Development of Clinical-Radiomics Nomogram for Predicting Post-Surgery Functional Improvement in High-Grade Glioma Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Defining the Kps-Flag
2.3. MRI and Preprocessing
2.4. Feature Selection Rad-Model
2.5. Machine Learning Approach for Rad-Model Training and Testing
2.6. Developing Clinical-Radiomic-Model
3. Results
3.1. Study Population
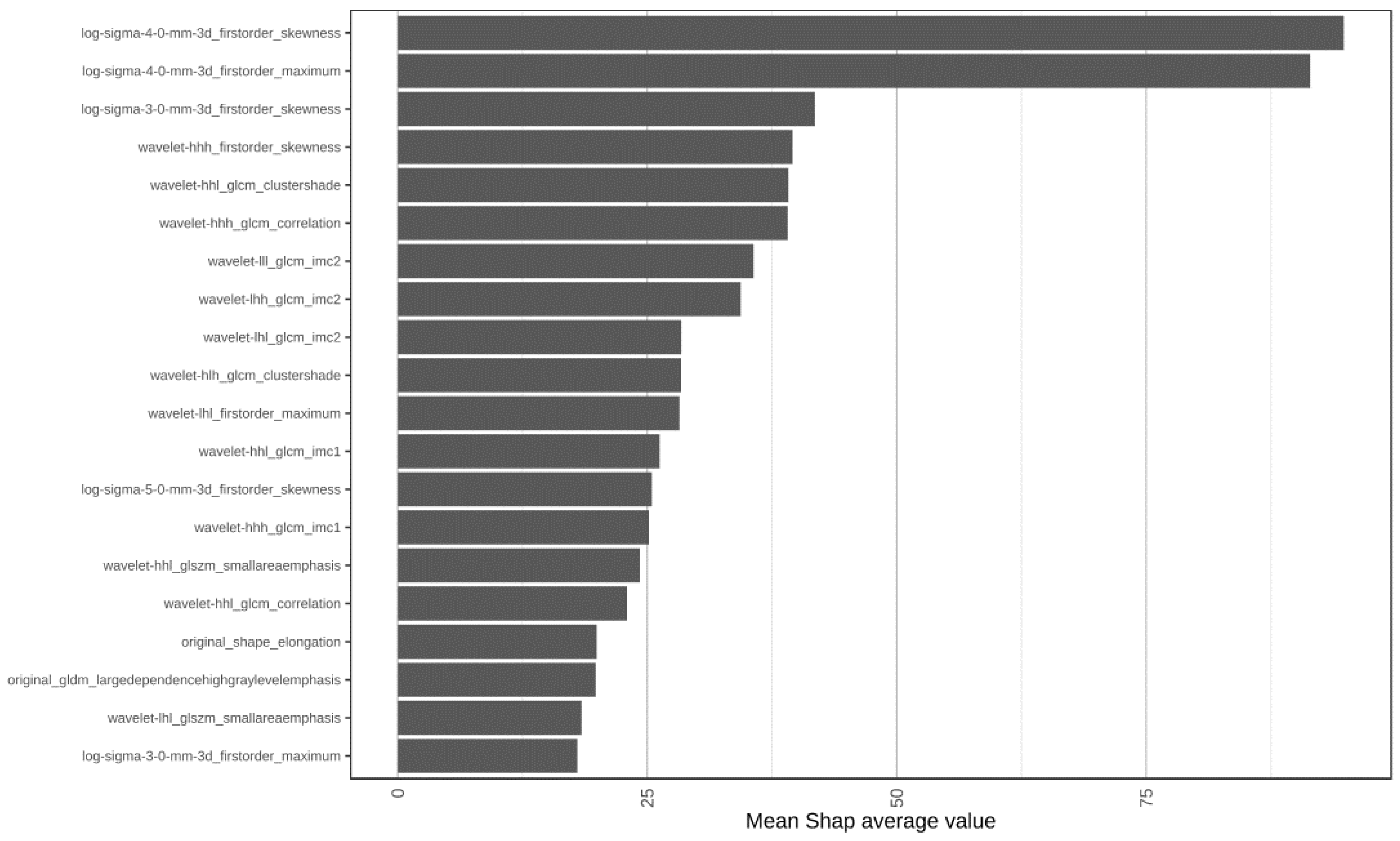
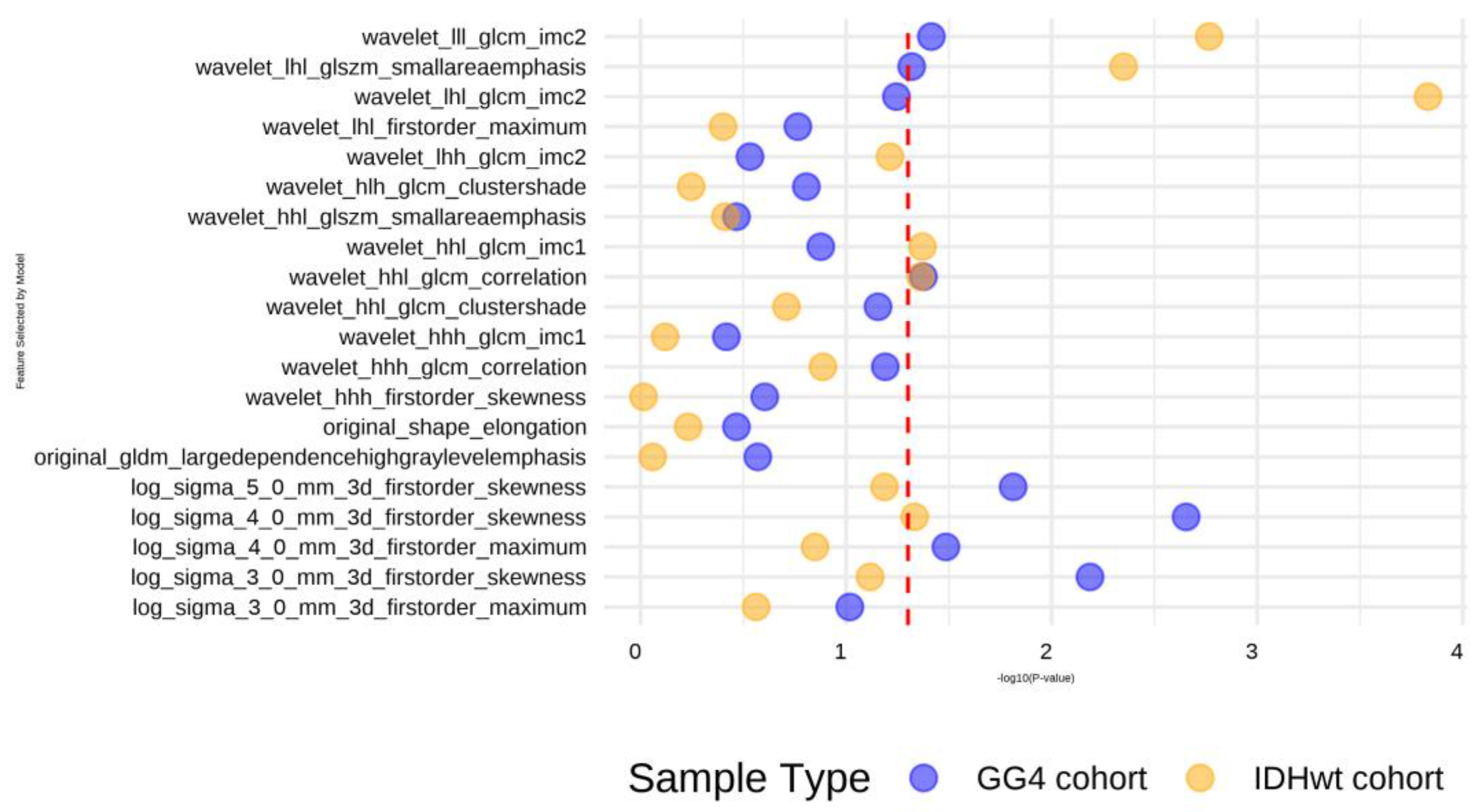
3.2. Constructing a Machine Learning Model of the Radiomics Signature
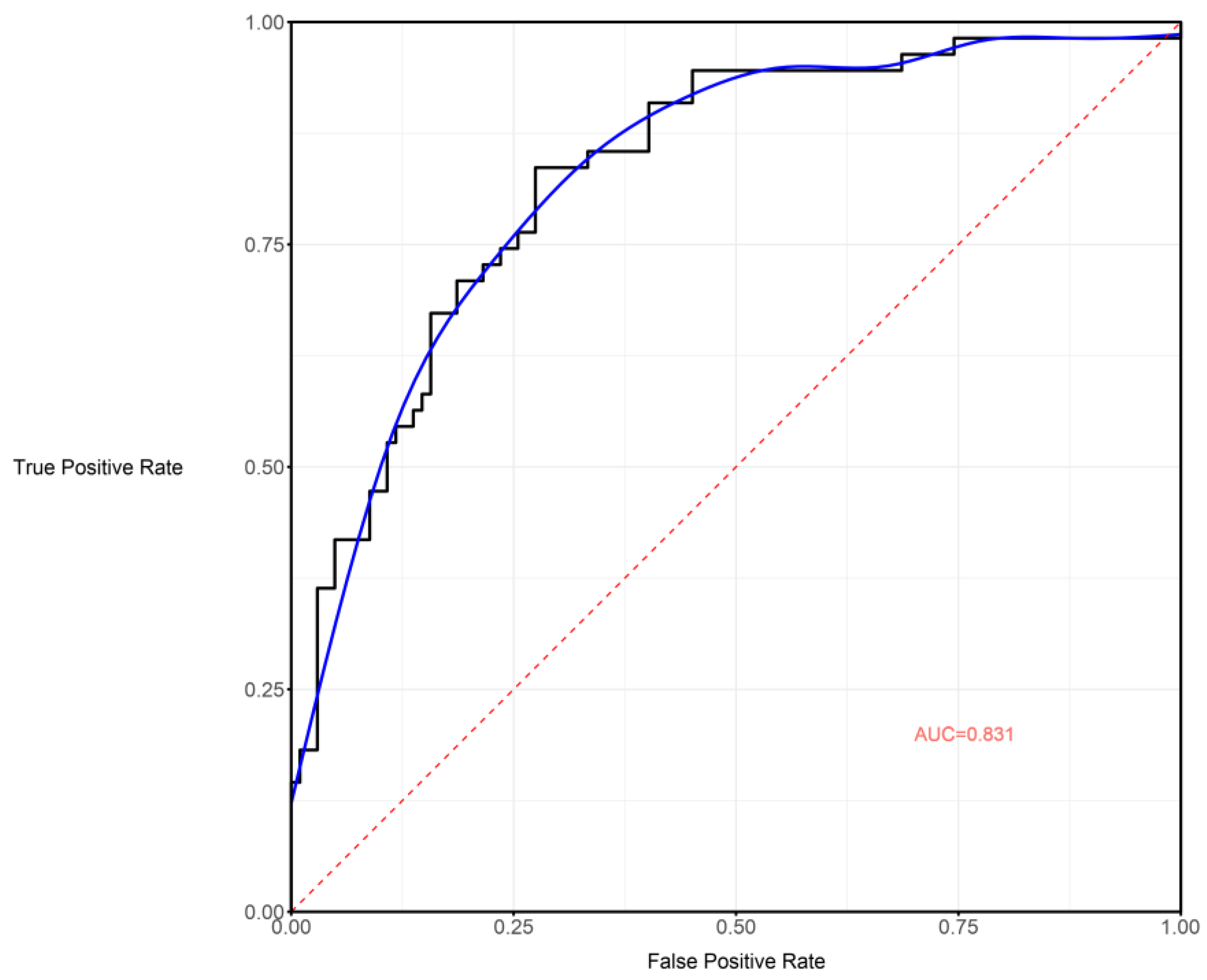
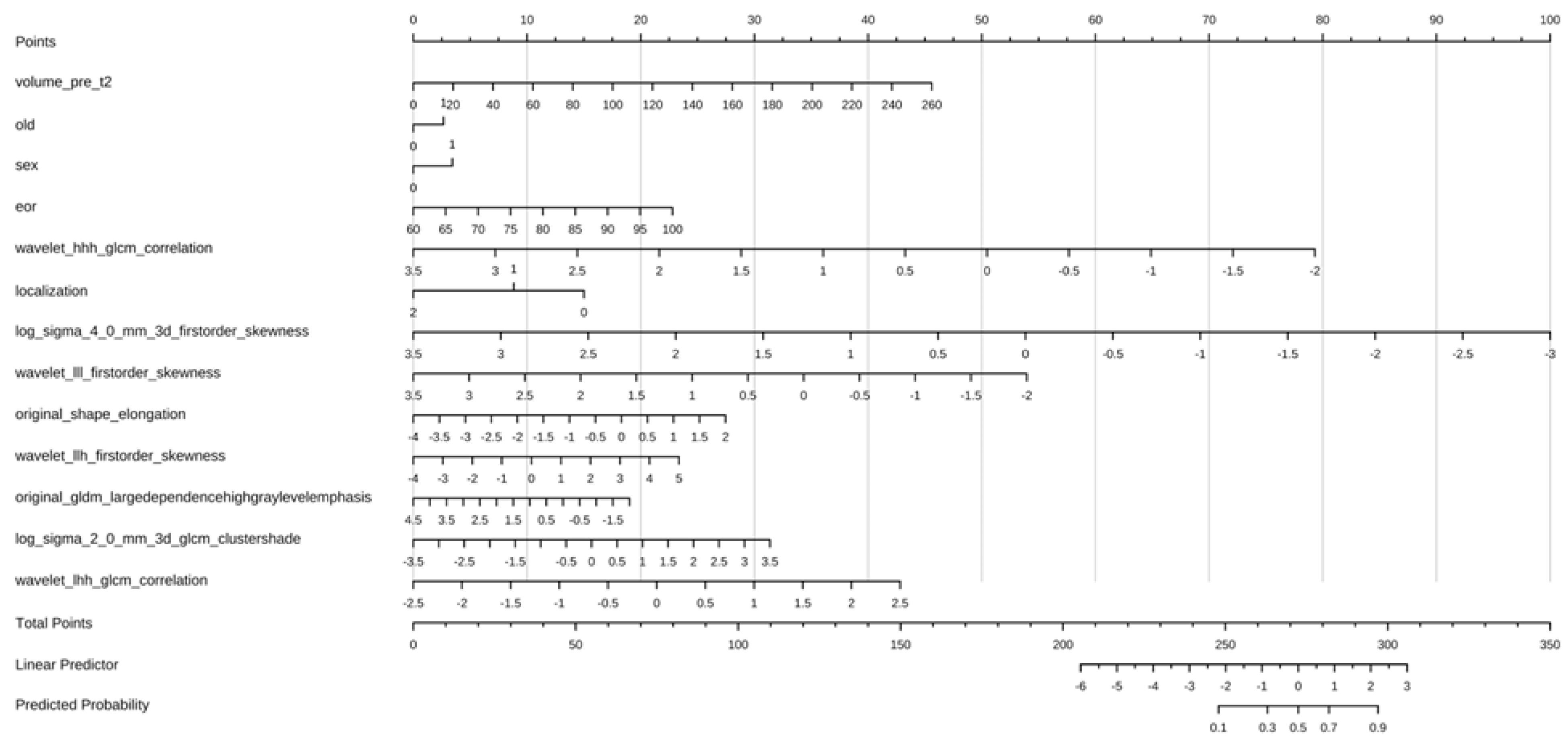
3.3. Performance of Clinical–Radiological Model and Nomogram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosenthal, M.; Wen, P.Y.; Stupp, R.; et al. Glioma. Nat. Rev. Dis. Primer 2015, 1, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Youssef, G.; Wen, P.Y. Updated Response Assessment in Neuro-Oncology (RANO) for Gliomas. Curr. Neurol. Neurosci. Rep. 2024, 24, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ius, T.; Sabatino, G.; Panciani, P.P.; Fontanella, M.M.; Rudà, R.; Castellano, A.; Barbagallo, G.M.V.; Belotti, F.; Boccaletti, R.; Catapano, G.; et al. Surgical Management of Glioma Grade 4: Technical Update from the Neuro-Oncology Section of the Italian Society of Neurosurgery (SINch®): A Systematic Review. J. Neurooncol. 2023, 162, 267–293. [Google Scholar] [CrossRef]
- Roh, T.H.; Kim, S.-H. Supramaximal Resection for Glioblastoma: Redefining the Extent of Resection Criteria and Its Impact on Survival. Brain Tumor Res. Treat. 2023, 11, 166–172. [Google Scholar] [CrossRef]
- Ndirangu, B.; Bryan, K.; Nduom, E. Extent of Resection and Outcomes of Patients with Primary Malignant Brain Tumors. Curr. Treat. Options Oncol. 2023, 24, 1948–1961. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, M.M.; Sankey, E.W.; Ryan, K.J.; Chongsathidkiet, P.; Lorrey, S.J.; Wilkinson, D.S.; Fecci, P.E. Immune Suppression in Gliomas. J. Neurooncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Wang, H.; Sun, Q.; Wang, Y.; Liu, H.; Liang, P.; Lv, Z. The Involvement of Brain Regions Associated with Lower KPS and Shorter Survival Time Predicts a Poor Prognosis in Glioma. Front. Neurol. 2023, 14, 1264322. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, X. Which Parameter Is More Important for the Prognosis of New-Onset Adult Glioblastoma: Residual Tumor Volume or Extent of Resection? World Neurosurg. 2018, 116, e444–e451. [Google Scholar] [CrossRef]
