Effects of Opioids in Cancer Pain: An Interplay Among Genetic Factors, Immune Response, and Clinical Outcomes—A Scoping Review
Simple Summary
Abstract
1. Introduction
2. Methodology
3. Pain Perception, Opioid Use, and Survival
4. Refractory Cancer Pain: Mechanisms and Consequences
4.1. Mechanisms Affecting the Response to Analgesics
4.2. Consequences of Pain Refractory Pain to Conventional Treatment
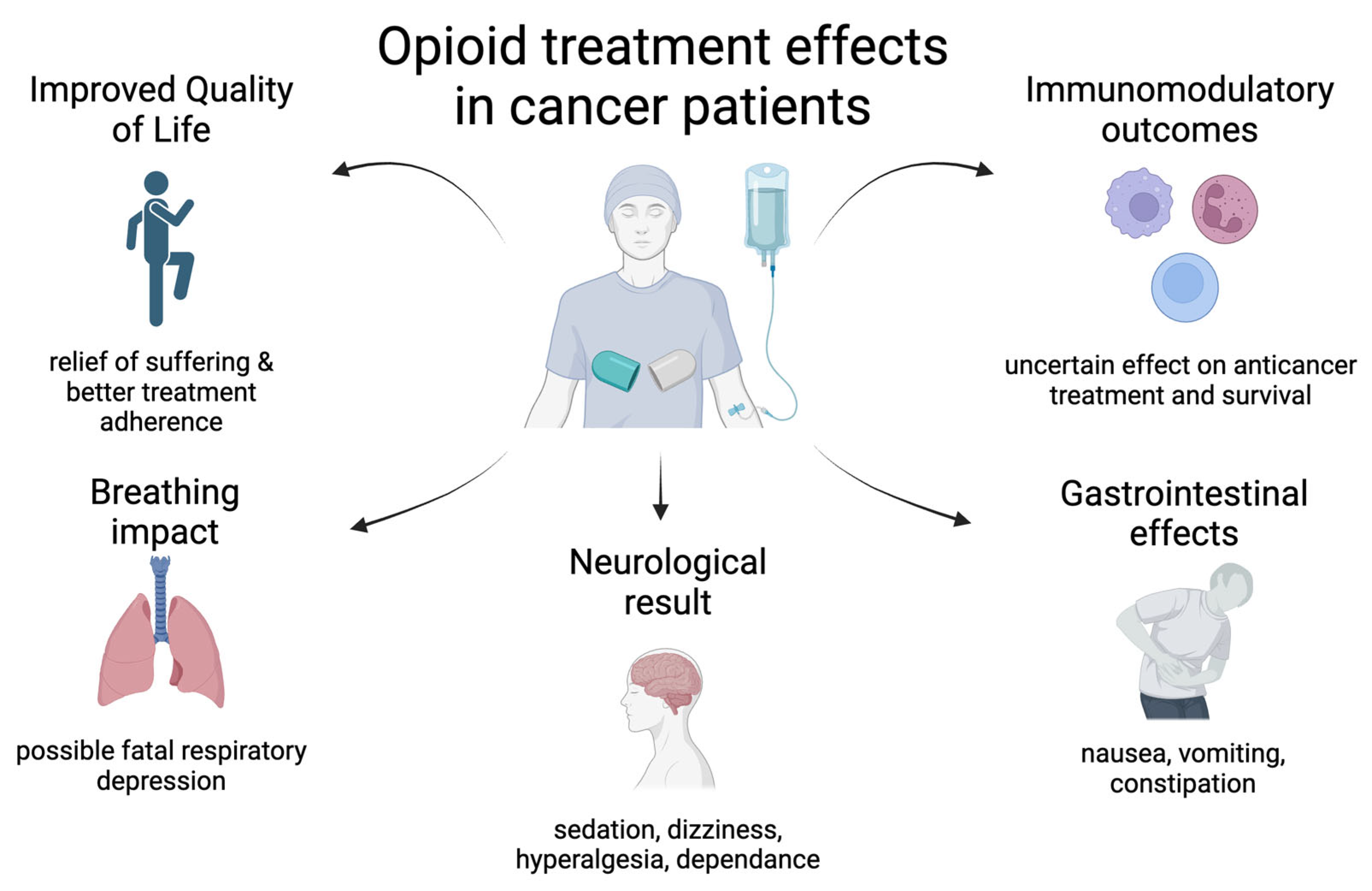
5. Immunosuppression Due to Opioid Treatment
6. Genetic Profiling
6.1. OPRM1, OPRD1, and OPRK1 Genes
6.2. NOP Gene of the Nociceptin/Orphanin FQ Receptor
6.3. CACNA1B Gene
6.4. BCL2 and BAX (Apoptosis-Related Genes)
6.5. FAAH (Fatty Acid Amide Hydrolase) Gene
6.6. KCNJ6 (GIRK2) Gene
6.7. CYP3A4 (Cytochrome P450 3A4) Gene
6.8. CYP3A5 (Cytochrome P450 3A5) Gene
6.9. CYP2C19 (Cytochrome P450 2C19) Gene
6.10. UGT2B7 (UDP Glucuronosyltransferase Family 2 Member B7) Gene
6.11. COMT (Catechol-O-Methyltransferase) Gene
6.12. ABCB1 (ATP-Binding Cassette Sub-Family B Member-1) Gene
6.13. SLC6A3 and SLC6A4 (Solute Carrier Family 6 Member 3 and 4) Gene
6.14. DRD2 (Dopamine Receptor D2) Gene
6.15. NLRs (NOD-like Receptors) Gene
6.16. PTGS2 (Prostaglandin-Endoperoxide Synthase 2) Gene
7. Clinical Applications and Treatment Implications
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zylla, D.; Kuskowski, M.A.; Gupta, K.; Gupta, P. Association of Opioid Requirement and Cancer Pain with Survival in Advanced Non-Small Cell Lung Cancer. Br. J. Anaesth. 2014, 113, i109–i116. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, J.; Wang, W.; Zhou, H.; Cai, S.; Zhu, L.; Qian, X.; Wang, J.; Lu, Z.; Huang, C. The Impact of Pain and Opioids Use on Survival in Cancer Patients. Medicine 2020, 99, e19306. [Google Scholar] [CrossRef] [PubMed]
- Giese-Davis, J.; Collie, K.; Rancourt, K.M.S.; Neri, E.; Kraemer, H.C.; Spiegel, D. Decrease in Depression Symptoms Is Associated with Longer Survival in Patients with Metastatic Breast Cancer: A Secondary Analysis. J. Clin. Oncol. 2011, 29, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Satin, J.R.; Linden, W.; Phillips, M.J. Depression as a Predictor of Disease Progression and Mortality in Cancer Patients. Cancer 2009, 115, 5349–5361. [Google Scholar] [CrossRef]
- Kumar, S.; Kundra, P.; Ramsamy, K.; Chandrasekaran, A. Pharmacogenetics of Opioids: A Narrative Review. Anaesthesia 2019, 74, 1456–1470. [Google Scholar] [CrossRef]
- Frangakis, S.G. Association of Genetic Variants with Postsurgical Pain: A Systematic Review and Meta-Analyses. Anesthesiology 2023, 139, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Zylla, D.; Steele, G.; Gupta, P. A Systematic Review of the Impact of Pain on Overall Survival in Patients with Cancer. Support. Care Cancer 2017, 25, 1687–1698. [Google Scholar] [CrossRef]
- Reyes-Gibby, C.C.; Anderson, K.O.; Merriman, K.W.; Todd, K.H.; Shete, S.; Hanna, E.Y. Survival Patterns in Squamous Cell Carcinoma of the Head and Neck: Pain as an Independent Prognostic Factor for Survival. J. Pain 2014, 15, 1015–1022. [Google Scholar] [CrossRef]
- Boland, J.W.; Bennett, M. State of the Science: Opioids and Survival in Cancer Pain Management. BMJ Support. Palliat. Care 2020, 10, 379–380. [Google Scholar] [CrossRef]
- Janjan, N.A. Improving Cancer Pain Control with NCCN Guideline-Based Analgesic Administration: A Patient-Centered Outcome. J. Natl. Compr. Cancer Netw. 2014, 12, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Kavgaci, G. Impact of Opioid Analgesics on Survival in Cancer Patients Receiving Immune Checkpoint Inhibitors. Support. Care Cancer 2024, 32, 467. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, W.Z.; Mehaffey, J.H.; Desai, R.P.; Beller, J.P.; Balkrishnan, R.; Walters, D.M.; Martin, L.W. Prolonged Opioid Use Associated with Reduced Survival After Lung Cancer Resection. Ann. Thorac. Surg. 2021, 111, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Gao, Z.; Liu, X.; Zhou, H.; Wang, R.; Zheng, C.; Dong, D.-S.; Zhu, Z.; Liu, C.-G. The Negative Impact of Opioids on Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J. Cancer Res. Clin. Oncol. 2022, 149, 2699–2708. [Google Scholar] [CrossRef]
- Zylberberg, H.M.; Woodrell, C.; Rustgi, S.D.; Aronson, A.; Kessel, E.; Amin, S.; Lucas, A.L. Opioid Prescription Is Associated with Increased Survival in Older Adult Patients with Pancreatic Cancer in the United States: A Propensity Score Analysis. JCO Oncol Pract. 2022, 18, e659–e668. [Google Scholar] [CrossRef]
- Bradley, A.; Boland, J.W. Effects of Opioids on Immune and Endocrine Function in Patients with Cancer Pain. Curr. Treat. Options Oncol. 2023, 24, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-J.; Liu, W.-S.; Guan, B.-Q.; Hao, J.-L.; Ji, K.; Cheng, X.-J.; Wang, K. Contribution of Opiate Analgesics to the Development of Infections in Advanced Cancer Patients. Clin. J. Pain 2017, 33, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Montagna, G.; Gupta, H.; Hannum, M.; Tan, K.S.; Lee, J.; Scarpa, J.R.; Plitas, G.; Irie, T.; McCormick, P.J.; Fischer, G.W.; et al. Intraoperative Opioids Are Associated with Improved Recurrence-Free Survival in Triple-Negative Breast Cancer. Br. J. Anaesth. 2021, 126, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.G.; Tan, K.S.; Mastrogiacomo, B.; Dycoco, J.; Caso, R.; Jones, G.D.; McCormick, P.J.; Sánchez-Vega, F.; Irie, T.; Scarpa, J.R.; et al. Intraoperative Opioid Exposure, Tumour Genomic Alterations, and Survival Differences in People with Lung Adenocarcinoma. Br. J. Anaesth. 2021, 127, 75–84. [Google Scholar] [CrossRef]
- Schmidt, B.L. The Neurobiology of Cancer Pain. Neuroscientist 2014, 20, 546–562. [Google Scholar] [CrossRef]
- Eskander, M.A.; Ruparel, S.; Green, D.P.; Chen, P.B.; Por, E.D.; Jeske, N.A.; Gao, X.; Flores, E.R.; Hargreaves, K.M. Persistent Nociception Triggered by Nerve Growth Factor (NGF) Is Mediated by TRPV1 and Oxidative Mechanisms. J. Neurosci. 2015, 35, 8593–8603. [Google Scholar] [CrossRef] [PubMed]
