Modern Therapeutic Approaches in Anaplastic Thyroid Cancer: A Meta-Analytic Review of Randomised and Single Arm Studies on Efficacy and Survival
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Intervention
2.2. Inclusion and Exclusion Criteria
2.3. Search Approach
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Methods
3. Results
3.1. Overall Response Rates (ORR) and Disease Control Rates (DCR)
3.2. Survival Outcomes
3.3. Biomarker Analysis
- -
- PIK3CA status was mentioned in 4 studies (8.5%) and was positive in 10 patients (1.0%).
- -
- RAS (KRAS and NRAS) status was reported in 4 studies (8.5%) and was positive in 11 patients (1.1%).
- -
- NTRK1/3 was mentioned in 1 study (2.1%) and was positive in 7 patients (0.7%).
- -
- P53 status was reported in 3 studies (6.3%) and was detected in 10 patients (1.0%).
- -
- Programmed death ligand-1 (PDL-1) status was reported in 10 studies (21.3%) and was positive in 90 patients (9.2%).
3.4. Toxicity Profile
4. Discussion
4.1. Next-Generation Sequencing (NGS) and Molecular-Driven Treatment
4.2. PD-L1 Expression in ATC
4.3. Radiation Therapy in ATC
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATC | Anaplastic thyroid cancer |
| ORR | Overall response rate |
| DCR | Disease control rate |
| MD | Mean difference |
| DT | Dabrafenib/trametinib |
| LP | Lenvatinib/pembrolizumab |
| OS | Overall survival |
| PFS | Progression-free survival |
| TKI | Tyrosine kinase inhibitors |
| PD/PDL1 | Programmed death/ programmed death ligand 1 |
| IO | Immunotherapy |
| PRISMA | Preferred reporting items for systemic reviews and meta-analyses |
| MOOSE | Meta analyses for observational studies in epidemiology |
| CR | Complete response |
| PR | Partial response |
| SD | Stable disease |
| IPW | Inverse probability weighting |
| HypoRT | Hypofractionated radiotherapy |
| MINORS | Mythological index for non-randomised studies |
Appendix A
| Study/Year/Nature | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yamazaki H, et al. [18] | Yes | Yes | Yes | Yes | Yes | Yes | unclear | Yes | Yes | Yes | Include |
| Sparano C, et al. [19] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Park J, et al. [20] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Iwasaki H, et al. [21] | Yes | Yes | Yes | Yes | Yes | Yes | unclear | Yes | Yes | Yes | Include |
| Ishihara S, et al. [22] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Fukuda, N et al. [23] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Kim SY, et al. [24] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Iyer PC, et al. [26] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Lorimer C, et al. [8] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Bueno F, et al. [38] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| da Silva TN, et al. [39] | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Include |
| Zhao X, et al. [40] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Hatashima A, et al. [42] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Dierks C, et al. [45] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Iyer PC, et al. [47] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Harris EJ, et al. [50] | Yes | Yes | Yes | Yes | Yes | Yes | unclear | Yes | Yes | Yes | Include |
| Song Y, et al. [59] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Soll D, et al. [56] | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Include |
| Evans LK, et al. [55] | Yes | Yes | Yes | Yes | Yes | Yes | unclear | Yes | Yes | Yes | Include |
| Tan JSH, et al. [53] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Hamidi S, et al. [52] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Include |
| Study/Year/Nature | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Higashiyama T, et al. (HOPE) [16] | 2 | 2 | 2 | 0 | 2 | 2 | 1 | 2 | 13 |
| Wirth LJ, et al. [17] | 2 | 1 | 2 | 0 | 2 | 1 | 2 | 2 | 12 |
| Takahashi S, et al. [25] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Iyer PC, et al. [47] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Ito Y et al. [27] | 2 | 2 | 1 | 2 | 0 | 2 | 1 | 0 | 10 |
| Savvides P, et al. [28] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 14 |
| Bible KC, et al. [29] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Pennell NA, et al. [31] | 2 | 2 | 1 | 2 | 0 | 2 | 1 | 0 | 10 |
| Zhao Q, et al. [32] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 16 |
| Doebele, et al. [51] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 16 |
| Ha HT, et al. [33] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Mooney CJ, et al. [34] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 16 |
| Sosa JA, et al. [35] | 2 | 2 | 1 | 2 | 0 | 2 | 1 | 0 | 10 |
| Subbiah V, et al. [36] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 16 |
| ROAR Study [37] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 16 |
| Hyman DM, et al. [41] | 2 | 1 | 2 | 0 | 2 | 1 | 2 | 2 | 12 |
| Hatashima A, et al. [42] | 2 | 1 | 2 | 0 | 2 | 1 | 2 | 2 | 12 |
| Canabillas ME, et al. [57] | 2 | 1 | 2 | 0 | 2 | 1 | 2 | 2 | 12 |
| Capdevila J, et al. [43] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 16 |
| Lorch JH, et al. [44] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 16 |
| ATLEP trial [46] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 16 |
