Simple Summary
Salivary gland cancers are rare but can be aggressive, making early detection and effective treatment crucial. Understanding the genetic and molecular alterations opens the door for targeted treatments that can be more effective than traditional therapies. The review discusses the molecular landscape of different salivary gland cancer subtypes, including their diagnostic biomarkers and potential therapeutic targets. For example, HER2 and androgen receptor positivity in salivary duct carcinoma and the ETV6-NTRK3 fusion in secretory carcinoma are important for treatment planning. New methods, like liquid biopsies, offer a minimally invasive approach to monitor tumor dynamics and assess treatment response. These biomarkers can aid in early diagnosis, prognosis, and differentiation between malignant and benign tumors. However, the rarity and heterogeneity of these cancers pose challenges in treatment and require specific molecularly stratified clinical trials to improve patient outcomes.
Abstract
Background: Salivary gland cancers (SGCs) are a rare and heterogeneous group of malignancies, accounting for approximately 5% of head and neck cancers. Despite their rarity, advances in molecular profiling have revealed a variety of genetic and molecular pathways, many of which are potentially actionable with targeted therapies. Methods: We reviewed the current literature involving the molecular landscape of SGCs, encompassing the diagnostic and prognostic value of tissue and liquid biomarkers and the potential therapeutic targets across various histological subtypes. Results: Our review highlights key molecular diagnostic findings such as the CRTC1-MAML2 fusion in mucoepidermoid carcinoma and MYB-NFIB rearrangements in adenoid cystic carcinoma, but also targetable alterations such as HER2 and AR positivity in salivary duct carcinoma and ETV6-NTRK3 fusion in secretory carcinoma. Liquid biopsy (both blood- or salivary-based), including circulating tumor DNA, circulating tumor cells, and miRNAs, offers novel, noninvasive approaches for disease monitoring and personalized treatment. Emerging therapies such as HER2 inhibitors, androgen deprivation therapy, and TRK inhibitors underscore the shift towards precision oncology in managing these malignancies. Conclusions: Despite promising advances, challenges remain due to the rarity and phenotypic heterogeneity of SGCs, emphasizing the need for molecularly stratified clinical trials. This review presents an overview of tissue and liquid biomarkers, focusing on molecular targets and therapeutic innovations that lay the foundation for improved diagnostic and treatment strategies for SGCs.
1. Introduction
Salivary gland cancers (SGCs) are a rare and complex group of neoplasms, representing approximately 5% of head and neck cancers (HNCs) in Europe. Globally, their annual incidence ranges from 0.57 to 0.69 cases per 100,000 individuals, with a slight male prevalence and a higher rate of diagnosis in the sixth–seventh decades of life [1,2]. The broad histological diversity of these tumors, including over 20 recognized malignant subtypes classified by the World Health Organization (WHO), leads to heterogeneous biological behaviors, prognoses, and therapeutic implications, underscoring the importance of a detailed histopathological and molecular characterization [3].
The integration of molecular diagnostics, including fluorescence in situ hybridization (FISH), reverse transcription–polymerase chain reaction (RT-PCR), and next-generation sequencing (NGS), has significantly implemented an accurate classification of SGCs. These tools are not only helpful for making accurate histological diagnoses but also for identifying actionable mutations, which allow for the use of targeted therapies [4].
The histologic grade is a strong predictor of outcome: low- and intermediate-grade SGCs have a 5-year survival of 85–90%, while high-grade SGCs report less than 40% [5,6].
Tumor size, nodal status, the presence of extracapsular spread, and margin status are additional predictors of poor outcomes [6].
The localized/locoregional disease is managed with surgery with or without post-operative radiation therapy (RT) in case of advanced T stage (T3-T4 disease), high/intermediate grade, incomplete resection margins, perineural invasion, and nodal involvement [7]. Retrospective series tried to establish a role for adjuvant chemotherapy in addition to RT, but with no apparent clinical benefit, even if high-risk features were present [7].
More than 70% of patients develop a recurrent/metastatic (R/M) disease, varying in incidence among different histological subtypes [8]. Conventional chemotherapy is mostly indicated, although the rarity of these conditions and their heterogeneity hinder large randomized clinical trials. Moreover, the majority of studies included all SGC subtypes, and we do not have information about the efficacy of each treatment in the specific histopathological subtype [9,10].
Our review highlights the molecular landscape of SGCs, encompassing the diagnostic and prognostic value of tissue and liquid biomarkers and the potential therapeutic targets across various histological entities.
2. Tissue Biomarkers Across Various Salivary Gland Subtypes: Diagnostic and Prognostic Roles
In the following sections, we provide an overview of the specific tissue markers and molecular alterations associated with selected SGC subtypes, focusing on their diagnostic utility, prognostic value, and potential as therapeutic targets. Not every histological subtype has been included despite exhibiting distinctive molecular alterations.
Table 1 provides a summary of recurrent immunohistochemical tumor markers, detectable mutations and molecular alterations observed in SGC subtypes.

Table 1.
Classification of recurrent histopathological features and molecular alterations in different salivary gland cancers subtypes *.
2.1. Mucoepidermoid Carcinoma (MEC)
Mucoepidermoid carcinoma (MEC) is the most common tumor type, accounting for 10–15% of SGCs and commonly occurring in the parotid gland, especially the high-grade type [11]. MEC is traditionally characterized as a “triphasic” tumor consisting of varying proportions of mucus-producing cells (which contribute the “muco” in “mucoepidermoid”), epidermoid (squamous-like) cells (responsible for the “epidermoid” component), and intermediate cells. Additionally, columnar cells are often present, and all these cell types may undergo clear cell or oncocytic changes. In most cases, MEC can be easily diagnosed based on routine histological examination alone, without the need for additional testing [12]. The presence of immunohistochemical p63 or p40 expression, combined with the absence of S100/SOX10 staining, can assist in distinguishing MEC from other SGCs [13].
Some studies have suggested that elevated MUC-1 expression may serve as a potential indicator of poor prognosis in high-grade MEC and could be explored as a molecular target to enhance patients’ outcomes in the future [14,15]. Moreover, high expression of HER2 and EGFR is typical of high-grade tumors, reported in 45% and 14% of cases, respectively, and is associated with a poor prognosis [16]. Amphiregulin (AREG), an EGFR ligand, has been shown to be a downstream target of CRTC1-MAML2 fusion and, when overexpressed in immunohistochemistry (IHC), showed a correlation with longer disease-free survival [17].
High-grade MECs often harbor additional molecular alterations, including TP53 mutations, which are linked to worse outcomes [18].
Most MEC are characterized by a translocation t(11;19)(q14-21;p12-13) resulting in CRTC1-MAML2 oncogene fusion [19]. The other translocation, t(11;19)(q21;q26), leads to a CRTC3-MAML2 fusion product that is detected in 6% of cases [20].
An additional uncommon alteration is the t(6;22)(p21;q12) translocation, which drives the formation of the ESWR1–POU5F1 fusion [21] and is associated with a favorable prognosis in low-grade MECs. The detection of these rearrangements may provide information to distinguish the oncocytic variant of MEC from oncocytoma and oncocytic carcinoma [22] or the “Warthin-like” MEC from the Warthin tumor [23].
CRTC1-MAML2 and CRTC3-MAML2 fusions appear to be associated with low-grade tumors, favorable clinical behavior, younger age (<60 years), and better prognosis [24,25]; on the other hand, fusion-negative tumors present a more aggressive biological behavior, characterized by coexisting TP53 mutations [25,26]. CDKN2A deletions in CRTC1-MAML2 fusion-positive MECs have been associated with an unfavorable prognosis [27].
Recently, microRNA have been described in MEC: the miR-17-92 cluster is upregulated in MEC with a poor prognosis [28], and miR-205-5p and miR22 upregulation, increasing cellular invasion potential, are linked to worse OS rates [29]. Conversely, the upregulation of miR-34a, involved in the suppression of c-Kit and β-catenin, is associated with improved OS [30]. Furthermore, specific miRNAs were tied to MEC grading, such as miR-4324, which is significantly lower in high-grade MEC and correlates with poorer OS [29].
2.2. Adenoid Cystic Carcinoma
Adenoid cystic carcinoma (ACC) accounts for approximately 10% of SGCs and ranks as the second most prevalent malignancy in these glands, following MEC [31]. It is the most common tumor of minor salivary glands. While ACC is recognized as a histopathological subtype with slow growth, it has a propensity for recurrence, often exhibiting perineural invasion and distant metastases, particularly to the lungs [32]. ACC is a biphasic epithelial tumor comprising myoepithelial and ductal cells, and due to its biological variability, it remains a challenging diagnosis [33]. Ductal cells are characterized by eosinophilic cytoplasm and uniformly round nuclei (positive for CK7 and CAM 5.2), while myoepithelial cells exhibit clear cytoplasm and hyperchromatic, angular nuclei (positive for calponin, p63, SOX10, S100, and SMA). The overexpression of c-Kit (CD117) is restricted to inner epithelial cells, and it is a prognostic factor of aggressive behavior, development of distant metastasis, and worse survival outcome [34,35]. MYB protein through IHC is widely accessible but does not offer optimal specificity [36].
Alterations in PI3K/AKT/mTOR, TP53 and TERT promoter mutations have been reported [37].
Furthermore, NOTCH mutations are associated with an aggressive subgroup of ACC, characterized by a higher rate of liver and bone metastasis, shorter relapse-free survival, and OS compared to NOTCH wild-type ACC [38].
Ho et al. also observed common alterations in genes involved in chromatin remodeling in R/M ACCs, including KDM6A, KMT2C/MLL3, ARID1A, ARID1B, BCOR, MLL2/KMT2D, and CREBBP, with a higher frequency compared to primary tumors [39].
Rearrangements involving MYB or MYBL1 are highly specific for ACC and constitute valuable diagnostic hallmarks, usually identified with FISH or NGS, not only for salivary gland ACC but also for ACC arising in other anatomic sites (lung, breast, trachea, sinonasal cavity, lacrimal glands, and skin) [40,41,42]. MYB is an oncogene controlling proliferation and differentiation acting as a DNA-binding transcription regulator and is not observed in normal salivary gland parenchyma [43]. Most ACC present a MYB-NFIB fusion, but in a not negligible percentage of cases, other chromosomal translocations have been observed [38].
Regarding miRNA, recent evidence showed that miR-6835-3p, miR-4676, and miR-1180 are predictive factors of reduced survival rates [44], and another study associated miR-20a and miR-17 with poor outcomes [38]. Additionally, miRNA profiles in ACC revealed altered expressions of miR-4487, miR-4430, miR-486-3p, miR-5191, miR-3131, and miR-211-3p during metastatic progression [45]. MiR-582-5p downregulation has been linked to reduced invasion and migration in ACC [46].
2.3. Acinic Cell Carcinoma
Acinic cell carcinoma (AciCC) is generally considered a low-grade tumor with an overall favorable prognosis, but a high recurrence rate has been reported based on the presence of poor prognostic factors. AciCC is usually positive for CK7 and CAM 5.2 and negative for S100. DOG1, well known for its expression in gastrointestinal stromal tumors (GIST), can be detected by IHC to identify well-differentiated AciCC; in fact, poorly differentiated tumors show a moderate to poor expression of DOG1 [47,48]. Moreover, AciCC is usually immunonegative for mammaglobin, which is useful in its distinction from secretory carcinoma.
Genetic profiling in AciCC assessed that CDKN2A and CDKN2B mutations are strongly associated with the presence of metastatic or relapsed disease (up to 90% of cases) and also with high-grade tumors (nearly 60%), highlighting them as a negative prognostic indicator [49,50]. Additionally, other genetic alterations have been observed in advanced AciCC: the most common rearrangements involved ATM, PTEN, FBXW7, and TP53, whereas BRAF, NF1, HRAS, NOTCH1, TERT, ARID2, BIRC3, MTAP, and FAT1 mutations were less frequent [49,50].