- Prato, V.; Taberner, F.J.; Hockley, J.R.F.; Callejo, G.; Arcourt, A.; Tazir, B.; Hammer, L.; Schad, P.; Heppenstall, P.A.; Smith, E.S.; et al. Functional and Molecular Characterization of Mechanoinsensitive “Silent” Nociceptors. Cell Rep. 2017, 21, 3102–3115. [Google Scholar] [CrossRef] [PubMed]
- Shillo, P.; Yiangou, Y.; Donatien, P.; Greig, M.; Selvarajah, D.; Wilkinson, I.D.; Anand, P.; Tesfaye, S. Nerve and Vascular Biomarkers in Skin Biopsies Differentiate Painful from Painless Peripheral Neuropathy in Type 2 Diabetes. Front. Pain Res. 2021, 2, 731658. [Google Scholar] [CrossRef] [PubMed]
- Melemedjian, O.K.; Asiedu, M.N.; Tillu, D.V.; Peebles, K.A.; Yan, J.; Ertz, N.; Dussor, G.O.; Price, T.J. IL-6- and NGF-Induced Rapid Control of Protein Synthesis and Nociceptive Plasticity via Convergent Signaling to the eIF4F Complex. J. Neurosci. 2010, 30, 15113–15123. [Google Scholar] [CrossRef]
- Salvo, E.; Campana, W.M.; Scheff, N.N.; Nguyen, T.H.; Jeong, S.; Wall, I.; Wu, A.K.; Zhang, S.; Kim, H.; Bhattacharya, A.; et al. Peripheral Nerve Injury and Sensitization Underlie Pain Associated with Oral Cancer Perineural Invasion. Pain 2020, 161, 2592–2602. [Google Scholar] [CrossRef]
- Nijs, J.; Leysen, L.; Adriaenssens, N.; Aguilar Ferrándiz, M.E.; Devoogdt, N.; Tassenoy, A.; Ickmans, K.; Goubert, D.; van Wilgen, C.P.; Wijma, A.J.; et al. Pain following cancer treatment: Guidelines for the clinical classification of predominant neuropathic, nociceptive and central sensitization pain. Acta Oncol. 2016, 55, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Kuner, R.; Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2017, 18, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Urch, C.E.; Donovan-Rodriguez, T.; Dickenson, A.H. Alterations in dorsal horn neurones in a rat model of cancer-induced bone pain. Pain 2003, 106, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, H.; Li, T.; Luo, H.; Gu, X.; Lü, N.; Ji, R.-R.; Zhang, Y. Delayed Activation of Spinal Microglia Contributes to the Maintenance of Bone Cancer Pain in Female Wistar Rats via P2X7 Receptor and IL-18. J. Neurosci. 2015, 35, 7950–7963. [Google Scholar] [CrossRef]
- Huang, G.; Zhou, Z.; Yang, J.; Su, C. Successful Treatment of Refractory Cancer Pain and Depression with Continuous Intrathecal Administration of Dexmedetomidine and Morphine: A Case Report. Pain Ther. 2020, 9, 797–804. [Google Scholar] [CrossRef]
- Porzio, G.; Capela, A.; Giusti, R.; Bianco, F.L.; Moro, M.M.; Ravoni, G.; Zułtak-Baczkowska, K. Multidisciplinary Approach, Continuous Care and Opioid Management in Cancer Pain: Case Series and Review of the Literature. Drugs Context 2023, 12, 2022-11-7. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Voltz, R.; Gaertner, J. Cancer Pain Management and Bone Metastases: An Update for the Clinician. Breast Care 2012, 7, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-P.; Lin, W.-Y.; Lin, F.-S.; Lee, Y.-S.; Jeng, C.-S.; Sun, W. Efficacy of Intrathecal Drug Delivery System for Refractory Cancer Pain Patients: A Single Tertiary Medical Center Experience. J. Formos. Med. Assoc. 2012, 111, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Navratilova, E.; Vagnerova, B.; Watanabe, M.; Kopruszinski, C.; Moreira de Souza, L.H.; Yue, X.; Ikegami, D.; Moutal, A.; Patwardhan, A.; et al. Chronic Pain Recruits Hypothalamic Dynorphin/Kappa Opioid Receptor Signalling to Promote Wakefulness and Vigilance. Brain 2023, 146, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Tegegn, H.G.; Gebreyohannes, E.A. Cancer Pain Management and Pain Interference with Daily Functioning among Cancer Patients in Gondar University Hospital. Pain Res. Manag. 2017, 2017, 5698640. [Google Scholar] [CrossRef]
- Abruquah, A.A.; Biney, R.P.; Osei-Bonsu, E.B.; Boamah, K.M.; Woode, E. Adequacy of Pain Management in Oncology Patients at a Tertiary Hospital in Ghana. Int. J. Basic Clin. Pharmacol. 2017, 6, 251–256. [Google Scholar] [CrossRef]
- Hong, S.H.; Roh, S.Y.; Kim, S.Y.; Shin, S.W.; Kim, C.S.; Choi, J.H.; Kim, S.Y.; Yim, C.Y.; Sohn, C.H.; Song, H.S.; et al. Change in Cancer Pain Management in Korea Between 2001 and 2006: Results of Two Nationwide Surveys. J. Pain Symptom Manag. 2011, 41, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Javier, F.O.; Irawan, C.; Mansor, M.B.; Sriraj, W.; Tan, K.H.; Thinh, D.H.Q. Cancer Pain Management Insights and Reality in Southeast Asia: Expert Perspectives from Six Countries. J. Glob. Oncol. 2016, 2, 235–243. [Google Scholar] [CrossRef]
- Vieira, C.M.P.; Fragoso, R.M.; Pereira, D.; Medeiros, R. Pain Polymorphisms and Opioids: An Evidence Based Review. Mol. Med. Rep. 2019, 19, 1423–1434. [Google Scholar] [CrossRef]
- Bissell, B.D.; Sturgill, J.L.; Bruno, M.E.C.; Lewis, E.D.; Starr, M.E. Assessment of Opioid-Induced Immunomodulation in Experimental and Clinical Sepsis. Crit. Care Explor. 2023, 5, e0849. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, J.J.; Friedman, B.C. Opioids and Immunosuppression: Clinical Evidence, Mechanisms of Action, and Potential Therapies. Palliat. Med. Rep. 2024, 5, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Stein, C. Opioid Receptors. Annu. Rev. Med. 2016, 67, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Pathan, H.; Williams, J. Basic Opioid Pharmacology: An Update. Br. J. Pain 2012, 6, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Ninković, J.; Roy, S. Role of the Mu-Opioid Receptor in Opioid Modulation of Immune Function. Amino Acids 2013, 45, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Stein, C. Targeting Pain and Inflammation by Peripherally Acting Opioids. Front. Pharmacol. 2013, 4, 123. [Google Scholar] [CrossRef] [PubMed]
