The Effect of Blood Selenium Level on the pCR Rate in Breast Cancer Patient Receiving Neoadjuvant Chemotherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
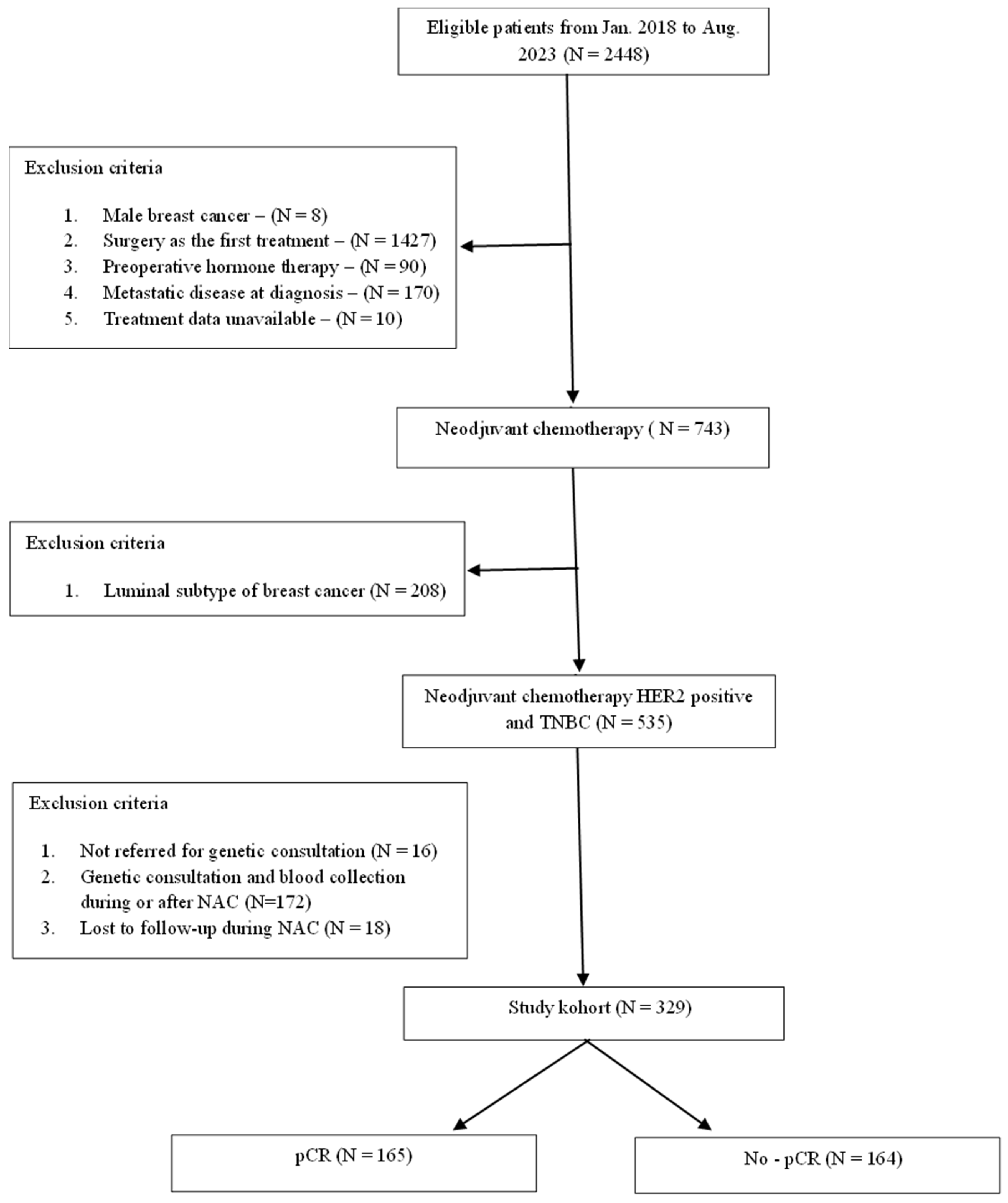
2.1. Study Population
2.2. Analytical Procedures
2.3. Statistical Analysis
3. Results
General Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bonadonna, G.; Valagussa, P.; Brambilla, C.; Ferrari, L. Preoperative chemotherapy in operable breast cancer. Lancet 1993, 341, 1485. [Google Scholar] [CrossRef]
- Mauri, D.; Pavlidis, N.; Ioannidis, J.P.A. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 188–194. [Google Scholar] [CrossRef]
- Smith, B.L. Neoadjuvant versus adjuvant systemic therapy for operable breast cancer: Equivalent outcomes? Ann. Surg. 2013, 257, 180–181. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Buchholz, T.A.; Tucker, S.L.; Meric-Bernstam, F.; Kuerer, H.M.; Gonzalez-Angulo, A.M.; Bedrosian, I.; Babiera, G.V.; Hoffman, K.; Yi, M.; et al. Impact of chemotherapy sequencing on local-regional failure risk in breast cancer patients undergoing breast-conserving therapy. Ann. Surg. 2013, 257, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Killelea, B.K.; Yang, V.Q.; Mougalian, S.; Horowitz, N.R.; Pusztai, L.; Chagpar, A.B.; Lannin, D.R. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: Results from the national cancer database. J. Am. Coll. Surg. 2015, 220, 1063–1069. [Google Scholar] [CrossRef]
- Boughey, J.C.; McCall, L.M.; Ballman, K.V.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Flippo-Morton, T.; Hunt, K.K. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer. Ann. Surg. 2014, 260, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Mamtani, A.; Barrio, A.V.; King, T.A.; Van Zee, K.J.; Plitas, G.; Pilewskie, M.; El-Tamer, M.; Gemignani, M.L.; Heerdt, A.S.; Sclafani, L.M.; et al. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? results of a prospective study. Ann. Surg. Oncol. 2016, 23, 3467–3474. [Google Scholar] [CrossRef]
- Chehade, H.E.H.; Headon, H.; El Tokhy, O.; Heeney, J.; Kasem, A.; Mokbel, K. Is sentinel lymph node biopsy a viable alternative to complete axillary dissection following neoadjuvant chemotherapy in women with node-positive breast cancer at diagnosis? An updated meta-analysis involving 3398 patients. Am. J. Surg. 2016, 212, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Fisher, B.; Bryant, J.; Wolmark, N.; Mamounas, E.; Brown, A.; Fisher, E.R.; Wickerham, D.L.; Begovic, M.; DeCillis, A.; Robidoux, A.; et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J. Clin. Oncol. 1998, 41, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Lee, S.-J.; Ohtani, S.; Im, Y.-H.; Lee, E.-S.; Yokota, I.; Kuroi, K.; Im, S.-A.; Park, B.-W.; Kim, S.-B.; et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N. Engl. J. Med. 2017, 376, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.; Bergh, J.; Burstein, H.; Cardoso, M.; Carey, L.; Dawood, S.; Del Mastro, L.; et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef]