Haller et al. identified the t(4;9)(q13;q31) rearrangement, which leads to the fusion of the secretory Ca-binding phosphoprotein (SCPP) gene cluster (including STATH, HTN1, HTN3, ODAM, FDCSP, and MUC7) with the NR4A3 gene (in 80% of cases), which acts as an oncogenic driver. This particular translocation is exclusive to AciCC, helping differentiate it from secretory carcinoma, especially in high-grade transformation cases [51].
The second most frequent fusion involves the HTN3 and MSANTD3 genes (t(4;9)(q13.3;q31.1)), which has been observed in a small number of cases (4–8%) [52,53,54]. Recent molecular studies have identified PON3-LCN1 fusion in a subset of AciCC cases. While this fusion is not yet targetable, its presence serves to differentiate AciCC from other SGCs [55].
2.4. Salivary Duct Carcinoma
Salivary duct carcinoma (SDC) is an aggressive epithelial tumor originating from intralobular and interlobular excretory ducts. Histologically, it mostly resembles invasive ductal breast carcinoma (DBC) but with marked cell atypia and high mitotic count and is frequently associated with distant metastasis [54]. CK7 shows consistent positivity, while S100 and SOX10 are negative. Staining for p63 can assist in identifying the intraductal component by highlighting the basal/myoepithelial cells around the neoplastic cells [56,57,58].
Approximately 70% of these tumors are androgen receptor (AR)-positive, revealed by IHC staining [59] (Figure 1). About 30% of SDC present HER2 overexpression by IHC or HER2 amplification by FISH or next-generation sequencing (NGS) [59], both associated with poor prognosis. These alterations are more frequently observed in SDCs ex-pleomorphic adenoma (SDC ex-PA) [60].
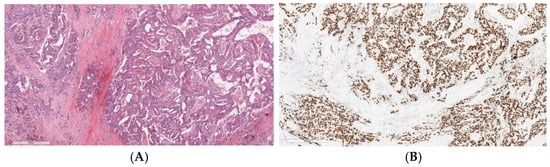
Figure 1.
Invasive salivary duct carcinoma: (A) Histological image stained with hematoxylin and eosin of an invasive salivary duct carcinoma, showing a complex architecture with cribriform gland formation, a Roman-bridge pattern, and solid nests. The tumor cells display large, pleomorphic nuclei with coarse chromatin, prominent nucleoli, and abundant eosinophilic cytoplasm, which is typically apocrine in nature. (B) Strong immunoreactivity of the neoplastic cells for androgen receptors.
Notably, while HRAS mutations are prevalent in de novo lesions, they are rare in SDC ex-PAs.
In recent years, there has been significant progress in understanding the genetics of SDC, although it has yet to be thoroughly explored. The tumor mutation burden is notably high in most SDC cases compared to other SGCs. Genetic fusions are not commonly observed in this subtype, but somatic mutations are significantly more frequent [61,62]. The most common alterations include PIK3CA, HRAS, NRAS, BRAF, EGFR, AKT1, and ERBB2 (HER2) rearrangements, some of which are associated with poor prognosis [56,61,62,63].
2.5. Other Subtypes
Secretory carcinoma (Figure 2A), previously known as mammary analog secretory carcinoma (MASC), predominantly arises from the parotid gland. ETV6-NTRK3 gene translocation t(12:15)(p13;q25) has been identified in the majority of secretory carcinoma and comes to be considered as a distinctive molecular feature, helpful as an additional diagnostic marker and providing a therapeutic target [64]. Consequently, IHC is frequently positive for pan-TRK (Figure 2B), which makes it a great biomarker to differentiate secretory carcinoma from other low-grade SGCs [65].
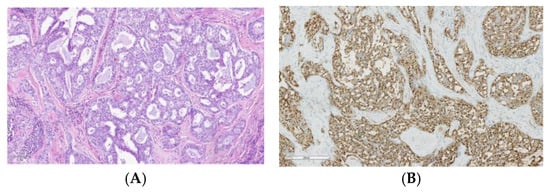
Figure 2.
Secretory carcinoma: (A) Histological image stained with hematoxylin and eosin of a secretory carcinoma showing a lobulated growth pattern, divided by fibrous septa, and consists of a combination of microcystic, tubular, follicular, and papillary-cystic structures containing characteristic luminal secretions. The tumor cells feature low-grade, round to oval nuclei with finely granular chromatin and prominent central nucleoli. The cytoplasm is pale pink with a granular or vacuolated appearance. Cellular atypia is generally mild, and mitotic activity is absent. (B) Strong nuclear staining for pan-TRK.
Even if ETV6-NTRK3 represents the most common rearrangement, additional ETV6 fusion partners have been identified, such as ETV6-MAML3 [66], ETV6-MET [67], and ETV6-RET [68].
NTRK gene fusions are important driver mutations that have led to the development of therapeutic agents [69].
Polymorphous adenocarcinoma (PAC) is a low-grade tumor primarily affecting minor salivary glands, and it can be accurately diagnosed using morphology and immunophenotype. Typical markers include diffuse positivity for CK-7, S100, CEA, GFAP, and SOX10, patchy expression of p63, and typical negativity for p40 [70]. PAC consists of a single cell type, likely representing a progenitor cell that precedes both luminal and myoepithelial differentiation. These cells originate from the intercalated duct and exclusively express DOG1, particularly in areas where lumen formation occurs and in the apical region of the cell [71].
Most PACs are associated with PRKD1 mutations. These mutations involve hotspot alterations leading to constitutive activation of protein kinase D1, which promotes tumor cell proliferation. PRKD1 mutations are highly specific to PAC and can aid in differentiating it from other low-grade subtypes [72]. Interestingly, in cribriform adenocarcinoma (CA), PRKD1-3 fusions are the most frequent [73]. CA is considered an aggressive variant of PAC, with a high likelihood of nodal metastasis at presentation. Among the fusion partners, ARID1A, ATL2, DDX3X, PPP2R2A, PRKAR2A, SNX9, and STRN3 (in cases with high-grade transformation) are particularly noteworthy [74,75,76]. However, the type of genomic alteration is not exclusive to any specific adenocarcinoma subtype [77].
Hyalinizing clear cell carcinoma (HCCC) is a malignant epithelial tumor of salivary gland origin with squamous differentiation, most commonly arising in the minor salivary glands of the oral cavity and the base of the tongue. HCCC typically presents as infiltrative cords of cells with eosinophilic to clear cytoplasm and round to raisinoid nuclei set within a prominent fibroblastic or hyalinized stroma [78]. The tumor exhibits a squamoid immunophenotype, usually showing diffuse positivity for p40 and CK5/6, though mucin production may also be observed. Some tumors express p16, which can create a diagnostic challenge when the tumor originates in the oropharynx; however, they do not contain transcriptionally active HPV. Myoepithelial markers such as S100, SMA, and calponin are negative. Most HCCC cases are characterized by the EWSR1::ATF1 fusion, which is also found in clear cell sarcoma [79]. The identification of this fusion has established HCCC as a distinct entity rather than a catch-all category for carcinomas with clear cells. Due to consistent breakpoints, EWSR1 FISH is a reliable and widely available method for confirming HCCC. A subset of cases is associated with the EWSR1::CREM fusion [80]. Identifying an EWSR1 fusion can definitively confirm the diagnosis when squamous cell carcinoma is part of the differential diagnosis.
Intraductal carcinoma (IDC). IDC most commonly occurs in the parotid gland and has been referred to by various names, such as low-grade salivary duct carcinoma and low-grade cribriform cystadenocarcinoma [81]. Initially, IDC was thought to represent a noninvasive neoplasm, similar to breast ductal carcinoma in situ, based on its benign behavior and histopathological features, which mirrored those of breast ductal carcinoma in situ—proliferating ducts filling rounded spaces and encased by a continuous layer of flattened myoepithelial cells. However, the current perspective is more nuanced [82]. The IDC category encompasses four distinct, though sometimes overlapping, subtypes. Intercalated duct IDC is characterized by small amphophilic to eosinophilic cells with oval nuclei. Apocrine IDC consists of large cells with bubbly eosinophilic cytoplasm, often displaying snouting and decapitation secretions. Oncocytic IDC features cells with abundant granular eosinophilic cytoplasm and round nuclei with prominent nucleoli, while mixed IDC presents a combination of these characteristics. Intercalated duct and oncocytic IDCs typically exhibit low-grade morphology with minimal mitotic activity, whereas apocrine IDC can range from low to high grade. The surrounding tissue frequently shows degen-erative alterations, including fibrosis, inflammation, cholesterol clefts, and hemorrhage. Invasive growth with the loss of myoepithelial cells may occasionally be observed in all IDC subtypes.
It is now understood that a significant portion of IDC cases harbor gene fusions. The most common fusion is NCOA4::RET, which can occur in any IDC subtype [83]. TRIM33::RET is primarily found in oncocytic IDC, while TRIM27::RET is associated with mixed types [84]. Although these are the most frequently identified fusions, the list of known fusions is expanding (Table 1). It is increasingly evident that fusion-positive IDCs are not carcinoma in situ but rather biphasic tumors [12].
Epithelial–myoepithelial carcinoma (EMC) is composed of two distinct cell populations that form a double-layer structure: inner ductal cells and outer myoepithelial cells. EMC can present in various histological subtypes, including sebaceous, oncocytic, and double-clear, which can complicate the differential diagnosis [85]. EMC is more common in females than in males, with the parotid gland and submandibular gland being the most frequently affected site. Typically, it presents as a slow-growing, painless mass. Histologically, low-molecular-weight cytokeratins are strongly positive in the ductal component and are less intense in the myoepithelial component. Myoepithelial markers include SMA, HHF35, p63, and calponin. S100 stains both the myoepithelial and the ductal components.
HRAS mutations (27–87%) are the most frequently observed genetic alterations in EMC [86], although they were not found in EMCs arising from pleomorphic adenomas (PAs) [87]. Molecular alterations in PIK3CA and AKT1 are relatively common in EMC [88].
Carcinoma ex-pleomorphic adenoma (CA ex PA) is a rare form of primary SGC that develops from a pre-existing PA. It is estimated that 5–15% of benign PAs undergo malignant transformation into carcinoma [89]. The presence of benign tumor components can sometimes lead to misdiagnosis, though rapid tumor growth and other associated symptoms should raise suspicion of malignancy. While the most common malignant components of Ca ex PA are SDC, myoepithelial carcinoma (MECA), and adenocarcinoma not otherwise specified (NOS), other types of SGCs have also been identified. Genetic alterations in the pleomorphic adenoma gene 1 (PLAG1) and the high-mobility group AT-hook 2 (HMGA2) genes are frequently seen in both PAs and Ca ex PAs, although these alterations are not typical of primary SDC, MECA, or adenocarcinoma NOS [90,91]. The alterations reported in the SDC subtype are amplification in HMGA2, MDM2, and ERBB2 (HER2) [92].
Myoepithelial carcinoma (MECA) accounts for only 2% of all SGCs, and most cases occur in the parotid gland. MECA has a propensity for distant metastases. It can either occur as a de novo tumor or result from the malignant transformation of a pre-existing PA or myoepithelioma [93]. Studies suggest that MECA arising from PAs is more commonly detected than de novo cases [94]. However, it remains unclear which form exhibits more aggressive behavior or poorer outcomes. Due to the rarity of salivary gland MECA, limited genetic studies have been conducted. Dalin et al. analyzed 40 tumors, dividing them into MECA de novo and MECA ex PA, as well as cases with and without recurrence. They found that MECA ex PA tumors exhibited more genetic alterations, including fusions, somatic mutations, and copy number variations (CNVs). The authors suggested that CNVs are involved in the malignant transformation of PA into MECA ex PA and are associated with worse prognosis [95]. The most common fusion identified in MECA ex PA was PLAG1-FGFR1 (18%), followed by PLAG1- TGFBR3, though these fusions did not show prognostic significance. EWSR1-ATF1 was found only in de novo MECA cases, with or without recurrence. EWSR1 rearrangements were frequently seen in the clear cell component of MECA [94,95].