- Glare, P.; Walsh, D.; Sheehan, D. The Adverse Effects of Morphine: A Prospective Survey of Common Symptoms during Repeated Dosing for Chronic Cancer Pain. Am. J. Hosp. Palliat. Med. 2006, 23, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid Complications and Side Effects. Pain Physician 2008, 11, S105–S120. [Google Scholar] [CrossRef] [PubMed]
- Khosrow-Khavar, F.; Kurteva, S.; Cui, Y.; Filion, K.B.; Douros, A. Opioids and the Risk of Infection: A Critical Appraisal of the Pharmacologic and Clinical Evidence. Expert Opin. Drug Metab. Toxicol. 2019, 15, 565–575. [Google Scholar] [CrossRef]
- Boland, J.W.; Pockley, A.G. Influence of Opioids on Immune Function in Patients with Cancer Pain: From Bench to Bedside. Br. J. Pharmacol. 2018, 175, 2726–2736. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Jiang, Y.; Liang, S.; Cheng, Z.; Zhu, X.; Guo, Q. Opioids Regulate the Immune System: Focusing on Macrophages and Their Organelles. Front. Pharmacol. 2021, 12, 814241. [Google Scholar] [CrossRef]
- Roy, S.; Ninkovic, J.; Banerjee, S.; Charboneau, R.G.; Das, S.; Dutta, R.; Kirchner, V.A.; Koodie, L.; Ma, J.; Meng, J.; et al. Opioid Drug Abuse and Modulation of Immune Function: Consequences in the Susceptibility to Opportunistic Infections. J. Neuroimmune Pharmacol. 2011, 6, 442–465. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.J.; McHugh, D.P.; Magister, M.J.; Zagon, I.S. Endogenous Opioid Inhibition of Proliferation of T and B Cell Subpopulations in Response to Immunization for Experimental Autoimmune Encephalomyelitis. BMC Immunol. 2015, 16, 24. [Google Scholar] [CrossRef]
- Skiba, D.; Jaskuła, K.; Nawrocka, A.; Poznański, P.; Łazarczyk, M.; Szymański, Ł.; Żera, T.; Sacharczuk, M.; Cudnoch-Jędrzejewska, A.; Gaciong, Z. The Role of Opioid Receptor Antagonists in Regulation of Blood Pressure and T-Cell Activation in Mice Selected for High Analgesia Induced by Swim Stress. Int. J. Mol. Sci. 2024, 25, 2618. [Google Scholar] [CrossRef] [PubMed]
- Boland, J.W.; Ziegler, L.; Boland, E.G.; McDermid, K.; Bennett, M.I. Is Regular Systemic Opioid Analgesia Associated with Shorter Survival in Adult Patients with Cancer? A Systematic Literature Review. Pain 2015, 156, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, J.; Huang, S. Effect of Repeated Intraperitoneal Injections of Different Concentrations of Oxycodone on Immune Function in Mice. Front. Pharmacol. 2024, 15, 1370663. [Google Scholar] [CrossRef] [PubMed]
- McIlvried, L.A.; Matos, A.A.M.; Yuan, M.M.; Atherton, M.A.; Obuekwe, F.; Nilsen, M.L.; Nikpoor, A.R.; Talbot, S.; Bruno, T.C.; Taggart, D.N.; et al. Morphine Treatment Restricts Response to Immunotherapy in Oral Squamous Cell Carcinoma. J. ImmunoTherapy Cancer 2024, 12, e009962. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Jia, X.; Jiang, P.; Wang, Q.; Zhang, Y.; Li, Y.; Fu, X.; Jiao, M.; Jiang, L.; Liu, Z.; et al. Effect of Concomitant Use of Analgesics on Prognosis in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 861723. [Google Scholar] [CrossRef]
- Plein, L.M.; Rittner, H.L. Opioids and the Immune System—Friend or Foe. Br. J. Pharmacol. 2018, 175, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Kc, B.; Bhattarai, H.B.; Shah, S.; Bhattarai, M.; Uprety, M.; Jha, A.; Rayamajhi, S.; Pant, S.; Limbu, C.P.; Shrestha, B.R. Herpes Simplex Encephalitis in a Patient Abusing Morphine: A Case Report from Nepal. Ann. Med. Surg. 2023, 85, 1216. [Google Scholar] [CrossRef] [PubMed]
- Puzhko, S.; Eisenberg, M.J.; Filion, K.B.; Windle, S.B.; Hébert-Losier, A.; Gore, G.; Paraskevopoulos, E.; Martel, M.O.; Kudrina, I. Effectiveness of Interventions for Prevention of Common Infections Among Opioid Users: A Systematic Review of Systematic Reviews. Front. Public Health 2022, 10, 749033. [Google Scholar] [CrossRef] [PubMed]
- Kelty, E.; Rae, K.; Jantzie, L.L.; Wyrwoll, C.S.; Preen, D.B. Prenatal Opioid Exposure and Immune-Related Conditions in Children. JAMA Netw. Open 2024, 7, e2351933. [Google Scholar] [CrossRef] [PubMed]
- Kudrina, I.; Page, M.G.; Choinière, M.; Shir, Y.; Eisenberg, M.J.; Ben-Sasson, M.; Lebouché, B.; Puzhko, S. Risk of Infections among Persons Treated with Opioids for Chronic Pain: A Systematic Review and Meta-Analysis Protocol. BMJ Open 2024, 14, e083791. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, J.R.; Montagna, G.; Plitas, G.; Gulati, A.; Fischer, G.W.; Mincer, J.S. Opioids and Immune Checkpoint Inhibitors Differentially Regulate a Common Immune Network in Triple-Negative Breast Cancer. Front. Oncol. 2023, 13, 1267532. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Garcia, A.; Hall, O.M.; Jeha, G.M.; Cramer, K.D.; Granier, A.L.; Kallurkar, A.; Cornett, E.M.; Urman, R.D. Update on the Pharmacogenomics of Pain Management. Pharmacogenomics Pers. Med. 2019, 12, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wacker, D.; Mileni, M.; Katritch, V.; Han, G.W.; Vardy, E.; Liu, W.; Thompson, A.A.; Huang, X.-P.; Carroll, F.I.; et al. Structure of the Human κ-Opioid Receptor in Complex with JDTic. Nature 2012, 485, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Knapman, A.; Santiago, M.; Connor, M. A 6 V Polymorphism of the Human μ-opioid Receptor Decreases Signalling of Morphine and Endogenous Opioids in Vitro. Br. J. Pharmacol. 2015, 172, 2258–2272. [Google Scholar] [CrossRef]
- Vidic, Z.; Goricar, K.; Strazisar, B.; Besic, N.; Dolzan, V. Association of OPRM1, MIR23B, and MIR107 Genetic Variability with Acute Pain, Chronic Pain and Adverse Effects after Postoperative Tramadol and Paracetamol Treatment in Breast Cancer. Radiol. Oncol. 2023, 57, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Oh, C.-S.; Choi, J.M.; Park, S.; Kim, S.-H. Mu-Opioid Receptor Polymorphisms and Breast Cancer Recurrence in Adult Korean Women Undergoing Breast Cancer Surgery: A Retrospective Study. Int. J. Med. Sci. 2020, 17, 2941–2946. [Google Scholar] [CrossRef] [PubMed]