- Coates, A.S.; Colleoni, M.; Goldhirsch, A. Is adjuvant chemotherapy useful for women with luminal a breast cancer? J. Clin. Oncol. 2012, 30, 1260–1263. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef]
- Gori, S.; Fabi, A.; Angiolini, C.; Turazza, M.; Salvini, P.; Ferretti, G.; Cretella, E.; Gianni, L.; Bighin, C.; Toss, A.; et al. Neoadjuvant Systemic Therapy in Early Breast Cancer: Results of a Prospective Observational Multicenter BRIDE Study. Cancers 2023, 15, 4852. [Google Scholar] [CrossRef] [PubMed]
- Schott, A.F.; Hayes, D.F. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J. Clin. Oncol. 2012, 30, 1747–1749. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, V.; Broglio, K.; Kau, S.-W.; Cristofanilli, M.; Buzdar, A.U.; Valero, V.; Buchholz, T.; Meric, F.; Middleton, L.; Hortobagyi, G.N.; et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J. Clin. Oncol. 2006, 24, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Truin, W.; Vugts, G.; Roumen, R.M.H.; Maaskant-Braat, A.J.G.; Nieuwenhuijzen, G.A.P.; Loo, M.v.d.H.-V.d.; Tjan-Heijnen, V.C.G.; Voogd, A.C. Differences in Response and Surgical Management with Neoadjuvant Chemotherapy in Invasive Lobular Versus Ductal Breast Cancer. Ann. Surg. Oncol. 2016, 23, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lips, E.H.; I-SPY TRIAL Investigators; Mukhtar, R.A.; Yau, C.; de Ronde, J.J.; Livasy, C.; Carey, L.A.; Loo, C.E.; Vrancken-Peeters, M.-J.T.F.D.; Sonke, G.S.; et al. Lobular histology and response to neoadjuvant chemotherapy in invasive breast cancer. Breast Cancer Res. Treat. 2012, 136, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Precht, L.M.; Lowe, K.A.; Atwood, M.; Beatty, J.D. Neoadjuvant chemotherapy of breast cancer: Tumor markers as predictors of pathologic response, recurrence, and survival. Breast J. 2010, 16, 362–368. [Google Scholar] [CrossRef]
- Denkert, C.; Loibl, S.; Müller, B.M.; Eidtmann, H.; Schmitt, W.D.; Eiermann, W.; Gerber, B.; Tesch, H.; Hilfrich, J.; Huober, J.; et al. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: A translational investigation in the neoadjuvant GeparTrio trial. Ann. Oncol. 2013, 24, 2786–2793. [Google Scholar] [CrossRef]
- Sueta, A.; Yamamoto, Y.; Hayashi, M.; Yamamoto, S.; Inao, T.; Ibusuki, M.; Murakami, K.; Iwase, H. Clinical significance of pretherapeutic Ki67 as a predictive parameter for response to neoadjuvant chemotherapy in breast cancer; is it equally useful across tumor subtypes? Surgery 2014, 155, 927–935. [Google Scholar] [CrossRef] [PubMed]
