤ 0.001). The patients with an abnormal karyotype had worse OS than the patients with a normal karyotype in the younger (71 mo. vs. NE, p ≤ 0.0001) (Figure 3C) and older age group (2.4 mo. vs. 12 mo., p ≤ 0.0001) (Figure 3D).
On further analyses of survival in the patients with ELN2022 favorable risk (NPM1MT without and cytogenetically normal AML (CN-AML)), the OS benefit of NPM1 mutation was still restricted to the ≤65-year age group (NE vs. 38 mo., p = 0.001) and did not extend to the >65-year group (12 mo. vs. 14.6 mo., p = 0.5) (Figure 3E).
3.5. OS Based on Clonal Dominance
The patients with a dominant NPM1MT had a higher and statistically significant OS in all comers (74.6 mo. vs. 19.87 mo., p = 0.02), and FLT3-ITDPOS (NE vs. 16.8 mo., p ≤ 0.001) population, but not in FLT3-ITDNEG (46.5 vs. 21.7 mo., p = 0.43), (Figure 4A).
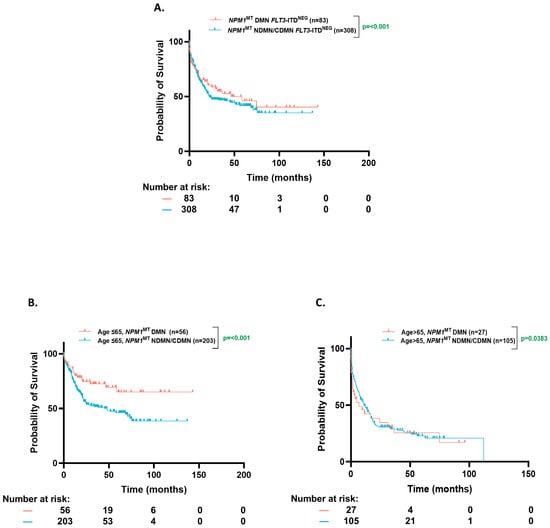
Figure 4.
Overall survival based on clonal dominance. (A). Kaplan–Meier estimates of OS for all NPM1MT-dominant and NPM1MT-non-dominant/co-dominant patients according to Variant Allele Frequency (VAF). (B,C). Kaplan–Meier estimates of OS for NPM1MT-dominant and NPM1MT-non-dominant/co-dominant patients according to VAF [age ≤ 65 (B) and age > 65 (C)]. DMN= dominant; NDMN = non-dominant; CDMN = co-dominant.
The median VAF of NPM1 was ~38% (Interquartile range (IQR), 30–45%) in both the younger and older cohorts. However, the ≤65-year group was more enriched with dominant NPM1MT when compared to the >65-year group (28.8% vs. 18%, p = 0.01). The NPM1MT-dominant patients trended towards a better numerical OS in the ≤65-year group (NE vs. 75.2 mo. p = 0.21), whereas this trend was absent in the >65-year group (6.8 mo. vs. 10.3 mo., p = 0.6), although this comparison did not reach statistical significance (Figure 4B,C).
3.6. OS Based on Treatment Intensity
A higher proportion of patients received non-intensive induction therapy (53% vs. 6%, p < 0.0001) and a lower fraction received allo-HSCT (21% vs. 55%, p < 0.0001) in the older population (n = 72) compared to the younger age group (n = 82) in the CCF and KCI patient cohorts (Figure 5A,C). Only 4% of the younger patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of ≥2 compared to 30% in the older population (p = 0.01). Comparable results were observed in the BEAT-AML dataset [non-intensive therapy in 46% vs. 7%, p < 0.0001; and allo-HSCT in 7% vs. 32%, p = 0.03; in older (n = 32) vs. younger (n = 55) patients, respectively] (Figure 5B,D). In the older group, the patients who received non-intensive therapy had a worse OS than those receiving intensive treatment (KCI/CCF cohort: 4.1 mo. vs. 13 mo., p = 0.0006; BEAT-AML cohort: 0.5 mo. vs. 23 mo., p = 0.03) (Figure 5A,B). Furthermore, the older patients who received allo-HSCT had a better prognosis than those who did not (KCI/CCF cohort: 24.9 mo. vs. 4.9 mo., p = 0.002; BEAT AML cohort: NE vs. 12.1 mo., p = 0.05) (Figure 5C,D).
