Primary Intrathoracic Synovial Sarcoma: An Analysis of Outcomes of This Rare Disease
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Method
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mankin, H.J.; Hornicek, F.J. Diagnosis, classification, and management of soft tissue sarcomas. Cancer Control 2005, 12, 5–21. [Google Scholar] [CrossRef]
- Thway, K.; Fisher, C. Synovial sarcoma: Defining features and diagnostic evolution. Ann. Diagn. Pathol. 2014, 18, 369–380. [Google Scholar] [CrossRef]
- Lan, T.; Chen, H.; Xiong, B.; Zhou, T.; Peng, R.; Chen, M.; Ye, F.; Yao, J.; He, X.; Wang, Y. Primary pleuropulmonary and mediastinal synovial sarcoma: A clinicopathologic and molecular study of 26 genetically confirmed cases in the largest institution of southwest China. Diagn. Pathol. 2016, 11, 62. [Google Scholar] [CrossRef]
- Hartel, P.H.; Fanburg-Smith, J.C.; Frazier, A.A.; Galvin, J.R.; Lichy, J.H.; Shilo, K.; Franks, T.J. Primary pulmonary and mediastinal synovial sarcoma: A clinicopathologic study of 60 cases and comparison with five prior series. Mod. Pathol. 2007, 20, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Hisaoka, M.; Daa, T.; Hatakeyama, K.; Iwamasa, T.; Hashimoto, H. Primary pulmonary synovial sarcoma: A clinicopathologic, immunohistochemical, and molecular study of 11 cases. Hum. Pathol. 2004, 35, 850–856. [Google Scholar] [CrossRef]
- Suster, S.; Moran, C.A. Primary synovial sarcomas of the mediastinum: A clinicopathologic, immunohistochemical, and ultrastructural study of 15 cases. Am. J. Surg. Pathol. 2005, 29, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Essary, L.R.; Vargas, S.O.; Fletcher, C.D. Primary pleuropulmonary synovial sarcoma: Reappraisal of a recently described anatomic subset. Cancer 2002, 94, 459–469. [Google Scholar] [CrossRef]