- Bloch, O.; Han, S.J.; Cha, S.; Sun, M.Z.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; Parsa, A.T. Impact of Extent of Resection for Recurrent Glioblastoma on Overall Survival: Clinical Article. J. Neurosurg. 2012, 117, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Choi, J.; Khalafallah, A.M.; Price, C.; Bettegowda, C.; Lim, M.; Gallia, G.; Weingart, J.; Brem, H.; Mukherjee, D. A Systematic Review and Meta-Analysis of Supratotal versus Gross Total Resection for Glioblastoma. J. Neurooncol. 2020, 148, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Mondragon-Soto, M.G.; Altawalbeh, G.; Baumgart, L.; Gempt, J.; Bernhardt, D.; Combs, S.E.; Meyer, B.; Aftahy, A.K. Enhancing Outcomes: Neurosurgical Resection in Brain Metastasis Patients with Poor Karnofsky Performance Score - a Comprehensive Survival Analysis. Front. Oncol. 2024, 13, 1343500. [Google Scholar] [CrossRef]
- Sharifian, A.; Kazemian, A.; Farzin, M.; Amirkhani, N.; Farazmand, B.; Naderi, S.; Khalilian, A.; Pourrashidi, A.; Amjad, G.; Kolahdouzan, K.; et al. Postoperative NEOadjuvant TEMozolomide Followed by Chemoradiotherapy versus Upfront Chemoradiotherapy for Glioblastoma Multiforme (NEOTEM) Trial: Interim Results. Neuro-Oncol. Adv. 2024, 6, vdae195. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.M.J.; De Swart, M.E.; Ardon, H.; Barkhof, F.; Bello, L.; Berger, M.S.; Bouwknegt, W.; Van den Brink, W.A.; Conti Nibali, M.; Eijgelaar, R.S.; et al. Timing of Glioblastoma Surgery and Patient Outcomes: A Multicenter Cohort Study. Neuro-Oncol. Adv. 2021, 3, vdab053. [Google Scholar] [CrossRef]
- Young, J.S.; Al-Adli, N.N.; Muster, R.; Chandra, A.; Morshed, R.A.; Pereira, M.P.; Chalif, E.J.; Hervey-Jumper, S.L.; Theodosopoulos, P.V.; McDermott, M.W.; et al. Does Waiting for Surgery Matter? How Time from Diagnostic MRI to Resection Affects Outcomes in Newly Diagnosed Glioblastoma. J. Neurosurg. 2024, 140, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.; Russo, R.; Beghella Bartoli, F.; Palumbo, I.; Sabatino, G.; Cannatà, M.C.; Gigli, R.; Longo, S.; Tran, H.E.; Boldrini, L.; et al. MRI-Derived Radiomics to Guide Post-Operative Management of Glioblastoma: Implication for Personalized Radiation Treatment Volume Delineation. Front. Med. 2023, 10, 1059712. [Google Scholar] [CrossRef]