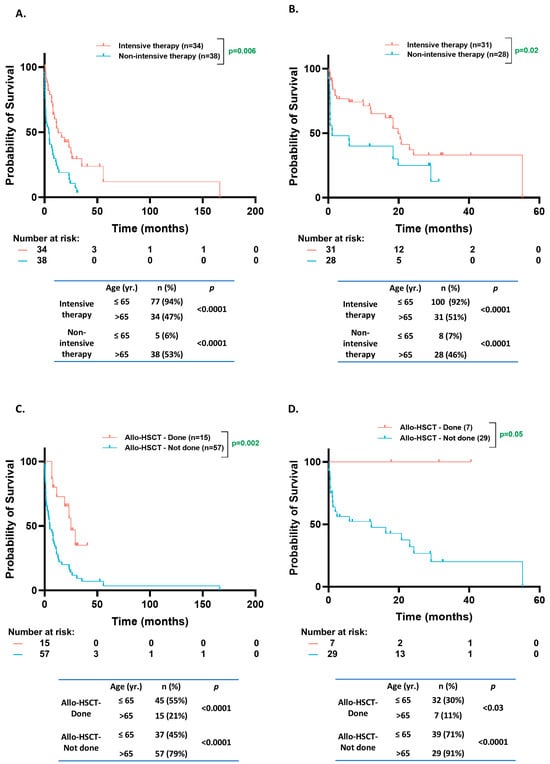
Figure 5.
Overall survival based on treatment intensity and transplant status. (A,B). Kaplan–Meier estimates of OS according to treatment intensity of induction therapy for NPM1MT patients from Karmanos Cancer Institute (KCI)/Cleveland Clinic Foundation (CCF) cohorts aged > 65 (A) and NPM1MT patients from BEAT-AML and cBioPortal cohorts age > 65 (B). (C,D). Kaplan–Meier estimates of OS according to allogeneic stem cell transplant status for NPM1MT patients from KCI/CCF age > 65 (C) and NPM1MT patients from BEAT-AML cBioPortal cohorts age > 65 (D).
4. Discussion
The survival benefit of NPM1MT is age-dependent and lost in the older population. In a combined analysis of 743 patients with CN-AML treated in SWOG and MCR/NCRI trials, Ostronoff et al. demonstrated that NPM1MT, FLT3-ITDNEG, and CN-AML patients in the 55–65-year age group had a better two-year OS as compared to those without this genotype (65% vs. 40%, p < 0.001). On the contrary, patients in the >65-year age group had no significant improvement in 2-year OS (36% vs. 26%, p = 0.062), which was comparable to the findings in our largest comprehensive analysis of patients primarily treated outside of clinical trials [10,17].
FLT3 is a strong predictor in determining the clinical course in AML, even in NPM1-mutated patients [17]. Besides FLT3, the difference in the molecular profile of the younger and older patients could partly explain the age-stratified impact on the prognosis. SRSF2 and ASXL1 mutations more frequently occur in the elderly, are associated with AML with MDS-related changes, and are recognized as adverse prognostic factors in the ELN 2022 risk classification [3,19]. On the other hand, the prognostic impact of TET2MT is controversial [3,20,21]. NRAS and WT1 mutations were more commonly seen in the ≤65 age group. Multiple studies show that NRAS mutations have no influence on prognosis [22,23]. Albeit WT1 mutations seem to be associated with a poor prognostic impact, it is still unaccounted for in the current ELN classification [24,25,26]. More recent evidence suggests conflicting roles of secondary-type/MDS-related mutations on NPM1MT AML where studies from Chan et al. and Zhao et al. suggest poor outcomes and Eckardt et al. suggest no impact on outcomes [18,27,28]. To ascertain the precise clinical relevance of secondary-type mutations in NPM1MT AML and validate the impact on OS, larger registry studies are needed. Recent work by Othman et al. identified a prognostically significant archetypal co-mutation triplet in NPM1MT AML characterized by FLT3-ITD, DNMT3AMT, and WT1MT mutations [18]. While their study reported poorer outcomes (in terms of higher MRD and shorter OS) when NPM1 co-occurred with any two mutations from this triplet, our analysis revealed that the negative impact was skewed towards the older compared to the younger patients. Notably, the younger NPM1MT patients had a significantly improved OS when co-mutated with DNMT3A or WT1. This age-specific divergence in WT1’s prognostic significance is particularly noteworthy, as Othman’s cohort (derived from AML17 and AML19 trials) predominantly included patients under 60 years. Our findings provide external validation and important context for understanding the prognostic value of this co-mutational triplet in an older population.
