Frozen Section Analysis in Submandibular Gland Tumors: Optimizing Intraoperative Decision-Making
Simple Summary
Abstract
1. Introduction
2. Material and Methods
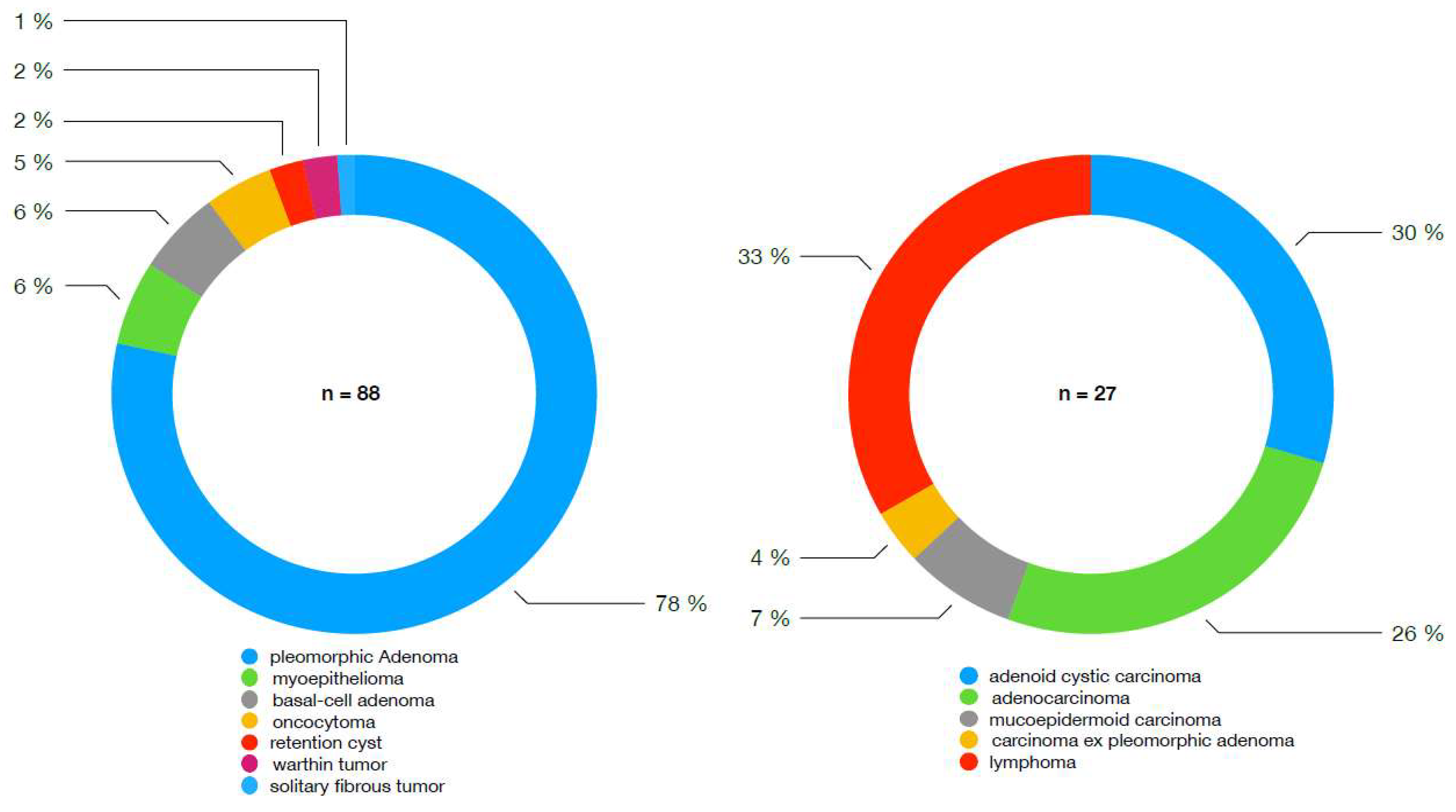
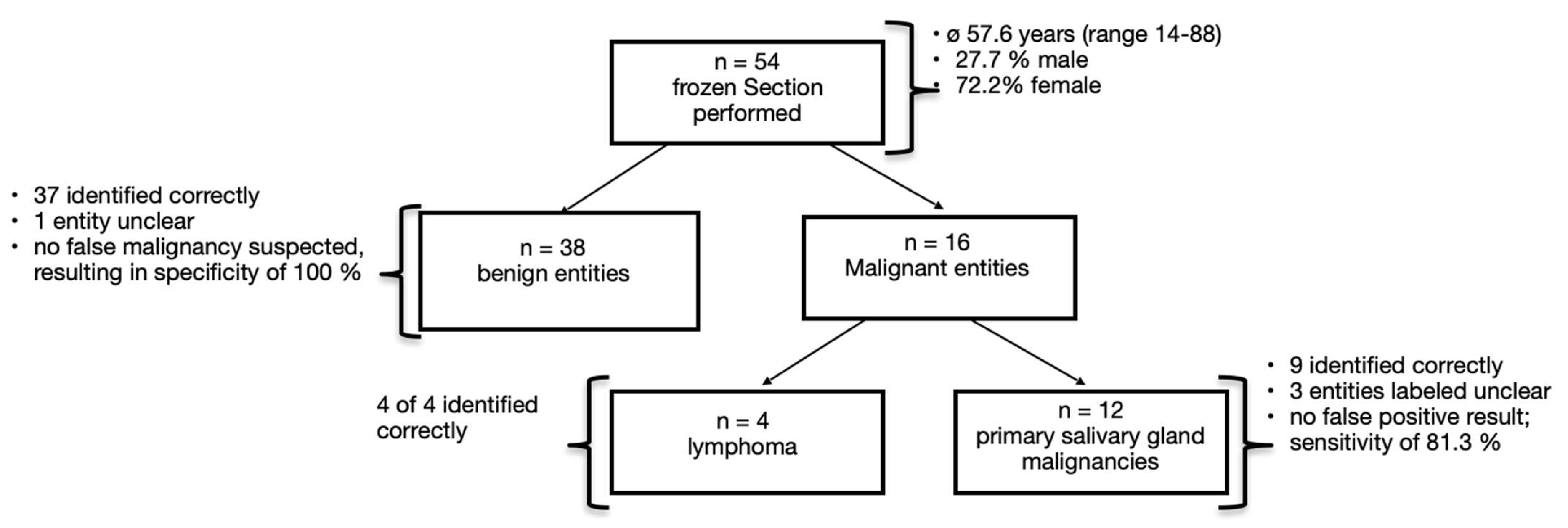
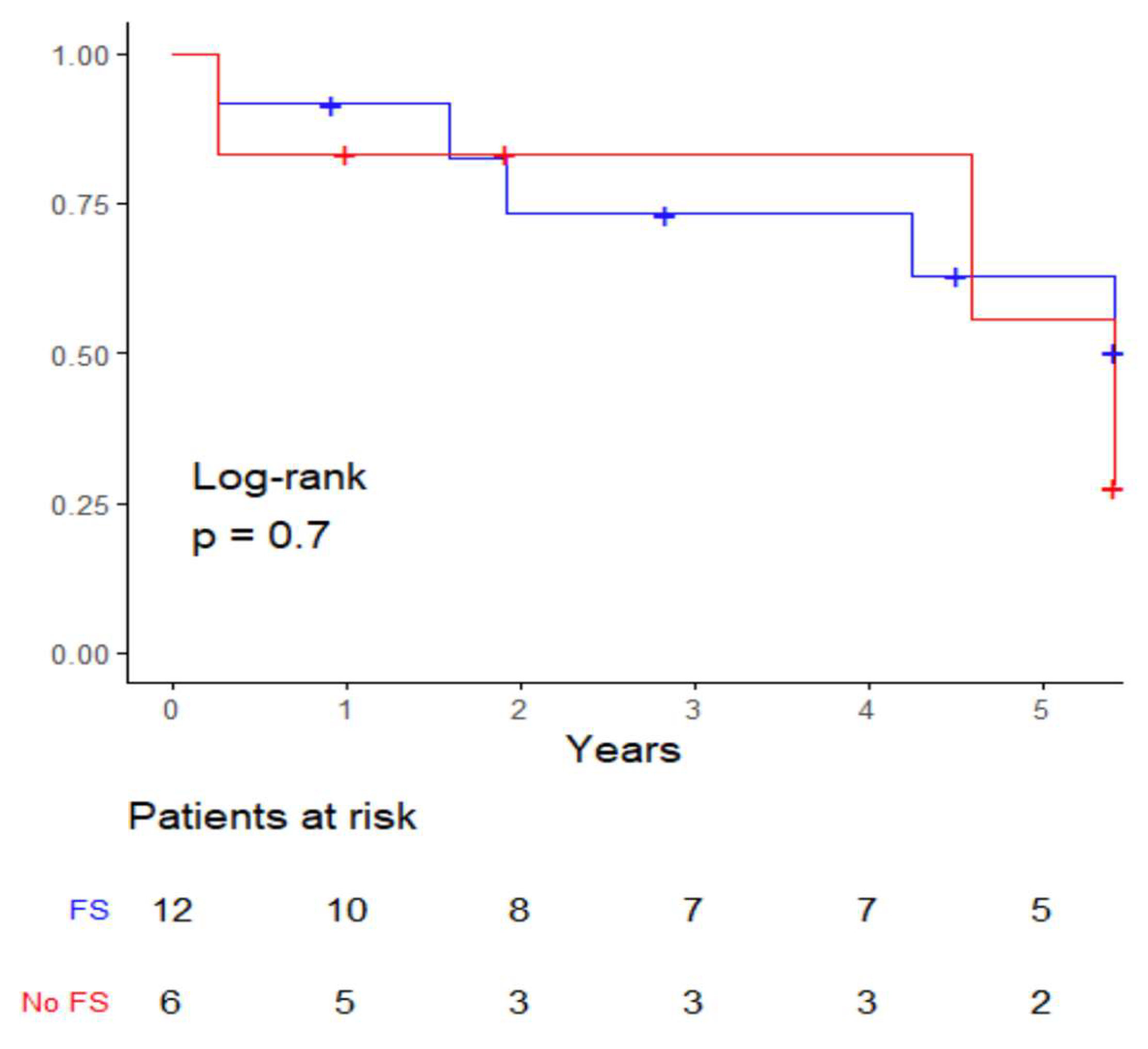
3. Results
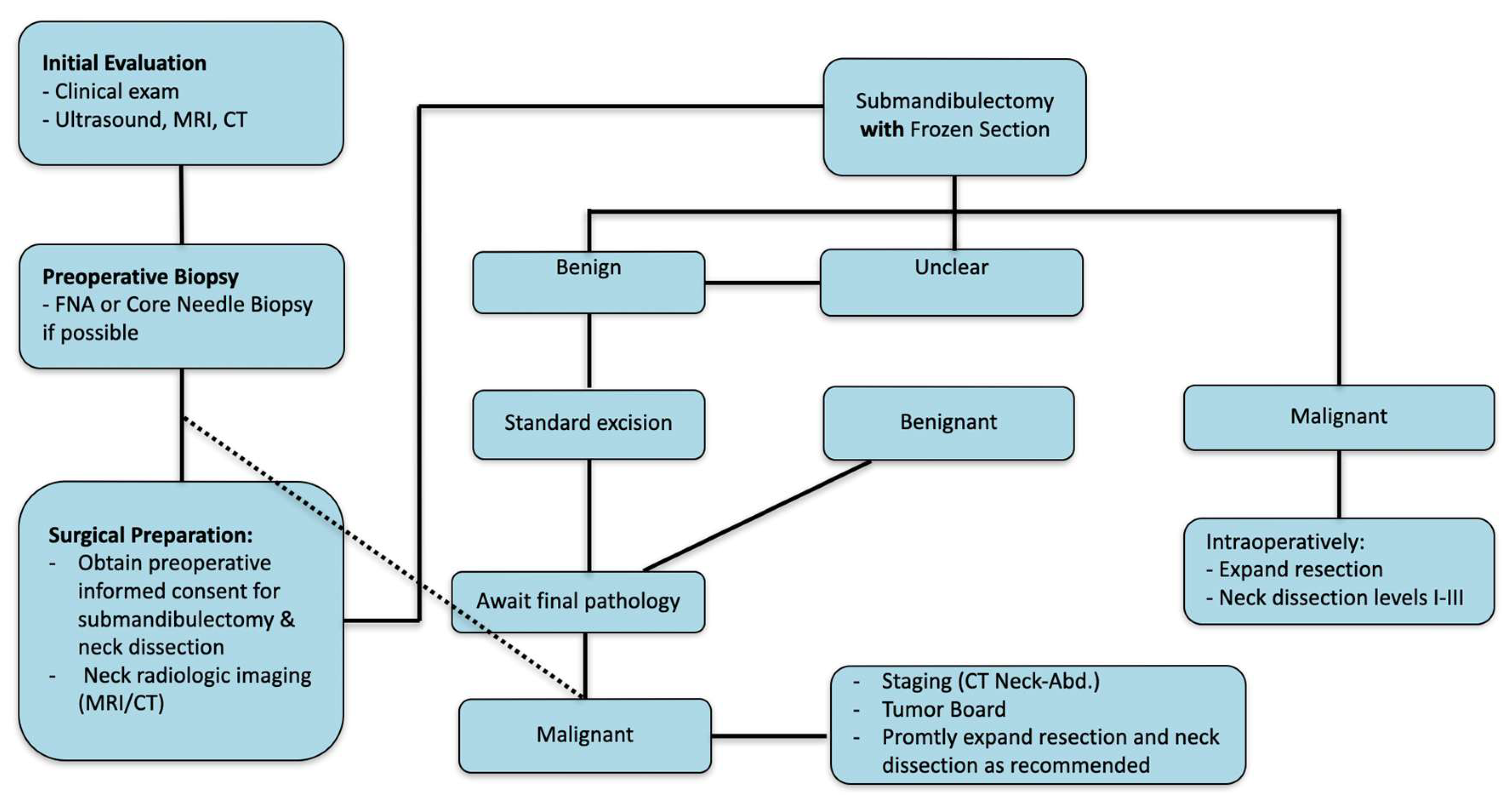
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skálová, A.; Hyrcza, M.D.; Leivo, I. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Salivary Glands. Head Neck Pathol. 2022, 16, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, M.; Locati, L.D.; Prott, F.J.; Gatta, G.; McGurk, M.; Licitra, L. Major and minor salivary gland tumors. Crit. Rev. Oncol. Hematol. 2010, 74, 134–148. [Google Scholar] [CrossRef]
- Hofauer, B.; Chaker, A.; Strenger, T.; Bas, M.; Mansour, N.; Knopf, A. Swelling of the submandibular and parotid glands: A†description of possible differential diagnoses. Hno 2016, 64, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Eveson, J.W. Salivary tumours. Periodontol. 2000 2011, 57, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Tan, A.P.; Tong, E.L.; Satyadev, N.; Christensen, R.E. Epidemiology, Prognostic Factors, and Treatment of Malignant Submandibular Gland Tumors: A Population-Based Cohort Analysis. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Waldron, C.A.; el-Mofty, S.K.; Gnepp, D.R. Tumors of the intraoral minor salivary glands: A demographic and histologic study of 426 cases. Oral. Surg. Oral. Med. Oral. Pathol. 1988, 66, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Spiro, R.H. Salivary neoplasms: Overview of a 35-year experience with 2807 patients. Head Neck Surg. 1986, 8, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Riemann, S.; Knopf, A. Frozen section biopsy for tumors of the submandibular triangle. Hno 2023, 71, 164–169. [Google Scholar] [CrossRef]