- van Mackelenbergh, M.T.; Denkert, C.; Nekljudova, V.; Karn, T.; Schem, C.; Marmé, F.; Stickeler, E.; Jackisch, C.; Hanusch, C.; Huober, J.; et al. Outcome after neoadjuvant chemotherapy in estrogen receptor-positive and progesterone receptor-negative breast cancer patients: A pooled analysis of individual patient data from ten prospectively randomized controlled neoadjuvant trials. Breast Cancer Res. Treat. 2018, 167, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Jeruss, J.S.; Mittendorf, E.A.; Tucker, S.L.; Gonzalez-Angulo, A.M.; Buchholz, T.A.; Sahin, A.A.; Cormier, J.N.; Buzdar, A.U.; Hortobagyi, G.N.; Hunt, K.K. Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J. Clin. Oncol. 2008, 26, 246–252. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Jeruss, J.S.; Tucker, S.L.; Kolli, A.; Newman, L.A.; Gonzalez-Angulo, A.M.; Buchholz, T.A.; Sahin, A.A.; Cormier, J.N.; Buzdar, A.U.; et al. Validation of a Novel Staging System for Disease-Specific Survival in Patients with Breast Cancer Treated with Neoadjuvant Chemotherapy. J. Clin. Oncol. 2011, 29, 1956–1962. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Roman, L.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.A.; et al. Efficacy and safety of neoadjuvant pertuzamab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomisedmulticentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Byrski, T.; Gronwald, J.; Huzarski, T.; Grzybowska, E.; Budryk, M.; Stawicka, M.; Mierzwa, T.; Szwiec, M.; Wisniowski, R.; Siolek, M.; et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J. Clin. Oncol. 2010, 28, 375–379. [Google Scholar] [CrossRef]
- Byrski, T.; Huzarski, T.; Dent, R.; Marczyk, E.; Jasiowka, M.; Gronwald, J.; Jakubowicz, J.; Cybulski, C.; Wisniowski, R.; Godlewski, D.; et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res. Treat. 2014, 147, 401–405. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B.; et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 2014, 15, 747e56. [Google Scholar] [CrossRef] [PubMed]
- Tomiczek-Szwiec, J.; Szwiec, M.; Falco, M.; Cybulski, C.; Wokolorczyk, D.; Jakubowska, A.; Gronwald, J.; Stawicka, M.; Godlewski, D.; Kilar, E.; et al. The impact of oophorectomy on survival from breast cancer in patients with CHEK2 mutations. Br. J. Cancer 2022, 127, 84–91. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B.; Xie, Y. Association between CHEK2 H371Y mutation and response to neoadjuvant chemotherapy in women with breast cancer. BMC Cancer 2015, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Karatas, F.; Erdem, G.U.; Sahin, S.; Aytekin, A.; Yuce, D.; Sever, A.R.; Babacan, T.; Ates, O.; Ozisik, Y.; Altundag, K. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast 2017, 32, 237–244. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, P.; Lan, N.; Kong, F.; Abdumijit, A.; Tu, S.; Li, Y.; Yuan, W. Metabolic Syndrome Predicts Response to Neoadjuvant Chemotherapy in Breast Cancer. Front. Oncol. 2022, 12, 899335. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lv, M.; Yuan, P.; Chen, X.; Liu, Z. Dyslipidemia is associated with a poor prognosis of breast cancer in patients receiving neoadjuvant chemotherapy. BMC Cancer 2023, 23, 208. [Google Scholar] [CrossRef] [PubMed]