- Duran-Moreno, J.; Kampoli, K.; Kapetanakis, E.I.; Mademli, M.; Koufopoulos, N.; Foukas, P.G.; Kostopanagiotou, K.; Tomos, P.; Koumarianou, A. Pericardial synovial sarcoma: Case report, literature review and pooled analysis. In Vivo 2019, 33, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Terra, S.B.; Aesif, S.W.; Maleszewski, J.J.; Folpe, A.L.; Boland, J.M. Mediastinal Synovial Sarcoma. Am. J. Surg. Pathol. 2018, 42, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Aubry, M.-C.; Bridge, J.A.; Wickert, R.; Tazelaar, H.D. Primary monophasic synovial sarcoma of the pleura: Five cases confirmed by the presence of SYT-SSX fusion transcript. Am. J. Surg. Pathol. 2001, 25, 776–781. [Google Scholar] [CrossRef]
- Cheng, Y.; Sheng, W.; Zhou, X.; Wang, J. Pericardial synovial sarcoma, a potential for misdiagnosis: Clinicopathologic and molecular cytogenetic analysis of three cases with literature review. Am. J. Clin. Pathol. 2012, 137, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, E.; Zeren, H.E.; Fleming, M.V.; Colby, T.V.; Travis, W.D. Biphasic synovial sarcomas arising in the pleural cavity: A clinicopathologic study of five cases. Am. J. Surg. Pathol. 1996, 20, 36–45. [Google Scholar] [CrossRef]
- Kaira, K.; Ishizuka, T.; Sunaga, N.; Hashimoto, K.; Yanagitani, N.; Nonaka, T.; Ebara, T.; Hisada, T.; Mori, M. Primary mediastinal synovial sarcoma: A report of 2 cases. J. Comput. Assist. Tomogr. 2008, 32, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, M.Y.; Koo, H.J.; Song, J.S.; Choi, C.-M. Primary pulmonary synovial sarcoma in a tertiary referral center: Clinical characteristics, CT, and 18F-FDG PET findings, with pathologic correlations. Medicine 2015, 94, e1392. [Google Scholar] [CrossRef] [PubMed]
- Baheti, A.D.; Sewatkar, R.; Hornick, J.L.; Saboo, S.S.; Jagannathan, J.P.; Ramaiya, N.H.; Tirumani, S.H. Imaging features of primary and recurrent intrathoracic synovial sarcoma: A single-institute experience. Clin. Imaging 2015, 39, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Bégueret, H.; Galateau-Salle, F.; Guillou, L.; Chetaille, B.; Brambilla, E.; Vignaud, J.-M.; Terrier, P.; Groussard, O.; Coindre, J.-M. Primary intrathoracic synovial sarcoma: A clinicopathologic study of 40 t (X; 18)-positive cases from the French Sarcoma Group and the Mesopath Group. Am. J. Surg. Pathol. 2005, 29, 339–346. [Google Scholar] [CrossRef]