| Lim SM, et al. [48] | 2 | 1 | 2 | 0 | 2 | 1 | 2 | 2 | 12 |
| Sehgal L, et al. [58] | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 12 |
| Tahara M, et al. [54] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 16 |
| Waguespack SG, et al. [9] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 16 |
| Hanna GJ, et al. [49] | 2 | 1 | 2 | 0 | 2 | 1 | 2 | 2 | 12 |
| Sehgal K, et al. [60] | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 16 |
| Study/Year/Nature | Female/Male | Mutation Analysed (n) | Median Follow Up (Months) | Progression (Events) | Deaths (Events) |
|---|---|---|---|---|---|
| Higashiyama T, et al. (HOPE) [16] Prospective 2022 | NR | NR | 12 | 11 | 37 |
| Wirth LJ, et al. [17] Prospective 2021 | 13/21 | NR | 14 | 26 | 23 |
| Yamazaki H, et al. [18] Retrospective 2021 | 9/11 | NR | 24 | 15 | 18 |
| Sparano C, et al. [19] Retrospective 2021 | 9/6 | NR | 12 | 8 | NR |
| Park J, et al. [20] Retrospective 2021 | NR | NR | 6 | 4 | 8 |
| Iwasaki H, et al. [21] Retrospective 2021 | 14/18 | NR | 60 | 12 | 25 |
| Ishihara S, et al. [22] Retrospective 2021 | 3/7 | NR | 14 | 4 | 11 |
| Fukuda, N et al. [23] Retrospective 2020 | 4/9 | NR | 14 | 4 | 6 |
| Kim M, et al. [24] Retrospective 2020 | 5/9 | NR | 14 | 1 | 6 |
| Takahashi S, et al. [25] Prospective 2019 | 6/11 | NR | 12 | 1 | NR |
| Iyer PC, et al. [26] Retrospective 2018 | NR | NR | 14 | 4 | 8 |
| Ito Y et al. [27] Prospective 2017 | 5/6 | NR | 12 | 4 | 9 |
| Savvides P, et al. [28] Prospective 2013 | 7/13 | NR | 24 | NR | 19 |
| Bible KC, et al. [29] Prospective 2012 | 10/5 | NR | 12 | 15 | 13 |
| NRG/RTOG 0912 [30] Prospective 2023 | 37/34 | NR | 12 | 15 Pazopanib plus XRT 11 in XRT | 33 Pazopanib plus XRT 30 in XRT |
| Pennell NA, et al. [31] Prospective 2008 | NR | NR | 17.5 | 5 | 5 |
| Zhao Q, et al. [32] Prospective 2022 | NR | NR | 10.7 | 2 | NR |
| Ha HT, et al. [33] Prospective 2010 | 5/6 | NR | 6 | 1 | 6 |
| Mooney CJ, et al. [34] Prospective 2009 | 10/16 | NR | 8 | 19 | 21 |
| Sosa JA, et al. [35] Prospective 2014 | NR | NR | 12 | NR | 20 CP/fosbretabulin 8 CP |
| Subbiah V, et al. [36] Prospective 2018 | 10/6 | BRAF V600 = 15 No mutation = 1 | 11.75 | 2 | NR |
| ROAR Study [37] Prospective 2022 | 20/16 | BRAF V600 = 33 | 11.1 | 22 | 24 |
| Lorimer C, et al. [8] Retrospective 2023 | NR | BRAF V600 = 17 | 12 | 3 | NR |
| Bueno F, et al. [38] Retrospective 2023 | 2/3 | BRAF V600 = 5 | 60 | 0 | 2 |
| da Silva TN, et al. [39] retrospective 2023 | NR | BRAF V600 = 27 | 12 | NR | 18 |
| Zhao X, et al. [40] Retrospective 2023 | 27/30 | BRAF V600 = 57 | 24 | 11 | 49 |
| Hyman DM, et al. [41] Prospective 2015 | 3/4 | BRAF V600 = 7 | 12 | 4 | NR |
| Hatashima A, et al. [42] Retrospective 2022 | 6/7 | BRAF V600 = 7 PDL1 = 10 | 13.5 | 11 | 8 |
| Capdevila J, et al. [43] Prospective 2020 | 15/23 | BRAF V600 = 12 | 26 | 10 | 22 |
| Lorch JH, et al. [44] Prospective 2020 | NR | PDL1 = 10 | 24 | NR | NR |
| Dierks C, et al. [45] Retrospective 2021 | 3/3 | PDL1 = 6 | 40 | 1 | 6 |
| ATLEP trial [46] Prospective 2022 | NR | PDL1 = 29 | 20 | 3 | 22 |
| Iyer PC, et al. [47] Retrospective 2018 | 4/8 | PDL1 = 10 | 40.5 | 11 | 7 |
| Lim SM, et al. [48] Prospective 2013 | NR | NR | 20 | 6 | 7 |
| Hanna GJ, et al. [49] Prospective 2018 | NR | PIK3CA = 7 | 25 | 5 | NR |
| Harris EJ, et al. [50] Retrospective 2019 | 1/4 | BRAF V600 = 1 PIK3CA = 1 | 16.9 | 3 | 5 |
| Waguespack SG, et al. [9] Prospective 2022 | 5/2 | NTRK1 = 3 NTRK3 = 4 | 24 | 5 | 5 |
| Doebele RC, et al. [51] Prospective 2020 | NR | NR | 12.9 | 5 | 5 |
| Hamidi S, et al. [52] Retrospective 2024 | NR | BRAF V600 = 71 | 110 | NR | NR |
| Tan JSH, et al. [53] Retrospective 2024 | 4/1 | BRAF V600 = 1 NRAS p.Q61K = 2 KRAS p.L19F = 1 TP53 p.M169 = 2 PIK3CA p.H1047R = 1 PDL1 = 3 | 32.6 | 3 | 3 |
| Tahara M, et al. [54] Prospective 2024 | NR | BRAF V600 = 5 | 11.5 | 4 | 4 |
| Evans LK, et al. [55] Retrospective 2025 | 22/19 | BRAF V600 = 15 KRAS p.G12R = 1 TP53 = 9 NRAS = 2 P63 = 2 PDL1 = 5 | 65 | NR | 33 |
| Soll D, et al. [56] Retrospective 2024 | 2/3 | BRAF V600 = 2 KRAS p.G12R = 1 TP53 p.P153fs = 1 PIK3CA p.E545K = 1 PDL1 = 3 | 12.7 | 1 | 4 |
| Cabanillas ME, et al. [57] Prospective 2024 | 22/20 | BRAF V600 = 18 MEK = 21 VEGF = 3 | 42.1 | 31 | 29 |
| Sehgal K, et al. [58] Prospective 2024 | NR | NR | NR | 18 | NR |
| Song Y, et al. [59] Retrospective 2024 | 11/7 | BRAF V600 = 9 PDL1= 9 | 14 | NR | 10 |
| Sehgal K, et al. [60] Prospective 2024 | NR | BRAF V600 = 3 NRAS codon 61 = 4 PDL1 = 5 | 24 | 8 | 4 |

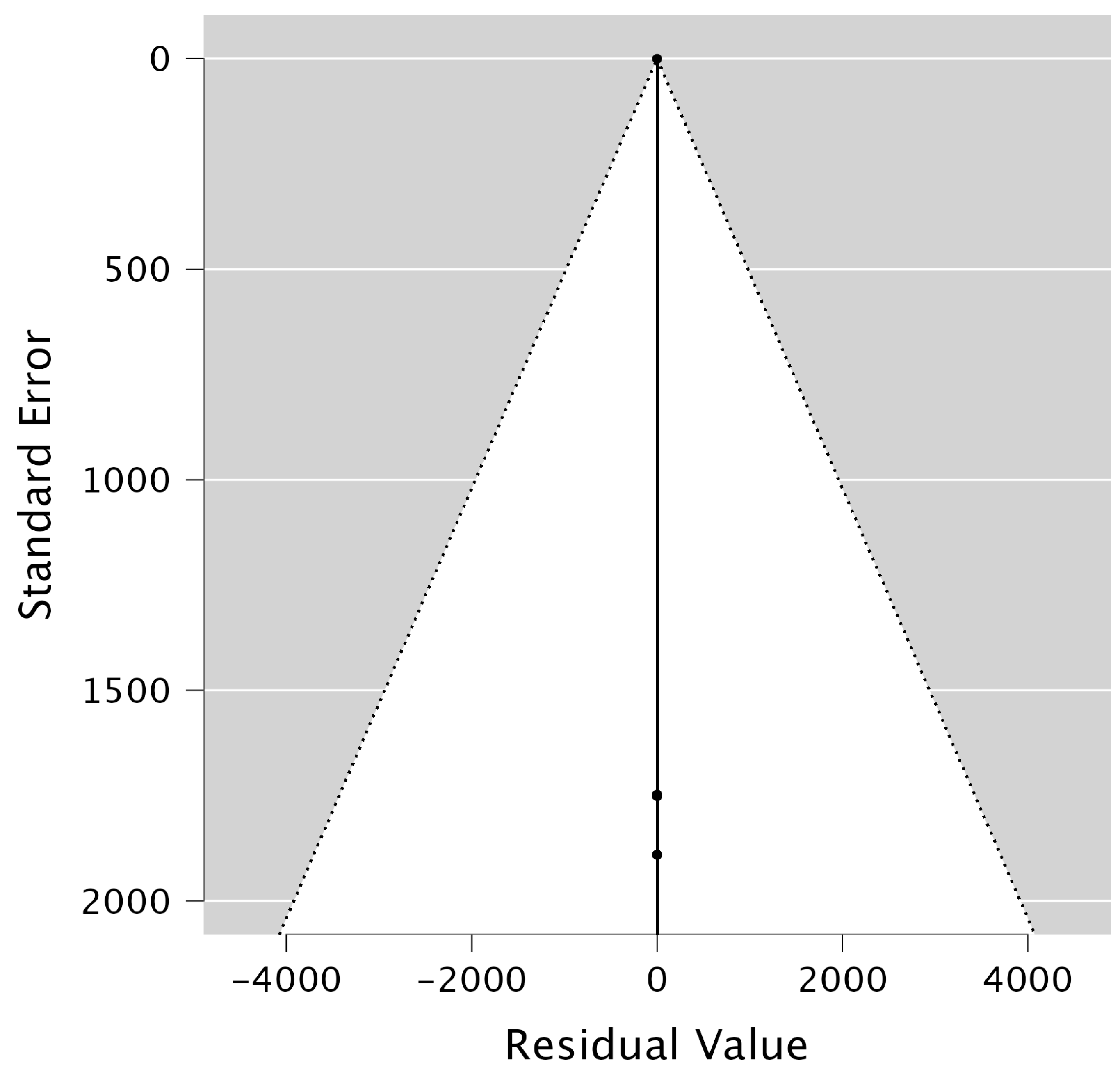
References
- Casali, P.G.; Trama, A. Rationale of the rare cancer list: A consensus paper from the Joint Action on Rare Cancers (JARC) of the European Union (EU). ESMO Open 2020, 5, e000666. [Google Scholar] [CrossRef]
- Molinaro, E.; Romei, C.; Biagini, A.; Sabini, E.; Agate, L.; Mazzeo, S.; Materazzi, G.; Sellari-Franceschini, S.; Ribechini, A.; Torregrossa, L.; et al. Anaplastic thyroid carcinoma: From clinicopathology to genetics and advanced therapies. Nat. Rev. Endocrinol. 2017, 13, 644–660. [Google Scholar] [CrossRef]
- Nagaiah, G.; Hossain, A.; Mooney, C.J.; Parmentier, J.; Remick, S.C. Anaplastic thyroid cancer: A review of epidemiology, pathogenesis, and treatment. J. Oncol. 2011, 2011, 542358. [Google Scholar] [CrossRef] [PubMed]