3. Liquid Biopsy for SGCs: Potential Clinical Applications and Future Perspectives
Liquid biopsy is the term used to refer to several technologies which enable the molecular analysis of bodily fluids (most commonly peripheral blood, but also pleural and peritoneal fluids, urine, saliva, and cerebrospinal fluid) and permit early diagnosis, prognosis assessment, and acquisition of information about tumor behavior and response to therapy [96]. In recent years, it has been shown to offer several advantages in medical oncology, including the capacity to overcome the limitations of traditional tissue biopsy through a minimally invasive and quick approach. Among the various biomarkers, the most studied are represented by circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), extracellular vesicles (EVs), and circulating microRNAs (miRNAs) (Figure 3).
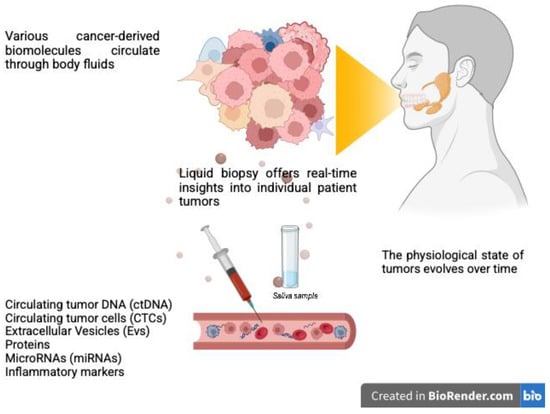
Figure 3.
Liquid biopsy for salivary gland cancer diagnosis and monitoring involves detecting cancer-derived biomaterials released into body fluids, such as blood and saliva. These biomolecules act as critical indicators, potentially providing real-time insights into the disease status. This innovative approach could support personalized treatment strategies for patients with salivary gland cancers. Created with BioRender.com.
Here, we provide an overview of liquid biopsy biomarkers that have been studied for SGCs. In the mentioned studies, SGCs were often examined as a whole, without specification according to histotype and/or in association with other HNCs.
ctDNA consists of a small fraction of cell-free DNA fragments ranging from 160 to 2000 base pairs (bp), which is released into bodily fluids from the primary tumor following tumor cell death or via active secretion and is characterized by tumor-specific gene rearrangements, as the one expressed in the originating tumor, potentially leading to the identification of targetable mutations. It is relatively easy to extract and can be quantified accurately using the appropriate techniques, even during the early stages of cancer [97,98]. Typically, ctDNA is more fragmented than extracellular DNA (with fragments around 90–150 bp in length) [99], and its half-life in peripheral blood ranges from 15 min to several hours, making it an ideal biomarker for real-time monitoring of tumor dynamics [97]. ctDNA levels had been investigated as a biomarker to predict radiological response in patients with SDCs from the CABO-ASAP trial. The results suggested a potential correlation between changes in ctDNA levels and radiological response in these patients. At baseline, ctDNA was detectable in four out of five patients, with levels varying significantly between individuals, highlighting the dynamic nature of ctDNA shedding by tumors. Notably, in two patients, an increase in ctDNA was detected before any radiological or clinical signs of disease progression, demonstrating the potential of ctDNA for early monitoring of disease progression [100].
CTCs are malignant cells that detach from primary tumors and circulate as individual cells or in clusters; in the bloodstream, they can travel to distant tissues and establish metastasis [101]. The presence and number of CTCs are emerging as potential predictors of recurrence [102]. CTCs also have utility for in vitro studies to conduct functional tests, including drug testing or creating experimental models like xenografts [103,104]. However, one major challenge of using CTCs in liquid biopsy is their low abundance in the bloodstream—usually only 1–10 cells per 10 mL of blood—which makes their detection and isolation technically difficult [97].
CTCs have been recognized as a potential marker for early metastasis, treatment response, and surveillance in HNCs [105,106], including SGCs. In a pilot study involving patients with ACC, CTCs were detected in three patients (3/8, 37.5%), all of whom had either recurrent local disease or known distant metastatic disease [107]. Additionally, cultures derived from CTCs closely mirror the histological and molecular characteristics of uncultured ex vivo samples. Notably, the generation of models suitable for small-scale drug screening took less than three months, a timeframe that could provide valuable treatment-relevant information for patients, although larger studies are required to systematically assess the effectiveness of CTC expansion in SGCs and the correlation between in vitro drug responses and patients’ outcomes [108].
EVs are membrane-bound vesicles secreted in various bodily fluids, also by cancer cells, and are crucial for intercellular communication [109,110]. They are classified into three types based on their size and origin: exosomes (40–150 nm), microvesicles (40–1000 nm), and apoptotic bodies (800–5000 nm). Among these, exosomes are the most studied due to their stability in circulation, ability to penetrate tissues, and role in promoting tumor progression [111,112,113,114]. Exosomes contain a variety of biomolecules, including RNAs and proteins, which can mediate oncological processes and support tumor growth, particularly in pre-metastatic niches. These exosomal RNAs include not only protein-coding RNAs but also regulatory miRNAs, long non-coding RNAs (lncRNAs), and small nucleolar RNAs (snoRNAs), which contribute to cancer progression [115,116,117]. Moreover, exosomes in SGCs are involved in tumor progression by tumor microenvironment modulation, immune response regulation, and promotion of metastasis [118].
Inflammatory markers have also been examined to distinguish parotid gland tumors from healthy controls [119]. IL-33 is a cytokine with a dual function, currently investigated along with its receptor, sST2, because of its association with negative prognosis in various cancer types, and it has also been identified in SGCs tissue; it has been observed that the serum IL-33 level was significantly elevated in patients with parotid gland tumors, and sST2 receptor levels were significantly higher in benign neoplasms such as pleomorphic adenoma but also in AciCC patients compared to the controls [119]. Another study identified significantly elevated carbohydrate antigen 19-9 (CA 19-9) in saliva from malignant parotid gland tumor cases, showing CA 19-9 as a new diagnostic tool in the preoperative differentiation between malignant and benign parotid tumors [120].
A study was conducted to assess the levels of carcinoembryonic antigen (CEA) and carcinoma-associated antigen-50 (CA-50) in patients with oral cancers and SGCs. Concentrations of salivary CEA and CA-50 were measured in 80 patients with these types of tumors, 40 patients with benign tumors, and 80 healthy controls. The results showed that measurements of salivary CEA and CA-50 were more sensitive than serum measurements. Salivary levels of both CEA and CA-50 were significantly higher in patients with malignant tumors compared to those with benign tumors and healthy controls (p-value < 0.001). In contrast, only 7 and 3 out of the 80 patients with malignant tumors exhibited elevated serum levels of CEA and CA-50, respectively [121].
Additionally, microRNAs (miRNAs) are small non-coding RNAs implied in the regulation of gene expression in cancer (i.e., enhancing protein-coding oncogenes or inhibiting tumor suppressors). They have emerged as promising circulating biomarkers for diagnostic and prognostic purposes both in HNCs and SGCs; in fact, circulating miRNAs contribute to intercellular signaling and are often dysregulated in different cancer types [122,123,124,125,126,127]. As for SGCs, 95 miRNAs have been assessed in the blood and saliva of patients with SGCs, and it has been shown that in malignant tumors, compared to the control group, plasma miR-30e was significantly upregulated [128]. On the other hand, two studies have reported variations in miRNA expression in saliva samples from patients with malignant or benign parotid gland tumors [129]. Furthermore, a four-miRNA combination (hsa-miR-132, hsa-miR-15b, mmu-miR-140, and hsa-miR-223) was able to distinguish saliva samples of patients with malignant tumors from those with benign parotid gland tumors, achieving a sensitivity of 69% and a specificity of 95% [130]. Another study investigated miRNA expression levels in saliva samples from patients with parotid gland neoplasms versus healthy controls and identified four miRNAs (hsa-miR-296-5p, hsa-miR-1233, hsa-miR-1267, and hsa-miR-1825) that were significantly upregulated in all saliva samples from patients with parotid gland neoplasms. Additionally, three miRNAs (hsa-miR-103a-3p, hsa-miR-211, and hsa-miR-425-5p) were significantly downregulated in tumor patients [131].
In saliva samples, other biomarkers have been found and described. A nuclear magnetic resonance (NMR)-based metabolomic analysis of saliva from patients with SGCs (n = 36) compared to healthy people (n = 23) has been conducted. The findings revealed that individuals with parotid tumors exhibit a distinct metabolomic profile, marked by abnormalities in the concentrations of several amino acids. Notably, alanine and leucine levels were particularly significant, suggesting disruptions in the metabolic pathways of glucogenic amino acids and ketone bodies [132].
A summary of liquid biomarkers is represented in Table 2.

Table 2.
Liquid biomarkers in salivary gland cancers.
4. Precision Oncology in SGCs: A Brief Overview of Emerging Therapeutic Options
The advent of targeted therapies has introduced a new era of precision oncology for SGCs, particularly for aggressive subtypes such as SDC and secretory carcinoma.
Established therapies, such as HER2 inhibitors, have shown efficacy in HER2-positive SGCs, mostly revealed in SDCs (in 30% of cases).
A Japanese phase II trial investigating the combination of trastuzumab and docetaxel in HER2-positive R/M SDCs reported an objective response rate (ORR) of 70% and a clinical benefit rate of 84%, with a median PFS (mPFS) and mOS of 8.9 and 39.7 months, respectively [133].
Beyond trastuzumab, other anti-HER2 agents, such as pertuzumab and Ado-trastuzumab emtansine (T-DM1), have been explored, particularly in cases of disease progression after first-line therapy [134]. More recently, emerging drugs, such as the ADC trastuzumab deruxtecan (T-DXd), have been evaluated. The DESTINY-PanTumor02 trial demonstrated durable clinical benefits for T-DXd in HER2-positive solid tumors, including SGCs, reporting an ORR of up to 61.3% in HER2 IHC 3+ populations (irrespective of prior HER2 therapy), with mOS exceeding 21 months even in heavily pre-treated patients. The greatest benefit was observed in the IHC 3+ subgroup, underscoring the potential role of T-DXd as a tumor-agnostic therapy for HER2-overexpressing solid tumors [135].
A further area of interest includes hormone therapies such as androgen deprivation therapy (ADT), particularly for R/M SDCs, due to the overexpression of AR. However, AR positivity is no longer a sufficient predictive biomarker of efficacy. It has been demonstrated that high levels of SRD5A1 mRNA are strongly predictive of better efficacy in AR-positive SDC patients treated with combined androgen blockade [136]. On the other hand, a high expression of EZH2, a histone methyltransferase of histone H3, and H3K27me3 exhibit a predictive value for poor efficacy of AR-targeted therapy [137].
ADT regimens typically involve non-steroidal anti-androgens, such as bicalutamide, either alone or in combination with luteinizing hormone-releasing hormone (LHRH) analogs.
A prospective phase II study assessed the efficacy and safety of combined androgen blockade with leuprorelin acetate and bicalutamide in SGCs, demonstrating equivalent efficacy and less toxicity compared with conventional chemotherapy in the unresectable, locally advanced or metastatic setting, with an ORR of 42% (11% CR) and an mPFS and an mOS of 8.8 and 30.5 months, respectively [138]. A phase II clinical trial demonstrated ORR of 21% and a disease control rate of 62.5% in AR-positive SGCs treated with abiraterone and an LHRH agonist who progressed on ADT in the second-line setting, with an mPFS of 3.65 months (consistent with the results of second-line chemotherapy) and an mOS of 22.47 months (better than observed with chemotherapy) [139]. However, the final results of EORTC1206 (NCT01969578) showed that ADT (a combination of triptorelin and bicalutamide) did not outperform chemotherapy as a first-line treatment for AR-expressing SDC patients. Interestingly, ADT could also be amenable in poor-risk AR-positive SDC in the adjuvant setting, as demonstrated in a retrospective series [140].
A currently recruiting randomized phase II trial, the DUCT study (NCT05513365), aims to evaluate the benefit of dutasteride in addition to combined androgen blockade in order to overcome therapy resistance in patients with AR-positive R/M SDC previously treated with ADT [141]. Given the rarity of SGCs and the lack of therapeutic options, ADT is a valid option for AR-positive subtypes, warranting further exploration.