The distinction between dominant and secondary mutant clones can have clinical implications for managing AML. Despite the inherent limitations in determining clonal hierarchy with VAF, it can still provide helpful information about the clonal architecture, prognosis, and response to treatment [29,30,31,32]. If clonal dominance alone sufficed a substantial prognostic impact, it would convey an unbiased effect on prognosis. However, we observed a numerically better OS with a dominant NPM1MT, and this potentially favorable impact was only limited to patients aged ≤ 65. In two studies of NPM1MT AML patients, NPM1 allelic burden in isolation did not correlate with prognosis, but clonal hierarchy was not described [33,34].
Intensive chemotherapy regimens are associated with higher rates of complete remission and more prolonged overall survival, particularly in younger AML patients or those with favorable risk factors [35,36]. However, other studies have found no significant difference in outcomes between intensive and less intensive treatment approaches, particularly in older patients or those with unfavorable risk factors [36]. Considering the varied treatment approaches for different age groups in NPM1MT patients, we studied the impact of induction therapy and allogenic transplant at second remission on OS. The treatment differences between the age groups might have contributed to a lack of survival benefit of NPM1MT in the elderly in our cohort. Ostronoff et al. described a lower CR rate and a higher one-year relapse rate but no difference in treatment-related mortality in the >65 vs. 55–65-year group, supporting a more intensive choice of therapy for the selected elderly patients [10]. In a UK NCRI study predominantly enrolling patients aged > 60 y, the CR rate in NPM1MT/FLT3-ITDNEG was better than NPM1WT/FLT3-ITDNEG patients with induction therapy, whether intensive (70% vs. 54%) or non-intensive (37% vs. 17%). However, the higher CR rate observed in the NPM1MT/FLT3-ITDNEG patients with intensive and non-intensive induction therapy did not translate into a survival benefit [37]. A smaller study of patients older than 60 demonstrated similar findings, with the NPM1MT patients receiving intensive induction therapy having a significantly higher CR rate than NPM1WT (80.0% vs. 40.5% p = 0.03). At the same time, the OS was not statistically different [38]. One common limitation of these studies is the lack of information on subsequent allo-HSCT. For instance, Jentzsch et al. described a low relapse rate and favorable outcomes of older NPM1MT/FLT3-ITDNEG AML patients consolidated with allogeneic stem cell transplant in CR1 [39].
While ECOG performance status does not provide a comprehensive assessment of a patient’s clinical fitness and comorbidities, a higher proportion of elderly patients in our data received non-intensive therapy, which cannot be explained by ECOG score alone [40]. Indeed, 53% received non-intensive treatment, but only 30% had an ECOG of ≥2. Further research is required to explore whether the choice of therapy in the older age group is guided by comorbidities or primarily determined by age-based stratification based on institutional practices and whether a careful selection of older patients for intensive chemotherapy may improve outcomes in this otherwise poorly performing population [41]. AML poses a significant treatment challenge, especially in the elderly. However, the guidelines recommend intensive antileukemic therapy in patients who can tolerate it [42,43]. Newer treatment options in AML, such as venetoclax in combination with hypomethylating agents (HMA), have shown improved outcomes in older NPM1MT AML patients [44,45]. Menin interaction inhibitors such as revumenib and ziftomenib have been used in relapsed/refractory settings in NPM1MT- and KMT2A-rearranged AML, with complete or complete remission with a partial hematologic recovery rate (CR/CRh) of around 30% [46,47,48]. Whether these agents can bode more favorable outcomes in older patients with NPM1MT AML remains to be seen. Menin interaction inhibitors in combination with other frontline agents currently being explored in clinical trials (NCT05735184, NCT04067336, NCT05848687, and NCT04065399) may shed more light on the outcomes [46,47,48].
We acknowledge the inherent selection bias related to any retrospective study. This notwithstanding, our study population broadly represents patients treated at different cancer centers and a snapshot of a “real-life” scenario. Comprehensive data on cytogenetic abnormalities for more granular classification into cytogenetic risk categories. Despite the attrition in our sample size due to missing molecular information, our analysis still provides a substantial age-stratified clinico-genomic report in NPM1MT AML patients. Our analysis also emphasizes the need for better tools to assess patient “fitness”, providing a more meaningful objective translation of the subjective assessment to determine treatment decisions.