- Smallridge, R.C.; Ain, K.B.; Asa, S.L.; Bible, K.C.; Brierley, J.D.; Burman, K.D.; Kebebew, E.; Lee, N.Y.; Nikiforov, Y.E.; Rosenthal, M.S.; et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2012, 22, 1104–1139. [Google Scholar] [CrossRef] [PubMed]
- Maniakas, A.; Dadu, R.; Busaidy, N.L.; Wang, J.R.; Ferrarotto, R.; Lu, C.; Williams, M.D.; Gunn, G.B.; Hofmann, M.C.; Cote, G.; et al. Evaluation of Overall Survival in Patients With Anaplastic Thyroid Carcinoma, 2000–2019. JAMA Oncol. 2020, 6, 1397–1404. [Google Scholar] [CrossRef]
- Wu, S.S.; Lamarre, E.D.; Yalamanchali, A.; Brauer, P.R.; Hong, H.; Reddy, C.A.; Yilmaz, E.; Woody, N.; Ku, J.A.; Prendes, B.; et al. Association of Treatment Strategies and Tumor Characteristics With Overall Survival Among Patients With Anaplastic Thyroid Cancer: A Single-Institution 21-Year Experience. JAMA Otolaryngol. Head. Neck Surg. 2023, 149, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhang, J.; Zheng, X.; Gao, M. Efficacy and Safety of Lenvatinib in Anaplastic Thyroid Carcinoma: A Meta-Analysis. Front. Endocrinol. 2022, 13, 920857. [Google Scholar] [CrossRef]
- Lorimer, C.; Cheng, L.; Chandler, R.; Garcez, K.; Gill, V.; Graham, K.; Grant, W.; Sardo Infirri, S.; Wadsley, J.; Wall, L.; et al. Dabrafenib and Trametinib Therapy for Advanced Anaplastic Thyroid Cancer—Real-World Outcomes From UK Centres. Clin. Oncol. (R Coll. Radiol.) 2023, 35, e60–e66. [Google Scholar] [CrossRef] [PubMed]
- Waguespack, S.G.; Drilon, A.; Lin, J.J.; Brose, M.S.; McDermott, R.; Almubarak, M.; Bauman, J.; Casanova, M.; Krishnamurthy, A.; Kummar, S.; et al. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. Eur. J. Endocrinol. 2022, 186, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Vodopivec, D.M.; Hu, M.I. RET kinase inhibitors for RET-altered thyroid cancers. Ther. Adv. Med. Oncol. 2022, 14, 17588359221101691. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Lin, B.; Wang, M.; Liang, X.; Su, L.; Okose, O.; Li, J. Immunotherapy in anaplastic thyroid cancer. Am. J. Transl. Res. 2020, 12, 974–988. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Faber, T.; Ravaud, P.; Riveros, C.; Perrodeau, E.; Dechartres, A. Meta-analyses including non-randomized studies of therapeutic interventions: A methodological review. BMC Med. Res. Methodol. 2016, 16, 35. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, Y.; Kwong, J.S.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, T.; Sugino, K.; Hara, H.; Ito, K.I.; Nakashima, N.; Onoda, N.; Tori, M.; Katoh, H.; Kiyota, N.; Ota, I.; et al. Phase II study of the efficacy and safety of lenvatinib for anaplastic thyroid cancer (HOPE). Eur. J. Cancer 2022, 173, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Wirth, L.J.; Brose, M.S.; Sherman, E.J.; Licitra, L.; Schlumberger, M.; Sherman, S.I.; Bible, K.C.; Robinson, B.; Rodien, P.; Godbert, Y.; et al. Open-Label, Single-Arm, Multicenter, Phase II Trial of Lenvatinib for the Treatment of Patients With Anaplastic Thyroid Cancer. J. Clin. Oncol. 2021, 39, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Iwasaki, H.; Suganuma, N.; Toda, S.; Masudo, K.; Nakayama, H.; Rino, Y.; Masuda, M. Inflammatory biomarkers and dynamics of neutrophil-to-lymphocyte ratio in lenvatinib treatment for anaplastic thyroid carcinoma. Gland Surg. 2021, 10, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Sparano, C.; Godbert, Y.; Attard, M.; Do Cao, C.; Zerdoud, S.; Roudaut, N.; Joly, C.; Berdelou, A.; Hadoux, J.; Lamartina, L.; et al. Limited efficacy of lenvatinib in heavily pretreated anaplastic thyroid cancer: A French overview. Endocr. Relat. Cancer 2021, 28, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jung, H.A.; Shim, J.H.; Park, W.Y.; Kim, T.H.; Lee, S.H.; Kim, S.W.; Ahn, M.J.; Park, K.; Chung, J.H. Multimodal treatments and outcomes for anaplastic thyroid cancer before and after tyrosine kinase inhibitor therapy: A real-world experience. Eur. J. Endocrinol. 2021, 184, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Toda, S.; Murayama, D.; Kato, S.; Matsui, A. Relationship between adverse events associated with lenvatinib treatment for thyroid cancer and patient prognosis. Mol. Clin. Oncol. 2021, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Onoda, N.; Noda, S.; Tauchi, Y.; Morisaki, T.; Asano, Y.; Kashiwagi, S.; Takashima, T.; Ohira, M. Treatment of anaplastic thyroid cancer with tyrosine kinase inhibitors targeted on the tumor vasculature: Initial experience in clinical practice. Endocr. J. 2021, 68, 63–68. [Google Scholar] [CrossRef]
- Fukuda, N.; Toda, K.; Fujiwara, Y.U.; Wang, X.; Ohmoto, A.; Urasaki, T.; Hayashi, N.; Sato, Y.; Nakano, K.; Yunokawa, M.; et al. Neutrophil-to-Lymphocyte Ratio as a Prognostic Marker for Anaplastic Thyroid Cancer Treated With Lenvatinib. In Vivo 2020, 34, 2859–2864. [Google Scholar] [CrossRef]
- Kim, M.; Ahn, J.; Song, D.E.; Yoon, J.H.; Kang, H.C.; Lim, D.J.; Kim, W.G.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; et al. Real-world experience of lenvatinib in patients with advanced anaplastic thyroid cancer. Endocrine 2021, 71, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Kiyota, N.; Yamazaki, T.; Chayahara, N.; Nakano, K.; Inagaki, L.; Toda, K.; Enokida, T.; Minami, H.; Imamura, Y.; et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol. 2019, 15, 717–726. [Google Scholar] [CrossRef]