- Schug, S.A.; Zech, D.; Grond, S.; Jung, H.; Meuser, T.; Stobbe, B. A Long-Term Survey of Morphine in Cancer Pain Patients. J Pain Symptom Manag. 1992, 7, 259–266. [Google Scholar] [CrossRef]
- Gagnon, B.; Scott, S.; Nadeau, L.; Lawlor, P.G. Patterns of Community-Based Opioid Prescriptions in People Dying of Cancer. J. Pain Symptom Manag. 2015, 49, 36–44.e1. [Google Scholar] [CrossRef]
- Preux, C.; Bertin, M.; Tarot, A.; Authier, N.; Pinol, N.; Brugnon, D.; Pereira, B.; Guastella, V. Prevalence of Opioid Use Disorder among Patients with Cancer-Related Pain: A Systematic Review. J. Clin. Med. 2022, 11, 1594. [Google Scholar] [CrossRef]
- Viet, C.T.; Dang, D.; Aouizerat, B.E.; Miaskowski, C.; Ye, Y.; Viet, D.T.; Ono, K.; Schmidt, B.L. OPRM1 Methylation Contributes to Opioid Tolerance in Cancer Patients. J. Pain 2017, 18, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.M.; Christrup, L.L.; Sato, H.; Drewes, A.M.; Olesen, A.E. Genetic Influences of OPRM1,OPRD1 and COMT on Morphine Analgesia in a Multi-Modal, Multi-Tissue Human Experimental Pain Model. Basic Clin. Pharmacol. Toxicol. 2017, 121, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kc, B.; Mohd Yusoff, Z.B.; Alrasheedy, A.A.; Othman, S.B. The Characteristics and the Pharmacological Management of Cancer Pain and Its Effect on the Patients’ Daily Activities and Their Quality of Life: A Cross—Sectional Study from Malaysia. J. Clin. Diagn. Res. 2013, 7, 1408. [Google Scholar] [CrossRef]
- Parás-Bravo, P.; Paz-Zulueta, M.; Boixadera-Planas, E.; Fradejas-Sastre, V.; Palacios-Ceña, D.; Fernández-de-las-Peñas, C.; Alonso-Blanco, C. Cancer Patients and Anxiety: A Gender Perspective. Int. J. Environ. Res. Public Health 2020, 17, 1302. [Google Scholar] [CrossRef]
- Julia Willems, A.A.; Kudrashou, A.F.; Theunissen, M.; Hoeben, A.; Van Everdingen, M.H. Measuring Pain in Oncology Outpatients: Numeric Rating Scale Versus Acceptable/Non Acceptable Pain. A Prospective Single Center Study. Pain Pract. 2021, 21, 871–876. [Google Scholar] [CrossRef]
- Zhang, Y.; Schalo, I.; Durand, C.D.; Standifer, K.M. Sex Differences in Nociceptin/Orphanin FQ Peptide Receptor-Mediated Pain and Anxiety Symptoms in a Preclinical Model of Post-Traumatic Stress Disorder. Front. Psychiatry 2019, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Daibani, A.E.; Che, T. Spotlight on Nociceptin/Orphanin FQ Receptor in the Treatment of Pain. Molecules 2022, 27, 595. [Google Scholar] [CrossRef] [PubMed]
- Calò, G.; Lambert, D.G. Nociceptin/Orphanin FQ Receptor Ligands and Translational Challenges: Focus on Cebranopadol as an Innovative Analgesic. Br. J. Anaesth. 2018, 121, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, O.N.A.; Awwad, H.O.; Zhang, Y.; Standifer, K.M. Therapeutic Potential of Nociceptin/Orphanin FQ Peptide (NOP) Receptor Modulators for Treatment of Traumatic Brain Injury, Traumatic Stress, and Their Co-Morbidities. Pharmacol. Ther. 2022, 231, 107982. [Google Scholar] [CrossRef]
- Sukhtankar, D.; Zaveri, N.T.; Husbands, S.M.; Ko, M. Effects of Spinally Administered Bifunctional Nociceptin/Orphanin FQ Peptide Receptor/μ-Opioid Receptor Ligands in Mouse Models of Neuropathic and Inflammatory Pain. J. Pharmacol. Exp. Ther. 2013, 346, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Ding, H.; Ko, M. Central N/Ofq-Nop Receptor System in Pain Modulation. Adv. Pharmacol. 2016, 75, 217–243. [Google Scholar] [CrossRef] [PubMed]
- Khroyan, T.V.; Polgar, W.E.; Orduna, J.; Montenegro, J.L.C.; Jiang, F.; Zaveri, N.T.; Toll, L. Differential Effects of Nociceptin/Orphanin FQ (NOP) Receptor Agonists in Acute Versus Chronic Pain: Studies with Bifunctional NOP/μ Receptor Agonists in the Sciatic Nerve Ligation Chronic Pain Model in Mice. J. Pharmacol. Exp. Ther. 2011, 339, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Sliepen, S.H.J.; Korioth, J.; Christoph, T.; Tzschentke, T.; Diaz-delCastillo, M.; Heegaard, A.; Rutten, K. The Nociceptin/Orphanin FQ Receptor System as a Target to Alleviate Cancer-induced Bone Pain in Rats: Model Validation and Pharmacological Evaluation. Br. J. Pharmacol. 2020, 178, 1995–2007. [Google Scholar] [CrossRef]
- Popiołek-Barczyk, K.; Rojewska, E.; Jurga, A.M.; Makuch, W.; Zador, F.; Borsodi, A.; Piotrowska, A.; Przewłocka, B.; Mika, J. Minocycline Enhances the Effectiveness of Nociceptin/Orphanin FQ During Neuropathic Pain. Biomed Res. Int. 2014, 2014, 762930. [Google Scholar] [CrossRef]
- Yiangou, Y.; Anand, U.; Mukerji, G.; Sinisi, M.; Fox, M.; McQuillan, A.; Quick, T.; Korchev, Y.; Hein, P. Nociceptin/Orphanin FQ Receptor Expression in Clinical Pain Disorders and Functional Effects in Cultured Neurons. Pain 2016, 157, 1960–1969. [Google Scholar] [CrossRef]
- Gavioli, E.C.; Calò, G. Nociceptin/Orphanin FQ Receptor Antagonists as Innovative Antidepressant Drugs. Pharmacol. Ther. 2013, 140, 10–25. [Google Scholar] [CrossRef]
- Phan, N.N.; Wang, C.; Chen, C.; Sun, Z.; Lai, M.; Lin, Y. Voltage-Gated Calcium Channels: Novel Targets for Cancer Therapy. Oncol. Lett. 2017, 14, 2059–2074. [Google Scholar] [CrossRef] [PubMed]
- López Soto, E.J.; Lipscombe, D. Cell-Specific Exon Methylation and CTCF Binding in Neurons Regulate Calcium Ion Channel Splicing and Function. eLife 2020, 9, e54879. [Google Scholar] [CrossRef] [PubMed]
- Gandini, M.A.; Souza, I.A.; Raval, D.; Xu, J.; Pan, Y.X.; Zamponi, G.W. Differential Regulation of Cav2.2 Channel Exon 37 Variants by Alternatively Spliced Μ-Opioid Receptors. Mol. Brain 2019, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.; Sarmento-Ribeiro, A.B.; Andrade, J.P.; Dourado, M. Apoptosis and (In) Pain—Potential Clinical Implications. Biomedicines 2022, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Hosseinzadeh, H. Investigating the Ameliorative Effect of Alpha-mangostin on Development and Existing Pain in a Rat Model of Neuropathic Pain. Phytother. Res. 2020, 34, 3211–3225. [Google Scholar] [CrossRef]