- Graziano, V.; Grassadonia, A.; Iezzi, L.; Vici, P.; Pizzuti, L.; Barba, M.; Quinzii, A.; Camplese, A.; Di Marino, P.; Peri, M.; et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast 2019, 44, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Jakubowska, A.; Debniak, T.; Falco, M.; Kladny, J.; et al. Serum selenium levels predict survival after breast cancer. Breast Cancer Res. Treat. 2018, 167, 591–598. [Google Scholar] [CrossRef]
- Szwiec, M.; Marciniak, W.; Derkacz, R.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Dębniak, T.; Jakubowska, A.; Lener, M.; Falco, M.; et al. Serum Selenium Level Predicts 10-Year Survival after Breast Cancer. Nutrients 2021, 13, 953. [Google Scholar] [CrossRef]
- Short, S.P.; Williams, C.S. Selenoproteins in Tumorigenesis and Cancer Progression. Adv. Cancer Res. 2017, 136, 49–83. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Davis, C.D.; Tsuji, P.A.; Milner, J.A. Selenoproteins and cancer prevention. Annu. Rev. Nutr. 2012, 32, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Wallenberg, M.; Misra, S.; Wasik, A.M.; Marzano, C.; Björnstedt, M.; Gandin, V.; Fernandes, A.P. Selenium induces a multi-targeted cell death process in addition to ROS formation. J. Cell. Mol. Med. 2014, 18, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B. Sodium Selenite as an Anticancer Agent. Anticancer Agents Med. Chem. 2017, 17, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Brodin, O.; Eksborg, S.; Wallenberg, M.; Asker-Hagelberg, C.; Larsen, E.H.; Mohlkert, D.; Lenneby-Helleday, C.; Jacobsson, H.; Linder, S.; Misra, S.; et al. Pharmacokinetics and Toxicity of Sodium Selenite in the Treatment of Patients with Carcinoma in a Phase I Clinical Trial: The SECAR Study. Nutrients 2015, 7, 4978–4994. [Google Scholar] [CrossRef]
- Song, M.; Kumaran, M.N.; Gounder, M.; Gibbon, D.G.; Nieves-Neira, W.; Vaidya, A.; Hellmann, M.; Kane, M.P.; Buckley, B.; Shih, W.; et al. Phase I trial of selenium plus chemotherapy in gynecologic cancers. Gynecol. Oncol. 2018, 150, 478–486. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.; Zhou, X.; Ren, X.; Ma, X.; Zhang, W.; Yang, R.; Song, T.; Liu, Y. Real-world study of trastuzumab and pertuzumab combined with chemotherapy in neoadjuvant treatment for patients with HER2-positive breast cancer. Medicine 2022, 101, e30892. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; Perou, C.M.; et al. Estrogen receptor and Progesterone receptor testing in breast cancer ASCO/CAP guideline update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Esserman, L.J.; Berry, D.A.; DeMichele, A.; Carey, L.; Davis, S.E.; Buxton, M.; Hudis, C.; Gray, J.W.; Perou, C.; Yau, C.; et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: Results from the I-SPY 1 TRIAL-CALGB 150007/150012, ACRIN 6657. J. Clin. Oncol. 2012, 30, 3242–3249. [Google Scholar] [CrossRef] [PubMed]