- Iyer, P.C.; Dadu, R.; Ferrarotto, R.; Busaidy, N.L.; Habra, M.A.; Zafereo, M.; Gross, N.; Hess, K.R.; Gule-Monroe, M.; Williams, M.D.; et al. Real-World Experience with Targeted Therapy for the Treatment of Anaplastic Thyroid Carcinoma. Thyroid 2018, 28, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Onoda, N.; Ito, K.I.; Sugitani, I.; Takahashi, S.; Yamaguchi, I.; Kabu, K.; Tsukada, K. Sorafenib in Japanese Patients with Locally Advanced or Metastatic Medullary Thyroid Carcinoma and Anaplastic Thyroid Carcinoma. Thyroid 2017, 27, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Savvides, P.; Nagaiah, G.; Lavertu, P.; Fu, P.; Wright, J.J.; Chapman, R.; Wasman, J.; Dowlati, A.; Remick, S.C. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid 2013, 23, 600–604. [Google Scholar] [CrossRef]
- Bible, K.C.; Suman, V.J.; Menefee, M.E.; Smallridge, R.C.; Molina, J.R.; Maples, W.J.; Karlin, N.J.; Traynor, A.M.; Kumar, P.; Goh, B.C.; et al. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2012, 97, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.J.; Harris, J.; Bible, K.C.; Xia, P.; Ghossein, R.A.; Chung, C.H.; Riaz, N.; Gunn, G.B.; Foote, R.L.; Yom, S.S.; et al. Radiotherapy and paclitaxel plus pazopanib or placebo in anaplastic thyroid cancer (NRG/RTOG 0912): A randomised, double-blind, placebo-controlled, multicentre, phase 2 trial. Lancet Oncol. 2023, 24, 175–186. [Google Scholar] [CrossRef]
- Pennell, N.A.; Daniels, G.H.; Haddad, R.I.; Ross, D.S.; Evans, T.; Wirth, L.J.; Fidias, P.H.; Temel, J.S.; Gurubhagavatula, S.; Heist, R.S.; et al. A phase II study of gefitinib in patients with advanced thyroid cancer. Thyroid 2008, 18, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Feng, H.; Yang, Z.; Liang, J.; Jin, Z.; Chen, L.; Zhan, L.; Xuan, M.; Yan, J.; Kuang, J.; et al. The central role of a two-way positive feedback pathway in molecular targeted therapies-mediated pyroptosis in anaplastic thyroid cancer. Clin. Transl. Med. 2022, 12, e727. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.T.; Lee, J.S.; Urba, S.; Koenig, R.J.; Sisson, J.; Giordano, T.; Worden, F.P. A phase II study of imatinib in patients with advanced anaplastic thyroid cancer. Thyroid 2010, 20, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Mooney, C.J.; Nagaiah, G.; Fu, P.; Wasman, J.K.; Cooney, M.M.; Savvides, P.S.; Bokar, J.A.; Dowlati, A.; Wang, D.; Agarwala, S.S.; et al. A phase II trial of fosbretabulin in advanced anaplastic thyroid carcinoma and correlation of baseline serum-soluble intracellular adhesion molecule-1 with outcome. Thyroid 2009, 19, 233–240. [Google Scholar] [CrossRef]
- Sosa, J.A.; Elisei, R.; Jarzab, B.; Balkissoon, J.; Lu, S.P.; Bal, C.; Marur, S.; Gramza, A.; Yosef, R.B.; Gitlitz, B.; et al. Randomized safety and efficacy study of fosbretabulin with paclitaxel/carboplatin against anaplastic thyroid carcinoma. Thyroid 2014, 24, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.C.; Cabanillas, M.E.; Boran, A.; et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: Updated analysis from the phase II ROAR basket study. Ann. Oncol. 2022, 33, 406–415. [Google Scholar] [CrossRef]
- Bueno, F.; Smulever, A.; Califano, I.; Guerra, J.; Del Grecco, A.; Carrera, J.M.; Giglio, R.; Rizzo, M.; Lingua, A.; Voogd, A.; et al. Dabrafenib plus trametinib treatment in patients with anaplastic thyroid carcinoma: An Argentinian experience. Endocrine 2023, 80, 134–141. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.N.; Rodrigues, R.; Saramago, A.; Pires, C.; Rito, M.; Horta, M.; Martins, C.; Leite, V.; Cavaco, B.M. Target therapy for BRAF mutated anaplastic thyroid cancer: A clinical and molecular study. Eur. J. Endocrinol. 2023, 188, lvac011. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, J.R.; Dadu, R.; Busaidy, N.L.; Xu, L.; Learned, K.O.; Chasen, N.N.; Vu, T.; Maniakas, A.; Eguia, A.A.; et al. Surgery After BRAF-Directed Therapy Is Associated with Improved Survival in BRAFV600EMutant Anaplastic Thyroid Cancer: A Single-Center Retrospective Cohort Study. Thyroid 2023, 33, 484–491. [Google Scholar] [CrossRef]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Hatashima, A.; Archambeau, B.; Armbruster, H.; Xu, M.; Shah, M.; Konda, B.; Lott Limbach, A.; Sukrithan, V. An Evaluation of Clinical Efficacy of Immune Checkpoint Inhibitors for Patients with Anaplastic Thyroid Carcinoma. Thyroid 2022, 32, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Wirth, L.J.; Ernst, T.; Ponce Aix, S.; Lin, C.C.; Ramlau, R.; Butler, M.O.; Delord, J.P.; Gelderblom, H.; Ascierto, P.A.; et al. PD-1 Blockade in Anaplastic Thyroid Carcinoma. J. Clin. Oncol. 2020, 38, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Lorch, J.H.; Barletta, J.A.; Nehs, M.; Uppaluri, R.; Alexander, E.K.; Haddad, R.I. A phase II study of nivolumab (N) plus ipilimumab (I) in radioidine refractory differentiated thyroid cancer (RAIR DTC) with exploratory cohorts in anaplastic (ATC) and medullary thyroid cancer (MTC). J. Clin. Oncol. 2020, 38, 6513. [Google Scholar] [CrossRef]
- Dierks, C.; Seufert, J.; Aumann, K.; Ruf, J.; Klein, C.; Kiefer, S.; Rassner, M.; Boerries, M.; Zielke, A.; la Rosee, P.; et al. Combination of Lenvatinib and Pembrolizumab Is an Effective Treatment Option for Anaplastic and Poorly Differentiated Thyroid Carcinoma. Thyroid 2021, 31, 1076–1085. [Google Scholar] [CrossRef]