- Schmidt, R.L.; Hunt, J.P.; Hall, B.J.; Wilson, A.R.; Layfield, L.J. A systematic review and meta-analysis of the diagnostic accuracy of frozen section for parotid gland lesions. Am. J. Clin. Pathol. 2011, 136, 729–738. [Google Scholar] [CrossRef]
- Atula, T.; Panigrahi, J.; Tarkkanen, J.; Mäkitie, A.; Aro, K. Preoperative evaluation and surgical planning of submandibular gland tumors. Head Neck 2017, 39, 1071–1077. [Google Scholar] [CrossRef]
- Olsen, K.D.; Moore, E.J.; Lewis, J.E. Frozen section pathology for decision making in parotid surgery. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Györi, E.; Przestrzelski, C.; Pona, I.; Hagmann, M.; Rath, T.; Radtke, C.; Tzou, C.H.J. Quality of life and functional assessment of facial palsy patients: A questionnaire study. Int. J. Surg. 2018, 55, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Bruins, T.E.; van Veen, M.M.; Kleiss, I.J.; Broekstra, D.C.; Dijkstra, P.U.; Ingels, K.; Werker, P. Interpreting Quality-of-Life Questionnaires in Patients with Long-Standing Facial Palsy. Facial Plast. Surg. Aesthet. Med. 2022, 24, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Jethwa, A.R.; Khariwala, S.S.; Johnson, J.; Shin, J.J. Sensitivity, Specificity, and Posttest Probability of Parotid Fine-Needle Aspiration: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2016, 154, 9–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmidt, R.L.; Hall, B.J.; Layfield, L.J. A systematic review and meta-analysis of the diagnostic accuracy of ultrasound-guided core needle biopsy for salivary gland lesions. Am. J. Clin. Pathol. 2011, 136, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Novoa, E.; Gürtler, N.; Arnoux, A.; Kraft, M. Role of ultrasound-guided core-needle biopsy in the assessment of head and neck lesions: A meta-analysis and systematic review of the literature. Head Neck 2012, 34, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, J.; Ridder, G.J. Diagnostic value of ultrasound-guided core needle biopsy