- Zhang, H.; Li, N.; Li, Z.; Li, Y.; Yu, Y.; Zhang, L. The Involvement of Caspases in Neuroinflammation and Neuronal Apoptosis in Chronic Pain and Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 898574. [Google Scholar] [CrossRef]
- Liao, M.; Lu, K.-T.; Hsu, J.-C.; Lee, C.-H.; Cheng, M.; Ro, L. The Role of Autophagy and Apoptosis in Neuropathic Pain Formation. Int. J. Mol. Sci. 2022, 23, 2685. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Fu, C.; Wang, Z.; Zhang, Z.; Wang, H.; Liu, Y. Mangiferin Attenuates Contusive Spinal Cord Injury in Rats Through the Regulation of Oxidative Stress, Inflammation and the BCL-2 and Bax Pathway. Mol. Med. Rep. 2015, 12, 7132–7138. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, T.; Yue, L.; Hu, L. Exogenous Melatonin Alleviates Neuropathic Pain-Induced Affective Disorders by Suppressing NF-κB/ NLRP3 Pathway and Apoptosis. Sci. Rep. 2023, 13, 2111. [Google Scholar] [CrossRef] [PubMed]
- Amin, B.; Taheri, M.M.H.; Hosseinzadeh, H. Effects of Intraperitoneal Thymoquinone on Chronic Neuropathic Pain in Rats. Planta Medica 2014, 80, 1269–1277. [Google Scholar] [CrossRef]
- Kharrazi, A.E. Positive Correlation Between Bax and BCL-2 Gene Polymorphisms with the Risk of Endometriosis: A Case-Control Study. Int. J. Reprod. Biomed. 2024, 22, 451. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Yue, J.; Li, X.; Wu, Q.; Yang, G.; Li, S.; Sun, Q.; Jiang, S. Electroacupuncture Alleviates Neuropathic Pain Through Regulating miR-206-3p Targeting BDNF After CCI. Neural Plast. 2022, 2022, 1489841. [Google Scholar] [CrossRef]
- Yang, P.; Chen, H.; Li, L.; Shi, H.; Li, J.; He, Y.; Su, S.F. Electroacupuncture Attenuates Chronic Inflammatory Pain and Depression Comorbidity by Inhibiting Hippocampal Neuronal Apoptosis via the PI3K/Akt Signaling Pathway. Neurosci. Lett. 2023, 812, 137411. [Google Scholar] [CrossRef] [PubMed]
- Sadhasivam, S.; Zhang, X.; Chidambaran, V.; Mavi, J.; Pilipenko, V.; Mersha, T.B.; Meller, J.; Kaufman, K.M.; Martin, L.J.; McAuliffe, J. Novel Associations between FAAH Genetic Variants and Postoperative Central Opioid-Related Adverse Effects. Pharmacogenomics J. 2015, 15, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Bruehl, S.; Denton, J.S.; Lonergan, D.; Koran, M.E.; Chont, M.; Sobey, C.; Fernando, S.; Bush, W.S.; Mishra, P.; Thornton-Wells, T.A. Associations between KCNJ6 (GIRK2) Gene Polymorphisms and Pain-Related Phenotypes. Pain 2013, 154, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Cajanus, K.; Holmström, E.; Wessman, M.; Anttila, V.; Kaunisto, M.A.; Kalso, E. Effect of Endocannabinoid Degradation on Pain. Pain 2016, 157, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Mikaeili, H.; Habib, A.M.; Yeung, C.W.; Santana-Varela, S.; Luiz, A.P.; Panteleeva, K.; Zuberi, S.; Athanasiou-Fragkouli, A.; Houlden, H.; Wood, J.N.; et al. Molecular Basis of FAAH-Out-Associated Human Pain Insensitivity. Brain 2023, 146, 3851–3865. [Google Scholar] [CrossRef] [PubMed]
- Nasirinezhad, F.; Jergova, S.; Pearson, J.P.; Sagen, J. Attenuation of Persistent Pain-Related Behavior by Fatty Acid Amide Hydrolase (FAAH) Inhibitors in a Rat Model of HIV Sensory Neuropathy. Neuropharmacology 2015, 95, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ozberk, D.; Haywood, A.; Sutherland, H.G.; Yu, C.; Albury, C.L.; Zunk, M.; George, R.; Good, P.; Griffiths, L.R.; Hardy, J.; et al. Association of KCNJ6 Rs2070995 and Methadone Response for Pain Management in Advanced Cancer at End-of-Life. Sci. Rep. 2022, 12, 17422. [Google Scholar] [CrossRef]
- Elens, L.; Norman, E.; Matić, M.; Rane, A.; Fellman, V.; Schaik, R.H.V. Genetic Predisposition to Poor Opioid Response in Preterm Infants: Impact of KCNJ6 and COMT Polymorphisms on Pain Relief After Endotracheal Intubation. Ther. Drug Monit. 2016, 38, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Langford, D.J.; West, C.; Elboim, C.; Cooper, B.A.; Abrams, G.; Paul, S.M.; Schmidt, B.L.; Levine, J.D.; Merriman, J.D.; Dhruva, A.; et al. Variations in Potassium Channel Genes Are Associated with Breast Pain in Women Prior to Breast Cancer Surgery. J. Neurogenet. 2014, 28, 122–135. [Google Scholar] [CrossRef]
- Smith, P.A. K+ Channels in Primary Afferents and Their Role in Nerve Injury-Induced Pain. Front. Cell. Neurosci. 2020, 14, 566418. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, D.; Sánchez-Rodríguez, I.; Jiménez-Díaz, L.; Navarro-López, J.D. Therapeutic Potential of Targeting G Protein-Gated Inwardly Rectifying Potassium (GIRK) Channels in the Central Nervous System. Pharmacol. Ther. 2021, 223, 107808. [Google Scholar] [CrossRef]
- Bony, A.R.; McArthur, J.R.; Finol-Urdaneta, R.K.; Adams, D.J. Analgesic A-conotoxins Modulate Native and Recombinant GIRK1/2 Channels via Activation of GABAB Receptors and Reduce Neuroexcitability. Br. J. Pharmacol. 2021, 179, 179–198. [Google Scholar] [CrossRef]
- Obeng, A.O.; Hamadeh, I.; Smith, M.A. Review of Opioid Pharmacogenetics and Considerations for Pain Management. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Feierman, D.E.; Lasker, J.M. Metabolism of Fentanyl, a Synthetic Opioid Analgesic, by Human Liver Microsomes. Role of CYP3A4. Drug Metab. Dispos. 1996, 24, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Kharasch, E.D.; Hoffer, C.; Whittington, D.; Sheffels, P. Role of Hepatic and Intestinal Cytochrome P450 3A and 2B6 in the Metabolism, Disposition, and Miotic Effects of Methadone. Clin. Pharmacol. Ther. 2004, 76, 250–269. [Google Scholar] [CrossRef]
- Lamba, J.K.; Lin, Y.S.; Schuetz, E.G.; Thummel, K.E. Genetic Contribution to Variable Human CYP3A-Mediated Metabolism. Adv. Drug Deliv. Rev. 2002, 54, 1271–1294. [Google Scholar] [CrossRef] [PubMed]