- Dierks, C.; Seufert, J.; Aumann, K.; Ruf, J.; Klein, C.; Kiefer, S.; Rassner, M.; Boerries, M.; Zielke, A.; la Rosee, P.; et al. Phase II ATLEP trial: Final results for lenvatinib/pembrolizumab in metastasized anaplastic and poorly differentiated thyroid carcinoma. Ann. Oncol. 2022, 33 (Suppl. S7), S750–S757. [Google Scholar] [CrossRef]
- Iyer, P.C.; Dadu, R.; Gule-Monroe, M.; Busaidy, N.L.; Ferrarotto, R.; Habra, M.A.; Zafereo, M.; Williams, M.D.; Gunn, G.B.; Grosu, H.; et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J. Immunother. Cancer 2018, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Chang, H.; Yoon, M.J.; Hong, Y.K.; Kim, H.; Chung, W.Y.; Park, C.S.; Nam, K.H.; Kang, S.W.; Kim, M.K.; et al. A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Ann. Oncol. 2013, 24, 3089–3094. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Busaidy, N.L.; Chau, N.G.; Wirth, L.J.; Barletta, J.A.; Calles, A.; Haddad, R.I.; Kraft, S.; Cabanillas, M.E.; Rabinowits, G.; et al. Genomic Correlates of Response to Everolimus in Aggressive Radioiodine-refractory Thyroid Cancer: A Phase II Study. Clin. Cancer Res. 2018, 24, 1546–1553. [Google Scholar] [CrossRef]
- Harris, E.J.; Hanna, G.J.; Chau, N.; Rabinowits, G.; Haddad, R.; Margalit, D.N.; Schoenfeld, J.; Tishler, R.B.; Barletta, J.A.; Nehs, M.; et al. Everolimus in Anaplastic Thyroid Cancer: A Case Series. Front. Oncol. 2019, 9, 106. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, S.; Iyer, P.C.; Dadu, R.; Gule-Monroe, M.K.; Maniakas, A.; Zafereo, M.E.; Wang, J.R.; Busaidy, N.L.; Cabanillas, M.E. Checkpoint Inhibition in Addition to Dabrafenib/Trametinib for BRAFV600E-Mutated Anaplastic Thyroid Carcinoma. Thyroid 2024, 34, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.H.; Tay, T.K.Y.; Ong, E.H.W.; Fehlings, M.; Tan, D.S.; Sukma, N.B.; Chen, E.X.; Sng, J.H.; Yip, C.S.P.; Lim, K.H.; et al. Combinatorial Hypofractionated Radiotherapy and Pembrolizumab in Anaplastic Thyroid Cancer. Eur. Thyroid J. 2024, 13, e230144. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Kiyota, N.; Imai, H.; Takahashi, S.; Nishiyama, A.; Tamura, S.; Shimizu, Y.; Kadowaki, S.; Ito, K.I.; Toyoshima, M.; et al. A Phase 2 Study of Encorafenib in Combination with Binimetinib in Patients with Metastatic BRAF-Mutated Thyroid Cancer in Japan. Thyroid 2024, 34, 467–476. [Google Scholar] [CrossRef]
- Evans, L.K.; Chen, H.; Taki Labib, M.; Cronkite, D.A.; Yu, A.C.; Ashendouek, M.; Elashoff, D.; Chai-Ho, W.; Wong, D.J.; St John, M. Improved Survival of Advanced-Stage Anaplastic Thyroid Cancer With Systemic Therapy. Laryngoscope 2025, 135, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Soll, D.; Bischoff, P.; Frisch, A.; Jensen, M.; Karadeniz, Z.; Mogl, M.T.; Horst, D.; Penzkofer, T.; Spranger, J.; Keilholz, U.; et al. First effectiveness data of lenvatinib and pembrolizumab as first-line therapy in advanced anaplastic thyroid cancer: A retrospective cohort study. BMC Endocr. Disord. 2024, 24, 25. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Dadu, R.; Ferrarotto, R.; Gule-Monroe, M.; Liu, S.; Fellman, B.; Williams, M.D.; Zafereo, M.; Wang, J.R.; Lu, C.; et al. Rare Tumor Initiative Team. Anti-Programmed Death Ligand 1 Plus Targeted Therapy in Anaplastic Thyroid Carcinoma: A Nonrandomized Clinical Trial. JAMA Oncol. 2024, 10, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, K.; Serritella, A.; Liu, M.; ONeill, A.; Nangia, C.; Pappa, T.; Demeure, M.J.; Worden, F.P.; Haddad, R.; Lorch, J. A phase I/II trial of sapanisertib in advanced anaplastic and radioiodine refractory differentiated thyroid carcinoma. J. Clin. Endocrinol. Metab. 2024, dgae443. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Y.; Bai, Y.; Wang, T.; Xu, G.; Ma, X.; Fei, K.; Zhang, B. Combination kinase inhibitors and immunotherapy for unresectable anaplastic thyroid carcinoma: A retrospective single-center study. Oral Oncol. 2024, 159, 107067. [Google Scholar] [CrossRef]
- Sehgal, K.; Pappa, T.; Shin, K.Y.; Schiantarelli, J.; Liu, M.; Ricker, C.; Besson, N.R.; Jones, S.M.; Welsh, E.L.; Pfaff, K.L.; et al. Dual Immune Checkpoint Inhibition in Patients With Aggressive Thyroid Carcinoma: A Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2024, 10, 1663–1671. [Google Scholar] [CrossRef]
- Silver Karcioglu, A.; Iwata, A.J.; Pusztaszeri, M.; Abdelhamid Ahmed, A.H.; Randolph, G.W. The American Thyroid Association (ATA) integrates molecular testing into its framework for managing patients with anaplastic thyroid carcinoma (ATC): Update on the 2021 ATA ATC guidelines. Cancer Cytopathol. 2022, 130, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, S.; Dadu, R.; Zafereo, M.E.; Ferrarotto, R.; Wang, J.R.; Maniakas, A.; Gunn, G.B.; Lee, A.; Spiotto, M.T.; Iyer, P.C.; et al. Initial Management of BRAF V600E-Variant Anaplastic Thyroid Cancer: The FAST Multidisciplinary Group Consensus Statement. JAMA Oncol. 2024, 10, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Jannin, A.; Escande, A.; Al Ghuzlan, A.; Blanchard, P.; Hartl, D.; Chevalier, B.; Deschamps, F.; Lamartina, L.; Lacroix, L.; Dupuy, C.; et al. Anaplastic Thyroid Carcinoma: An Update. Cancers 2022, 14, 1061. [Google Scholar] [CrossRef]