in patients with salivary gland masses. Int. J. Oral. Maxillofac. Surg. 2012, 41, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Wu, C.T.; Lin, G.; Chuang, W.Y.; Yeow, K.M.; Wan, Y.L. Comparison of ultrasonographically guided fine-needle aspiration and core needle biopsy in the diagnosis of parotid masses. J. Clin. Ultrasound 2012, 40, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Novoa, E.; Gürtler, N.; Arnoux, A.; Kraft, M. Diagnostic value of core needle biopsy and fine-needle aspiration in salivary gland lesions. Head Neck 2016, 38 (Suppl. S1), E346–E352. [Google Scholar] [CrossRef] [PubMed]
- Krarup Sigaard, R.; Wennervaldt, K.; Munksgaard, L.; Rahbek Gjerdrum, L.M.; Homøe, P. Core needle biopsy is an inferior tool for diagnosing cervical lymphoma compared to lymph node excision. Acta Oncol. 2021, 60, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Douville, N.J.; Bradford, C.R. Comparison of ultrasound-guided core biopsy versus fine-needle aspiration biopsy in the evaluation of salivary gland lesions. Head Neck 2013, 35, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Song, I.H.; Song, J.S.; Sung, C.O.; Roh, J.L.; Choi, S.H.; Nam, S.Y.; Kim, S.Y.; Lee, J.H.; Baek, J.H.; Cho, K. Accuracy of Core Needle Biopsy Versus Fine Needle Aspiration Cytology for Diagnosing Salivary Gland Tumors. J. Pathol. Transl. Med. 2015, 49, 136–143. [Google Scholar] [CrossRef] [PubMed]
- van Herpen, C.; Vander Poorten, V.; Skalova, A.; Terhaard, C.; Maroldi, R.; van Engen, A.; Baujat, B.; Locati, L.D.; Jensen, A.D.; Smeele, L.; et al. Salivary gland cancer: ESMO-European Reference Network on Rare Adult Solid Cancers (EURACAN) Clinical Practice Guideline for diagnosis, treatment and follow-up. ESMO Open 2022, 7, 100602. [Google Scholar] [CrossRef] [PubMed]
- Bolooki, A.; Stenzl, A.; Weusthof, C.; Hofauer, B. Indications for Submandibulectomy Within a 20-Year Period. Ear Nose Throat J. 2024. [Google Scholar] [CrossRef]