The MYB-NFIB fusion, a specific genetic hallmark of ACC, has emerged as a promising target for therapy. Preclinical models using all-trans retinoic acid (ATRA) have demonstrated effective suppression of tumor growth and proliferation in ACC patient-derived xenograft models. These findings have led to ongoing clinical trials aimed at validating ATRA’s efficacy in SGC patients [142].
Gamma-secretase inhibitors, which target NOTCH mutations frequently found in aggressive ACC, are currently being evaluated in early-phase clinical trials. Preliminary evidence from these studies suggests that such inhibitors may contribute to disease stability, offering a novel approach for this challenging subtype [143,144].
Overexpression of EGFR, observed in 17–100% of SGCs across all histological subtypes (most commonly in ACC, but also MEC and SDC), has been explored as a therapeutic target with controversial results. In SGCs, cetuximab achieved a clinical benefit rate of 50% but failed to determine objective responses in a phase II study [145]. However, case reports have documented CRs or PRs in metastatic SGC patients treated with EGFR inhibitors combined with chemotherapy, highlighting the potential role of such combinations in specific clinical contexts [146,147].
BRAF V600E mutations, known drivers of oncogenesis, detected via NGS or IHC, are occasionally present in SGCs and have shown responsiveness to targeted therapies with BRAF plus MEK inhibitors [148].
The PI3K/AKT/mTOR pathway plays a crucial role in cellular survival, proliferation, and resistance to therapy. Mutations in PIK3CA have been identified in various SGC subtypes, particularly in SDC. Preclinical studies and case reports demonstrated tumor responses to PI3K inhibitors, underlining their potential as a therapeutic strategy in this defined molecular subgroup [149,150].
The efficacy of immunotherapy in SGCs remains controversial. High-grade MEC and SDC can be considered to be endowed with an “immune-hot” tumor microenvironment [151]. A retrospective experience showed promising clinical efficacy of PD-1 inhibitors in SGCs (especially in CA ex PA and SDC) [152], whereas pembrolizumab, an anti-PD-1 antibody, alone or in combination with radiotherapy demonstrated unflattering results in a phase II study [153]. These differences can be explained by the complexity of the tumor microenvironment, which is highly variable between different SGC subtypes and deeply influences the response to immunotherapy [154]. However, immune checkpoint inhibitors lack clinical efficacy in SGCs, with an ORR inferior to 20% as monotherapy [153]. The combination of nivolumab, an anti-PD-1, and ipilimumab, an anti-CTLA4, in patients with R/M SGCs showed an ORR of 16%, meeting the primary efficacy endpoint of the study. While the treatment showed limited efficacy in ACC, with few responses, it appeared promising for non-ACC SGCs, especially for SDC [155].
Recently, NTRK inhibitors have been evaluated in the case of TRK fusions. Larotrectinib, a selective TRK inhibitor, has been investigated in patients with SGCs from two clinical trials (NCT02122913 and NCT02576431). Tumor histology consisted of secretory carcinoma in 54%, adenocarcinoma in 21%, and MEC in 13%, with additional cases of ACC, glandular sarcomatoid carcinoma, and adenocarcinoma NOS. All patients exhibited an ETV6-NTRK3 gene fusion [156]. The ORR to larotrectinib was 92%, including 79% partial responses (PRs) and 13% complete responses (CRs). The median response time was 1.84 months (range: 0.99–5.98 months), with treatment durations spanning from 0.95 to over 60.4 months. The PFS rate at 36 months was 66%, while the OS rate was 91%. Moreover, larotrectinib exhibited a favorable safety profile, with predominantly low-grade adverse events and no treatment discontinuations due to drug-related toxicities [156].
Similarly, entrectinib, a pan-TRK inhibitor, has demonstrated efficacy in NTRK fusion-positive tumors. A single case study involving a patient with former MASC reported significant tumor regression following treatment with entrectinib [157].
Both larotrectinib and entrectinib received Food and Drug Administration (FDA) and European Medicine Agency (EMA) tissue-agnostic approval.
Second-generation TRK inhibitors, including selitrectinib (BAY 2731954, LOXO-195) and repotrectinib (TPX-0005), were developed to address on-target resistance mechanisms. Results from a phase I trial of selitrectinib indicated a 45% ORR in patients with confirmed on-target resistance mutations; 10% of cases investigated were secretory carcinoma [158].
While data for repotrectinib remain limited, ongoing phase I/II trials have reported a confirmed partial response in a patient with secretory carcinoma who had progressed on entrectinib [159].
Unremarkable results were obtained from the combination of pembrolizumab and the pan-histone deacetylases (HDAC) inhibitor vorinostat in a recent phase II trial [160]. Although not fully successful in metastatic patients, attempts are being made to move immunotherapy to the neoadjuvant setting: a phase II trial is currently examining the efficacy of neoadjuvant therapy with carboplatin, nab-paclitaxel and the anti-PD-1 antibody toripalimab, in SGCs [NCT04825938].
Recently, a study showed that around 60% of ACCs have high levels of TROP2 expression on IHC, providing the biological rationale for using anti-TROP2 antibody–drug conjugate (ADC) such as Sacituzumab govitecan, widely used in breast cancer and evaluated in HNSCC, as novel potential therapy [161,162].
Overall, these data underline the importance of adequate molecular profiling to suggest new treatment targets and the need to design future clinical trials with the aim of improving the prognosis and survival outcomes of these rare malignancies.
Table 3 summarizes the association between recurrent alterations and targeted therapies in SGCs.

Table 3.
Main associations of recurrent alterations and targeted therapies for salivary gland cancers.
5. Conclusions
In conclusion, this review presents an overview of tissue and liquid biomarkers in SGCs. Histopathological features, along with detectable mutations, molecular alterations, and miRNAs, provide valuable insights into the heterogeneous landscape of SGCs, aiding in the diagnosis, prognosis, and development of targeted therapy. The integration of molecular profiling with targeted therapies, such as HER2 inhibitors, NTRK inhibitors, and ADT, underscores the shift towards precision oncology in SGC management.
Liquid biopsy, encompassing ctDNA, CTCs, and exosomes, represents a promising, minimally invasive approach for real-time disease monitoring and personalized therapeutic strategies in clinical oncology, and SGCs are no exception. Although it may not fully replace traditional tissue biopsies at this stage, liquid biopsy offers several advantages, such as less invasive early detection, disease monitoring, and enhanced treatment personalization. The future of liquid biopsy in SGCs will depend on the refinement of analytical techniques, the discovery of more specific biomarkers, and the clinical validation of findings. As research and technology progress, liquid biopsy has the potential to become a key component of the diagnostic and therapeutic approach for patients with these rare and complex tumors.
Despite these advances, the rarity and phenotypic heterogeneity of SGCs necessitate further research to validate biomarkers and optimize treatment strategies. The future lies in well-designed, molecularly stratified clinical trials to improve outcomes for patients with these complex malignancies.
Author Contributions
Conceptualization, D.M.F., E.B. and F.C.; methodology, D.M.F., E.B. and M.F.; data curation, D.M.F., E.B. and G.Q.; writing—original draft preparation, F.C., E.B., S.C., M.B. and A.G.; writing—review and editing, D.M.F., G.Q. and F.C.; visualization, D.M.F.; supervision, D.M.F., G.Q. and M.F.; project administration, D.M.F., E.B., M.F. and G.Q.; funding acquisition, E.B. and D.M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today, Version 1.1; International Agency for Research on Cancer: Lyon, France, 2024. Available online: https://gco.iarc.who.int/today (accessed on 30 January 2025).
- Cavalieri, S.; Filippini, D.M.; Ottini, A.; Bergamini, C.; Resteghini, C.; Colombo, E.; Lombardo, R.; Nuzzolese, I.; Alfieri, S.; Licitra, L.; et al. Immunotherapy in head and neck squamous cell carcinoma and rare head and neck malignancies. Explor. Target. Anti-tumor Ther. 2021, 2, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Filippini, D.M.; Carosi, F.; Querzoli, G.; Fermi, M.; Ricciotti, I.; Molteni, G.; Presutti, L.; Foschini, M.P.; Locati, L.D. Rare Head and Neck Cancers and Pathological Diagnosis Challenges: A Comprehensive Literature Review. Diagnostics 2024, 14, 2365. [Google Scholar] [CrossRef] [PubMed]
- Skálová, A.; Stenman, G.; Simpson, R.H.; Hellquist, H.; Slouka, D.; Svoboda, T.; Bishop, J.A.; Hunt, J.L.; Nibu, K.-I.; Rinaldo, A.; et al. The Role of Molecular Testing in the Differential Diagnosis of Salivary Gland Carcinomas. Am. J. Surg. Pathol. 2018, 42, e11–e27. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.A.; Tavares, M.R.; Dias, F.L.; Kligerman, J.; Nascimento, M.F.; Barbosa, M.M.; Cernea, C.R.; Soares, J.R.; Santos, I.C.; Salviano, S. Clinical prognostic factors in malignant parotid gland tumors. Otolaryngol. Head Neck Surg. 2005, 133, 702–708. [Google Scholar] [CrossRef]
- Walvekar, R.R.; Filho, P.A.A.; Seethala, R.R.; Gooding, W.E.; Heron, D.E.; Johnson, J.T.; Ferris, R.L. Clinicopathologic features as stronger prognostic factors than histology or grade in risk stratification of primary parotid malignancies. Head Neck 2011, 33, 225–231. [Google Scholar] [CrossRef]
- van Herpen, C.; Poorten, V.V.; Skalova, A.; Terhaard, C.; Maroldi, R.; van Engen, A.; Baujat, B.; Locati, L.; Jensen, A.; Smeele, L.; et al. Salivary gland cancer: ESMO–European Reference Network on Rare Adult Solid Cancers (EURACAN) Clinical Practice Guideline for diagnosis, treatment and follow-up. ESMO Open 2022, 7, 100602. [Google Scholar] [CrossRef]
- Nam, S.J.; Roh, J.-L.; Cho, K.-J.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Risk Factors and Survival Associated with Distant Metastasis in Patients with Carcinoma of the Salivary Gland. Ann. Surg. Oncol. 2016, 23, 4376–4383. [Google Scholar] [CrossRef] [PubMed]