- Suter, Y.; Knecht, U.; Alão, M.; Valenzuela, W.; Hewer, E.; Schucht, P.; Wiest, R.; Reyes, M. Radiomics for Glioblastoma Survival Analysis in Pre-Operative MRI: Exploring Feature Robustness, Class Boundaries, and Machine Learning Techniques. Cancer Imaging 2020, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Waldo-Benítez, G.; Padierna, L.C.; Cerón, P.; Sosa, M.A. Machine Learning in Magnetic Resonance Images of Glioblastoma: A Review. Curr. Med. Imaging 2024. [Google Scholar] [CrossRef]
- Gatta, R.; Depeursinge, A.; Ratib, O.; Michielin, O.; Leimgruber, A. Integrating Radiomics into Holomics for Personalised Oncology: From Algorithms to Bedside. Eur. Radiol. Exp. 2020, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, M.A.; Sawaya, R.; Shi, W.; Thall, P.F.; Leeds, N.E. Prognostic Significance of Preoperative MRI Scans in Glioblastoma Multiforme. J. Neurooncol. 1996, 27, 65–73. [Google Scholar] [CrossRef]
- Capobianco, E.; Dominietto, M. Assessment of Brain Cancer Atlas Maps with Multimodal Imaging Features. J. Transl. Med. 2023, 21, 385. [Google Scholar] [CrossRef] [PubMed]
- Hooper, G.W.; Ginat, D.T. MRI Radiomics and Potential Applications to Glioblastoma. Front. Oncol. 2023, 13, 1134109. [Google Scholar] [CrossRef]
- Parmar, C.; Grossmann, P.; Bussink, J.; Lambin, P.; Aerts, H.J.W.L. Machine Learning Methods for Quantitative Radiomic Biomarkers. Sci. Rep. 2015, 5, 13087. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lafata, K.; Sun, W.; Wang, C.; Chang, Z.; Kirkpatrick, J.P.; Yin, F.-F. An Investigation of Machine Learning Methods in Delta-Radiomics Feature Analysis. PloS One 2019, 14, e0226348. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Z.; He, L.; Chen, X.; Pan, D.; Ma, Z.; Liang, C.; Tian, J.; Liang, C. Radiomics Signature: A Potential Biomarker for the Prediction of Disease-Free Survival in Early-Stage (I or II) Non-Small Cell Lung Cancer. Radiology 2016, 281, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Kocak, B.; Baessler, B.; Bakas, S.; Cuocolo, R.; Fedorov, A.; Maier-Hein, L.; Mercaldo, N.; Müller, H.; Orlhac, F.; Pinto Dos Santos, D.; et al. CheckList for EvaluAtion of Radiomics Research (CLEAR): A Step-by-Step Reporting Guideline for Authors and Reviewers Endorsed by ESR and EuSoMII. Insights Imaging 2023, 14, 75. [Google Scholar] [CrossRef]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.