5. Conclusions
The favorable prognostic impact of NPM1MT is lost in older people (>65 years) regardless of the cytogenetic background, which can be partially explained by differences in molecular profile and co-mutational patterns. Treatment selection significantly influences outcomes, and careful consideration of the clonal hierarchy of NPM1 mutations may provide valuable insights when additional genomic alterations are present. Our findings suggest that age-adapted therapeutic approaches are crucial for the aging population. Improved risk stratification and patient selection for intensive chemotherapy versus allogeneic hematopoietic stem cell transplantation, optimized timing and dosing of venetoclax-based regimens, and the integration of emerging targeted therapies directed at NPM1 or cooperating mutations may collectively enhance outcomes in elderly NPM1-mutated AML patients. Future prospective studies incorporating comprehensive age-stratified genomic profiling and minimal residual disease monitoring will be essential to develop personalized treatment algorithms for this biologically distinct subgroup of AML.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers17061020/s1, Figure S1: Patient selection; Figure S2: Co-existing mutations in NPM1MT patients aged ≤ 65 and >65; Figure S3: DNMT3A, WT1, and FLT3-ITD co-mutations in NPM1MT patients aged ≤ 65 and >65; Supplemental Methods.
Author Contributions
Conceptualization, V.D., A.M.K. and S.K.B.; methodology, V.D., A.M.K. and S.K.B.; software, G.D. and D.C.; validation, V.D., A.M.K. and S.K.B.; formal analysis, V.D., A.M.K., J.J.M.A. and S.N.R.; investigation, V.D., A.M.K., M.M.A., T.K., W.B. and C.G.; resources, S.K.B.; data curation, V.D., A.M.K., J.J.M.A., J.B., J.Y. and S.N.R.; writing—original draft preparation, V.D. and A.M.K.; writing—review and editing, V.D., A.M.K., C.G., V.V., J.M. and S.K.B.; visualization, project administration, S.K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the NIH Cancer Center Support Grant 5P30CA022453-43.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the retrospective nature of published data.
Informed Consent Statement
Due to the retrospective analysis of publicly available data, patient consent was waived.
Data Availability Statement
The data that supports the findings of this study are available upon reasonable request.
Conflicts of Interest
Authors declare no conflict of interest relevant to this article.
Abbreviations
The following abbreviations are used in this manuscript:
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Suzuki, T.; Kiyoi, H.; Ozeki, K.; Tomita, A.; Yamaji, S.; Suzuki, R.; Kodera, Y.; Miyawaki, S.; Asou, N.; Kuriyama, K. Clinical characteristics and prognostic implications of NPM1 mutations in acute myeloid leukemia. Blood 2005, 106, 2854–2861. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood J. Am. Soc. Hematol. 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood J. Am. Soc. Hematol. 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.; Chan, S.; Minden, M.; Murphy, T.; Shlush, L.; Schimmer, A. Biological and clinical consequences of NPM1 mutations in AML. Leukemia 2017, 31, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Kayser, S.; Levis, M.J. The clinical impact of the molecular landscape of acute myeloid leukemia. Haematologica 2023, 108, 308. [Google Scholar] [CrossRef]
- Döhner, K.; Schlenk, R.F.; Habdank, M.; Scholl, C.; Rücker, F.G.; Corbacioglu, A.; Bullinger, L.; Fröhling, S.; Döhner, H.; Group, A.S. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: Interaction with other gene mutations. Blood 2005, 106, 3740–3746. [Google Scholar] [CrossRef]