- Lassche, G.; van Boxtel, W.; Ligtenberg, M.J.; van Engen-van Grunsven, A.C.H.; van Herpen, C.M. Advances and challenges in precision medicine in salivary gland cancer. Cancer Treat. Rev. 2019, 80, 101906. [Google Scholar] [CrossRef] [PubMed]
- Filippini, D.M.; Marret, G.; Bastien, E.; Sanchez, R.; Borcoman, E.; Le Tourneau, C. Phase I trials of single-agent new drugs in head and neck cancer: A scoping review. Chin. Clin. Oncol. 2024, 13, 73. [Google Scholar] [CrossRef]
- Pires, F.R.; Pringle, G.A.; de Almeida, O.P.; Chen, S.-Y. Intra-oral minor salivary gland tumors: A clinicopathological study of 546 cases. Oral Oncol. 2007, 43, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.A. Fusions in salivary gland neoplasms: A review of practical diagnostic applications. J. Clin. Pathol. 2024. [Google Scholar] [CrossRef]
- Sams, R.N.; Gnepp, D.R. P63 Expression can be used in differential diagnosis of salivary gland acinic cell and mucoepidermoid carcinomas. Head Neck Pathol. 2013, 7, 64–68. [Google Scholar] [CrossRef]
- Siyi, L.; Shengwen, L.; Min, R.; Wenjun, Y.; Lizheng, W.; Chenping, Z. Increased expression of MUC-1 has close relation with patient survivor in high-grade salivary gland mucoepidermoid carcinoma. J. Oral Pathol. Med. 2014, 43, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Honjo, K.; Hiraki, T.; Higashi, M.; Noguchi, H.; Nomoto, M.; Yoshimura, T.; Batra, S.K.; Yonezawa, S.; Semba, I.; Nakamura, N.; et al. Immunohistochemical expression profiles of mucin antigens in salivary gland mucoepidermoid carcinoma: MUC4-and MUC6-negative expression predicts a shortened survival in the early postoperative phase. Histol. Histopathol. 2018, 33, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Cros, J.; Sbidian, E.; Hans, S.; Roussel, H.; Scotte, F.; Tartour, E.; Brasnu, D.; Laurent-Puig, P.; Bruneval, P.; Blons, H.; et al. Expression and mutational status of treatment-relevant targets and key oncogenes in 123 malignant salivary gland tumours. Ann. Oncol. 2013, 24, 2624–2629. [Google Scholar] [CrossRef]
- Shinomiya, H.; Ito, Y.; Kubo, M.; Yonezawa, K.; Otsuki, N.; Iwae, S.; Inagaki, H.; Nibu, K.-I. Expression of amphiregulin in mucoepidermoid carcinoma of the major salivary glands: A molecular and clinicopathological study. Hum. Pathol. 2016, 57, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ramirez, C.; Zhang, Z.; Warner, K.A.; Herzog, A.E.; Mantesso, A.; Zhang, Z.; Yoon, E.; Wang, S.; Wicha, M.S.; Nör, J.E. p53 Inhibits Bmi-1-driven Self-Renewal and Defines Salivary Gland Cancer Stemness. Clin. Cancer Res. 2022, 28, 4757–4770. [Google Scholar] [CrossRef] [PubMed]
- Tirado, Y.; Williams, M.D.; Hanna, E.Y.; Kaye, F.J.; Batsakis, J.G.; El-Naggar, A.K. CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin’s tumors: Implications for histogenesis and biologic behavior. Genes Chromosom. Cancer 2007, 46, 708–715. [Google Scholar] [CrossRef]
- Skálová, A.; Hyrcza, M.D.; Leivo, I. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Salivary Glands. Head Neck Pathol. 2022, 16, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Mehta, S.; Vanik, S.; Trivedi, P.; Banerjee, N.; Dhar, H.; Datta, S.; Karanjai, S. The evolving role of molecular pathology in the diagnosis of salivary gland tumours with potential pitfalls. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 3769–3783. [Google Scholar] [CrossRef] [PubMed]
- García, J.J.; Hunt, J.L.; Weinreb, I.; McHugh, J.B.; Barnes, E.L.; Cieply, K.; Dacic, S.; Seethala, R.R. Fluorescence in situ hybridization for detection of MAML2 rearrangements in oncocytic mucoepidermoid carcinomas: Utility as a diagnostic test. Hum. Pathol. 2011, 42, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.D.; Ito, Y.D.; Masaki, A.; Fujii, K.D.; Beppu, S.; Sakakibara, T.C.; Takino, H.C.; Takase, H.B.; Ijichi, K.; Shimozato, K.D.; et al. Warthin-like Mucoepidermoid Carcinoma: A Combined Study of Fluorescence In Situ Hybridization and Whole-slide Imaging. Am. J. Surg. Pathol. 2015, 39, 1479–1487. [Google Scholar] [CrossRef]
- Schwarz, S.; Stiegler, C.; Müller, M.; Ettl, T.; Brockhoff, G.; Zenk, J.; Agaimy, A. Salivary gland mucoepidermoid carcinoma is a clinically, morphologically and genetically heterogeneous entity: A clinicopathological study of 40 cases with emphasis on grading, histological variants and presence of the t(11;19) translocation. Histopathology 2011, 58, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Murase, T.; Okumura, Y.; Ueda, K.; Sakamoto, Y.; Masaki, A.; Kawakita, D.; Tada, Y.; Nibu, K.; Shibuya, Y.; et al. Clinicopathological significance of EGFR pathway gene mutations and CRTC1/3–MAML2 fusions in salivary gland mucoepidermoid carcinoma. Histopathology 2020, 76, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.; Werenicz, S.; Trocchi, P.; Falk, M.; Friedrich, R.E.; Stammler, A.; Stang, A.; Oesterling, F.; Khil, L.; Stenman, G.; et al. Mucoepidermoid carcinoma of the salivary glands revisited with special reference to histologic grading and CRTC1/3-MAML2 genotyping. Virchows Arch. 2021, 479, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Anzick, S.L.; Chen, W.; Park, Y.; Meltzer, P.; Bell, D.; El-Naggar, A.K.; Kaye, F.J. Unfavorable prognosis of CRTC1-MAML2 positive mucoepidermoid tumors with CDKN2A deletions. Genes, Chromosom. Cancer 2010, 49, 59–69. [Google Scholar] [CrossRef]
- Mitani, Y.; Roberts, D.B.; Fatani, H.; Weber, R.S.; Kies, M.S.; Lippman, S.M.; El-Naggar, A.K. MicroRNA Profiling of Salivary Adenoid Cystic Carcinoma: Association of miR-17-92 Upregulation with Poor Outcome. PLoS ONE 2013, 8, e66778. [Google Scholar] [CrossRef]
- Naakka, E.; Barros-Filho, M.C.; Adnan-Awad, S.; Al-Samadi, A.; Marchi, F.A.; Kuasne, H.; Korelin, K.; Suleymanova, I.; Brown, A.L.; Scapulatempo-Neto, C.; et al. miR-22 and miR-205 Drive Tumor Aggressiveness of Mucoepidermoid Carcinomas of Salivary Glands. Front. Oncol. 2022, 11, 786150. [Google Scholar] [CrossRef] [PubMed]
- Kerche, L.E.; de Sousa, E.A.; Squarize, C.H.; Oliveira, K.K.; Marchi, F.A.; Bettim, B.B.; Kowalski, L.P.; Soares, F.A.; Lourenço, S.V.; Coutinho-Camillo, C.M. EMT in salivary gland tumors: The expression of microRNAs miR-155 and miR-200c is associated with clinical-pathological parameters. Mol. Biol. Rep. 2022, 49, 2157–2167. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Rodrigo, J.P.; Bradley, P.J.; Poorten, V.V.; Triantafyllou, A.; Hunt, J.L.; Strojan, P.; Rinaldo, A.; Haigentz, M., Jr.; Takes, R.P.; et al. Adenoid cystic carcinoma of the head and neck—An update. Oral Oncol. 2015, 51, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Cantù, G. Adenoid cystic carcinoma. An indolent but aggressive tumour. Part A: From aetiopathogenesis to diagnosis. Acta Otorhinolaryngol. Ital. 2021, 41, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Jaso, J.; Malhotra, R. Adenoid cystic carcinoma. Arch. Pathol. Lab. Med. 2011, 135, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-L.; Fan, Y.-L.; Jiang, J.; Li, K.-D.; Zheng, M.; Chen, W.; Ma, X.-R.; Geng, N.; Chen, Q.-M.; Chen, Y.; et al. C-kit induces epithelial-mesenchymal transition and contributes to salivary adenoid cystic cancer progression. Oncotarget 2014, 5, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Jia, D.; Yan, W.; Zhang, X.; Wang, C.; Li, Y.; Chen, H.; Huang, W.; Li, Z.; Zhang, X. KIT/PDGFRA/KDR amplification defines a novel molecular subtype of adenoid cystic carcinoma patients who may benefit from treatment with tyrosine kinase inhibitors. Transl. Cancer Res. 2020, 9, 4703–4714. [Google Scholar] [CrossRef] [PubMed]
- Swid, M.A.; Li, L.; Drahnak, E.M.; Idom, H.; Quinones, W. Updated Salivary Gland Immunohistochemistry: A Review. Arch. Pathol. Lab. Med. 2023, 147, 1383–1389. [Google Scholar] [CrossRef]
- Moore, A.; Bar, Y.; Maurice-Dror, C.; Ospovat, I.; Sarfaty, M.; Korzets, Y.; Goldvaser, H.; Gordon, N.; Billan, S.; Gutfeld, O.; et al. Next-generation sequencing in salivary gland carcinoma: Targetable alterations lead to a therapeutic advantage—Multicenter experience. Head Neck 2020, 42, 599–607. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Mitani, Y.; Diao, L.; Guijarro, I.; Wang, J.; Zweidler-McKay, P.; Bell, D.; William, W.N.; Glisson, B.S.; Wick, M.J.; et al. Activating NOTCH1 Mutations Define a Distinct Subgroup of Patients With Adenoid Cystic Carcinoma Who Have Poor Prognosis, Propensity to Bone and Liver Metastasis, and Potential Responsiveness to Notch1 Inhibitors. J. Clin. Oncol. 2017, 35, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Ochoa, A.; Jayakumaran, G.; Zehir, A.; Mayor, C.V.; Tepe, J.; Makarov, V.; Dalin, M.G.; He, J.; Bailey, M.; et al. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J. Clin. Investig. 2019, 129, 4276–4289. [Google Scholar] [CrossRef]
- Nordkvist, A.; Mark, J.; Gustafsson, H.; Bang, G.; Stenman, G. Non-random chromosome rearrangements in adenoid cystic carcinoma of the salivary glands. Genes, Chromosom. Cancer 1994, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Davies, H.R.; Mitani, Y.; Van Loo, P.; Shlien, A.; Tarpey, P.S.; Papaemmanuil, E.; Cheverton, A.; Bignell, G.R.; Butler, A.P.; et al. Whole exome sequencing of adenoid cystic carcinoma. J. Clin. Investig. 2013, 123, 2965–2968. [Google Scholar] [CrossRef]
- Mitani, Y.; Liu, B.; Rao, P.H.; Borra, V.J.; Zafereo, M.; Weber, R.S.; Kies, M.; Lozano, G.; Futreal, P.A.; Caulin, C.; et al. Novel MYBL1 Gene Rearrangements with Recurrent MYBL1–NFIB Fusions in Salivary Adenoid Cystic Carcinomas Lacking t(6;9) Translocations. Clin. Cancer Res. 2016, 22, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Roberts, D.; Karpowicz, M.; Hanna, E.Y.; Weber, R.S.; El-Naggar, A.K. Clinical significance of Myb protein and downstream target genes in salivary adenoid cystic carcinoma. Cancer Biol. Ther. 2011, 12, 569–573. [Google Scholar] [CrossRef]
- Andreasen, S.; Tan, Q.; Agander, T.K.; Hansen, T.V.O.; Steiner, P.; Bjørndal, K.; Høgdall, E.; Larsen, S.R.; Erentaite, D.; Olsen, C.H.; et al. MicroRNA dysregulation in adenoid cystic carcinoma of the salivary gland in relation to prognosis and gene fusion status: A cohort study. Virchows Arch. 2018, 473, 329–340. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, X.; Dong, Z.; Cao, G.; Zhang, S. Identification of microRNA profiles in salivary adenoid cystic carcinoma cells during metastatic progression. Oncol. Lett. 2014, 7, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-W.; Chen, B.; Lei, C.-B.; Liu, G.-X.; Wang, Y.-G.; Yi, C.; Wang, Y.-Y.; Zhang, S.-Y. miR-582-5p inhibits invasion and migration of salivary adenoid cystic carcinoma cells by targeting FOXC1. Jpn. J. Clin. Oncol. 2017, 47, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, V.; Straccia, P.; Tralongo, P.; Musarra, T.; Pierconti, F.; Martini, M.; Fadda, G.; Rossi, E.D.; Larocca, L.M. DOG1 as an Immunohistochemical Marker of Acinic Cell Carcinoma: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 9711. [Google Scholar] [CrossRef] [PubMed]