-C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Ius, T.; Pignotti, F.; Della Pepa, G.M.; La Rocca, G.; Somma, T.; Isola, M.; Battistella, C.; Gaudino, S.; Polano, M.; Dal Bo, M.; et al. A Novel Comprehensive Clinical Stratification Model to Refine Prognosis of Glioblastoma Patients Undergoing Surgical Resection. Cancers 2020, 12, 386. [Google Scholar] [CrossRef]
- Pirkl, C.M.; Nunez-Gonzalez, L.; Kofler, F.; Endt, S.; Grundl, L.; Golbabaee, M.; Gómez, P.A.; Cencini, M.; Buonincontri, G.; Schulte, R.F.; et al. Accelerated 3D Whole-Brain T1, T2, and Proton Density Mapping: Feasibility for Clinical Glioma MR Imaging. Neuroradiology 2021, 63, 1831–1851. [Google Scholar] [CrossRef]
- Smith, J.S.; Chang, E.F.; Lamborn, K.R.; Chang, S.M.; Prados, M.D.; Cha, S.; Tihan, T.; Vandenberg, S.; McDermott, M.W.; Berger, M.S. Role of Extent of Resection in the Long-Term Outcome of Low-Grade Hemispheric Gliomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-Based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Khalid, S.; Khalil, T.; Nasreen, S. A Survey of Feature Selection and Feature Extraction Techniques in Machine Learning. In Proceedings of the 2014 Science and Information Conference, London, UK, 27–29 August 2014; pp. 372–378. [Google Scholar]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016. [Google Scholar]
- Lohmann, P.; Galldiks, N.; Kocher, M.; Heinzel, A.; Filss, C.P.; Stegmayr, C.; Mottaghy, F.M.; Fink, G.R.; Jon Shah, N.; Langen, K.-J. Radiomics in Neuro-Oncology: Basics, Workflow, and Applications. Methods San Diego Calif 2021, 188, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Raschka, S. Model Evaluation, Model Selection, and Algorithm Selection in Machine Learning. arXiv 2020, arXiv:1811.12808. [Google Scholar]
- Demircioğlu, A. The Effect of Data Resampling Methods in Radiomics. Sci. Rep. 2024, 14, 2858. [Google Scholar] [CrossRef] [PubMed]