- Mahdiannasser, M.; Khazaei, S.; Akhavan Rahnama, M.; Soufi-Zomorrod, M.; Soutodeh, F.; Parichehreh-Dizaji, S.; Rakhsh-Khorshid, H.; Samimi, H.; Haghpanah, V. Illuminating the role of lncRNAs ROR and MALAT1 in cancer stemness state of anaplastic thyroid cancer: An exploratory study. Noncoding RNA Res. 2023, 8, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kim, T.H.; Kim, S.W.; Ki, C.S.; Jang, H.W.; Kim, J.S.; Kim, J.H.; Choe, J.H.; Shin, J.H.; Hahn, S.Y.; et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr. Relat. Cancer 2017, 24, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Crosta, S.; Boldorini, R.; Bono, F.; Brambilla, V.; Dainese, E.; Fusco, N.; Gianatti, A.; L’Imperio, V.; Morbini, P.; Pagni, F. PD-L1 Testing and Squamous Cell Carcinoma of the Head and Neck: A Multicenter Study on the Diagnostic Reproducibility of Different Protocols. Cancers 2021, 13, 292. [Google Scholar] [CrossRef]
- Oliinyk, D.; Augustin, T.; Koehler, V.F.; Rauch, J.; Belka, C.; Spitzweg, C.; Käsmann, L. Hypofractionated Radiotherapy for Anaplastic Thyroid Cancer: Systematic Review and Pooled Analysis. Cancers 2020, 12, 2506. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.B.; Ge, Y.; Zhang, W.X.; Lin, G.H.; Kuang, B.H.; Wang, B.C. The efficacy and safety of antiangiogenesis tyrosine kinase inhibitors in patients with advanced anaplastic thyroid cancer: A meta-analysis of prospective studies. Medicine 2024, 103, e38679. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, J.; Ye, T.; Tang, W.; Pan, X.; Wang, S.; Liu, J. Efficacy and safety of anlotinib-based chemotherapy for locally advanced or metastatic anaplastic thyroid carcinoma. Endocrine 2023, 81, 540–546. [Google Scholar] [CrossRef]
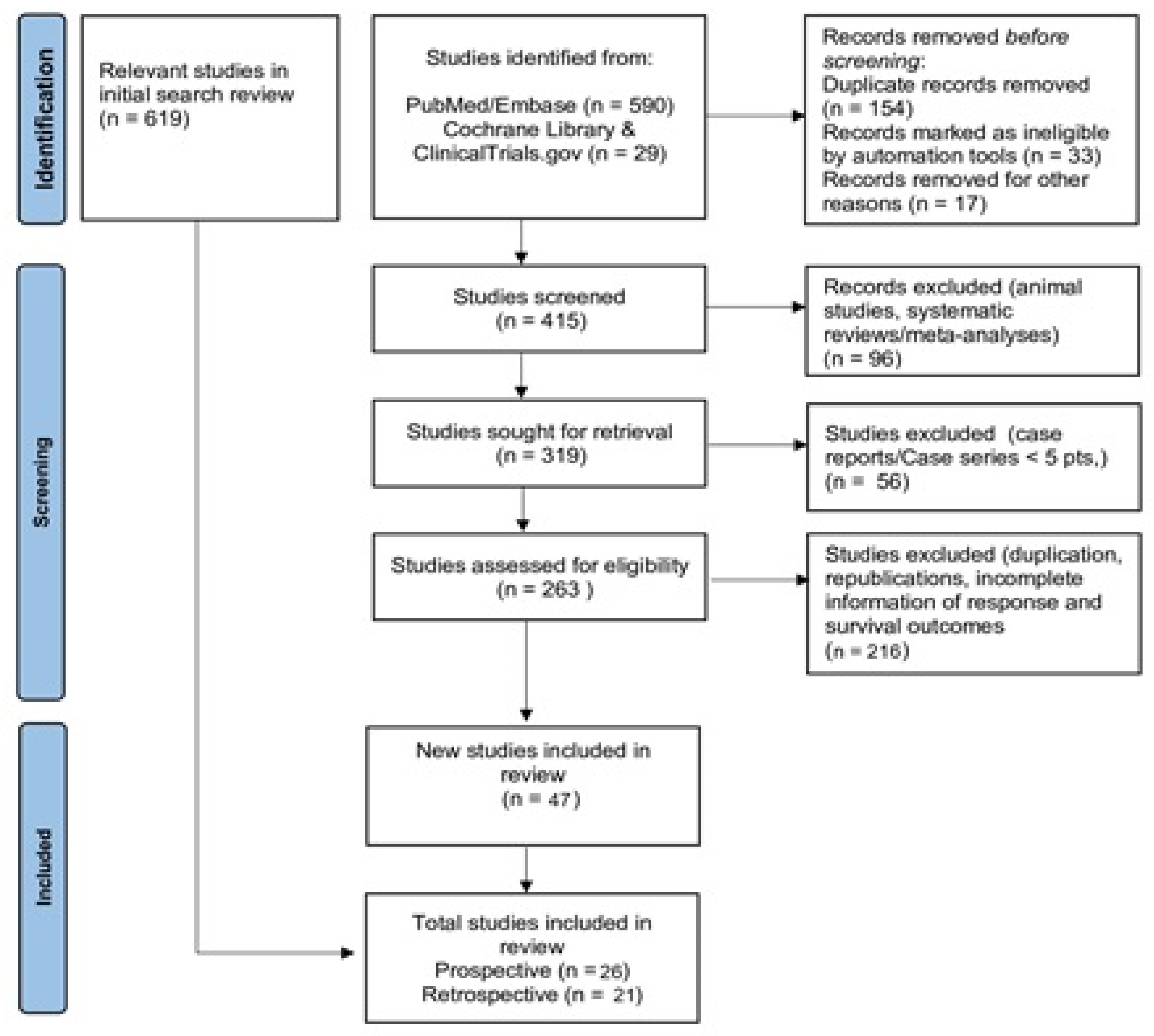



| Study/Year/ Nature | Country | N (Median Age) | TNM (n) | Previous Treatment (%) | Intervention | Endpoints | ORR (%) | Median PFS (Months) | Median OS (Months) |
|---|---|---|---|---|---|---|---|---|---|
| Higashiyama T, et al. (HOPE) [16] Prospective 2022 | Japan | 42 (73y) | T4 = 37 N1a = 3 N1b = 23 M0 = 17 M1 = 21 | CTH = 40 XRT = 21.4 Sx = 54.7 | Lenvatinib | Primary: OS Secondary: PFS, ORR, DCR, safety | 5 (11.9) | 16.5 | 18.5 |
| Wirth LJ, et al. [17] Prospective 2021 | US, UK, France, Italy, Australia, | 34 (65y) | M1 = 34 | CTH = 62 TKI = 9 IO = 9 XRT = 65 Sx = 71 | Lenvatinib | Primary: ORR, safety Secondary: PFS, OS | 1 (2.9) | 2.6 (1.4–2.8) | 10.6 (3.8–19.8) |
| Yamazaki H, et al. [18] Retrospective 2021 | Japan | 20 (73.6y) | T4 = 14 | Sx = 6 | Lenvatinib | - | 2 (10.0) | 4.1 (1.1–12.2) | - |
| Sparano C, et al. [19] Retrospective 2021 | France, Italy | 15 (63y) | Recurrent/ M1 = 15 | XRT = 71 CTH = 73.2 TKI = 26.5 | Lenvatinib | - | 5 (33.3) | 2.7 (1.9–3.5) | 3.1 (0.6–5.5) |
| Park J, et al. [20] Retrospective 2021 | South Korea | 11 (66.4y) | T4/ M1 = 11 | Sx = 64.2 XRT = 53.3 CTH = 24.2 TKI = 15.8 | Lenvatinib | - | 3 (27.3) | 2.7 (0.1–12.7) | 12.4 (1.7–47.7) |
| Iwasaki H, et al. [21] Retrospective 2021 | Japan | 32 (77y) | T4/ M1 = 32 | - | Lenvatinib | - | 6 (18.8) | - | 3.2 (0.5–28.9) |
| Ishihara S, et al. [22] Retrospective 2021 | Japan | 11 (74y) | Recurrent = 5 T4 = 4 T3b = 2 | CTH = 63.6 XRT = 63.6 TKI = 27.3 | Lenvatinib | - | 3 (30.0) | - | 4.7 (1.9–13.1) |
| Fukuda, N et al. [23] Retrospective 2020 | Japan | 13 (68y) | T4 = 10 N1 = 7 M1 = 11 | Sx = 77 XRT = 46 CTH = 31 | Lenvatinib | - | 3 (23.0) | 3.8 (1.8–6.4) | 10.2 (3.7–17.6) |
| Kim M, et al. [24] Retrospective 2020 | South Korea | 14 (64.9y) | T4 = 14 | Sx = 33 CTH = 100 XRT = 100 | Lenvatinib | - | 4 (28.6) | 5.7 (2.2–8.3) | 6.7 (3.0–8.4) |
| Takahashi S, et al. [25] Prospective 2019 | Japan, US | 17 (65y) | T4 = 17 | Sx = 82 CTH = 41 XRT = 53 | Lenvatinib | Primary: Safety Secondary: PFS, ORR, DCR, OS | 4 (23.5) | 7.4 (1.7–12.9) | 10.6 (3.8–19.8) |
| Iyer PC, et al. [26] Retrospective 2018 | US | 10 (67y) | T3-4/N1 = 4 M1 = 6 | Sx = 50 CTH = 60 XRT = 44 | Lenvatinib | - | 3 (30.0) | 2.6 (2.8-NR) | 3.9 (2.5-NR) |
| Ito Y et al. [27] Prospective 2017 | Japan | 10 (72y) | M1 = 8 T4 = 2 | Sx = 70 CTH = 60 XRT = 70 | Sorafenib | Primary: Safety Secondary: PFS, ORR, DCR, OS | 3 (40.0) | 2.8 (0.7–5.6) | 5 (0.7–5.7) |
| Savvides P, et al. [28] Prospective 2013 | US | 20 (59y) | M1 = 20 | CTH = 100 XRT = 90 Sx = 90 | Sorafenib | Primary: ORR, DCR Secondary: PFS, OS, safety | 2 (10.0) | 1.9 (1.3–3.6) | 3.9 (2.2–7.1) |
| Bible KC, et al. [29] Prospective 2012 | US | 15 (63) | T4 = 2 N1 = 1 M1 = 12 | CTH = 100 XRT = 90 Sx = 90 | Pazopanib | Primary: ORR, DCR Secondary: PFS, OS, safety | 5 (33.3) | 2.1 (NR) | 3.7 (0.5–35) |
| NRG/RTOG 0912 [30] Prospective 2023 | US | 71 (63y) | T4 = 71 N1 = 52 M1 = 26 | Sx = 57.5 | a. Pazopanib + weekly Paclitaxel +IMRT 66 Gy/33 fractions (36pts) b. Weekly Paclitaxel +IMRT 66 Gy/33 fractions | Primary: OS Secondary: PFS, safety | 11 (30.5) 11 (31.4) | - | 5.7 (4.0–12.8) 7·3 (4.3–10.6) |
| Pennell NA, et al. [31] Prospective 2008 | US | 5/27 (65y) | T4/M1 = 5 | CTH = 22 Sx = 94 XRT = 85 | Gefitinib | Primary: ORR, DCR Secondary: PFS, OS, safety | 2 (40.0) | 3.7 | 17.5 |
| Zhao Q, et al. [32] Prospective 2022 | China | 17 (61y) | T4 = 3 N1 = 7 M1 = 6 | - | Apatinib | Primary: ORR, DCR | 7 (41.1) | - | - |
| Ha HT, et al. [33] Prospective 2010 | US | 11 (65y) | Recurrent = 7 M1 = 7 | Sx = 64 CTH = 55 | Imatinib | Primary: ORR | 2 (18.2) | 5.8 (2–29) | 3.2 (2–37) |