- Sheng, D.; Zhang, Y.; Xue, T.; Zhou, X.-Y.; Li, X.-Q. Identification of LMO2 as a new marker for acinic cell carcinoma of salivary gland. Diagn. Pathol. 2022, 17, 15. [Google Scholar] [CrossRef]
- Ross, J.S.; Gay, L.M.; Wang, K.; Vergilio, J.-A.; Suh, J.; Ramkissoon, S.; Somerset, H.; Johnson, J.M.; Russell, J.; Ali, S.; et al. Comprehensive genomic profiles of metastatic and relapsed salivary gland carcinomas are associated with tumor type and reveal new routes to targeted therapies. Ann. Oncol. 2017, 28, 2539–2546. [Google Scholar] [CrossRef]
- Dogan, S.; Xu, B.; Rana, S.; Chen, H.; Ghossein, R.A.; Berger, M.F.; Ho, A.L.; Katabi, N. Loss of CDKN2A/B is a Molecular Marker of High-grade Histology and is Associated with Aggressive Behavior in Acinic Cell Carcinoma. Mod. Pathol. 2023, 36, 100150. [Google Scholar] [CrossRef]
- Haller, F.; Bieg, M.; Will, R.; Körner, C.; Weichenhan, D.; Bott, A.; Ishaque, N.; Lutsik, P.; Moskalev, E.A.; Mueller, S.K.; et al. Enhancer hijacking activates oncogenic transcription factor NR4A3 in acinic cell carcinomas of the salivary glands. Nat. Commun. 2019, 10, 368. [Google Scholar] [CrossRef]
- Wong, K.S.; Mariño-Enriquez, A.; Hornick, J.L.; Jo, V.Y. NR4A3 Immunohistochemistry Reliably Discriminates Acinic Cell Carcinoma from Mimics. Head Neck Pathol. 2021, 15, 425–432. [Google Scholar] [CrossRef]
- Haller, F.; Skálová, A.; Ihrler, S.; Märkl, B.; Bieg, M.; Moskalev, E.A.; Erber, R.; Blank, S.; Winkelmann, C.; Hebele, S.; et al. Nuclear NR4A3 Immunostaining Is a Specific and Sensitive Novel Marker for Acinic Cell Carcinoma of the Salivary Glands. Am. J. Surg. Pathol. 2019, 43, 1264–1272. [Google Scholar] [CrossRef]
- Andreasen, S.; Varma, S.; Barasch, N.; Thompson, L.D.; Miettinen, M.; Rooper, L.; Stelow, E.B.; Agander, T.K.; Seethala, R.R.; Chiosea, S.I.; et al. The HTN3-MSANTD3 Fusion Gene Defines a Subset of Acinic Cell Carcinoma of the Salivary Gland. Am. J. Surg. Pathol. 2019, 43, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Sun, L.; Zhang, Y.; Liu, X.; Li, X.M.; Zhou, Z.; Cui, Y.M.; Zhou, C.-X.; Li, T.-J. PON3::LCN1 and HTN3::MSANTD3 Gene Fusions With NR4A3/NR4A2 Expression in Salivary Acinic Cell Carcinoma. Am. J. Surg. Pathol. 2024, 48, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Nakaguro, M.; Tada, Y.; Faquin, W.C.; Sadow, P.M.; Wirth, L.J.; Nagao, T. Salivary duct carcinoma: Updates in histology, cytology, molecular biology, and treatment. Cancer Cytopathol. 2020, 128, 693–703. [Google Scholar] [CrossRef]
- Santana, T.; Pavel, A.; Martinek, P.; Steiner, P.; Grossmann, P.; Baněčková, M.; Skálová, A. Biomarker immunoprofile and molecular characteristics in salivary duct carcinoma: Clinicopathological and prognostic implications. Hum. Pathol. 2019, 93, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.H.W. Salivary duct carcinoma: New developments—Morphological variants including pure in situ high grade lesions; Proposed molecular classification. Head Neck Pathol. 2013, 7, S48–S58. [Google Scholar] [CrossRef] [PubMed]
- Filippini, D.M.; Pagani, R.; Tober, N.; Lorini, L.; Riefolo, M.; Molinari, G.; Burato, A.; Alfieri, S.; Bossi, P.; Presutti, L. HER2-targeted therapies for salivary gland cancers. Oral Oncol. 2024, 148, 106612. [Google Scholar] [CrossRef] [PubMed]
- Glisson, B.; Colevas, A.D.; Haddad, R.; Krane, J.; El-Naggar, A.; Kies, M.; Costello, R.; Summey, C.; Arquette, M.; Langer, C.; et al. HER2 expression in salivary gland carcinomas: Dependence on histological subtype. Clin. Cancer Res. 2004, 10, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.A.; Gauthier, M.-E.A.; Blackburn, J.; Grady, J.P.; Kraitsek, S.; Hajdu, E.; Dettmer, M.S.; Dahlstrom, J.E.; Lee, C.S.; Luk, P.P.; et al. Molecular patterns in salivary duct carcinoma identify prognostic subgroups. Mod. Pathol. 2020, 33, 1896–1909. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, S.; Tada, Y.; Ando, M.; Nakaguro, M.; Shirai, Y.; Ueno, T.; Kojima, S.; Hirai, H.; Saigusa, N.; Kano, S.; et al. Identification of novel prognostic and predictive biomarkers in salivary duct carcinoma via comprehensive molecular profiling. NPJ Precis. Oncol. 2022, 6, 82. [Google Scholar] [CrossRef]
- Dalin, M.G.; Desrichard, A.; Katabi, N.; Makarov, V.; Walsh, L.A.; Lee, K.-W.; Wang, Q.; Armenia, J.; West, L.; Dogan, S.; et al. Comprehensive Molecular Characterization of Salivary Duct Carcinoma Reveals Actionable Targets and Similarity to Apocrine Breast Cancer. Clin. Cancer Res. 2016, 22, 4623–4633. [Google Scholar] [CrossRef] [PubMed]
- Skálová, A.; Vanecek, T.; Sima, R.; Laco, J.; Weinreb, I.; Perez-Ordonez, B.; Starek, I.; Geierova, M.; Simpson, R.H.; Passador-Santos, F.; et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto undescribed salivary gland tumor entity. Am. J. Surg. Pathol. 2010, 34, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.T.; Fowler, E.; Krings, G.; Chen, Y.-Y.; Bean, G.R.; Vincent-Salomon, A.; Fuhrmann, L.; Barnick, S.E.; Chen, B.; Hosfield, E.M.; et al. Pan-TRK Immunohistochemistry: A Useful Diagnostic Adjunct For Secretory Carcinoma of the Breast. Am. J. Surg. Pathol. 2019, 43, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Guilmette, J.; Dias-Santagata, D.; Nosé, V.; Lennerz, J.K.; Sadow, P.M. Novel gene fusions in secretory carcinoma of the salivary glands: Enlarging the ETV6 family. Hum. Pathol. 2019, 83, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Rooper, L.M.; Karantanos, T.; Ning, Y.; Bishop, J.A.; Gordon, S.W.; Kang, H. Salivary Secretory Carcinoma With a Novel ETV6-MET Fusion: Expanding the Molecular Spectrum of a Recently Described Entity. Am. J. Surg. Pathol. 2018, 42, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Black, M.; Liu, C.Z.; Onozato, M.; Iafrate, A.J.; Darvishian, F.; Jour, G.; Cotzia, P. Concurrent Identification of Novel EGFR–SEPT14 Fusion and ETV6–RET Fusion in Secretory Carcinoma of the Salivary Gland. Head Neck Pathol. 2020, 14, 817–821. [Google Scholar] [CrossRef]
- Drilon, A. TRK inhibitors in TRK fusion-positive cancers. Ann. Oncol. 2019, 30, viii23–viii30. [Google Scholar] [CrossRef]
- Rooper, L.; Sharma, R.; Bishop, J.A. Polymorphous low grade adenocarcinoma has a consistent p63+/p40− immunophenotype that helps distinguish it from adenoid cystic carcinoma and cellular pleomorphic adenoma. Head Neck Pathol. 2015, 9, 79–84. [Google Scholar] [CrossRef]
- de Andrade, E.P.; Teixeira, L.N.; Montalli, V.A.M.; Garcia, F.d.M.; Passador-Santos, F.; Soares, A.B.; de Araújo, V.C. Epithelial membrane antigen and DOG1 expression in minor salivary gland tumours. Ann. Diagn. Pathol. 2019, 43, 151408. [Google Scholar] [CrossRef] [PubMed]
- Katabi, N.; Xu, B. Polymorphous Adenocarcinoma. Surg. Pathol. Clin. 2021, 14, 127–136. [Google Scholar] [CrossRef]
- Hahn, E.; Xu, B.; Katabi, N.; Dogan, S.; Smith, S.M.; Perez-Ordonez, B.; Patel, P.B.; MacMillan, C.; Lubin, D.J.; Gagan, J.; et al. Comprehensive Molecular Characterization of Polymorphous Adenocarcinoma, Cribriform Subtype: Identifying Novel Fusions and Fusion Partners. Mod. Pathol. 2023, 36, 100305. [Google Scholar] [CrossRef] [PubMed]
- de Jager, V.D.; de Visscher, S.A.H.J.; Schuuring, E.; Doff, J.J.; van Kempen, L.C. A novel PPP2R2A::PRKD1 fusion in a cribriform adenocarcinoma of salivary gland. Genes, Chromosom. Cancer 2023, 62, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Owosho, A.A.; Baker, E.; Wood, C.B.; Jain, R. A novel STRN3::PRKD1 fusion in a cribriform adenocarcinoma of salivary gland with high-grade transformation. Genes Chromosom. Cancer 2023, 62, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Freiberger, S.N.; Brada, M.; Fritz, C.; Höller, S.; Vogetseder, A.; Horcic, M.; Bihl, M.; Michal, M.; Lanzer, M.; Wartenberg, M.; et al. SalvGlandDx—A comprehensive salivary gland neoplasm specific next generation sequencing panel to facilitate diagnosis and identify therapeutic targets. Neoplasia 2021, 23, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Barbieri, A.L.; Bishop, J.A.; Chiosea, S.I.; Dogan, S.; Di Palma, S.; Faquin, W.C.; Ghossein, R.; Hyrcza, M.; Jo, V.Y.; et al. Histologic Classification and Molecular Signature of Polymorphous Adenocarcinoma (PAC) and Cribriform Adenocarcinoma of Salivary Gland (CASG): An International Interobserver Study. Am. J. Surg. Pathol. 2020, 44, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Milchgrub, S.; Gnepp, D.R.; Vuitch, F.; Delgado, R.; Albores-Saavedra, J. Hyalinizing clear cell carcinoma of salivary gland. Am. J. Surg. Pathol. 1994, 18, 74–82. [Google Scholar] [CrossRef]
- Antonescu, C.R.; Katabi, N.; Zhang, L.; Sung, Y.S.; Seethala, R.R.; Jordan, R.C.; Perez-Ordoñez, B.; Have, C.; Asa, S.L.; Leong, I.T.; et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosom. Cancer 2011, 50, 559–570. [Google Scholar] [CrossRef]
- Chapman, E.; Skalova, A.; Ptakova, N.; Martinek, P.; Goytain, A.; Tucker, T.; Xiong, W.; Leader, M.; Kudlow, B.A.; Haimes, J.D.; et al. Molecular Profiling of Hyalinizing Clear Cell Carcinomas Revealed a Subset of Tumors Harboring a Novel EWSR1-CREM Fusion: Report of 3 Cases. Am. J. Surg. Pathol. 2018, 42, 1182–1189. [Google Scholar] [CrossRef]
- Bishop, J.A. IDK what’s next for IDC: The unfolding saga of intraductal carcinoma of salivary glands. Cancer Cytopathol. 2021, 129, 926–927. [Google Scholar] [CrossRef]
- Bishop, J.A. Proceedings of the North American Society of Head and Neck Pathology, Los Angeles, CA, March 20, 2022: Emerging Entities in Salivary Gland Tumor Pathology. Head Neck Pathol. 2022, 16, 179–189. [Google Scholar] [CrossRef]
- Thompson, L.D.R.; Bishop, J.A. Salivary Gland Intraductal Carcinoma: How Do 183 Reported Cases Fit Into a Developing Classification. Adv. Anat. Pathol. 2023, 30, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Skálová, A.; Vanecek, T.; Uro-Coste, E.; Bishop, J.A.; Weinreb, I.; Thompson, L.D.; de Sanctis, S.; Schiavo-Lena, M.; Laco, J.; Badoual, C.; et al. Molecular Profiling of Salivary Gland Intraductal Carcinoma Revealed a Subset of Tumors Harboring NCOA4-RET and Novel TRIM27-RET Fusions: A Report of 17 cases. Am. J. Surg. Pathol. 2018, 42, 1445–1455. [Google Scholar] [CrossRef]