- van Ramshorst, M.S.; van der Voort, A.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Dezentjé, V.O.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1630–1640. [Google Scholar] [CrossRef]
- Yang, Z.J.; Xin, F.; Chen, Z.J.; Yu, Y.; Wang, X.; Cao, X.C. Real-world data on neoadjuvant chemotherapy with dual-anti HER2 therapy in HER2 positive breast cancer. BMC Cancer 2024, 24, 134. [Google Scholar] [CrossRef]
- Tanioka, M.; Sasaki, M.; Shimomura, A.; Fujishima, M.; Doi, M.; Matsuura, K.; Sakuma, T.; Yoshimura, K.; Saeki, T.; Ohara, M.; et al. Pathologic complete response after neoadjuvant chemotherapy in HER2-overexpressing breast cancer according to hormonal receptor status. Breast 2014, 4, 466–472. [Google Scholar] [CrossRef]
- Hall, B.J.; Bhojwani, A.A.; Wong, H.; Law, A.; Flint, H.; Ahmed, E.; Innes, H.; Cliff, J.; Malik, Z.; O’Hagan, J.E.; et al. Neoadjuvant Trastuzumab and Pertuzumab for Early HER2-Positive Breast Cancer: A Real World Experience. Breast J. 2022, 2022, 7146172. [Google Scholar] [CrossRef] [PubMed]
- González-Santiago, S.; Saura, C.; Ciruelos, E.; Alonso, J.L.; de la Morena, P.; Santisteban Eslava, M.; Gallegos Sancho, M.I.; de Luna, A.; Dalmau, E.; Servitja, S.; et al. Real-world effectiveness of dual HER2 blockade with pertuzumab and trastuzumab for neoadjuvant treatment of HER2-positive early breast cancer (The NEOPETRA Study). Breast Cancer Res. Treat. 2020, 184, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, F.; Chen, X. Impact of body mass index in therapeutic response for HER2 positive breast cancer treated with neoadjuvant targeted therapy: A multi-center study and meta-analysis. NPJ Breast Cancer 2023, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.R.; McKenzie, R.C.; Beckett, G.J. Selenium in the immune system. J. Nutr. 2003, 133 (Suppl. S1), 1457S–1459S. [Google Scholar] [CrossRef] [PubMed]
- Köse, S.A.; Nazıroğlu, M. Selenium reduces oxidative stress and calcium entry through TRPV1 channels in the neutrophils of patients with polycystic ovary syndrome. Biol. Trace Elem. Res. 2014, 158, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Kiremidjian-Schumacher, L.; Roy, M.; Wishe, H.I. Supplementation with selenium augments the functions of natural killer and lymphokine-activated killer cells. Biol. Trace Elem. Res. 1996, 52, 227–239. [Google Scholar] [CrossRef]
- Gautam, P.K.; Kumar, S.; Tomar, M.; Singh, R.K.; Acharya, A.; Ram, B. Selenium nanoparticles induce suppressed function of tumor associated macrophages and inhibit Dalton’s lymphoma proliferation. Biochem. Biophys. Rep. 2017, 12, 172–184. [Google Scholar] [CrossRef]
