Targeting the Leukemia Inhibitory Factor/Leukemia Inhibitory Factor Receptor Axis Reduces the Growth of Inflammatory Breast Cancer by Promoting Ferroptosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
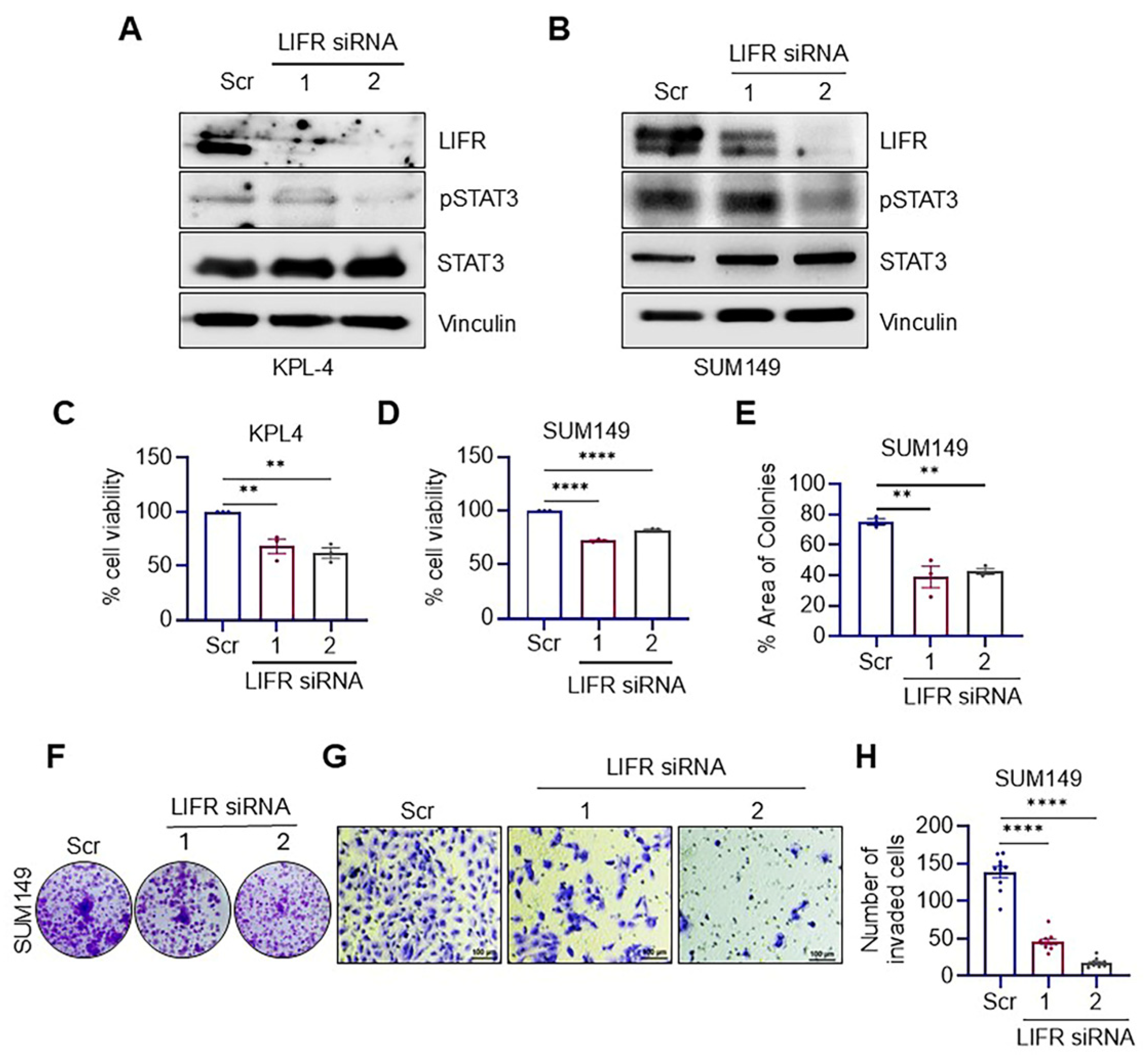
2.2. LIFR-Knockdown
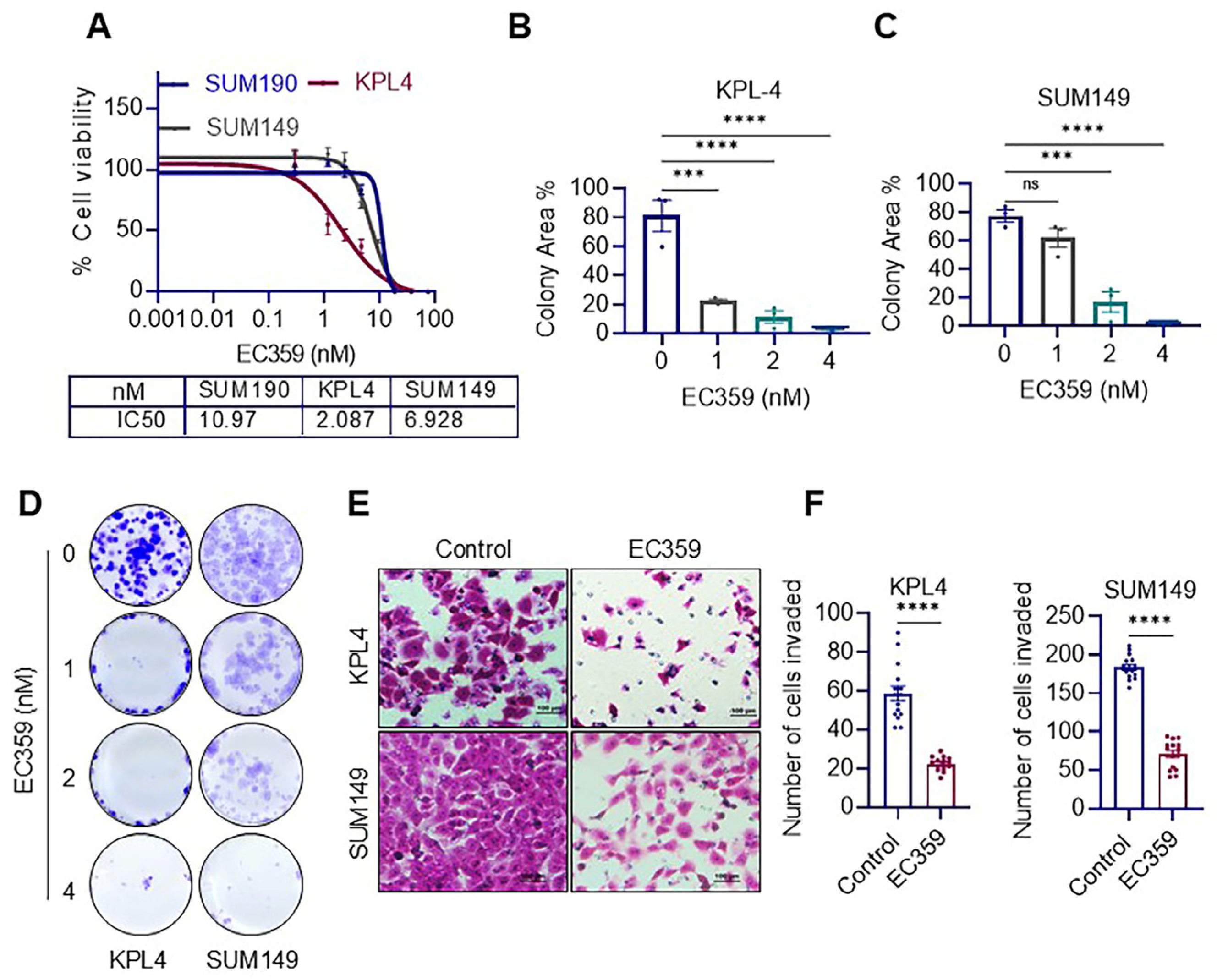
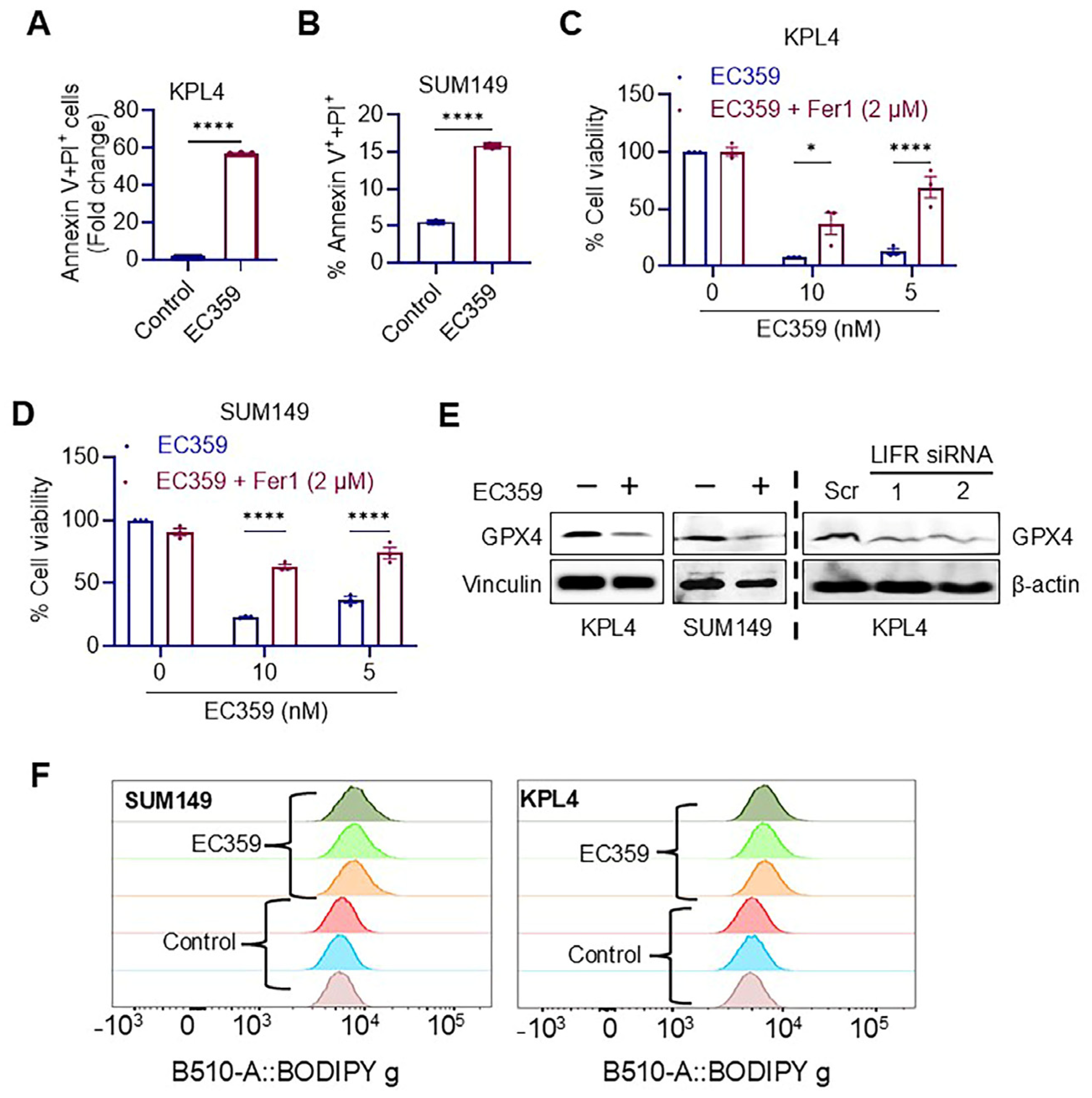
2.3. Cell Viability, Clonogenicity, Apoptosis, and Invasion Assays
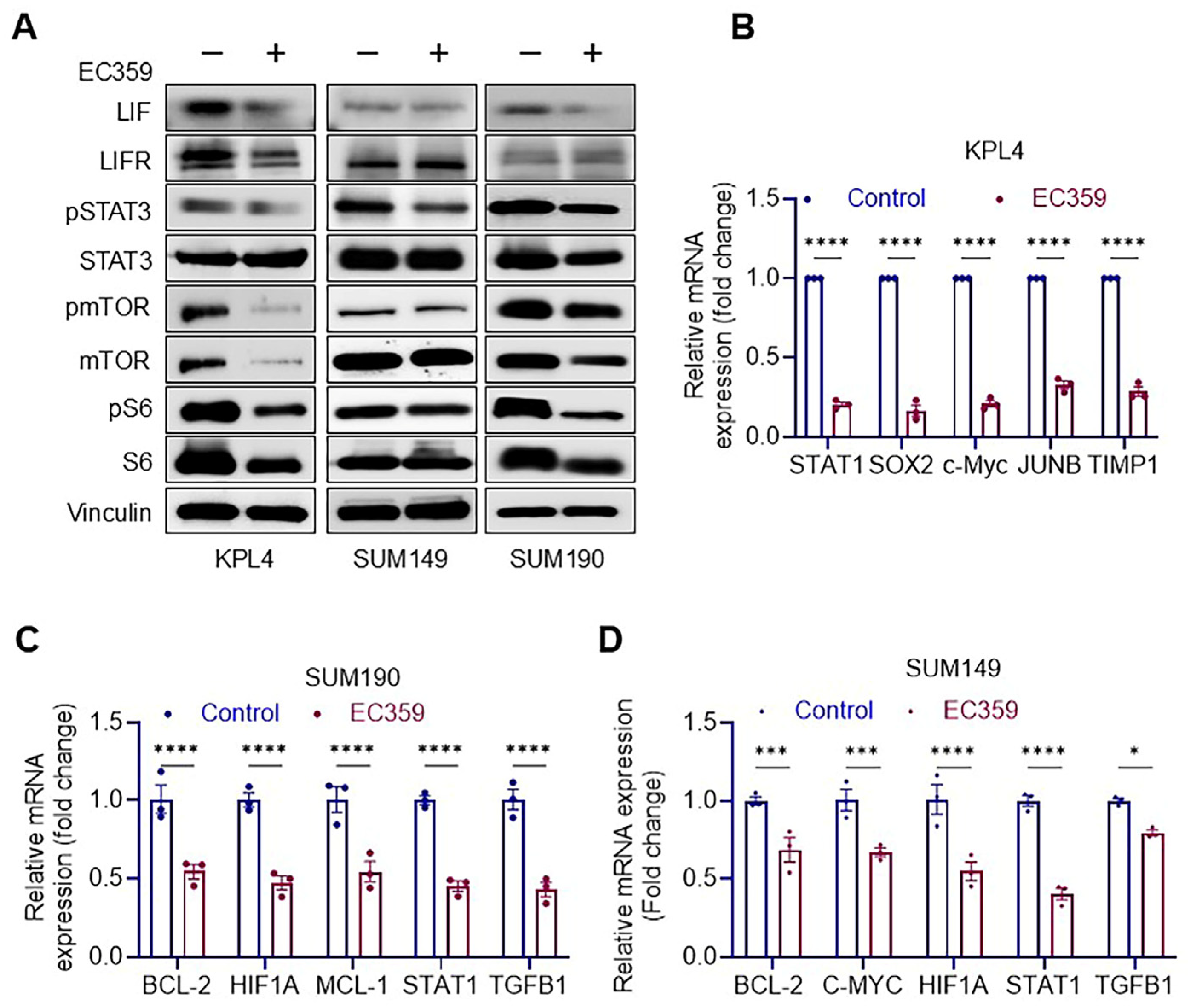
2.4. Western Blotting
2.5. RT-qPCR
2.6. Lipid Peroxidation
2.7. In Vivo Xenograft Studies
2.8. Immunohistochemistry
2.9. Statistical Analysis
3. Results
3.1. LIFR Knockdown Reduced the Clonogenicity and Invasion of IBC Cells
3.2. LIFR Inhibitor EC359 Reduced the Cell Viability, Clonogenicity and Invasion of IBC Cells
3.3. EC359 Reduced the Activation of LIFR Down Stream Signaling
3.4. EC359 Induced Apoptosis in IBC Cells Through the Ferroptosis Mechanism
3.5. LIFR Inhibitor EC359 Reduced the Growth of IBC Xenografts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Woodward, W.A. Inflammatory breast cancer: Unique biological and therapeutic considerations. Lancet Oncol. 2015, 16, e568–e576. [Google Scholar] [CrossRef] [PubMed]
- Dirix, L.Y.; Van Dam, P.; Prové, A.; Vermeulen, P.B. Inflammatory breast cancer: Current understanding. Curr. Opin. Oncol. 2006, 18, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Van Laere, S.J.; Ueno, N.T.; Finetti, P.; Vermeulen, P.; Lucci, A.; Robertson, F.M.; Marsan, M.; Iwamoto, T.; Krishnamurthy, S.; Masuda, H.; et al. Uncovering the molecular secrets of inflammatory breast cancer biology: An integrated analysis of three distinct affymetrix gene expression datasets. Clin. Cancer Res. 2013, 19, 4685–4696. [Google Scholar] [CrossRef] [PubMed]