- He, H.; Yang, L.; Peng, Y.; Liu, L.; Liu, L.; Xue, Q.; Gao, S. The value of multidisciplinary team (MDT) management in the diagnosis and treatment of primary intrathoracic synovial sarcomas: A single-center experience. J. Thorac. Dis. 2021, 13, 600. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Mankin, H.J.; Singer, S. Synovial sarcoma: The importance of size and location for survival. Clin. Orthop. Relat. Res. 2004, 419, 155–161. [Google Scholar] [CrossRef]
- Ferrari, A.; Bisogno, G.; Alaggio, R.; Cecchetto, G.; Collini, P.; Rosolen, A.; Meazza, C.; Indolfi, P.; Garaventa, A.; De Sio, L. Synovial sarcoma of children and adolescents: The prognostic role of axial sites. Eur. J. Cancer 2008, 44, 1202–1209. [Google Scholar] [CrossRef]
- El Beaino, M.; Hayea, W.T.; Li, T.L.; Connors, K.M.; Joseph, M.A.; Lin, P.P. Four-Decade Epidemiological Trends of Synovial Sarcoma: An Analysis of the Surveillance, Epidemiology, and End Results Program. In Proceedings of the Musculoskeletal Tumor Society Virtual Annual Meeting, Virtual, 8–9 September 2020. [Google Scholar]
- Cates, J.M. The AJCC 8th edition staging system for soft tissue sarcoma of the extremities or trunk: A cohort study of the SEER database. J. Natl. Compr. Cancer Netw. 2018, 16, 144–152. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Amini, B.; Wagner, M.J.; Nowell, E.N.; Lazar, A.J.; Lin, P.P.; Benjamin, R.S.; Araujo, D.M. Synovial sarcoma of the head and neck: A single institution review. Sarcoma 2017, 2017, 2016752. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; Lupo, P.J.; Bishop, A.J.; Lin, P.P.; Delclos, G.L.; Lazar, A.J.; Benjamin, R.S.; Araujo, D.M. Synovial sarcoma of the hand and foot: An institutional review. Am. J. Clin. Oncol. 