- Juliusson, G.; Jädersten, M.; Deneberg, S.; Lehmann, S.; Möllgård, L.; Wennström, L.; Antunovic, P.; Cammenga, J.; Lorenz, F.; Ölander, E. The prognostic impact of FLT3-ITD and NPM1 mutation in adult AML is age-dependent in the population-based setting. Blood Adv. 2020, 4, 1094–1101. [Google Scholar] [CrossRef]
- Falini, B.; Mecucci, C.; Tiacci, E.; Alcalay, M.; Rosati, R.; Pasqualucci, L.; La Starza, R.; Diverio, D.; Colombo, E.; Santucci, A. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 2005, 352, 254–266. [Google Scholar] [CrossRef]
- Becker, H.; Marcucci, G.; Maharry, K.; Margeson, D.; Radmacher, M.; Whitman, S.; Mrózek, K.; Baer, M.; Larson, R.; Bloomfield, C. NPM1 mutations as an independent prognosticator for older cytogenetically normal acute myeloid leukemia (CN AML). J. Clin. Oncol. 2009, 27, 7000. [Google Scholar] [CrossRef]
- Ostronoff, F.; Othus, M.; Lazenby, M.; Estey, E.; Appelbaum, F.R.; Evans, A.; Godwin, J.; Gilkes, A.; Kopecky, K.J.; Burnett, A. Prognostic significance of NPM1 mutations in the absence of FLT3–internal tandem duplication in older patients with acute myeloid leukemia: A SWOG and UK National Cancer Research Institute/Medical Research Council Report. J. Clin. Oncol. 2015, 33, 1157–1164. [Google Scholar] [CrossRef]
- Falini, B.; Brunetti, L.; Sportoletti, P.; Martelli, M.P. NPM1-mutated acute myeloid leukemia: From bench to bedside. Blood J. Am. Soc. Hematol. 2020, 136, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Awada, H.; Durmaz, A.; Gurnari, C.; Kishtagari, A.; Meggendorfer, M.; Kerr, C.M.; Kuzmanovic, T.; Durrani, J.; Shreve, J.; Nagata, Y. Machine learning integrates genomic signatures for subclassification beyond primary and secondary acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2021, 138, 1885–1895. [Google Scholar] [CrossRef]
- Nagata, Y.; Makishima, H.; Kerr, C.M.; Przychodzen, B.P.; Aly, M.; Goyal, A.; Awada, H.; Asad, M.F.; Kuzmanovic, T.; Suzuki, H. Invariant patterns of clonal succession determine specific clinical features of myelodysplastic syndromes. Nat. Commun. 2019, 10, 5386. [Google Scholar] [CrossRef] [PubMed]
- Tyner, J.W.; Tognon, C.E.; Bottomly, D.; Wilmot, B.; Kurtz, S.E.; Savage, S.L.; Long, N.; Schultz, A.R.; Traer, E.; Abel, M. Functional genomic landscape of acute myeloid leukaemia. Nature 2018, 562, 526–531. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: A joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.L.; Schumacher, J.A.; Frizzell, K.; Sorrells, S.; Shen, W.; Clayton, A.; Jattani, R.; Kelley, T.W. Coexisting and cooperating mutations in NPM1-mutated acute myeloid leukemia. Leuk. Res. 2017, 56, 7–12. [Google Scholar] [CrossRef]
- Arber, D.A.; Erba, H.P. Diagnosis and treatment of patients with acute myeloid leukemia with myelodysplasia-related changes (AML-MRC). Am. J. Clin. Pathol. 2020, 154, 731–741. [Google Scholar] [CrossRef]
- Othman, J.; Potter, N.; Ivey, A.; Tazi, Y.; Papaemmanuil, E.; Jovanovic, J.; Freeman, S.D.; Gilkes, A.; Gale, R.; Rapoz-D’Silva, T. Molecular, clinical, and therapeutic determinants of outcome in NPM1-mutated AML. Blood 2024, 144, 714–728. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-C.; Chou, S.-C.; Liu, C.-Y.; Chen, C.-Y.; Hou, H.-A.; Kuo, Y.-Y.; Lee, M.-C.; Ko, B.-S.; Tang, J.-L.; Yao, M. TET2 mutation is an unfavorable prognostic factor in acute myeloid leukemia patients with intermediate-risk cytogenetics. Blood J. Am. Soc. Hematol. 2011, 118, 3803–3810. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Cassady, K.; Zou, Z.; Zhang, X. TET2 function in hematopoietic malignancies, immune regulation, and DNA repair. Front. Oncol. 2019, 9, 210. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Z.; Li, T.; Li, Y.; Wang, W.; Hao, Q.; Xie, X.; Wan, D.; Jiang, Z.; Wang, C. Mutational spectrum and prognosis in NRAS-mutated acute myeloid leukemia. Sci. Rep. 2020, 10, 12152. [Google Scholar] [CrossRef]