| Mooney CJ, et al. [34] Prospective 2009 | US | 26 (59y) | Recurrent = 24 M1 = 7 | CTH = 50 Sx = 61 XRT = 61 | Fosbretabulin | Primary: Safety Secondary: | 8 (30.7) | - | 4.7 (2.5–6.4) |
| Sosa JA, et al. [35] Prospective 2014 | US | 80 (63y) | Recurrent = 20 | Sx = 25 XRT = 13.8 CTH =5 | Fosbretabulin +CP Or CP alone | Primary: OS | 13 (32.5) 13 (32.5) | 3.3 (2.3–5.6) 3.1 (2.7–5.4) | 5.2 (3.1–9) 4.0 (2.8–6.2) |
| Subbiah V, et al. [36] Prospective 2018 | US | 16 (72y) | Recurrent = 16 | Sx = 88 XRT = 81 CTH = 38 | DT | Primary: ORR, DCR Secondary: PFS, OS, safety | 11 (68.7) | NA | NA |
| ROAR Study [37] Prospective 2022 | US, France, south Korea | 36 (71y) | T4 = 1 M1 = 35 | Sx = 83 XRT = 83 CTH = 42 RAI = 31 TKI = 19 IO = 11 | DT | Primary: ORR Secondary: PFS, OS, DOR | 18 (50) | 6.7 (4.7–13.8) | 14.5 (6.8–23.2) |
| Lorimer C, et al. [8] Retrospective 2023 | UK | 17 (68y) | T4/M1 = 17 | Sx = 59 CTH = 12 RAI = 12 XRT = 18 | DT | - | 14 (82.2) | 4.7 (1.4–7.8) | 6.9 (2.46-NR) |
| Bueno F, et al. [38] Retrospective 2023 | Argentina | 5 (70y) | M1 = 5 | Sx = 60 XRT = 20 | DT | - | 4 (80.0) | - | 20 (18-NR) |
| da Silva TN, et al. [39] retrospective 2023 | Portugal | 27 (77y) | Recurrent/ M1 = 27 | Sx = 40.7 XRT = 40.7 CTH = 25.9 TKI = 7.4 | DT | - | 8 (29.6) | 9.0 (4.9–13.0) | 17.9 (15.9–19.8) |
| Zhao X, et al. [40] Retrospective 2023 | US | 57 (67.2y) | T4 = 20 M1 = 37 | IO = 75.4 XRT = 47.4 | DT | - | 18 (31.5) | 34.2 (15.8–NA) | 39.2 (NR) |
| Hyman DM, et al. [41] Prospective 2015 | US, UK, Germany, France, Spain | 7/122 (65y) | Recurrent/ M1 = 7 | Any = 78 XRT = 67 | Vemurafenib | Primary: ORR Secondary: PFS, OS | 2 (29.0) | - | - |
| Hatashima A, et al. [42] Retrospective 2022 | US | 13 (70y) | T4 = 2 M1 = 11 | - | Pembrolizumab (12 patients) Nivolumab (1 patient) | - | 2 (16.0) | 1.9 | 4.4 (4.0–29) |
| Capdevila J, et al. [43] Prospective 2020 | US, Canada, Germany, Italy, Switzerland, France, Poland | 42 (62.5y) | Recurrent/M1 = 42 | XRT = 71.4 Sx = 66.7 | Spartalizumab | Primary: ORR Secondary: Safety | 7 (16.6) | 1.7 (1.2–1.9) | 5.9 (2.4-NR) |
| Lorch JH, et al. [44] Prospective 2020 | US | 10/49 (65y) | T4/M1 = 10 | - | Ipilimumab + Nivolumab | Primary: ORR | 3 (30.0) | - | - |
| Dierks C, et al. [45] Retrospective 2021 | Germany | 6 (63.5y) | Recurrent/ M1 = 6 | Sx = 100 XRT = 87.5 CTH = 75 RAI = 25 | LP | - | 4 (66.0) | 17.7 | 18.5 |
| ATLEP trial [46] Prospective 2022 | Germany | 29 (63y) | N1/M1 = 29 | Sx = 90 XRT = 90 CTH = 90 | LP | 12 (41.2) | 9.5 | 10.25 (NR) | |
| Iyer PC, et al. [47] Retrospective 2018 | US | 11 (67y) | T4/M1 = 11 | Sx = 50 XRT = 44 CTH = 69 | LP | - | 5 (42.0) | 2.9 (2.2–3.7) | 6.93 (1.7–12.1) |
| Lim SM, et al. [48] Prospective 2013 | South Korea | 6/40 (61y) | T4/M1 = 6 | Sx = 68 CTH = 22 XRT = 15 TKI = 2 | Everolimus | Primary: ORR Secondary: PFS, OS | 1 (16.0) | 2.3 (1.1–3.7) | - |
| Hanna GJ, et al. [49] Prospective 2018 | US | 7/50 (62y) | Recurrent/ M1 = 7 | Sx = 71 XRT = 57 RAI = 28 CTH = 28 TKI = 14 | Everolimus | Primary: ORR Secondary: Safety | 1 (14.2) | - | 4.6 (<1–29.9) |
| Harris EJ, et al. [50] Retrospective 2019 | US | 5 (75y) | Recurrent/ M1 = 5 | XRT = 60 CTH = 60 RAI = 40 | Everolimus | - | 1 (20.0) | - | 7.4 (<1–40) |
| Waguespack SG, et al. [9] Prospective 2022 | US, Canada, Ireland, Italy, Germany, S. Korea | 7/29 (60y) | Recurrent/ T4 = 7 | Sx =100 XRT = 71 CTH = 43 | Larotrectinib | Primary: ORR Secondary: PFS, OS, Safety | 2 (29.0) | 2.2 (<1–6) | 2.5 (<1–6) |
| Doebele RC, et al. [51] Prospective 2020 | US, UK, Australia, Italy, Hong Kong, Spain, S. Korea, Poland, Netherlands | 5/54 (58y) | - | - | Entrectinib | Primary: ORR | 1 (20.0) | - | - |
| Hamidi S, et al. [52] Retrospective 2024 | US | 71 | T4 = 23 M1 = 48 | Sx/XRT | 23 = DT 48 = DTP | Primary: OS Secondary: PFS | 7 (30.4) 15 (31.3) | DT = 4 DTP =11 | DT = 9 DTP = 17 |
| Tan JSH, et al. [53] Retrospective 2024 | Singapore | 5 (63.6y) | T4 = 3 M1 = 2 | - | Quad-XRT/LP = 2. Quad-XRT/P = 3 | Primary: OS Secondary: PFS, ORR | 2 (40.0) | 7.6 | 6.2 |
| Tahara M, et al. [54] Prospective 2024 | Japan | 5/22 | T4/M1 = 5 | - | Encorafenib plus binimetinib | Primary: OS Secondary: PFS, ORR | 4 (80.0) | 11.5 | 11.5 |
| Evans LK, et al. [55] Retrospective 2025 | US | 41 (67.4y) | Recurrent/T4/M1 = 41 | - | LP = 18 DT = 9 | Primary: OS | 6 (33.3) 3 (33.3) | - | 7.6 |
| Soll D, et al. [56] Retrospective 2024 | Germany | 5 (65y) | M1 = 5 | - | LP | Primary: OS Secondary: PFS | 1 (20.0) | 4.7 | 6.4 |
| Cabanillas ME, et al. [57] Prospective 2024 | US | 39 | T4 = 12 M1 = 30 | XRT = 12 CTH = 38.1 | VCA = 18 CA = 21 | Primary: OS Secondary: PFS | 2 (11.1) 1 (5.7) | VCA = 13.9 CA = 8.7 BA = 6.2 | VCA = 43 CA = 13.9 BA = 8.7 |
| Sehgal K, et al. [58] Prospective 2024 | US | 20/26 | Recurrent /M1 = 20 | TKI = 20 | Sapanisertib | Primary: ORR, PFS | 6 (30.0) | 1.6 | - |
| Song Y, et al. [59] Retrospective 2024 | China | 18 | T4/M1 | - | DTP, LP, anlotinib sintilimab, or camrelizumab | Primary: ORR, PFS, OS | 11 (61.1) | - | 14 |
| Sehgal K, et al. [60] Prospective 2024 | US | 10/49 (65y) | Recurrent/metastatic/T4 = 10 | XRT =80 TKI = 70 | Ipilimumab/ nivolumab | Primary: ORR Secondary: PFS, OS, Safety | 3 (30.0) | - | - |