- Hance, K.W.; Anderson, W.F.; Devesa, S.S.; Young, H.A.; Levine, P.H. Trends in inflammatory breast carcinoma incidence and survival: The surveillance, epidemiology, and end results program at the National Cancer Institute. J. Natl. Cancer Inst. 2005, 97, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Ali, S.M.; Wang, K.; Khaira, D.; Palma, N.A.; Chmielecki, J.; Palmer, G.A.; Morosini, D.; Elvin, J.A.; Fernandez, S.V.; et al. Comprehensive genomic profiling of inflammatory breast cancer cases reveals a high frequency of clinically relevant genomic alterations. Breast Cancer Res. Treat. 2015, 154, 155–162. [Google Scholar] [CrossRef]
- Liang, X.; Vacher, S.; Boulai, A.; Bernard, V.; Baulande, S.; Bohec, M.; Bièche, I.; Lerebours, F.; Callens, C. Targeted next-generation sequencing identifies clinically relevant somatic mutations in a large cohort of inflammatory breast cancer. Breast Cancer Res. 2018, 20, 88. [Google Scholar] [CrossRef] [PubMed]
- Bièche, I.; Lerebours, F.; Tozlu, S.; Espie, M.; Marty, M.; Lidereau, R. Molecular profiling of inflammatory breast cancer: Identification of a poor-prognosis gene expression signature. Clin. Cancer Res. 2004, 10, 6789–6795. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.E.; Peluffo, G.; Qiu, X.; Temko, D.; Fassl, A.; Li, Z.; Trinh, A.; Seehawer, M.; Jovanović, B.; Alečković, M.; et al. JAK-STAT Signaling in Inflammatory Breast Cancer Enables Chemotherapy-Resistant Cell States. Cancer Res. 2023, 83, 264–284. [Google Scholar] [CrossRef]