- Bacher, U.; Haferlach, T.; Schoch, C.; Kern, W.; Schnittger, S. Implications of NRAS mutations in AML: A study of 2502 patients. Blood 2006, 107, 3847–3853. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.-A.; Huang, T.-C.; Lin, L.-I.; Liu, C.-Y.; Chen, C.-Y.; Chou, W.-C.; Tang, J.-L.; Tseng, M.-H.; Huang, C.-F.; Chiang, Y.-C. WT1 mutation in 470 adult patients with acute myeloid leukemia: Stability during disease evolution and implication of its incorporation into a survival scoring system. Blood J. Am. Soc. Hematol. 2010, 115, 5222–5231. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Mengge, G.; Wang, K.; Wang, Y.; Kong, J.; Sun, Y.; Zhao, X.; Huang, X.J. Prognostic impact of WT1 mutation on AML of different risk groups based on 2022 European Leukemianet (ELN) risk classification. Blood 2022, 140, 3216–3217. [Google Scholar] [CrossRef]
- Awada, H.; Durmaz, A.; Gurnari, C.; Kishtagari, A.; Zawit, M.; Pagliuca, S.; Visconte, V. Friend or foe? The case of Wilms’ Tumor 1 (WT1) mutations in acute myeloid leukemia. Blood Cells Mol. Dis. 2021, 88, 102549. [Google Scholar] [CrossRef]
- Chan, O.; Al Ali, N.; Tashkandi, H.; Ellis, A.; Ball, S.; Zhang, L.; Hussaini, M.; Song, J.; Yun, S.; Talati, C. Mutations highly specific for secondary AML are associated with poor outcomes in patients with NPM1-mutated ELN favorable risk AML. Blood 2021, 138, 686. [Google Scholar] [CrossRef]
- Zhao, D.; Zarif, M.; Eladl, E.; Capo-Chichi, J.-M.; Smith, A.C.; Atenafu, E.G.; Tierens, A.; Minden, M.D.; Schuh, A.; Chang, H. NPM1-mutated AML-MRC diagnosed on the basis of history of MDS or MDS/MPN frequently harbours secondary-type mutations and confers inferior outcome compared to AML with mutated NPM1. Leuk. Res. 2022, 118, 106869. [Google Scholar] [CrossRef]
- Eckardt, J.-N.; Bill, M.; Rausch, C.; Metzeler, K.; Spiekermann, K.; Stasik, S.; Sauer, T.; Scholl, S.; Hochhaus, A.; Crysandt, M. Secondary-type mutations do not impact outcome in NPM1-mutated acute myeloid leukemia–implications for the European LeukemiaNet risk classification. Leukemia 2023, 37, 2282–2285. [Google Scholar] [CrossRef]
- Lachowiez, C.; DiNardo, C.D.; Morita, K.; Furudate, K.; Wang, F.; Tanaka, T.; Wang, S.A.; Kadia, T.M.; Daver, N.; Short, N.J. Longitudinal next generation sequencing reveals the clonal hierarchy of IDH mutated clones and impact on survival in NPM1 mutated AML. Blood 2021, 138, 607. [Google Scholar] [CrossRef]
- Benard, B.; Leak, L.; Azizi, A.; Thomas, D.; Gentles, A.; Majeti, R. Clonal Architecture and Variant Allele Frequency Predict Clinical Outcomes and Drug Response in Acute Myeloid Leukemia. Blood 2020, 136, 2. [Google Scholar] [CrossRef]
- Benard, B.A.; Leak, L.B.; Azizi, A.; Thomas, D.; Gentles, A.J.; Majeti, R. Clonal architecture predicts clinical outcomes and drug sensitivity in acute myeloid leukemia. Nat. Commun. 2021, 12, 7244. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, L.; Shen, C.; Zhu, S.; Lang, W.; Luo, Y.; Zhang, H.; Yang, W.; Han, Y.; Ma, L. Impact of mutational variant allele frequency on prognosis in myelodysplastic syndromes. Am. J. Cancer Res. 2020, 10, 4476. [Google Scholar] [PubMed]
- Abbas, H.A.; Ravandi, F.; Loghavi, S.; Patel, K.P.; Borthakur, G.; Kadia, T.M.; Jabbour, E.; Takahashi, K.; Cortes, J.; Issa, G.C. NPM1 mutant variant allele frequency correlates with leukemia burden but does not provide prognostic information in NPM1-mutated AML. Am. J. Hematol. 2019, 94, E158. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg-Thurley, M.; Herold, T.; Görlich, D.; Sauerland, C.; Janke, H.; Prassek, V.V.; Konstandin, N.P.; Dufour, A.M.; Schneider, S.; Ksienzyk, B. NPM1 variant allele frequency and outcomes in AML. Blood 2018, 132, 1486. [Google Scholar] [CrossRef]