- Lobo, R.; Hawk, J.; Srinivasan, A. A Review of Salivary Gland Malignancies: Common Histologic Types, Anatomic Considerations, and Imaging Strategies. Neuroimaging Clin. 2018, 28, 171–182. [Google Scholar] [CrossRef]
- Aro, K.; Tarkkanen, J.; Saat, R.; Saarilahti, K.; Mäkitie, A.; Atula, T. Submandibular gland cancer: Specific features and treatment considerations. Head Neck 2018, 40, 154–162. [Google Scholar] [CrossRef]
- Lombardi, D.; McGurk, M.; Vander Poorten, V.; Guzzo, M.; Accorona, R.; Rampinelli, V.; Nicolai, P. Surgical treatment of salivary malignant tumors. Oral. Oncol. 2017, 65, 102–113. [Google Scholar] [CrossRef]
- Nobis, C.P.; Rohleder, N.H.; Wolff, K.D.; Wagenpfeil, S.; Scherer, E.Q.; Kesting, M.R. Head and neck salivary gland carcinomas—Elective neck dissection, yes or no? J. Oral Maxillofac. Surg. 2014, 72, 205–210. [Google Scholar] [CrossRef]
- Vander Poorten, V.L.; Balm, A.J.; Hilgers, F.J.; Tan, I.B.; Loftus-Coll, B.M.; Keus, R.B.; Hart, A.A. Prognostic factors for long term results of the treatment of patients with malignant submandibular gland tumors. Cancer 1999, 85, 2255–2264. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakaegawa, Y.; Kawase, T.; Ikeda, M.; Murono, S. The role of frozen section biopsy for submandibular gland tumors. Acta Otolaryngol. 2021, 141, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, N.; Santini, L.; Lagier, A.; Dessi, P.; Giovanni, A. Fine needle aspiration cytology and frozen section in the diagnosis of malignant parotid tumours. Int. J. Oral. Maxillofac. Surg. 2014, 43, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Alonso, I.; Lopez-Perez, L.; Guirado, J.C.M.; Cabrera-Umpierrez, M.F.; Arredondo, M.T.; Fico, G. Data analytics for predicting quality of life changes in head and neck cancer survivors: A scoping review. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 2262–2265. [Google Scholar]