2021, 44, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Zeren, H.; Moran, C.A.; Suster, S.; Fishback, N.F.; Koss, M.N. Primary pulmonary sarcomas with features of monophasic synovial sarcoma: A clinicopathological, immunohistochemical, and ultrastructural study of 25 cases. Hum. Pathol. 1995, 26, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-C.; Wu, X.-C.; Andrews, P.A.; Chen, V.W. Racial and ethnic disparities in the incidence and trends of soft tissue sarcoma among adolescents and young adults in the United States, 1995–2008. J. Adolesc. Young Adult Oncol. 2013, 2, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Oncology, T.L. Racial disparities in cancer care: Can we close the gap? Lancet Oncol. 2021, 22, 1643. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Staals, E.L.; Alberghini, M.; Zanella, L.; Ferrari, C.; Benassi, M.S.; Picci, P.; Mercuri, M.; Bacci, G.; Ferrari, S. Synovial sarcoma: Retrospective analysis of 250 patients treated at a single institution. Cancer 2009, 115, 2988–2998. [Google Scholar] [CrossRef]
- Lamm, W.; Schur, S.; Köstler, W.J.; Amann, G.; Pokrajac, B.; Funovics, P.; Panotopoulos, J.; Brodowicz, T. Initially localized synovial sarcoma in adults: A retrospective single-center analysis of 26 patients registered at the Department of Oncology, University of Vienna between 2004 and 2013. Oncology 2014, 87, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Van Tine, B.A. Synovial sarcoma: Current concepts and future perspectives. J. Clin. Oncol. 2018, 36, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.-Y.; von Mehren, M.; Jones, R.; Martin-Broto, J.; Stacchiotti, S.; Bauer, S.; Gelderblom, H.; Orbach, D.; Hindi, N.; Dei Tos, A. Synovial sarcoma: Characteristics, challenges, and evolving therapeutic strategies. ESMO Open 2023, 8, 101618. [Google Scholar] [CrossRef]
- Pervaiz, N.; Colterjohn, N.; Farrokhyar, F.; Tozer, R.; Figueredo, A.; Ghert, M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2008, 113, 573–581. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Patients with Localized Disease at Diagnosis (n = 63) |