- Robiony, M.; Politi, M.; Avellini, C.; Orsaria, M. Epithelial-myoepithelial carcinoma of the parotid gland: Clinicopathological aspect, diagnosis and surgical consideration. Ann. Maxillofac. Surg. 2014, 4, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Chiosea, S.I.; Miller, M.; Seethala, R.R. HRAS Mutations in epithelial–myoepithelial carcinoma. Head Neck Pathol. 2014, 8, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Nakaguro, M.; Tanigawa, M.D.; Hirai, H.; Yamamoto, Y.M.; Urano, M.; Takahashi, R.H.; Sukeda, A.; Okumura, Y.; Honda, S.; Tasaki, K.; et al. The Diagnostic Utility of RAS Q61R Mutation-specific Immunohistochemistry in Epithelial-Myoepithelial Carcinoma. Am. J. Surg. Pathol. 2021, 45, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, I.; Vollbrecht, C.; Meinrath, J.; Meyer, M.F.; Heukamp, L.C.; Drebber, U.; Quaas, A.; Beutner, D.; Hüttenbrink, K.-B.; Wardelmann, E.; et al. Targeted next generation sequencing of parotid gland cancer uncovers genetic heterogeneity. Oncotarget 2015, 6, 18224–18237. [Google Scholar] [CrossRef] [PubMed]
- Hellquist, H.; Paiva-Correia, A.; Poorten, V.V.; Quer, M.; Hernandez-Prera, J.C.; Andreasen, S.; Zbären, P.; Skalova, A.; Rinaldo, A.; Ferlito, A. Analysis of the Clinical Relevance of Histological Classification of Benign Epithelial Salivary Gland Tumours. Adv. Ther. 2019, 36, 1950–1974. [Google Scholar] [CrossRef]
- Stenman, G. Fusion oncogenes in salivary gland tumors: Molecular and Clinical consequences. Head Neck Pathol. 2013, 7, 12–19. [Google Scholar] [CrossRef]
- de Lima-Souza, R.A.D.; Altemani, A.; Michal, M.; Mariano, F.V.D.; Leivo, I.; Skálová, A. Expanding the Molecular Spectrum of Carcinoma Ex Pleomorphic Adenoma: An Analysis of 84 Cases With a Novel HMGA2::LINC02389 Fusion. Am. J. Surg. Pathol. 2024, 48, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Myers, J.N.; Rao, P.H.; El-Naggar, A.K. t(3;8) as the sole chromosomal abnormality in a myoepithelial carcinoma ex pleomorphic adenoma: A putative progression event. Head Neck 2013, 35, E181–E183. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Mneimneh, W.; Torrence, D.E.; Higgins, K.; Klimstra, D.; Ghossein, R.; Katabi, N. Misinterpreted Myoepithelial Carcinoma of Salivary Gland: A Challenging and Potentially Significant Pitfall. Am. J. Surg. Pathol. 2019, 43, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Dalin, M.G.; Katabi, N.; Persson, M.; Lee, K.-W.; Makarov, V.; Desrichard, A.; Walsh, L.A.; West, L.; Nadeem, Z.; Ramaswami, D.; et al. Multi-dimensional genomic analysis of myoepithelial carcinoma identifies prevalent oncogenic gene fusions. Nat. Commun. 2017, 8, 1197. [Google Scholar] [CrossRef]
- Skálová, A.M.; Weinreb, I.; Hyrcza, M.M.; Simpson, R.H.; Laco, J.M.; Agaimy, A.; Vazmitel, M.M.; Majewska, H.M.; Vanecek, T.R.; Talarčik, P.; et al. Clear cell myoepithelial carcinoma of salivary glands showing EWSR1 rearrangement: Molecular analysis of 94 salivary gland car-cinomas with prominent clear cell component. Am. J. Surg. Pathol. 2015, 39, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.; Freitas, C.; Fernandes, M.G.; Sousa, C.; Reboredo, C.; Cruz-Martins, N.; Mosquera, J.; Hespanhol, V.; Campelo, R. Liquid biopsy: The value of different bodily fluids. Biomarkers Med. 2022, 16, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Kuligina, E.S.; Yanus, G.A.; Imyanitov, E.N. Diversity of the Circulating Tumor Markers: Perspectives of a Multimodal Liquid Biopsy. Biochemistry 2024, 89, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Mouliere, F.; Chandrananda, D.; Piskorz, A.M.; Moore, E.K.; Morris, J.; Ahlborn, L.B.; Mair, R.; Goranova, T.; Marass, F.; Heider, K.; et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl. Med. 2018, 10, eaat4921. [Google Scholar] [CrossRef]
- Weijers, J.A.; Weijers, J.A.; Weijers, J.A.; de Bitter, T.J.; de Bitter, T.J.; de Bitter, T.J.; Verhaegh, G.W.; Verhaegh, G.W.; Verhaegh, G.W.; van Boxtel, W.; et al. Exploring the potential of circulating tumour DNA to monitor treatment response in salivary duct carcinoma patients of the CABO-ASAP trial. Oral Oncol. 2023, 147, 106620. [Google Scholar] [CrossRef] [PubMed]
- Visal, T.H.; Hollander, P.D.; Cristofanilli, M.; Mani, S.A. Circulating tumour cells in the -omics era: How far are we from achieving the ‘singularity’? Br. J. Cancer 2022, 127, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Vorobyeva, A.; Luan, Q.; Papautsky, I. Single Cell Analysis of Inertial Migration by Circulating Tumor Cells and Clusters. Micromachines 2023, 14, 787. [Google Scholar] [CrossRef]
- De Renzi, G.; De Marco, G.; De Meo, M.; Del Rosso, E.; Gazzaniga, P.; Nicolazzo, C. In vitro cultures of circulating tumor cells: A potential tool to unravel drug sensitivity. Cancer Drug Resist. 2022, 5, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Kahounová, Z.; Pícková, M.; Drápela, S.; Bouchal, J.; Szczyrbová, E.; Navrátil, J.; Souček, K. Circulating tumor cell-derived preclinical models: Current status and future perspectives. Cell Death Dis. 2023, 14, 530. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Tiwari, R.; Nauta, J.P.J.; Van der Waal, I.; Snow, G.B. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer 1993, 71, 452–456. [Google Scholar] [CrossRef]
- Weller, P.; Nel, I.; Hassenkamp, P.; Gauler, T.; Schlueter, A.; Lang, S.; Dountsop, P.; Hoffmann, A.-C.; Lehnerdt, G. Detection of circulating tumor cell subpopulations in patients with head and neck squamous cell carcinoma (HNSCC). PLoS ONE 2014, 9, e113706. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Tang, K.; Warkiani, M.; Punyadeera, C.; Batstone, M.D. A pilot study for presence of circulating tumour cells in adenoid cystic carcinoma. Int. J. Oral Maxillofac. Surg. 2021, 50, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Gužvić, N.S.; Lüke, F.; Treitschke, S.; Coluccio, A.; Hoffmann, M.; Feliciello, G.; Varadarajan, A.R.; Lu, X.; Weidele, K.; Botteron, C.; et al. Cellular liquid biopsy provides unique chances for disease monitoring, preclinical model generation and therapy adjustment in rare salivary gland cancer patients. Mol. Oncol. 2024. [Google Scholar] [CrossRef]
- Vaiaki, E.M.; Falasca, M. Comparative analysis of the minimal information for studies of extracellular vesicles guidelines: Advancements and implications for extracellular vesicle research. Semin. Cancer Biol. 2024, 101, 12–24. [Google Scholar] [CrossRef]
- Goberdhan, D.C.I. Large tumour-derived extracellular vesicles as prognostic indicators of metastatic cancer patient survival. Br. J. Cancer 2023, 128, 471–473. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, J.; Zhang, Z.; Qian, Y.; Wang, G.; Duan, M.; Zhao, H.; Yang, Z.; Jiang, X. Mesenchymal stem cell-derived exosome: A tumor regulator and carrier for targeted tumor therapy. Cancer Lett. 2022, 526, 29–40. [Google Scholar] [CrossRef]
- Cocks, A.; Martinez-Rodriguez, V.; Del Vecchio, F.; Schukking, M.; Broseghini, E.; Giannakopoulos, S.; Fabbri, M. Diverse roles of EV-RNA in cancer progression. Semin. Cancer Biol. 2021, 75, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, F.; Martinez-Rodriguez, V.; Schukking, M.; Cocks, A.; Broseghini, E.; Fabbri, M. Professional killers: The role of extracellular vesicles in the reciprocal interactions between natural killer, CD8+ cytotoxic T-cells and tumour cells. J. Extracell. Vesicles 2021, 10, e12075. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M.; et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom. 2013, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.; Mariscal, J.; Kim, M.; Guerra, G.; Victor, B.; Qian, C.; Broseghini, E.; Posadas, E.; Freeman, M.R.; Sharma, S.; et al. miR-1227 Targets SEC23A to Regulate the Shedding of Large Extracellular Vesicles. Cancers 2021, 13, 5850. [Google Scholar] [CrossRef]
- Durante, G.; Broseghini, E.; Comito, F.; Naddeo, M.; Milani, M.; Salamon, I.; Campione, E.; Dika, E.; Ferracin, M. Circulating microRNA biomarkers in melanoma and non-melanoma skin cancer. Expert Rev. Mol. Diagn. 2022, 22, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Nieszporek, A.; Wierzbicka, M.; Labedz, N.; Zajac, W.; Cybinska, J.; Gazinska, P. Role of Exosomes in Salivary Gland Tumors and Technological Advances in Their Assessment. Cancers 2024, 16, 3298. [Google Scholar] [CrossRef]
- Pawel, S.; Maciej, M.; Maciej, Z.; Bogdan, M.; Monika, A.-S. Novel interleukin-33 and its soluble ST2 receptor as potential serum biomarkers in parotid gland tumors. Exp. Biol. Med. 2018, 243, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Dyckhoff, G.; Warta, R.; Gonnermann, A.; Plinkert, P.K.; Flechtenmacher, C.; Volkmann, M. Carbohydrate Antigen 19-9 in Saliva: Possible Preoperative Marker of Malignancy in Parotid Tumors. Otolaryngol--head. Otolaryngol. Neck Surg. 2011, 145, 772–777. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, G.; Zhou, L.; Liu, Y. A joint detection of CEA and CA-50 levels in saliva and serum of patients with tumors in oral region and salivary gland. J. Cancer Res. Clin. Oncol. 2009, 135, 1315–1321. [Google Scholar] [CrossRef]
- Summerer, I.; Unger, K.; Braselmann, H.; Schuettrumpf, L.; Maihoefer, C.; Baumeister, P.; Kirchner, T.; Niyazi, M.; Sage, E.; Specht, H.M.; et al. Circulating microRNAs as prognostic therapy biomarkers in head and neck cancer patients. Br. J. Cancer 2015, 113, 76–82. [Google Scholar] [CrossRef]
- Broseghini, E.; Filippini, D.M.; Fabbri, L.; Leonardi, R.; Abeshi, A.; Molin, D.D.; Fermi, M.; Ferracin, M.; Fernandez, I.J. Diagnostic and Prognostic Value of microRNAs in Patients with Laryngeal Cancer: A Systematic Review. Non-Coding RNA 2023, 9, 9. [Google Scholar] [CrossRef]
- Kabzinski, J.; Maczynska, M.; Majsterek, I. MicroRNA as a Novel Biomarker in the Diagnosis of Head and Neck Cancer. Biomolecules 2021, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Dharmawardana, N.; Ooi, E.H.; Woods, C.; Hussey, D. Circulating microRNAs in head and neck cancer: A scoping review of methods. Clin. Exp. Metastasis 2019, 36, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.R.; Thachil, T.; Gee, H.; Milic, N. Circulating MicroRNAs as Prognostic Molecular Biomarkers in Human Head and Neck Cancer: A Systematic Review and Meta-Analysis. Dis. Markers 2019, 2019, 8632018. [Google Scholar] [CrossRef]