- Nicola, N.A.; Babon, J.J. Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev. 2015, 26, 533–544. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Yu, H.; Wu, L.; Zhao, Y.; Zhang, C.; Yue, X.; Liu, Z.; Wu, H.; Haffty, B.G.; et al. LIF promotes tumorigenesis and metastasis of breast cancer through the AKT-mTOR pathway. Oncotarget 2014, 5, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Viswanadhapalli, S.; Luo, Y.; Sareddy, G.R.; Santhamma, B.; Zhou, M.; Li, M.; Ma, S.; Sonavane, R.; Pratap, U.P.; Altwegg, K.A.; et al. EC359-A first-in-class small molecule inhibitor for targeting oncogenic LIFR signaling in triple negative breast cancer. Mol. Cancer Ther. 2019, 18, 1341–1354. [Google Scholar] [CrossRef]
- Thomas, C.; Karagounis, I.V.; Srivastava, R.K.; Vrettos, N.; Nikolos, F.; Francois, N.; Huang, M.; Gong, S.; Long, Q.; Kumar, S.; et al. Estrogen Receptor β-Mediated Inhibition of Actin-Based Cell Migration Suppresses Metastasis of Inflammatory Breast Cancer. Cancer Res. 2021, 81, 2399–2414. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Viswanadhapalli, S.; Pratap, U.P.; Rahul, G.; Yang, X.; Pitta Venkata, P.; Drel, V.; Santhamma, B.; Konda, S.; Li, X.; et al. Pharmacological inhibition of the LIF/LIFR autocrine loop reveals vulnerability of ovarian cancer cells to ferroptosis. npj Precis. Oncol. 