|---|---|
| Age at diagnosis (y) Median (range) | 43 (18–77) |
| <45 | 36 (57) |
| ≥45 | 27 (43) |
| Total | 63 |
| Sex | |
| Female | 36 (57) |
| Male | 27 (43) |
| Total | 63 |
| Race and ethnicity | |
| White | 57 (90) |
| Others * | 6 (10) |
| Total | 63 |
| Smoking history | |
| Never | 41 (65) |
| Yes | 19 (30) |
| Unknown | 3 (5) |
| Total | 63 |
| Primary site | |
| Lung | 43 (68) |
| Lung with involvement of pleura | 2 (3) |
| Chest wall | 3 (5) |
| Mediastinum | 15 (24) |
| Total | 63 |
| Tumor size (cm) Median (range) | 7 (1–18) |
| <5 | 15 (24) |
| 5–10 | 25 (40) |
| ≥10 | 15 (24) |
| Unknown | 8 (13) |
| Total | 63 |
| Subtype | |
| Monophasic | 47 (75) |
| Biphasic | 6 (10) |
| Poorly differentiated | 5 (8) |
| Unknown | 5 (8) |
| Total | 63 |
| Fusion gene | |
| Present ** | 40 (63) |
| Not tested | 23 (37) |
| Total | 63 |
| Stage | |
| Stage ll | 17 (27) |
| Stage lllA | 26 (41) |
| Stage lllB | 12 (19) |
| Stage lV | 0 |
| Unknown | 8 (13) |
| Total | 63 |
| Tumor resection | |
| Non-resected | 1 (2) |
| Resected | 62 (98) |
| Total | 63 |
| Final surgical resection margin | |
| Negative | 35 (56) |
| Positive | 13 (21) |
| Unknown | 14 (23) |
| Total (Patients who had resection) | 63 |
| Neo/adjuvant radiation therapy | |
| No | 44 (71) |
| Yes | 18 (29) |
| Total (Patients who had resection) | 63 |
| Timing of radiation therapy | |
| Neoadjuvant | 2 (11) |
| Adjuvant | 15 (83) |
| Unknown | 1 (6) |
| Total | 18 |
| Neo/adjuvant chemotherapy | |
| No | 19 (31) |
| Yes | 43 (69) |
| Total (Patients who had resection) | 62 |
| Timing of chemotherapy | |
| Neoadjuvant | 17 (40) |
| Adjuvant | 26 (60) |
| Total | 43 |
| Chemotherapy regimen | |
| Doxorubicin and ifosfamide | 34 (79) |
| High-dose ifosfamide | 1 (2) |
| Gemcitabine and docetaxel | 1 (2) |
| Other | 7 (16) |
| Total | 43 |
| Local recurrence | |
| No | 48 (77) |
| Yes | 14 (23) |