- Birdwell, K.A.; Decker, B.; Barbarino, J.M.; Peterson, J.F.; Stein, C.M.; Sadee, W.; Wang, D.; Vinks, A.A.; He, Y.; Swen, J.J.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin. Pharmacol. Ther. 2015, 98, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Benjeddou, M.; Peiró, A.M. Pharmacogenomics and Prescription Opioid Use. Pharmacogenomics 2021, 22, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.-G.; Wood, A.J.; Kim, R.B.; Stein, C.M.; Wilkinson, G.R. Genetic Variability in CYP3A5 and Its Possible Consequences. Pharmacogenomics 2004, 5, 243–272. [Google Scholar] [CrossRef] [PubMed]
- Kuip, E.J.M.; Zandvliet, M.L.; Koolen, S.L.W.; Mathijssen, R.H.J.; van der Rijt, C.C.D. A Review of Factors Explaining Variability in Fentanyl Pharmacokinetics; Focus on Implications for Cancer Patients. Br. J. Clin. Pharmacol. 2017, 83, 294–313. [Google Scholar] [CrossRef]
- Rosemary, J.; Adithan, C. The Pharmacogenetics of CYP2C9 and CYP2C19: Ethnic Variation and Clinical Significance. Curr. Clin. Pharmacol. 2007, 2, 93–109. [Google Scholar] [CrossRef]
- Ibeanu, G.C.; Blaisdell, J.; Ferguson, R.J.; Ghanayem, B.I.; Brosen, K.; Benhamou, S.; Bouchardy, C.; Wilkinson, G.R.; Dayer, P.; Goldstein, J.A. A Novel Transversion in the Intron 5 Donor Splice Junction of CYP2C19 and a Sequence Polymorphism in Exon 3 Contribute to the Poor Metabolizer Phenotype for the Anticonvulsant Drug S-Mephenytoin. J. Pharmacol. Exp. Ther. 1999, 290, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Yuan, L.-J.; Hu, X.-X.; Zhou, Q.; Wang, J.; Huang, X.-X.; Dai, D.-P.; Cai, J.-P.; Hu, G.-X. Effects of CYP2C19 Variants on Methadone Metabolism in Vitro. Drug Test. Anal. 2017, 9, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Samer, C.F.; Lorenzini, K.I.; Rollason, V.; Daali, Y.; Desmeules, J.A. Applications of CYP450 Testing in the Clinical Setting. Mol. Diagn. Ther. 2013, 17, 165–184. [Google Scholar] [CrossRef]
- Muraoka, W.; Nishizawa, D.; Fukuda, K.; Kasai, S.; Hasegawa, J.; Wajima, K.; Nakagawa, T.; Ikeda, K. Association between UGT2B7 Gene Polymorphisms and Fentanyl Sensitivity in Patients Undergoing Painful Orthognathic Surgery. Mol. Pain 2016, 12, 1744806916683182. [Google Scholar] [CrossRef]
- Meissner, K.M.; Meyer zu Schwabedissen, H.M.; Göpfert, C.G.; Ding, M.; Blood, J.B.; Frey, K.; Kim, H.; Kharasch, E. UDP Glucuronosyltransferase 2B7 Single Nucleotide Polymorphism (Rs7439366) Influences Heat Pain Response in Human Volunteers after i.v. Morphine Infusion. Crit. Care 2011, 15, 363. [Google Scholar] [CrossRef]
- Satkunananthan, S.E.; Suppiah, V.; Toh, G.-T.; Yow, H.-Y. Pharmacogenomics of Cancer Pain Treatment Outcomes in Asian Populations: A Review. J. Pers. Med. 2022, 12, 1927. [Google Scholar] [CrossRef]
- Sastre, J.A.; Varela, G.; Lopez, M.; Muriel, C.M.; González-Sarmiento, R. Influence of Uridine Diphosphate-Glucuronyltransferase 2B7 (UGT2B7) Variants on Postoperative Buprenorphine Analgesia. Pain Pract. 2013, 15, 22–30. [Google Scholar] [CrossRef]
- Ning, M.; Tao, Y.; Hu, X.; Guo, L.; Ni, J.; Hu, J.; Shen, H.; Chen, Y. Roles of UGT2B7 C802T Gene Polymorphism on the Efficacy of Morphine Treatment on Cancer Pain Among the Chinese Han Population. Niger. J. Clin. Pract. 2019, 22, 1319. [Google Scholar] [CrossRef] [PubMed]
- Margarit, C.; Roca, R.; Inda, M.-M.; Muriel, J.; Ballester, P.; Moreu, R.; Conte, A.L.; Gaviria, Á.; Morales, D.; Peiró, A.M. Genetic Contribution in Low Back Pain: A Prospective Genetic Association Study. Pain Pract. 2019, 19, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Gregori, M.D.; Garbin, G.; Gregori, S.D.; Minella, C.E.; Bugada, D.; Lisa, A.; Govoni, S.; Regazzi, M.; Allegri, M.; Ranzani, G.N. Genetic Variability at COMT but Not at OPRM1 and UGT2B7 Loci Modulates Morphine Analgesic Response in Acute Postoperative Pain. Eur. J. Clin. Pharmacol. 2013, 69, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, P.; Mei, Q.; Xia, S.; Tian, Y.; Hu, L.; Chen, Y. The Impact of UGT2B7 C802T and CYP3A4*1G Polymorphisms on Pain Relief in Cancer Patients Receiving Oxycontin. Support. Care Cancer 2018, 26, 2763–2767. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Tsurutani, J.; Chiba, Y.; Fujita, Y.; Sakai, K.; Yoshida, T.; Nakura, M.; Sakamoto, R.; Makimura, C.; Ohtake, Y.; et al. Morphine Versus Oxycodone for Cancer Pain Using a Catechol-O-Methyltransferase Genotype Biomarker: A Multicenter, Randomized, Open-Label, Phase III Clinical Trial (RELIEF Study). Oncologist 2022, 28, 278-e166. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yin, J.; Xiong, E.; Zhai, J.; Xie, L.; Li, Y.; Qin, X.; Wang, E.; Zhang, Q.; Zuo, Y.; et al. COMT Gene Variants and Β-Endorphin Levels Contribute to Ethnic Differences in Experimental Pain Sensitivity. Mol. Pain 2020, 16, 1744806920908474. [Google Scholar] [CrossRef]
- Khan, A. Unraveling Catechol-O-Methyltransferase Rs4680 SNP’s Role in Patients’ Response to Tramadol and Its Adverse Effects: A Pharmacogenetics Insight into Postoperative Pain Management. J. Clin. Med. 2023, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Korczeniewska, O.A.; Kuo, F.; Huang, C.Y.; Nasri-Heir, C.; Khan, J.; Benoliel, R.; Hirschberg, C.; Eliav, E.; Diehl, S.R. Genetic Variation in Catechol-O-methyltransferase Is Associated with Individual Differences in Conditioned Pain Modulation in Healthy Subjects. J. Gene Med. 2021, 23, e3374. [Google Scholar] [CrossRef] [PubMed]
- Vetterlein, A.; Monzel, M.; Reuter, M. Are Catechol-O-Methyltransferase Gene Polymorphisms Genetic Markers for Pain Sensitivity After All?—A Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2023, 148, 105112. [Google Scholar] [CrossRef] [PubMed]
- Cornett, E.M.; Turpin, M.A.C.; Pinner, A.; Thakur, P.; Sekaran, T.S.G.; Siddaiah, H.; Rivas, J.L.M.; Yates, A.; Huang, G.J.; Senthil, A.; et al. Pharmacogenomics of Pain Management: The Impact of Specific Biological Polymorphisms on Drugs and Metabolism. Curr. Oncol. Rep. 2020, 22, 18. [Google Scholar] [CrossRef]
- Fujita, Y.; Matsuoka, H.; Chiba, Y.; Tsurutani, J.; Yoshida, T.; Sakai, K.; Nakura, M.; Sakamoto, R.; Makimura, C.; Ohtake, Y.; et al. Novel Single Nucleotide Polymorphism Biomarkers to Predict Opioid Effects for Cancer Pain. Oncol. Lett. 2023, 26, 355. [Google Scholar] [CrossRef] [PubMed]