2024, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Stahl, N.; Boulton, T.G.; Farruggella, T.; Ip, N.Y.; Davis, S.; Witthuhn, B.A.; Quelle, F.W.; Silvennoinen, O.; Barbieri, G.; Pellegrini, S.; et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science 1994, 263, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Tsang, N.M.; Chiang, W.C.; Chang, K.P.; Hsueh, C.; Liang, Y.; Juang, J.L.; Chow, K.P.; Chang, Y.S. Leukemia inhibitory factor promotes nasopharyngeal carcinoma progression and radioresistance. J. Clin. Investig. 2013, 123, 5269–5283. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Park, S.H.; Jang, Y.K. Epigenetic up-regulation of leukemia inhibitory factor (LIF) gene during the progression to breast cancer. Mol. Cells 2011, 31, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, S.; Anido, J.; Prieto-Sanchez, R.M.; Folch, G.; Barba, I.; Cuartas, I.; Garcia-Dorado, D.; Poca, M.A.; Sahuquillo, J.; Baselga, J.; et al. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell 2009, 15, 315–327. [Google Scholar] [CrossRef]
- Wu, L.; Yu, H.; Zhao, Y.; Zhang, C.; Wang, J.; Yue, X.; Yang, Q.; Hu, W. HIF-2alpha mediates hypoxia-induced LIF expression in human colorectal cancer cells. Oncotarget 2015, 6, 4406–4417. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, W.; Lytle, N.K.; Huang, P.; Yuan, X.; Dann, A.M.; Ridinger-Saison, M.; DelGiorno, K.E.; Antal, C.E.; Liang, G.; et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 2019, 569, 131–135. [Google Scholar] [CrossRef]