- Petrie, H.T.; Klassen, L.W.; Klassen, P.S.; O’Dell, J.R.; Kay, H.D. Selenium and the immune response: 2. enhancement of murine cytotoxic t-lymphocyte and natural killer cell cytotoxicity in vivo. J. Leukoc. Biol. 1989, 45, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Pei, B.; Hu, Q.; Li, X.; Fang, X.; Huang, X.; Yang, Z.; Chen, J.; He, D.; Sun, G.; et al. Effects of selenium supplementation on concurrent chemoradiotherapy in patients with cervical cancer: A randomized, double-blind, placebo-parallel controlled phase II clinical trial. Front. Nutr. 2023, 10, 1094081. [Google Scholar] [CrossRef]
- Qi, Y.; Fu, X.; Xiong, Z.; Zhang, H.; Hill, S.M.; Rowan, B.G.; Dong, Y. Methylseleninic acid enhances paclitaxel efficacy for the treatment of triple-negative breast cancer. PLoS ONE 2012, 7, e31539. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Marciniec, K.; Nowakowska, J.; Domínguez-Álvarez, E.; Bielawski, K. Short Communication: Novel Di- and Triselenoesters as Effective Therapeutic Agents Inhibiting Multidrug Resistance Proteins in Breast Cancer Cells. Int. J. Mol. Sci. 2024, 25, 9732. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.H.; Shih, M.Y.; Chung, C.H.; Lin, Y.C.; Fan, C.T.; Peng, C.L.; Chen, P.C.; Hsia, S. Fish Oil and Selenium with Doxorubicin Modulates Expression of Fatty Acid Receptors and Selenoproteins, and Targets Multiple Anti-Cancer Signaling in Triple-negative Breast Cancer Tumors. Int. J. Med. Sci. 2022, 19, 2044–2057. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Kim, J.B.; Cho, T.; Yoo, E.H.; Moon, B.I.; Kwon, H.; Lim, W. Selenium inhibits growth of trastuzumab-resistant human breast cancer cells via downregulation of Akt and beclin-1. PLoS ONE 2021, 16, e0257298. [Google Scholar] [CrossRef] [PubMed]
- Bapat, P.; Sewell, D.G.; Boylan, M.; Sharma, A.K.; Spallholz, J.E. In Vitro Cytotoxicity of Trastuzumab (Tz) and Se-Trastuzumab (Se-Tz) against the Her/2 Breast Cancer Cell Lines JIMT-1 and BT-474. Int. J. Mol. Sci. 2021, 22, 4655. [Google Scholar] [CrossRef]
| Clinicopathological Parameters | n = 329 | % | Mean Selenium Level ± SD | p-Value | |
|---|---|---|---|---|---|
| Age mean (range) | 54.8 (25–85) | ||||
| ≤50 | 126 | 38.3 | 100.4 ± 13.9 | 0.18 | |
| >50 | 203 | 61.7 | 102.8 ± 17.0 | ||
| BRCA1 status | |||||
| Positive | 22 | 6.7 | 96.3 ± 22.4 | 0.09 | |
| Negative | 307 | 93.3 | 102.3 ± 15.3 | ||
| CHEK2 status | |||||
| Positive | 18 | 5.5 | 100.3 ± 15.0 | 0.67 | |
| Negative | 311 | 94.5 | 101.9 ± 16.0 | ||
| PALB2 status | |||||
| Positive | 8 | 2.4 | 95.5 ± 15.8 | 0.25 | |
| Negative | 321 | 97.6 | 102.0 ± 15.9 | ||
| Menopausal status | |||||
| Premenopusal | 137 | 41.6 | 100.2 ± 13.6 | 0.11 | |
| Postmenopauzal | 192 | 58.4 | 103.0 ± 17.3 | ||
| Smoking status | |||||
| Current | 72 | 21.9 | 99.7 ± 15.2 | 0.18 | |
| Past | 57 | 17.3 | 104.8 ± 17.9 | ||
| Never | 176 | 53.5 | 102.8 ± 15.7 | ||
| Missing | 24 | 7.3 | 95.6 ± 11.7 | ||
| BMI | |||||
| ≤24.9 | 138 | 41.9 | 100.5 ± 15.4 | 0.40 | |
| 25.0–29.9 | 105 | 31.9 | 103.3 ± 16.2 | ||
| ≥30 | 86 | 26.2 | 102.3 ±16.5 | ||
| Tumor size | |||||
| ≤20 mm | 28 | 8.5 | 102.3 ± 19.1 | 0.90 | |
| 21–50 mm | 202 | 61.4 | 101.5 ± 15.4 | ||