| G3/>Toxicity | Lenvatinib (n = 266) | Sorafenib (n = 30) | Pazopanib (n = 51) | Gefitinib (n = 5) | Apatinib (n = 17) | Imatinib (n = 11) | Chemotherapy (n = 81) | mTORi (n = 18) | BRAFi (n = 206) | IO (n = 232) | NTRKi (n = 11) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Loss of appetite | 29 (11.0%) | 1 (3.0%) | 2 (5.8%) | 2 (40.0%) | - | 1 (9.0%) | 2 (2.3%) | 5 (27.7%) | 10 (4.8%) | 4 (1.7%) | 1 (9.0%) |
| Weight loss | 23 (8.6%) | - | - | 1 (20.0%) | - | 1 (9.0%) | 1 (1.2%) | 4 (22.2%) | 8 (3.8%) | 4 (1.7%) | 1 (9.0%) |
| Fatigue | 21 (7.9%) | - | 7 (13.7%) | 1 (20.0%) | - | - | 1 (1.2%) | 1 (5.5%) | 18 (8.7%) | 4 (1.7%) | 3 (27.3%) |
| Hypertension | 37 (14.0%) | - | 1 (1.9%) | - | 6 (35.3%) | - | 2 (2.3%) | - | 14 (6.7%) | - | - |
| HFS | 20 (7.5%) | 3 (9.0%) | - | - | - | - | - | - | 4 (1.7%) | - | |
| Nausea | 12 (4.5%) | 2 (6.0%) | 4 (7.8%) | 1 (20.0%) | - | - | 11 (13.6%) | 5 (27.7%) | 8 (3.8%) | - | 1 (9.0%) |
| Diarrhoea | 24 (9.0%) | 3 (9.0%) | - | 3 (60.0%) | - | - | 12 (14.8%) | - | 8 (3.8%) | - | 1 (9.0%) |
| Anaemia | 3 (1.1%) | - | 2 (3.9%) | - | - | 2 (18.0%) | 8 (9.8%) | - | 23 (11.1%) | 2 (0.8%) | 1 (9.0%) |
| Thrombocytopenia | 8 (3.0%) | - | 1 (1.9%) | - | 1 (5.9%) | 5 (45.5%) | 14 (17.3%) | - | - | - | - |
| Lymphopenia | 4 (1.5%) | - | 5 (9.8%) | - | 1 (5.9%) | - | 8 (9.8%) | 2 (11.0%) | - | - | - |
| Neutropenia | 2 (1.0%) | - | 7 (13.7%) | - | 1 (5.9%) | 1 (9.0%) | 2 (2.3%) | 5 (27.7%) | 1 (0.5%) | - | - |
| Hypophosphatemia | - | 1 (3.0%) | 1 (1.9%) | - | - | - | 2 (11.0%) | - | 2 (0.8%) | - | |
| Hyperglycaemia | - | - | - | - | - | 1 (1.2%) | 2 (11.0%) | 8 (3.8%) | 2 (0.8%) | - | |
| Hyponatremia | - | 2 (6.0%) | - | - | - | - | - | - | 11 (5.3%) | 3 (1.3%) | - |
| Voice alteration | 3 (1.1%) | 3 (5.8%) | - | 5 (29.4%) | - | - | - | - | 1 (0.4%) | - | |
| Proteinuria | 9 (3.4%) | 1 (3.0%) | - | - | - | - | - | - | 1 (0.5%) | - | - |
| Eczema/dermatitis | 3 (1.1%) | 3 (9.0%) | 13 (25.5%) | - | - | - | - | - | 2 (0.9%) | 5 (2.1%) | - |
| Mucositis oral | 3 (1.1%) | 3 (9.0%) | 5 (9.8%) | - | - | - | 1 (1.2%) | 7 (38.8%) | - | - | - |
| Arthralgia | 2 (1.0%) | 1 (3.0%) | - | - | - | 2 (18.0%) | - | - | - | - | 1 (9.0%) |
| Non-tumour bleeding | 4 (1.5%) | - | - | - | - | - | - | - | - | 1 (0.4%) | |
| Vomiting | 11 (4.1%) | 3 (9.0%) | 1 (1.9%) | - | - | 1 (9.0%) | 1 (1.2%) | 1 (5.5%) | 6 (2.9%) | 1 (0.4%) | - |
| Skin ulceration | 5 (1.9%) | - | - | - | - | - | - | - | - | ||
| Aspartate aminotransferase increased | 8 (3.0%) | 1 (3.0%) | 12 (23.5%) | - | 1 (5.9%) | - | - | 2 (11.0%) | - | 11 (4.7%) | 1 (9.0%) |
| Alkaline phosphatase increased | - | - | 2 (3.9%) | - | - | - | - | - | - | - | 1 (9.0%) |
| Amylase/lipase elevated | - | - | - | - | - | - | - | - | - | 21 (9.1%) | - |
| Hypothyroidism | 7 (2.6%) | - | - | - | - | - | - | - | - | 3 (1.3%) | - |
| Hypophysitis | - | - | - | - | - | - | - | - | - | 2 (0.8%) | - |
| Dyspnoea | 7 (2.6%) | - | - | - | - | - | - | - | - | 4 (1.7%) | - |
| Tracheal fistula | 4 (1.5%) | - | - | - | - | - | - | - | - | 2 (0.8%) | - |
| Fistula | 2 (0.8%) | 1 (3.0%) | 1 (1.9%) | - | - | - | - | - | - | 3 (1.3%) | |
| Aspiration pneumonia/ pneumonitis | 6 (2.2%) | 1 (3.0%) | 3 (5.8%) | - | - | - | - | 1 (5.5%) | 14 (6.7%) | 1 (0.4%) | -- |
| Pneumothorax | 1 (0.4%) | - | - | - | - | - | - | - | - | 3 (1.3%) | - |
| Pancreatitis | 1 (0.4%) | - | - | - | - | - | - | - | - | - | - |
| ECG QT prolonged | 3 (1.1%) | - | - | - | - | - | 6 (7.4%) | - | - | - | - |
| Acute heart failure | 1 (0.4%) | - | - | - | - | 1 (9.0%) | 1 (1.2%) | - | - | 5 (2.1%) | - |
| Adrenal insufficiency | - | - | - | - | - | - | - | - | - | 7 (3.0%) | - |
| Dose modifications | 59 (22.2%) | 7 (23.0%) | 16 (31.4%) | - | - | - | 24 (29.6%) | 7 (38.8%) | 31 (15.0%) | - | 4 (36.4%) |
| Interruptions | 44 (16.5%) | 6 (20.0%) | 9 (17.6%) | - | - | - | 25 (30.8%) | 6 (33.3%) | 34 (16.5%) | - | - |
| Withdrawals | 17 (6.3%) | 1 (3.0%) | 12 (23.5%) | - | - | - | 12 (14.8%) | 1 (5.5%) | 9 (4.4%) | 5 (2.1%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tunio, M.A.; Hinder, D.; Emery, B.; Riaz, M.H.; Ibraheem, Y.A.; Nayak, K.K.; Mohamed, W. Modern Therapeutic Approaches in Anaplastic Thyroid Cancer: A Meta-Analytic Review of Randomised and Single Arm Studies on Efficacy and Survival. Cancers 2025, 17, 777. https://doi.org/10.3390/cancers17050777
Tunio MA, Hinder D, Emery B, Riaz MH, Ibraheem YA, Nayak KK, Mohamed W. Modern Therapeutic Approaches in Anaplastic Thyroid Cancer: A Meta-Analytic Review of Randomised and Single Arm Studies on Efficacy and Survival. Cancers. 2025; 17(5):777. https://doi.org/10.3390/cancers17050777
Chicago/Turabian StyleTunio, Mutahar A., Donna Hinder, Blaise Emery, Muhammad H. Riaz, Yusef A. Ibraheem, Krishnendu Kumar Nayak, and Wael Mohamed. 2025. "Modern Therapeutic Approaches in Anaplastic Thyroid Cancer: A Meta-Analytic Review of Randomised and Single Arm Studies on Efficacy and Survival" Cancers 17, no. 5: 777. https://doi.org/10.3390/cancers17050777
APA StyleTunio, M. A., Hinder, D., Emery, B., Riaz, M. H., Ibraheem, Y. A., Nayak, K. K., & Mohamed, W. (2025). Modern Therapeutic Approaches in Anaplastic Thyroid Cancer: A Meta-Analytic Review of Randomised and Single Arm Studies on Efficacy and Survival. Cancers, 17(5), 777. https://doi.org/10.3390/cancers17050777