| Total (Patients who had resection) | 62 |
| Metastasis | |
| No | 16 (25) |
| Yes | 47 (75) |
| Total | 63 |
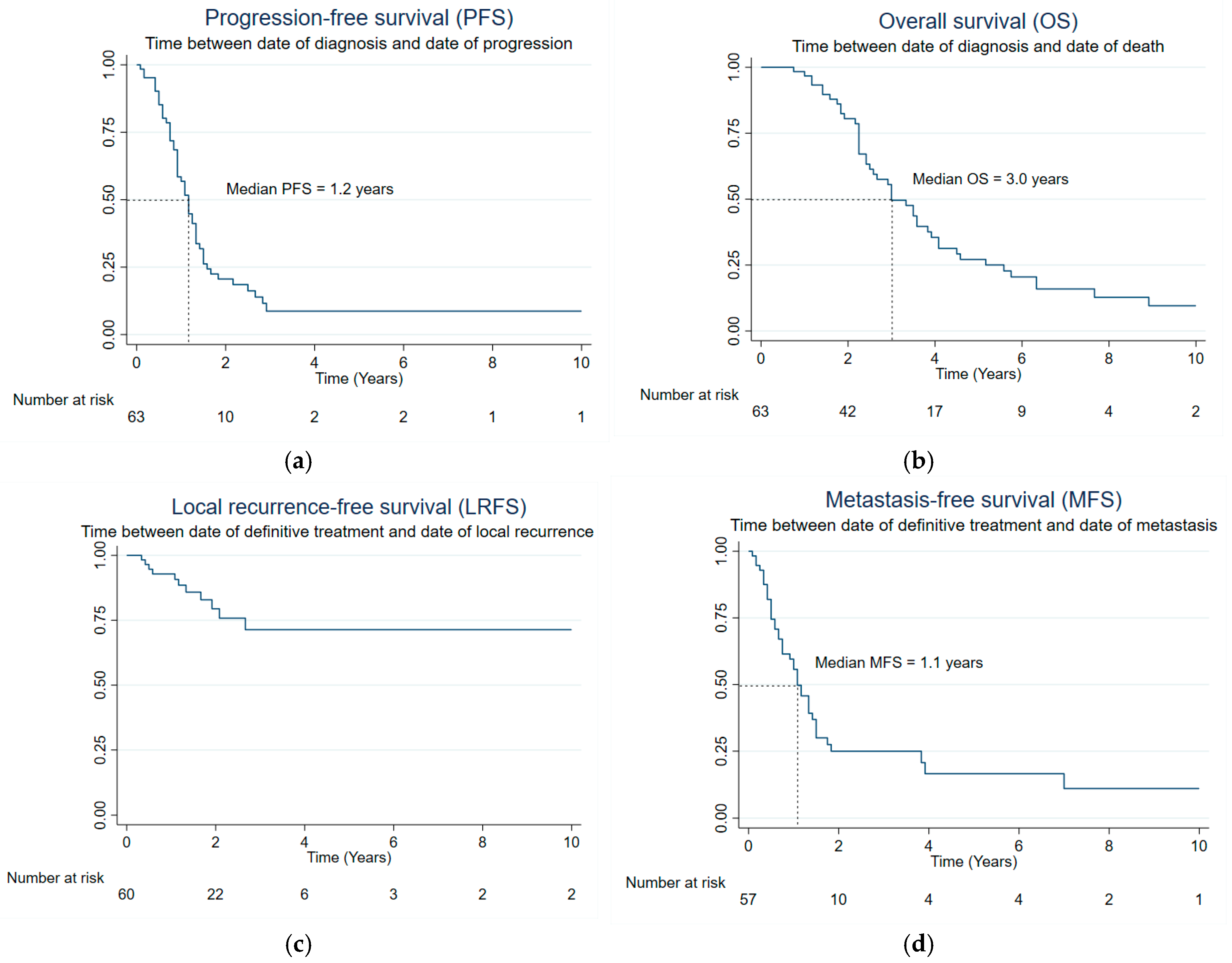
| Variables (Log-Rank p) | Median PFS (Years) | Median OS (Years) | Median MFS (Years) |
|---|---|---|---|
| Entire Cohort (n = 63) | |||
| Age at diagnosis (Years) | 0.565 | 0.461 | 0.942 |
| <45 | 0.92 | 3.00 | 1.00 |
| ≥45 | 1.25 | 3.58 | 1.33 |
| Sex | 0.292 | 0.880 | 0.992 |
| Female | 1.08 | 3.50 | 1.00 |
| Male | 1.17 | 3.00 | 1.33 |
| Race and ethnicity | 0.356 | 0.851 | 0.778 |
| Other | 0.92 | 2.42 | 1.08 |
| White | 1.17 | 3.50 | 1.08 |
| Smoking history | 0.768 | 0.294 | 0.538 |
| Never | 1.08 | 3.00 | 1.08 |
| Yes | 1.17 | 3.92 | 1.08 |
| Tumor origin | 0.191 | 0.716 | 0.490 |
| Lung | 1.08 | 3.33 | 1.08 |
| Lung with pleura | 0.83 | 2.25 | 0.67 |
| Chest wall | 1.33 | 3.58 | 1.08 |
| Mediastinum | 1.67 | 2.67 | 1.83 |
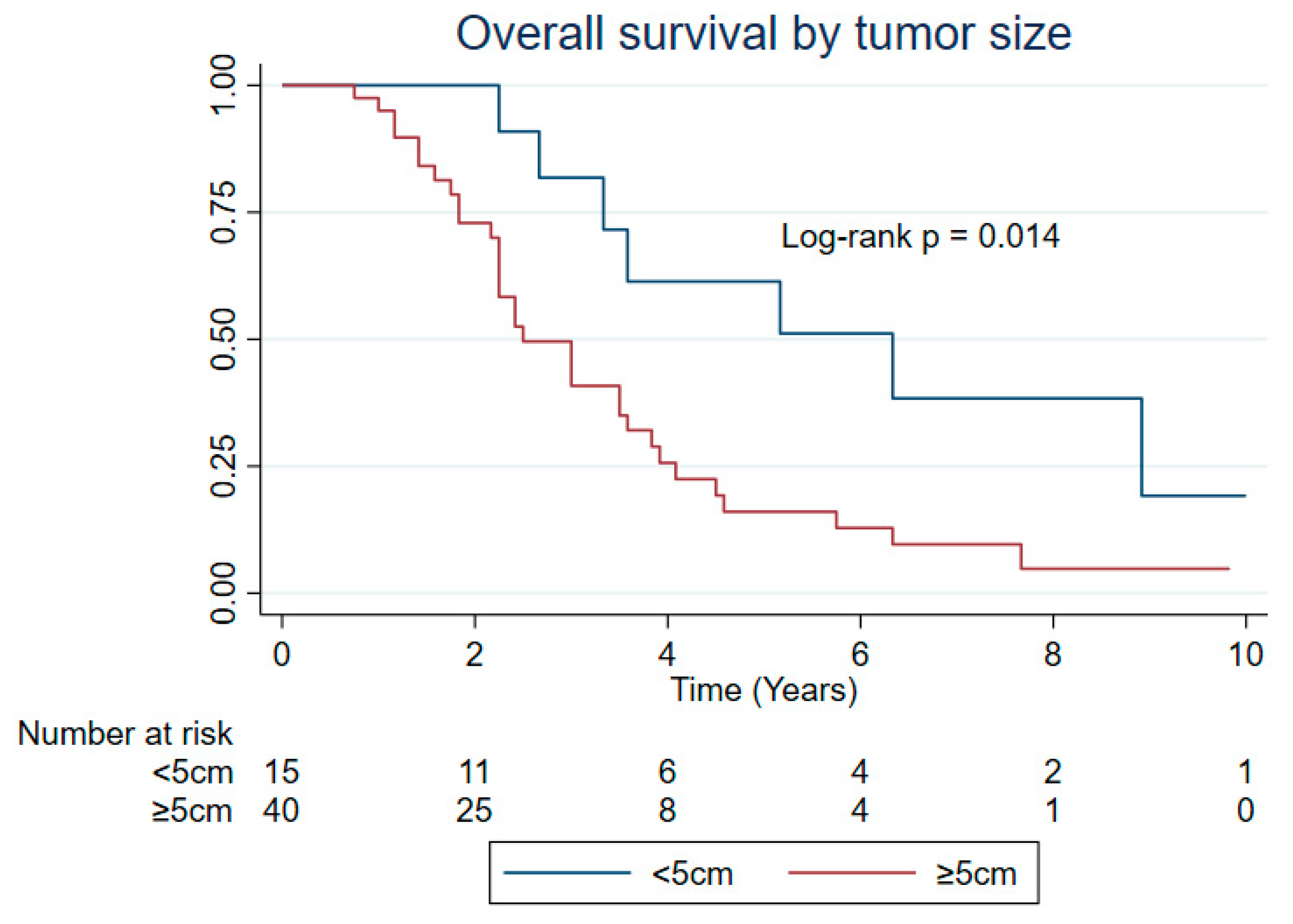
| Tumor size (cm) | 0.472 | 0.014 * | 0.875 |
| <5 | 1.25 | 6.33 | 1.33 |
| ≥5 | 1.17 | 2.50 | 1.17 |
| Subtype | 0.674 | 0.436 | 0.723 |
| Monophasic | 1.17 | 3.33 | 1.08 |
| Biphasic | 1.08 | 2.58 | 0.58 |
| Poorly differentiated | 2.92 | 2.67 | 0.75 |
| Tumor resection | 0.182 | 0.510 | - |
| No | - | - | - |
| Yes | 1.17 | 3.33 | - |
| Patients with surgical resection performed (n = 62) | |||
| Final resection margin | 0.459 | 0.044 * | 0.335 |
| Negative | 1.25 | 3.58 | 1.33 |
| Positive | 0.83 | 3.83 | 0.50 |
| Unknown | 1.08 | 2.25 | 1.00 |
| Neo/adj radiation therapy | 0.801 | 0.315 | 0.119 |
| No | 1.17 | 3.33 | 1.17 |
| Yes | 1.08 | 3.50 | 0.67 |
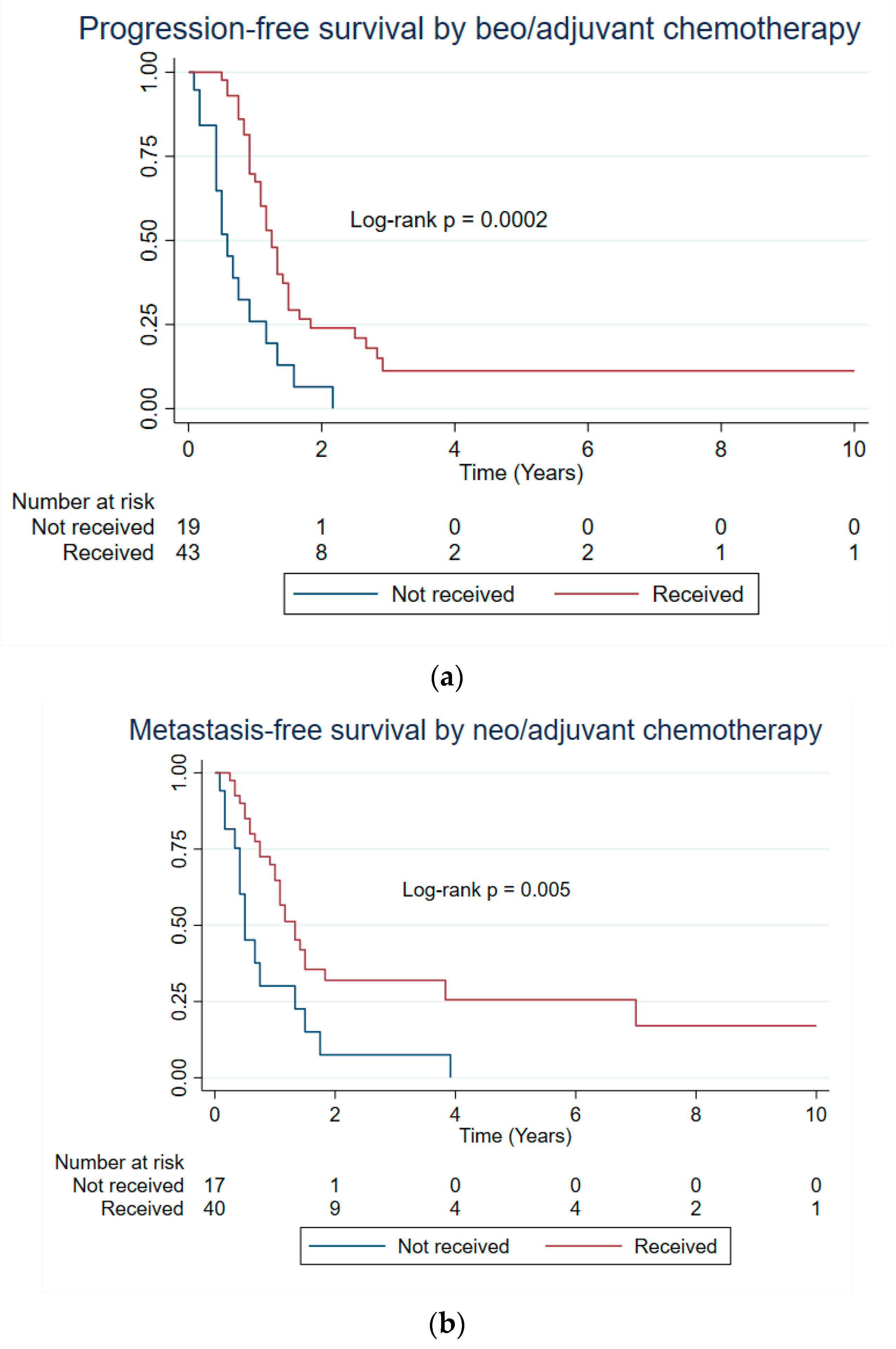
| Neo/adj chemotherapy | 0.0002 * | 0.187 | 0.005 * |
| No | 0.58 | 2.58 | 0.50 |
| Yes | 1.25 | 3.50 | 1.33 |
| Variables | PFS | OS | MFS | |||
|---|---|---|---|---|---|---|
| Univariate Analysis HR (95% CI) | Multivariable Analysis HR (95% CI) | Univariate Analysis HR (95% CI) | Multivariable Analysis HR (95% CI) | Univariate Analysis HR (95% CI) | Multivariable Analysis HR (95% CI) | |