- Ferrera, D.; Mercado, F.; Peláez, I.; Martínez-Íñigo, D.; Fernandes-Magalhaes, R.; Barjola, P.; Écija, C.; Díaz-Gil, G.; Gómez-Esquer, F. Fear of Pain Moderates the Relationship Between Self-Reported Fatigue and Methionine Allele of Catechol-O-Methyltransferase Gene in Patients with Fibromyalgia. PLoS ONE 2021, 16, e0250547. [Google Scholar] [CrossRef]
- Bartošová, O.; Polanecký, O.; Perlik, F.; Adámek, S.; Slanař, O. OPRM1 and ABCB1 Polymorphisms and Their Effect on Postoperative Pain Relief with Piritramide. Physiol. Res. 2015, 64, S521–S527. [Google Scholar] [CrossRef] [PubMed]
- Nojkov, J.; Kapталoв, A.; Kyзманoвска, Б.; Spiroska, T.; Seljmani, R.; Трајкoвски, Ѓ.; Matevska-Geshkovska, N.; Dimovski, A. Association of Single-Nucleotide Polymorhism C3435T in the ABCB1 Gene with Opioid Sensitivity in Treatment of Postoperative Pain. Prilozi 2016, 37, 73–80. [Google Scholar] [CrossRef]
- Liew, Y.; Capule, F.; Makmor-Bakry, M. Effects of Genetic Polymorphisms of ABCB1 on the Efficacy of Anesthetic and Analgesic Agents: A Systematic Review. Pharmacogenomics 2021, 22, 1099–1106. [Google Scholar] [CrossRef]
- Parchure, A.S.; Peng, Y.B. The Impact of Opioid Analgesics and the Pharmacogenomics of ABCB1 in Opioid Dependence and Pharmacotherapies: A Short Review. Open Pain J. 2020, 13, 7–21. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Chidambaran, V.; Zhang, X.; Meller, J.; Esslinger, H.; Zhang, K.; Martin, L.J.; McAuliffe, J.J. Opioid-Induced Respiratory Depression: ABCB1 Transporter Pharmacogenetics. Pharmacogenomics J. 2014, 15, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. Study on the Association Between Adverse Drug Reactions to Opioids and Gene Polymorphisms: A Case-Case–Control Study. BMC Pharmacol. Toxicol. 2023, 24, 64. [Google Scholar] [CrossRef] [PubMed]
- Nerenz, R.D.; Tsongalis, G.J. Pharmacogenetics of Opioid Use and Implications for Pain Management. J. Appl. Lab. Med. 2018, 2, 622–632. [Google Scholar] [CrossRef]
- Lloyd, R.A.; Hotham, E.; Hall, C.; Williams, M.; Suppiah, V. Pharmacogenomics and Patient Treatment Parameters to Opioid Treatment in Chronic Pain: A Focus on Morphine, Oxycodone, Tramadol, and Fentanyl. Pain Med. 2017, 18, 2369–2387. [Google Scholar] [CrossRef]
- Soultati, I.; Ntenti, C.; Tsaousi, G.; Pourzitaki, C.; Gkinas, D.; Thomaidou, E.; Alexandrakis, S.; Papavramidis, T.; Goulas, A. Effect of Common OPRM1, COMT, SLC6A4, ABCB1, and CYP2B6 Polymorphisms on Perioperative Analgesic and Propofol Demands on Patients Subjected to Thyroidectomy Surgery. Pharmacol. Rep. 2023, 75, 386–396. [Google Scholar] [CrossRef]
- Saiz-Rodríguez, M.; Valdez-Acosta, S.; Borobia, A.M.; Burgueño, M.; Gálvez-Múgica, M.A.; Acero, J.; Cabaleiro, T.; Muñoz-Guerra, M.F.; Puerro, M.; Llanos, L.; et al. Influence of Genetic Polymorphisms on the Response to Tramadol, Ibuprofen, and the Combination in Patients with Moderate to Severe Pain After Dental Surgery. Clin. Ther. 2021, 43, e86–e102. [Google Scholar] [CrossRef] [PubMed]
- Qadri, Y.J.; Bortsov, A.V.; Orrey, D.C.; Swor, R.A.; Peak, D.A.; Jones, J.S.; Rathlev, N.K.; Lee, D.H.; Domeier, R.M.; Hendry, P.L.; et al. Genetic Polymorphisms in the Dopamine Receptor 2 Predict Acute Pain Severity After Motor Vehicle Collision. Clin. J. Pain 2015, 31, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Horjales-Araújo, E.; Dahl, J.B. Is the Experience of Thermal Pain Genetics Dependent? Biomed Res. Int. 2015, 2015, 349584. [Google Scholar] [CrossRef]
- Jääskeläinen, S.; Lindholm, P.; Valmunen, T.; Pesonen, U.; Taiminen, T.; Virtanen, A.; Lamusuo, S.; Forssell, H.; Hagelberg, N.; Hietala, J.; et al. Variation in the Dopamine D2 Receptor Gene Plays a Key Role in Human Pain and Its Modulation by Transcranial Magnetic Stimulation. PAIN® 2014, 155, 2180–2187. [Google Scholar] [CrossRef]
- Zahari, Z.; Lee, C.; Ibrahim, M.; Musa, N.; Yasin, M.M.; Lee, Y.; Tan, S.; Mohamad, N.; Ismail, R. Influence of DRD2 Polymorphisms on the Clinical Outcomes of Opioiddependent Patients on Methadone Maintenance Therapy. J. Pharm. Bioallied Sci. 2020, 12, S787–S803. [Google Scholar] [CrossRef] [PubMed]
- Noble, E. D2 Dopamine Receptor Gene in Psychiatric and Neurologic Disorders and Its Phenotypes. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2003, 116, 103–125. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.M.; Strand, K.A.; Galer, E.L.; Urban, D.J.; Wang, X.; Baratta, M.V.; Fabisiak, T.J.; Anderson, N.D.; Cheng, K.; Greene, L.I.; et al. Morphine Paradoxically Prolongs Neuropathic Pain in Rats by Amplifying Spinal NLRP3 Inflammasome Activation. Proc. Natl. Acad. Sci. USA 2016, 113, E3441–E3450. [Google Scholar] [CrossRef] [PubMed]
- Zare, N. NLRs and Inflammasome Signaling in Opioid-Induced Hyperalgesia and Tolerance. Inflammopharmacology 2023, 32, 127–148. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C.; Averitt, D.L.; Basu, A. NLR Family Pyrin Domain Containing 3 (NLRP3) Inflammasomes and Peripheral Neuropathic Pain—Emphasis on microRNAs (miRNAs) as Important Regulators. Eur. J. Pharmacol. 2023, 955, 175901. [Google Scholar] [CrossRef] [PubMed]
- Martín-Vázquez, E.; Cobo-Vuilleumier, N.; López-Noriega, L.; Lorenzo, P.I.; Gauthier, B.R. The PTGS2/COX2-PGE2 Signaling Cascade in Inflammation: Pro or Anti? A Case Study with Type 1 Diabetes Mellitus. Int. J. Biol. Sci. 2023, 19, 4157–4165. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, H.; Wu, T.-X.; Wang, X.-M.; Dionne, R.A. Genetically Mediated Interindividual Variation in Analgesic Responses to Cyclooxygenase Inhibitory Drugs. Acute Pain 2006, 8, 141–142. [Google Scholar] [CrossRef]