| ≥51 mm | 99 | 30.1 | 102.4 ± 16.2 | ||
| Clinical node status | |||||
| Positive | 172 | 52.3 | 100.8 ± 14.9 | 0.22 | |
| Negative | 157 | 47.7 | 103.0 ± 16.9 | ||
| Histology | |||||
| Ductal, NST | 320 | 97.3 | 101.8 ± 15.8 | 0.61 | |
| Other | 9 | 2.7 | 104.5 ± 20.3 | ||
| Tumor grade | |||||
| G1 | 4 | 1.2 | 99.4 ± 9.7 | 0.74 | |
| G2 | 150 | 45.6 | 102.4 ± 15.2 | ||
| G3 | 169 | 51.4 | 101.1 ± 16.6 | ||
| Missing | 6 | 1.8 | 112.6 ± 17.8 | ||
| ER status | |||||
| Negative | 210 | 63.8 | 102.8 ± 16.4 | 0.15 | |
| Positive | 119 | 36.2 | 100.2 ± 15.0 | ||
| PR status | |||||
| Negative | 249 | 75.7 | 102.3 ± 15.9 | 0.37 | |
| Positive | 80 | 24.3 | 100.5 ± 15.9 | ||
| HER2 status | |||||
| Positive | 183 | 55.6 | 101.8 ± 14.7 | 0.92 | |
| Negative | 146 | 44.4 | 102.0 ± 17.3 | ||
| Ki 67 status | |||||
| 0–30% | 68 | 20.7 | 102.4 ± 17.8 | 0.96 | |
| 31–50% | 73 | 22.2 | 101.9 ± 13.9 | ||
| 51–100% | 185 | 56.2 | 101.8 ± 16.1 | ||
| Missing | 3 | 0.9 | 94.0 ± 1.6 | ||
| pCR status | |||||
| pCR | 165 | 50.2 | 104.0 ± 16.0 | 0.01 | |
| no pCR | 164 | 49.8 | 99.7 ± 15.6 | ||
| Clinicopathological Parameters | n = 329 | % PCR | Univariable Odds Ratio | p-Value | Multivariable Odds Ratio | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| OR | (95% OR) | OR | (95% OR) | ||||||
| Age (years) | |||||||||
| ≤40 | 52 | 65.4 | 2.01 | (1.03–3.93) | 0.04 | 1.39 | (0.65–2.96) | 0.40 | |
| 41–50 | 74 | 48.6 | 1.01 | (0.57–1.79) | 0.97 | 0.87 | (0.46–1.67) | 0.68 | |
| 51–60 | 77 | 44.2 | 0.84 | (0.48–1.49) | 0.56 | 0.67 | (0.36–1.27) | 0.22 | |
| ≥61 | 126 | 48.4 | Reference | Reference | |||||
| Tumor size | |||||||||
| ≤20 mm | 28 | 71.4 | 3.26 | (1.31–8.10) | 0.01 | 3.03 | (1.15–7.99) | 0.02 | |
| 21–50 mm | 202 | 50.5 | 1.33 | (0.82–2.16) | 0.25 | 1.61 | (0.94–2.74) | 0.08 | |
| ≥51 mm | 99 | 43.4 | Reference | Reference | |||||
| Breast cancer subtype | |||||||||
| HER2-positive | 183 | 54.1 | Reference | ||||||
| TNBC | 146 | 45.2 | 0.70 | (0.45–1.08) | 0.11 | ||||
| ER status | |||||||||
| Positive | 119 | 46.2 | Reference | ||||||
| Negative | 210 | 52.4 | 1.28 | (0.82–2.00) | 0.28 | ||||
| PR status | |||||||||
| Positive | 80 | 41.3 | Reference | Reference | |||||
| Negative | 249 | 53.0 | 1.61 | (0.97–2.68) | 0.07 | 1.67 | (0.95–2.96) | 0.08 | |
| Ki67 status | |||||||||
| 0–30% | 68 | 32.4 | Reference | Reference | |||||
| 31–50% | 73 | 53.4 | 2.40 | (1.21–4.76) | 0.012 | 2.18 | (1.04–4.55) | 0.04 | |
| 51–100% | 185 | 55.1 | 2.57 | (1.43–4.61) | 0.002 | 2.50 | (1.33–4.69) | 0.004 | |
| missing | 3 | 66.7 | |||||||
| Grade | |||||||||
| 1 | 4 | 25.0 | Reference | ||||||
| 2 | 150 | 48.7 | 2.84 | (0.29–27.97) | 0.37 | ||||
| 3 | 169 | 51.5 | 3.18 | (0.33–31.22) | 0.32 | ||||
| missing | 6 | 66.7 | |||||||
| Mutation status | |||||||||
| None | 281 | 48.8 | Reference | ||||||
| BRCA1 | 22 | 59.1 | 1.52 | (0.63–3.67) | 0.35 | ||||
| CHEK2 | 18 | 55.6 | 1.31 | (0.50–3.43) | 0.58 | ||||