- Lequertier, V.; Wang, T.; Fondrevelle, J.; Augusto, V.; Duclos, A. Hospital Length of Stay Prediction Methods: A Systematic Review. Med. Care 2021, 59, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Moubayed, S.P.; Sampalis, J.S.; Ayad, T.; Guertin, L.; Bissada, E.; Gologan, O.E.; Soulières, D.; Lambert, L.; Filion, E.; Nguyen-Tan, P.F.; et al. Predicting depression and quality of life among long-term head and neck cancer survivors. Otolaryngol. Head Neck Surg. 2015, 152, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Semple, C.J.; McKenna, G.; Parahoo, R.; Rogers, S.N.; Ehrsson, Y.T. Factors that affect quality of life for older people with head and neck cancer: A systematic review. Eur. J. Oncol. Nurs. 2023, 63, 102280. [Google Scholar] [CrossRef]
- Waljee, J.F.; Dimick, J.B. Do Patient-Reported Outcomes Correlate with Clinical Outcomes Following Surgery? Adv. Surg. 2017, 51, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Church, J. Quality of life and patient-reported outcomes. Br. J. Surg. 2018, 105, 157–158. [Google Scholar] [CrossRef] [PubMed]
- Kikuoka, Y.; Kawata, R.; Higashino, M.; Terada, T.; Haginomori, S.I. Operative technique for benign submandibular gland mass without identifying the mandibular branch of the facial nerve. Auris Nasus Larynx 2018, 45, 1221–1226. [Google Scholar] [CrossRef]
- Pang, J.; Houlton, J.J. Management of Malignant Salivary Gland Conditions. Surg. Clin. N. Am. 2022, 102, 325–333. [Google Scholar] [CrossRef]
- Knopf, A.; Cortolezis, N.; Bas, M.; Mansour, N.; Hofauer, B. Multimodal ultrasonographic algorithm in the differentiation of submandibular masses. Acta Otolaryngol. 2017, 137, 640–645. [Google Scholar] [CrossRef]
- Mantsopoulos, K.; Bessas, Z.; Sievert, M.; Müller, S.K.; Koch, M.; Agaimy, A.; Iro, H. Frozen Section of Parotid Gland Tumours: The Head and Neck Pathologist as a Key Member of the Surgical Team. J. Clin. Med. 2022, 11, 1249. [Google Scholar] [CrossRef]