- Cè, M.; Chiriac, M.D.; Cozzi, A.; Macrì, L.; Rabaiotti, F.L.; Irmici, G.; Fazzini, D.; Carrafiello, G.; Cellina, M. Decoding Radiomics: A Step-by-Step Guide to Machine Learning Workflow in Hand-Crafted and Deep Learning Radiomics Studies. Diagnostics 2024, 14, 2473. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Lundberg, S.M.; Erion, G.; Chen, H.; DeGrave, A.; Prutkin, J.M.; Nair, B.; Katz, R.; Himmelfarb, J.; Bansal, N.; Lee, S.-I. From Local Explanations to Global Understanding with Explainable AI for Trees. Nat. Mach. Intell. 2020, 2, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Q.; Mi, R.; Ye, H.; Zhang, H.; Chen, B.; Li, Y.; Huang, G.; Xia, J. Radiomics Nomogram Building From Multiparametric MRI to Predict Grade in Patients With Glioma: A Cohort Study. J. Magn. Reson. Imaging JMRI 2019, 49, 825–833. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Elkin, E.B.; Gonen, M. Extensions to Decision Curve Analysis, a Novel Method for Evaluating Diagnostic Tests, Prediction Models and Molecular Markers. BMC Med. Inform. Decis. Mak. 2008, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.F.; Brown, M.D.; Zhu, K.; Janes, H. Assessing the Clinical Impact of Risk Prediction Models With Decision Curves: Guidance for Correct Interpretation and Appropriate Use. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Buchner, J.A.; Kofler, F.; Mayinger, M.; Christ, S.M.; Brunner, T.B.; Wittig, A.; Menze, B.; Zimmer, C.; Meyer, B.; Guckenberger, M.; et al. Radiomics-Based Prediction of Local Control in Patients with Brain Metastases Following Postoperative Stereotactic Radiotherapy. Neuro-Oncol. 2024, 26, 1638–1650. [Google Scholar] [CrossRef]
- Della Pepa, G.M.; Caccavella, V.M.; Menna, G.; Ius, T.; Auricchio, A.M.; Chiesa, S.; Gaudino, S.; Marchese, E.; Olivi, A. Machine Learning-Based Prediction of 6-Month Postoperative Karnofsky Performance Status in Patients with Glioblastoma: Capturing the Real-Life Interaction of Multiple Clinical and Oncologic Factors. World Neurosurg. 2021, 149, e866–e876. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; He, X.; Li, Y.; Pang, P.; Shu, Z.; Gong, X. The Nomogram of MRI-Based Radiomics with Complementary Visual Features by Machine Learning Improves Stratification of Glioblastoma Patients: A Multicenter Study. J. Magn. Reson. Imaging JMRI 2021, 54, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Mammadov, O.; Akkurt, B.H.; Musigmann, M.; Ari, A.P.; Blömer, D.A.; Kasap, D.N.G.; Henssen, D.J.H.A.; Nacul, N.G.; Sartoretti, E.; Sartoretti, T.; et al. Radiomics for Pseudoprogression Prediction in High Grade Gliomas: Added Value of MR Contrast Agent. Heliyon 2022, 8, e10023. [Google Scholar] [CrossRef]