| PALB2 | 8 | 62.5 | 1.75 | (0.41–7.47) | 0.45 | ||||
| Lymph nodes | |||||||||
| Negative | 157 | 49.0 | 0.92 | (0.60–1.42) | 0.70 | ||||
| Positive | 172 | 51.2 | Reference | ||||||
| BMI | |||||||||
| <24.9 | 138 | 59.4 | 2.35 | (1.36–4.08) | 0.002 | 2.39 | (1.27–4.49) | 0.007 | |
| 25.0–29.9 | 105 | 47.6 | 1.46 | (0.82–2.61) | 0.20 | 1.57 | (0.84–2.94) | 0.16 | |
| ≥30 | 86 | 38.4 | Reference | Reference | |||||
| Selenium level * | |||||||||
| Tertile 1 | 110 | 39.0 | Reference | Reference | |||||
| Tertile 2 | 109 | 52.3 | 1.71 | (1.00–2.92) | 0.05 | 1.72 | (0.97–3.06) | 0.07 | |
| Tertile 3 | 110 | 59.0 | 2.25 | (1.31–3.86) | 0.003 | 2.75 | (1.53–4.95) | 0.001 | |
| NAC Regimens | n = 329 | % | Mean Selenium Level ± SD | p |
|---|---|---|---|---|
| 0.65 | ||||
| AC-Paclitaxel | 18 | 5.5 | 97.17 ± 15.02 | |
| AC-Paclitaxel + Carboplatin | 13 | 4.0 | 100.78 ± 24.00 | |
| AC(dd)-Paclitaxel | 55 | 16.7 | 102.64 ± 20.61 | |
| AC(dd)-Paclitaxel + Carboplatin | 48 | 14.6 | 104.58 ± 11.10 | |
| AC/EC-Docetaxel/Paclitaxel + Herceptin | 53 | 16.1 | 100.83 ± 13.76 | |
| AC/EC Docetaxel + Herceptin + Pertuzumab | 69 | 21.0 | 103.053±14.34 | |
| AC(dd)-Paclitaxel+Herceptin | 2 | 0.6 | 92.49±20.56 | |
| AC(dd)-Docetaxel/Paclitaxel+Herceptin+Pertuzumab | 31 | 9.4 | 102.55±15.16 | |
| Docetaxel+Caroplatin+Herceptin | 9 | 2.7 | 99.95±16.42 | |
| Docetaxel+Caroplatin+Herceptin+Pertuzumab | 16 | 4.9 | 103.38±16.92 | |
| Other | 15 | 4.6 | 95.18 ± 16.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwiec, M.; Tomiczek-Szwiec, J.; Marciniak, W.; Derkacz, R.; Huzarski, T.; Cybulski, C.; Gronwald, J.; Osowiecka, K.; Sibilski, R.; Narod, S.A.; et al. The Effect of Blood Selenium Level on the pCR Rate in Breast Cancer Patient Receiving Neoadjuvant Chemotherapy. Cancers 2025, 17, 839. https://doi.org/10.3390/cancers17050839
Szwiec M, Tomiczek-Szwiec J, Marciniak W, Derkacz R, Huzarski T, Cybulski C, Gronwald J, Osowiecka K, Sibilski R, Narod SA, et al. The Effect of Blood Selenium Level on the pCR Rate in Breast Cancer Patient Receiving Neoadjuvant Chemotherapy. Cancers. 2025; 17(5):839. https://doi.org/10.3390/cancers17050839
Chicago/Turabian StyleSzwiec, Marek, Joanna Tomiczek-Szwiec, Wojciech Marciniak, Róża Derkacz, Tomasz Huzarski, Cezary Cybulski, Jacek Gronwald, Karolina Osowiecka, Robert Sibilski, Steven A. Narod, and et al. 2025. "The Effect of Blood Selenium Level on the pCR Rate in Breast Cancer Patient Receiving Neoadjuvant Chemotherapy" Cancers 17, no. 5: 839. https://doi.org/10.3390/cancers17050839
APA StyleSzwiec, M., Tomiczek-Szwiec, J., Marciniak, W., Derkacz, R., Huzarski, T., Cybulski, C., Gronwald, J., Osowiecka, K., Sibilski, R., Narod, S. A., & Lubiński, J. (2025). The Effect of Blood Selenium Level on the pCR Rate in Breast Cancer Patient Receiving Neoadjuvant Chemotherapy. Cancers, 17(5), 839. https://doi.org/10.3390/cancers17050839