- Shailendra Kapoor, M.D. Affect of Genetic Polymorphisms on Pain Perception and Pain Management in Lung Cancer Survivors. J. Opioid Manag. 2012, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, P.; Liu, X.; Liao, H.; Chen, K.; Wang, Y.; Qin, J.; Yang, F. Sinomenine Alleviates Diabetic Peripheral Neuropathic Pain Through Inhibition of the Inositol-requiring Enzyme 1 Alpha–X-box Binding Protein 1 Pathway by Downregulating Prostaglandin-endoperoxide Synthase 2. J. Diabetes Investig. 2023, 14, 364–375. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wen, B.; Xu, L.; Huang, Y. Identification of Potential Inflammation-Related Genes and Key Pathways Associated with Complex Regional Pain Syndrome. Biomolecules 2023, 13, 772. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.; Tang, Y.; Zhang, M.J.; Hai, T.; Bhatnagar, A.; Srivastava, S.; Spite, M. Atf3 Negatively Regulates Ptgs2/Cox2 Expression During Acute Inflammation. Prostaglandins Other Lipid Mediat. 2015, 116–117, 49–56. [Google Scholar] [CrossRef] [PubMed]
| Category | Gene/ Citation | Role | Impact on Pain Perception | Effect on Analgesic Drugs |
|---|---|---|---|---|
| Opioid Signaling Pathway | OPRM1 OPRD1 OPRK1 | Encode classical opioid receptors μ-, δ-, and κ-, mediating opioid analgesic effects. | Variations may lead to differences in analgesic efficacy and pain control. | Influence opioid responsiveness, potentially causing inadequate pain relief or increased sensitivity. |
| NOP | Encodes nociceptin/orphanin FQ receptor, involved in non-classical opioid signaling. | Modulates nociceptive neurotransmission, reducing pain perception. | Potential target for novel analgesics with fewer side effects compared to traditional opioids. | |
| Pain Perception Modulators | CACNA1B | Encodes N-type calcium channel subunit, critical for neurotransmitter release. | Overexpression enhances pain sensation; linked to hyperalgesia. | Genetic variants may alter opioid efficacy; dysfunction affects analgesic responses. |
| BCL2 BAX | Regulate cellular apoptosis; BCL2 gene is anti-apoptotic, while BAX is pro-apoptotic. | Imbalance increases neuronal sensitivity to pain. | Targeting these pathways may reduce pain and modulate glial activation. | |
| FAAH | Enzyme degrading endocannabinoids, particularly anandamide. | Inhibition increases anandamide levels, reducing pain sensitivity. | FAAH 1 inhibitors may enhance analgesic effects; polymorphisms affect treatment responses. | |
| KCNJ6 | Encodes GIRK2 2 potassium channels, regulating neuronal excitability. | Variants influence pain sensitivity and opioid requirements. | Genetic variants may necessitate opioid dosage adjustments for effective analgesia. | |
| Drug Metabolism Genes | CYP2D6 CYP2B6 CYP3A4 CYP3A5 CYP2C19 | Enzymes involved in Phase I drug metabolism, including opioids. | No direct influence on pain perception. | Variants affect opioid metabolism, altering efficacy and risk of side effects like toxicity or inadequate relief. |
| UGT2B7 | Enzyme responsible for Phase II glucuronidation of opioids like morphine. | No direct influence on pain perception. | Polymorphisms impact opioid efficacy by altering metabolite levels; dosage adjustments may be required. | |
| COMT | Metabolizes catecholamines, affecting neurotransmitter levels. | Polymorphisms influence pain sensitivity and stress response. | Variants affect opioid dosage requirements; specific genotypes need higher or lower doses for effective pain control. | |
| Transport and Regulatory Genes | ABCB1 | Encodes P-glycoprotein, an efflux transporter affecting drug distribution and blood-brain barrier crossing. | Variants influence nociceptive processing and pain sensitivity. | Alters opioid transport, affecting drug efficacy and toxicity. |
| SLC6A3 SLC6A4 | Encode dopamine and serotonin transporters, regulating synaptic neurotransmitter levels. | Polymorphisms may influence pain modulation and emotional responses. | May affect efficacy of drugs modulating serotonergic or dopaminergic systems. | |
| DRD2 | Encodes dopamine D2 receptor, involved in dopaminergic signaling. | Variants affect pain sensitivity and stress responses. | May influence opioid efficacy and risk of addiction. | |
| NLRs | Encode NOD-like receptors 3, involved in immune and inflammatory responses. | Activation amplifies nociceptive signaling through pro-inflammatory cytokines. | Potential therapeutic targets for reducing opioid tolerance and opioid-induced hyperalgesia. | |
| Inflammatory Response Gene | PTGS2 | Encodes COX-2 4, essential for prostaglandin synthesis in inflammatory responses. | Variants linked to heightened pain sensitivity and inflammation. | Target for NSAIDs 5; polymorphisms may affect anti-inflammatory drug efficacy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, K.; Zuzda, K.; Jankowski, M.; Świerczyński, R.; Chudziński, K.; Czapski, B.; Szułdrzyński, K. Effects of Opioids in Cancer Pain: An Interplay Among Genetic Factors, Immune Response, and Clinical Outcomes—A Scoping Review. Cancers 2025, 17, 863. https://doi.org/10.3390/cancers17050863
Adamczyk K, Zuzda K, Jankowski M, Świerczyński R, Chudziński K, Czapski B, Szułdrzyński K. Effects of Opioids in Cancer Pain: An Interplay Among Genetic Factors, Immune Response, and Clinical Outcomes—A Scoping Review. Cancers. 2025; 17(5):863. https://doi.org/10.3390/cancers17050863
Chicago/Turabian StyleAdamczyk, Kamil, Konrad Zuzda, Miłosz Jankowski, Rafał Świerczyński, Kamil Chudziński, Bartosz Czapski, and Konstanty Szułdrzyński. 2025. "Effects of Opioids in Cancer Pain: An Interplay Among Genetic Factors, Immune Response, and Clinical Outcomes—A Scoping Review" Cancers 17, no. 5: 863. https://doi.org/10.3390/cancers17050863
APA StyleAdamczyk, K., Zuzda, K., Jankowski, M., Świerczyński, R., Chudziński, K., Czapski, B., & Szułdrzyński, K. (2025). Effects of Opioids in Cancer Pain: An Interplay Among Genetic Factors, Immune Response, and Clinical Outcomes—A Scoping Review. Cancers, 17(5), 863. https://doi.org/10.3390/cancers17050863