- Luckett, P.H.; Olufawo, M.O.; Park, K.Y.; Lamichhane, B.; Dierker, D.; Verastegui, G.T.; Lee, J.J.; Yang, P.; Kim, A.; Butt, O.H.; et al. Predicting Post-Surgical Functional Status in High-Grade Glioma with Resting State fMRI and Machine Learning. J. Neurooncol. 2024, 169, 175–185. [Google Scholar] [CrossRef]
- Sasagasako, T.; Ueda, A.; Mineharu, Y.; Mochizuki, Y.; Doi, S.; Park, S.; Terada, Y.; Sano, N.; Tanji, M.; Arakawa, Y.; et al. Postoperative Karnofsky Performance Status Prediction in Patients with IDH Wild-Type Glioblastoma: A Multimodal Approach Integrating Clinical and Deep Imaging Features. PLOS ONE 2024, 19, e0303002. [Google Scholar] [CrossRef] [PubMed]
- Decoux, A.; Duron, L.; Habert, P.; Roblot, V.; Arsovic, E.; Chassagnon, G.; Arnoux, A.; Fournier, L. Comparative Performances of Machine Learning Algorithms in Radiomics and Impacting Factors. Sci. Rep. 2023, 13, 14069. [Google Scholar] [CrossRef] [PubMed]
- Artzi, M.; Bressler, I.; Ben Bashat, D. Differentiation between Glioblastoma, Brain Metastasis and Subtypes Using Radiomics Analysis. J. Magn. Reson. Imaging JMRI 2019, 50, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Qian, Z.; Sun, Z.; Xu, K.; Wang, K.; Liu, S.; Fan, X.; Li, S.; Zhang, Z.; et al. A Radiomic Signature as a Non-Invasive Predictor of Progression-Free Survival in Patients with Lower-Grade Gliomas. NeuroImage Clin. 2018, 20, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, W.; Xie, J.; Lin, H.; Hu, X.; Li, C.; Shang, Y.; Wang, Y.; Jiang, Y.; Ding, M.; et al. Development and Validation of a Radiomics-Based Nomogram for Predicting a Major Pathological Response to Neoadjuvant Immunochemotherapy for Patients with Potentially Resectable Non-Small Cell Lung Cancer. Front. Immunol. 2023, 14, 1115291. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Hosseini, E.; Hajianfar, G.; Shiri, I.; Servaes, S.; Rosa-Neto, P.; Godoy, L.; Nasrallah, M.P.; O’Rourke, D.M.; Mohan, S.; et al. MRI-Based Radiomics Combined with Deep Learning for Distinguishing IDH-Mutant WHO Grade 4 Astrocytomas from IDH-Wild-Type Glioblastomas. Cancers 2023, 15, 951. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, S.-T.; Wei, J.