- Sorror, M.L.; Storer, B.E.; Fathi, A.T.; Brunner, A.; Gerds, A.T.; Sekeres, M.A.; Mukherjee, S.; Medeiros, B.C.; Wang, E.S.; Vachhani, P. Multisite 11-year experience of less-intensive vs intensive therapies in acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2021, 138, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Guyatt, G.H.; Teich, T.; Dawdy, J.L.; Shahid, S.; Altman, J.K.; Stone, R.M.; Sekeres, M.A.; Mukherjee, S.; LeBlanc, T.W. Intensive versus less-intensive antileukemic therapy in older adults with acute myeloid leukemia: A systematic review. PLoS ONE 2021, 16, e0249087. [Google Scholar] [CrossRef]
- Lazenby, M.; Gilkes, A.; Marrin, C.; Evans, A.; Hills, R.K.; Burnett, A. The prognostic relevance of flt3 and npm1 mutations on older patients treated intensively or non-intensively: A study of 1312 patients in the UK NCRI AML16 trial. Leukemia 2014, 28, 1953–1959. [Google Scholar] [CrossRef]
- Scholl, S.; Theuer, C.; Scheble, V.; Kunert, C.; Heller, A.; Mügge, L.O.; Fricke, H.J.; Höffken, K.; Wedding, U. Clinical impact of nucleophosmin mutations and Flt3 internal tandem duplications in patients older than 60 yr with acute myeloid leukaemia. Eur. J. Haematol. 2008, 80, 208–215. [Google Scholar] [CrossRef]
- Jentzsch, M.; Grimm, J.; Bill, M.; Goldmann, K.; Schulz, J.; Niederwieser, D.; Platzbecker, U.; Schwind, S. Outcomes of older patients with NPM1 mutated and FLT3-ITD negative acute myeloid leukemia receiving allogeneic transplantation. HemaSphere 2020, 4, e326. [Google Scholar] [CrossRef]
- Simcock, R.; Wright, J. Beyond performance status. Clin. Oncol. 2020, 32, 553–561. [Google Scholar] [CrossRef]
- Weisdorf, D. How old is too old for a transplant? Best Pract. Res. Clin. Haematol. 2021, 34, 101243. [Google Scholar] [CrossRef] [PubMed]
- Sekeres, M.A.; Guyatt, G.; Abel, G.; Alibhai, S.; Altman, J.K.; Buckstein, R.; Choe, H.; Desai, P.; Erba, H.; Hourigan, C.S. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020, 4, 3528–3549. [Google Scholar] [CrossRef]
- Wang, E.S. Treating acute myeloid leukemia in older adults. Hematol. Am. Soc. Hematol. Educ. Program 2014, 2014, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lachowiez, C.A.; Loghavi, S.; Kadia, T.M.; Daver, N.; Borthakur, G.; Pemmaraju, N.; Naqvi, K.; Alvarado, Y.; Yilmaz, M.; Short, N. Outcomes of older patients with NPM1-mutated AML: Current treatments and the promise of venetoclax-based regimens. Blood Adv. 2020, 4, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Brunetti, L.; Martelli, M.P. How I diagnose and treat NPM1-mutated AML. Blood J. Am. Soc. Hematol. 2021, 137, 589–599. [Google Scholar]
- Wang, R.; Xu, P.; Chang, L.-L.; Zhang, S.-Z.; Zhu, H.-H. Targeted therapy in NPM1-mutated AML: Knowns and unknowns. Front. Oncol. 2022, 12, 972606. [Google Scholar] [CrossRef]
- Issa, G.C.; Aldoss, I.; DiPersio, J.; Cuglievan, B.; Stone, R.; Arellano, M.; Thirman, M.J.; Patel, M.R.; Dickens, D.S.; Shenoy, S. The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia. Nature 2023, 615, 920–924. [Google Scholar] [CrossRef]
- Erba, H.P.; Fathi, A.T.; Issa, G.C.; Altman, J.K.; Montesinos, P.; Patnaik, M.M.; Foran, J.M.; De Botton, S.; Baer, M.R.; Schiller, G.J. Update on a phase 1/2 first-in-human study of the menin-KMT2A (MLL) inhibitor ziftomenib (KO-539) in patients with relapsed or refractory acute myeloid leukemia. Blood 2022, 140, 153–156. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).