- Sasaki, N.; Hirano, K.; Shichi, Y.; Gomi, F.; Yoshimura, H.; Matsushita, A.; Toyoda, M.; Ishiwata, T. Gp130-Mediated STAT3 Activation Contributes to the Aggressiveness of Pancreatic Cancer through H19 Long Non-Coding RNA Expression. Cancers 2022, 14, 2055. [Google Scholar] [CrossRef] [PubMed]
- Albrengues, J.; Bourget, I.; Pons, C.; Butet, V.; Hofman, P.; Tartare-Deckert, S.; Feral, C.C.; Meneguzzi, G.; Gaggioli, C. LIF mediates proinvasive activation of stromal fibroblasts in cancer. Cell Rep. 2014, 7, 1664–1678. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Wang, J.; Chang, C.Y.; Liu, J.; Yang, X.; Zhou, F.; Qiu, X.; Bhatt, V.; Guo, J.Y.; Su, X.; et al. Leukemia inhibitory factor drives glucose metabolic reprogramming to promote breast tumorigenesis. Cell Death Dis. 2022, 13, 370. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.T.; Fer, N.; Galeas, J.; Collisson, E.A.; Kim, S.E.; Sharib, J.; McCormick, F. Blockade of leukemia inhibitory factor as a therapeutic approach to KRAS driven pancreatic cancer. Nat. Commun. 2019, 10, 3055. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.R.; Cannon, A.; Thompson, C.; Santhamma, B.; Chavez-Riveros, A.; Bhatia, R.; Nair, H.B.; Nickisch, K.; Batra, S.K.; Kumar, S. Utilizing cell line-derived organoids to evaluate the efficacy of a novel LIFR-inhibitor, EC359 in targeting pancreatic tumor stroma. Genes Cancer 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Di Giorgio, C.; Lupia, A.; Marchiano, S.; Bordoni, M.; Bellini, R.; Massa, C.; Urbani, G.; Roselli, R.; Moraca, F.; Sepe, V.; et al. Repositioning Mifepristone as a Leukaemia Inhibitory Factor Receptor Antagonist for the Treatment of Pancreatic Adenocarcinoma. Cells 2022, 11, 3482. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.-Z.; Li, N.-Z.; Hu, X.-B.; Xie, Y.-Y.; Huang, Q.-H.; Zhang, J.; Chen, Z.; Chen, S.-J.; Wang, F.; Sun, X.-J. The LIFR-targeting small molecules EC330/EC359 are potent ferroptosis inducers. Genes Dis. 2023, 10, 735–738. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Zhou, Q.; Meng, Y.; Li, D.; Yao, L.; Le, J.; Liu, Y.; Sun, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis in cancer: From molecular mechanisms to therapeutic strategies. Signal Transduct. Target. Ther. 2024, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]

| Gene Name | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| STAT1 | CAGCTTGACTCAAAATTCCTGGA | TGAAGATTACGCTTGCTTTTCCT |
| SOX2 | TGCGAGCGCTGCACAT | TCATGAGCGTCTTGGTTTTCC |
| c-Myc | GGCTCCTGGCAAAAGGTCA | CTGCGTAGTTGTGCTGATGT |
| JUNB | ACGACTCATACACAGCTACGG | GCTCGGTTTCAGGAGTTTGTAGT |
| TIMP1 | CTTCTGCAATTCCGACCTCGT | ACGCTGGTATAAGGTGGTCTG |
| BCL-2 | GGTGGGGTCATGTGTGTGG | CGGTTCAGGTACTCAGTCATCC |
| HIF1A | CACCACAGGACAGTACAGGAT | CGTGCTGAATAATACCACTCACA |
| MCL-1 | GTAATAACACCAGTACGGACGG | CCACAAACCCATCCTTGGAAG |
| TGFB1 | CAATTCCTGGCGATACCTCAG | GCACAACTCCGGTGACATCAA |