-W.; Dong, D.; Wang, X.-C.; Yang, G.-Q.; Tian, J.; Zhang, H. A Radiomics Nomogram May Improve the Prediction of IDH Genotype for Astrocytoma before Surgery. Eur. Radiol. 2019, 29, 3325–3337. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Feng, W.; Duan, C.; Liu, Y.; Liu, J.; Liu, X. The Value of Enhanced MR Radiomics in Estimating the IDH1 Genotype in High-Grade Gliomas. BioMed Res. Int. 2020, 2020, 4630218. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Campbell, G.; Jones, W.D.; Campagne, F.; Wen, Z.; Walker, S.J.; Su, Z.; Chu, T.M.; Goodsaid, F.M.; Pusztai, L.; et al. The MicroArray Quality Control (MAQC)-II study of common practices for the development and validation of microarray-based predictive models. Nat. Biotechnol. 2010, 28, 827–838. [Google Scholar] [PubMed]
- Ren, J.; Zhai, X.; Yin, H.; Zhou, F.; Hu, Y.; Wang, K.; Yan, R.; Han, D. Multimodality MRI Radiomics Based on Machine Learning for Identifying True Tumor Recurrence and Treatment-Related Effects in Patients with Postoperative Glioma. Neurol. Ther. 2023, 12, 1729–1743. [Google Scholar] [CrossRef] [PubMed]
- Burka, D.; Puppe, C.; Szepesváry, L.; Tasnádi, A. Voting: A machine learning approach. Eur. J. Oper. Res. 2022, 299, 1003–1017. [Google Scholar] [CrossRef]
- Polano, M.; Chierici, M.; Bo, M.D.; Gentilini, D.; Di Cintio, F.; Baboci, L.; Gibbs, D.L.; Furlanello, C.; Toffoli, G. A Pan-Cancer Approach to Predict Responsiveness to Immune Checkpoint Inhibitors by Machine Learning. Cancers 2019, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Bergstra, J.; Yamins, D.; Cox, D. Making a Science of Model Search: Hyperparameter Optimization in Hundreds of Dimensions for Vision Architectures. In Proceedings of the 30th International Conference on Machine Learning, PMLR, Atlanta, GA, USA, 17–19 June 2013; pp. 115–123. [Google Scholar]
- Chicco, D.; Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genomics. 2020, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]


| Model | ACC CV (CI) | ACC Test | MCC CV (CI) | MCC Test |
|---|---|---|---|---|
| XGBoost | 0.802 (0.797–0.806) | 0.719 | 0.339 (0.330–0.3483) | 0.302 |
| RF | 0.633 (0.56–0.701) | 0.656 | 0.104 (−0.07–0.26) | 0.120 |
| Characteristic | OR 1 | 95% CI 1 | p-Value |
|---|---|---|---|
| Preoperative Volume | 1.01 | 1.01 to 1.02 | 0.001 |