- Mostaan, L.V.; Yazdani, N.; Madani, S.Z.; Borghei, H.; Mortazavi, S.; Ojani, L.; Mokhtari, Z. Frozen section as a diagnostic test for major salivary gland tumors. Acta Med. Iran. 2012, 50, 459–462. [Google Scholar] [PubMed]
- Cohen, A.N.; Damrose, E.J.; Huang, R.Y.; Nelson, S.D.; Blackwell, K.E.; Calcaterra, T.C. Adenoid cystic carcinoma of the submandibular gland: A 35-year review. Otolaryngol. Head Neck Surg. 2004, 131, 994–1000. [Google Scholar] [CrossRef]
- Nascimento, A.G.; Amaral, A.L.; Prado, L.A.; Kligerman, J.; Silveira, T.R. Adenoid cystic carcinoma of salivary glands. A study of 61 cases with clinicopathologic correlation. Cancer 1986, 57, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Thielker, J.; Wahdan, A.; Buentzel, J.; Kaftan, H.; Boeger, D.; Mueller, A.H.; Wittig, A.; Schultze-Mosgau, S.; Ernst, T.; Guntinas-Lichius, O. Long-Term Facial Nerve Outcome in Primary Parotid Cancer Surgery: A Population-Based Analysis. Laryngoscope 2021, 131, 2694–2700. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, G.R.; Morais-Perdigão, A.L.; Lopez-de-Cáceres, C.V.B.; de Almeida, O.P.; Vargas, P.A.; Roman-Tager, E.M.J.; de Andrade, B.A.B.; Soares, C.D.; de Oliveira Ramos, C.C.; de Andrade, M.M.P.; et al. Lymphomas affecting the submandibular glands. Med. Oral. Patol. Oral. Cir. Bucal 2024, 29, e78–e86. [Google Scholar] [CrossRef]
- Al-Shahrestani, F.; Al-Khafaf, A.E.; Asheer, Z.; Jelicic, J.; Chanchiri, I.; Blocher, C.E.; Sørensen, A.K.A.; Pedersen, L.M.; Gjerdrum, L.M.R.; Heegaard, S.; et al. Lymphomas of the submandibular gland: A nationwide cohort study. Eur. Arch. Oto-Rhino-Laryngol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Herd, M.K.; Woods, M.; Anand, R.; Habib, A.; Brennan, P.A. Lymphoma presenting in the neck: Current concepts in diagnosis. Br. J. Oral. Maxillofac. Surg. 2012, 50, 309–313. [Google Scholar] [CrossRef]