- Filippini, D.M.; Broseghini, E.; Carosi, F.; Molin, D.D.; Riefolo, M.; Fabbri, L.; Abeshi, A.; Fernandez, I.J.; Ferracin, M. A Systematic Review of Diagnostic and Prognostic Biomarkers for Head and Neck Cancer of Unknown Primary: An Unmet Clinical Need. Diagnostics 2023, 13, 1492. [Google Scholar] [CrossRef] [PubMed]
- Cinpolat, O.; Unal, Z.N.; Ismi, O.; Gorur, A.; Unal, M. Comparison of microRNA profiles between benign and malignant salivary gland tumors in tissue, blood and saliva samples: A prospective, case-control study. Braz. J. Otorhinolaryngol. 2017, 83, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.S.; Normando, A.G.C.; Scarini, J.F.; Crescencio, L.R.; de Lima-Souza, R.A.; Mariano, F.V.; Leme, A.F.P. Diagnostic and prognostic value of miRNAs on salivary gland tumors: A systematic review and meta-analysis. Oral Maxillofac. Surg. 2021, 25, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Matse, J.H.; Yoshizawa, J.; Wang, X.; Elashoff, D.; Bolscher, J.G.M.; Veerman, E.C.I.; Bloemena, E.; Wong, D.T.W. Discovery and prevalidation of salivary extracellular microRNA biomarkers panel for the noninvasive detection of benign and malignant parotid gland tumors. Clin. Cancer Res. 2013, 19, 3032–3038. [Google Scholar] [CrossRef]
- Matse, J.H.; Yoshizawa, J.; Wang, X.; Elashoff, D.; Bolscher, J.G.M.; Veerman, E.C.I.; Leemans, C.R.; Pegtel, M.D.; Wong, D.T.W.; Bloemena, E. Human Salivary Micro-RNA in Patients with Parotid Salivary Gland Neoplasms. PLoS ONE 2015, 10, e0142264. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, M.; Palisi, A.; Rossi, G.; Stillitano, I.; Faiella, F.; Montoro, P.; Rodriquez, M.; Palladino, R.; D’ursi, A.M.; Romano, R. Saliva of patients affected by salivary gland tumour: An NMR metabolomics analysis. J. Pharm. Biomed. Anal. 2018, 160, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Tada, Y.; Saotome, T.; Akazawa, K.; Ojiri, H.; Fushimi, C.; Masubuchi, T.; Matsuki, T.; Tani, K.; Osamura, R.Y.; et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2–Positive salivary duct carcinoma. J. Clin. Oncol. 2019, 37, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Shen, R.; Buonocore, D.; Olah, Z.T.; Ni, A.; Ginsberg, M.S.; Ulaner, G.A.; Offin, M.; Feldman, D.; Hembrough, T.; et al. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J. Clin. Oncol. 2018, 36, 2532–2537. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Lassche, G.; Tada, Y.; van Herpen, C.M.L.; Jonker, M.A.; Nagao, T.; Saotome, T.; Hirai, H.; Saigusa, N.; Takahashi, H.; Ojiri, H.; et al. Predictive and Prognostic Biomarker Identification in a Large Cohort of Androgen Receptor-Positive Salivary Duct Carcinoma Patients Scheduled for Combined Androgen Blockade. Cancers 2021, 13, 3527. [Google Scholar] [CrossRef]
- Saigusa, N.; Hirai, H.; Tada, Y.; Kawakita, D.; Nakaguro, M.; Tsukahara, K.; Kano, S.; Ozawa, H.; Kondo, T.; Okami, K.; et al. The Role of the EZH2 and H3K27me3 Expression as a Predictor of Clinical Outcomes in Salivary Duct Carcinoma Patients: A Large-Series Study With Emphasis on the Relevance to the Combined Androgen Blockade and HER2-Targeted Therapy. Front. Oncol. 2021, 11, 779882. [Google Scholar] [CrossRef]
- Fushimi, C.; Tada, Y.; Takahashi, H.; Nagao, T.; Ojiri, H.; Masubuchi, T.; Matsuki, T.; Miura, K.; Kawakita, D.; Hirai, H.; et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann. Oncol. 2018, 29, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Cavalieri, S.; Bergamini, C.; Resteghini, C.; Colombo, E.; Calareso, G.; Mariani, L.; Quattrone, P.; Alfieri, S.; Bossi, P.; et al. Abiraterone Acetate in Patients With Castration-Resistant, Androgen Receptor–Expressing Salivary Gland Cancer: A Phase II Trial. J. Clin. Oncol. 2021, 39, 4061–4068. [Google Scholar] [CrossRef] [PubMed]
- van Boxtel, W.; Locati, L.; van Engen-van Grunsven, A.C.H.; Bergamini, C.; Jonker, M.; Fiets, E.; Cavalieri, S.; Tooten, S.; Bos, E.; Quattrone, P.; et al. Adjuvant androgen deprivation therapy for poor-risk, androgen receptor–positive salivary duct carcinoma. Eur. J. Cancer 2019, 110, 62–70. [Google Scholar] [CrossRef]
- Weijers, J.A.M.; Verhaegh, G.W.; Lassche, G.; van Engen-van Grunsven, A.C.H.; Driessen, C.M.L.; van Erp, N.P.; Jonker, M.A.; Schalken, J.A.; van Herpen, C.M.L. A randomized phase II trial on the addition of dutasteride to combined androgen blockade therapy versus combined androgen blockade therapy alone in patients with advanced or metastatic salivary duct carcinoma—The DUCT study protocol. BMC Cancer 2024, 24, 1174. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum, J.; Shestopalov, I.A.; Henderson, R.E.; Chau, N.G.; Knoechel, B.; Wick, M.J.; Zon, L.I. Zebrafish blastomere screen identifies retinoic acid suppression of MYB in adenoid cystic carcinoma. J. Exp. Med. 2018, 215, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Ferrarotto, R.; Eckhardt, G.; Patnaik, A.; LoRusso, P.; Faoro, L.; Heymach, J.; Kapoun, A.; Xu, L.; Munster, P. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann. Oncol. 2018, 29, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Stathis, A.; Lopez-Miranda, E.; Racca, F.; Quon, D.; Leyvraz, S.; Hess, D.; Keam, B.; Rodon, J.; Ahn, M.-J.; et al. A Phase I Study of the Pan-Notch Inhibitor CB-103 for Patients with Advanced Adenoid Cystic Carcinoma and Other Tumors. Cancer Res. Commun. 2023, 3, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.; Bossi, P.; Perrone, F.; Potepan, P.; Crippa, F.; Mariani, L.; Casieri, P.; Orsenigo, M.; Losa, M.; Bergamini, C.; et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: A phase II study. Oral Oncol. 2009, 45, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Amoroso, V.; Buglione, M.; Rosati, A.; Gatta, R.; Pizzocaro, C.; Ferrari, V.D.; Marini, G. Cetuximab in the treatment of metastatic mucoepidermoid carcinoma of the salivary glands: A case report and review of literature. J. Med. Case Rep. 2008, 2, 320. [Google Scholar] [CrossRef]
- Kawahara, K.; Hiraki, A.; Yoshida, R.; Arita, H.; Matsuoka, Y.; Yamashita, T.; Koga, K.-I.; Nagata, M.; Hirosue, A.; Fukuma, D.; et al. Salivary duct carcinoma treated with cetuximab-based targeted therapy: A case report. Mol. Clin. Oncol. 2017, 6, 886–892. [Google Scholar] [CrossRef]
- Lin, V.T.; Nabell, L.M.; Spencer, S.A.; Carroll, W.R.; Harada, S.; Yang, E.S. First-Line Treatment of Widely Metastatic BRAF-Mutated Salivary Duct Carcinoma With Combined BRAF and MEK Inhibition. J. Natl. Compr. Cancer Netw. 2018, 16, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G.; Kang, S.; Vogt, P.K. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Sheth, H.; Kumar, P.; Shreenivas, A.; Sambath, J.; Pragya, R.; Madre, C.; Athikari, N.; Khandare, H.; Peshattiwar, V.; Datar, R.; et al. Excellent Response With Alpelisib and Bicalutamide for Advanced Salivary Duct Carcinoma With PIK3CA Mutation and High Androgen Receptor Expression—A Case Report. JCO Precis. Oncol. 2021, 5, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Yamaki, H.; Komatsuda, H.; Wakisaka, R.; Inoue, T.; Kumai, T.; Takahara, M. Exploring Immunological Effects and Novel Immune Adjuvants in Immunotherapy for Salivary Gland Cancers. Cancers 2024, 16, 1205. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Kumai, T.; Ishida, Y.; Yuasa, R.; Kubota, A.; Wakisaka, R.; Komatsuda, H.; Yamaki, H.; Wada, T.; Harabuchi, Y. The efficacy of PD-1 inhibitors in patients with salivary gland carcinoma: A retrospective observational study. Laryngoscope Investig. Otolaryngol. 2022, 7, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, U.; Bang, A.; Chen, Y.-H.; Mak, R.H.; Lorch, J.H.; Hanna, G.J.; Nishino, M.; Manuszak, C.; Thrash, E.M.; Severgnini, M.; et al. A Randomized Phase 2 Study of Pembrolizumab With or Without Radiation in Patients With Recurrent or Metastatic Adenoid Cystic Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, S.; Tasoulas, J.; Masaoutis, C.; Kokkali, S.; Klijanienko, J. Salivary gland cancer in the era of immunotherapy: Can we exploit tumor microenvironment? Expert Opin. Ther. Targets 2020, 24, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Vos, J.L.; Burman, B.; Jain, S.; Fitzgerald, C.W.R.; Sherman, E.J.; Dunn, L.A.; Fetten, J.V.; Michel, L.S.; Kriplani, A.; Ng, K.K.; et al. Nivolumab plus ipilimumab in advanced salivary gland cancer: A phase 2 trial. Nat. Med. 2023, 29, 3077–3089. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Baik, C.; Bauman, J.; Gilbert, J.; Brose, M.S.; Grilley-Olson, J.E.; Patil, T.; McDermott, R.; Raez, L.E.; Johnson, J.M.; et al. Larotrectinib treatment for patients With TRK fusion-positive salivary gland cancers. Oncol. 2022, 29, e779–e788. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Li, G.; Dogan, S.; Gounder, M.; Shen, R.; Arcila, M.; Wang, L.; Hyman, D.M.; Hechtman, J.; Wei, G.; et al. What hides behind the MASC: Clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann. Oncol. 2016, 27, 920–926. [Google Scholar] [CrossRef]
- Hyman, D.; Kummar, S.; Farago, A.; Geoerger, B.; Mau-Sorensen, M.; Taylor, M.; Garralda, E.; Nagasubramanian, R.; Natheson, M.; Song, L.; et al. Abstract CT127: Phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi). Cancer Res. 2019, 79, CT127. [Google Scholar] [CrossRef]
- Drilon, A.; Ou, S.-H.I.; Cho, B.C.; Kim, D.-W.; Lee, J.; Lin, J.J.; Zhu, V.W.; Ahn, M.-J.; Camidge, D.R.; Nguyen, J.; et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov. 2018, 8, 1227–1236. [Google Scholar] [CrossRef]
- Rodriguez, C.P.; Wu, Q.; Voutsinas, J.; Fromm, J.R.; Jiang, X.; Pillarisetty, V.G.; Lee, S.M.; Santana-Davila, R.; Goulart, B.; Baik, C.S.; et al. A Phase II Trial of Pembrolizumab and Vorinostat in Recurrent Metastatic Head and Neck Squamous Cell Carcinomas and Salivary Gland Cancer. Clin. Cancer Res. 2020, 26, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Filippini, D.M.; Le Tourneau, C. The potential roles of antibody-drug conjugates in head and neck squamous cell carcinoma. Curr. Opin. Oncol. 2024, 36, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.M.; Mitani, Y.; Marques-Piubelli, M.; Hoff, C.; Bonini, F.; Purushothaman, A.; McGrail, D.; El-Naggar, A.; Ferrarotto, R. 909P TROP2 expression in salivary gland adenoid cystic carcinoma (ACC): A new potential therapeutic target for the non-solid subtype. Ann. Oncol. 2023, 34, S578. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).