| 18S rRNA | GCTTAATTTGACTCAACACGGGA | AGCTATCAATCTGTCAATCCTGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romo, B.; Fuentes, Z.; Randolph, L.; Mahajan, M.; Aller, E.J.; Ebrahimi, B.; Santhamma, B.; Pratap, U.P.; Subbarayalu, P.; Nagandla, H.; et al. Targeting the Leukemia Inhibitory Factor/Leukemia Inhibitory Factor Receptor Axis Reduces the Growth of Inflammatory Breast Cancer by Promoting Ferroptosis. Cancers 2025, 17, 790. https://doi.org/10.3390/cancers17050790
Romo B, Fuentes Z, Randolph L, Mahajan M, Aller EJ, Ebrahimi B, Santhamma B, Pratap UP, Subbarayalu P, Nagandla H, et al. Targeting the Leukemia Inhibitory Factor/Leukemia Inhibitory Factor Receptor Axis Reduces the Growth of Inflammatory Breast Cancer by Promoting Ferroptosis. Cancers. 2025; 17(5):790. https://doi.org/10.3390/cancers17050790
Chicago/Turabian StyleRomo, Bianca, Zenaida Fuentes, Lois Randolph, Megharani Mahajan, Emily J. Aller, Behnam Ebrahimi, Bindu Santhamma, Uday P. Pratap, Panneerdoss Subbarayalu, Harika Nagandla, and et al. 2025. "Targeting the Leukemia Inhibitory Factor/Leukemia Inhibitory Factor Receptor Axis Reduces the Growth of Inflammatory Breast Cancer by Promoting Ferroptosis" Cancers 17, no. 5: 790. https://doi.org/10.3390/cancers17050790
APA StyleRomo, B., Fuentes, Z., Randolph, L., Mahajan, M., Aller, E. J., Ebrahimi, B., Santhamma, B., Pratap, U. P., Subbarayalu, P., Nagandla, H., Thomas, C., Nair, H. B., Vadlamudi, R. K., & Viswanadhapalli, S. (2025). Targeting the Leukemia Inhibitory Factor/Leukemia Inhibitory Factor Receptor Axis Reduces the Growth of Inflammatory Breast Cancer by Promoting Ferroptosis. Cancers, 17(5), 790. https://doi.org/10.3390/cancers17050790