- Neskoromna-Jędrzejczak, A.; Tyndorf, M.; Arkuszewski, P.; Kobos, J. Head and neck lymphomas--diagnostic difficulties. Pol. Przegl Chir. 2012, 84, 113–118. [Google Scholar] [CrossRef]
- Zapater, E.; Bagán, J.V.; Carbonell, F.; Basterra, J. Malignant lymphoma of the head and neck. Oral. Dis. 2010, 16, 119–128. [Google Scholar] [CrossRef]
- Pang, P.; Duan, W.; Liu, S.; Bai, S.; Ma, Y.; Li, R.; Liu, F.; Sun, C. Clinical study of tuberculosis in the head and neck region-11 years’ experience and a review of the literature. Emerg. Microbes Infect. 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McAllister, K.A.; MacGregor, F.B. Diagnosis of tuberculosis in the head and neck. J. Laryngol. Otol. 2011, 125, 603–607. [Google Scholar] [CrossRef] [PubMed]
| Revision Yes | Revision No | ||
|---|---|---|---|
| Frozen section | 3 (27% of patients with FS performed) | 8 (73% of patients with FS performed) | 11 |
| Non-frozen section | 6 | 0 | 6 (100% of patients without FS performed) |
| 9 | 8 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolooki, A.; Johnson, F.; Stenzl, A.; Zhu, Z.; Hofauer, B.G. Frozen Section Analysis in Submandibular Gland Tumors: Optimizing Intraoperative Decision-Making. Cancers 2025, 17, 895. https://doi.org/10.3390/cancers17050895
Bolooki A, Johnson F, Stenzl A, Zhu Z, Hofauer BG. Frozen Section Analysis in Submandibular Gland Tumors: Optimizing Intraoperative Decision-Making. Cancers. 2025; 17(5):895. https://doi.org/10.3390/cancers17050895
Chicago/Turabian StyleBolooki, Amir, Felix Johnson, Anna Stenzl, Zhaojun Zhu, and Benedikt Gabriel Hofauer. 2025. "Frozen Section Analysis in Submandibular Gland Tumors: Optimizing Intraoperative Decision-Making" Cancers 17, no. 5: 895. https://doi.org/10.3390/cancers17050895
APA StyleBolooki, A., Johnson, F., Stenzl, A., Zhu, Z., & Hofauer, B. G. (2025). Frozen Section Analysis in Submandibular Gland Tumors: Optimizing Intraoperative Decision-Making. Cancers, 17(5), 895. https://doi.org/10.3390/cancers17050895