| Age eldery | >0.99 | ||
| <70 | — | — | |
| >70 | 1.00 | 0.36 to 2.71 | |
| Gender | 0.62 | ||
| Male | — | — | |
| Female | 1.27 | 0.50 to 3.27 | |
| Extent of Resection (EOR) | 1.01 | 0.96 to 1.07 | 0.67 |
| wavelet_hhh_glcm_correlation | 0.32 | 0.14 to 0.69 | 0.003 |
| localization | 0.010 | ||
| precentral | — | — | |
| postcentral | 0.63 | 0.20 to 1.88 | |
| temporoinsular | 0.23 | 0.08 to 0.61 | |
| log_sigma_4_0_mm_3d_firstorder_skewness | 0.24 | 0.10 to 0.48 | <0.001 |
| wavelet_lll_firstorder_skewness | 0.44 | 0.22 to 0.84 | 0.012 |
| original_shape_elongation | 1.65 | 1.08 to 2.60 | 0.021 |
| wavelet_llh_firstorder_skewness | 1.41 | 0.92 to 2.18 | 0.11 |
| original_gldm_largedependencehighgraylevelemphasis | 0.65 | 0.40 to 1.00 | 0.051 |
| log_sigma_2_0_mm_3d_glcm_clustershade | 1.62 | 0.93 to 2.88 | 0.088 |
| wavelet_lhh_glcm_correlation | 1.72 | 0.81 to 3.80 | 0.16 |
| No. Obs. | 157 | ||
| AIC | 184 | ||
| BIC | 230 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ius, T.; Polano, M.; Dal Bo, M.; Bagatto, D.; Bertani, V.; Gentilini, D.; Lombardi, G.; D’agostini, S.; Skrap, M.; Toffoli, G. Development of Clinical-Radiomics Nomogram for Predicting Post-Surgery Functional Improvement in High-Grade Glioma Patients. Cancers 2025, 17, 758. https://doi.org/10.3390/cancers17050758
Ius T, Polano M, Dal Bo M, Bagatto D, Bertani V, Gentilini D, Lombardi G, D’agostini S, Skrap M, Toffoli G. Development of Clinical-Radiomics Nomogram for Predicting Post-Surgery Functional Improvement in High-Grade Glioma Patients. Cancers. 2025; 17(5):758. https://doi.org/10.3390/cancers17050758
Chicago/Turabian StyleIus, Tamara, Maurizio Polano, Michele Dal Bo, Daniele Bagatto, Valeria Bertani, Davide Gentilini, Giuseppe Lombardi, Serena D’agostini, Miran Skrap, and Giuseppe Toffoli. 2025. "Development of Clinical-Radiomics Nomogram for Predicting Post-Surgery Functional Improvement in High-Grade Glioma Patients" Cancers 17, no. 5: 758. https://doi.org/10.3390/cancers17050758
APA StyleIus, T., Polano, M., Dal Bo, M., Bagatto, D., Bertani, V., Gentilini, D., Lombardi, G., D’agostini, S., Skrap, M., & Toffoli, G. (2025). Development of Clinical-Radiomics Nomogram for Predicting Post-Surgery Functional Improvement in High-Grade Glioma Patients. Cancers, 17(5), 758. https://doi.org/10.3390/cancers17050758