| Age at diagnosis (Years) | ||||||
| <45 | Reference | - | Reference | - | Reference | - |
| ≥45 | 0.85 (0.49, 1.49) | - | 1.24 (0.69, 2.26) | - | 1.02 (0.55, 1.89) | - |
| Sex | ||||||
| Female | Reference | - | Reference | - | Reference | - |
| Male | 1.34 (0.76, 2.35) | - | 0.96 (0.52, 1.75) | - | 1.00 (0.54, 1.87) | - |
| Race and ethnicity | ||||||
| Other | Reference | - | Reference | - | Reference | - |
| White | 0.68 (0.29, 1.60) | - | 0.91 (0.32, 2.56) | - | 0.88 (0.34, 2.25) | - |
| Smoking history | ||||||
| Never | Reference | - | Reference | - | Reference | - |
| Yes | 1.09 (0.60, 1.98) | - | 0.71 (0.37, 1.37) | - | 1.23 (0.63, 2.40) | - |
| Tumor origin | ||||||
| Lung | Reference | Reference | Reference | Reference | Reference | Reference |
| Lung with pleura | 2.00 (0.47, 8.54) | 2.94 (0.66, 13.00) | 2.28 (0.53, 9.89) | 2.11 (0.27, 16.21) | 2.15 (0.50, 9.31) | 3.27 (0.72, 14.85) |
| Chest wall | 0.88 (0.27, 2.91) | 1.27 (0.37, 4.35) | 1.11 (0.33, 3.70) | 1.02 (0.31, 3.42) | 1.59 (0.48, 5.29) | 2.37 (0.67, 8.37) |
| Mediastinum | 0.54 (0.27, 1.07) | 0.80 (0.38, 1.66) | 1.11 (0.54, 2.29) | 0.86 (0.38, 2.04) | 0.77 (0.35, 1.70) | 1.11 (0.47, 2.61) |
| Tumor size (cm) | ||||||
| <5 | Reference | - | Reference | Reference | Reference | - |
| ≥5 | 1.28 (0.64, 2.53) | - | 2.68 (1.17, 6.15) | 2.66 (1.16, 6.11) | 1.06 (0.51, 2.22) | - |
| Subtype | ||||||
| Monophasic | Reference | - | Reference | - | Reference | - |
| Biphasic | 1.13 (0.44, 2.89) | - | 1.60 (0.62, 4.12) | - | 1.46 (0.56, 3.77) | - |
| Poorly differentiated | 0.62 (0.19, 2.03) | - | 1.64 (0.57, 4.70) | - | 1.07 (0.25, 4.48) | - |
| Tumor resection | ||||||
| No | Reference | - | Reference | - | Reference | - |
| Yes | - | - | 0.52 (0.07, 3.87) | - | - | - |
| Final resection margin | ||||||
| Negative | Reference | - | Reference | - | Reference | - |
| Positive | 1.48 (0.75, 2.94) | - | 1.38 (0.65, 2.96) | - | 1.72 (0.77, 3.75) | - |
| Unknown | 1.29 (0.65, 2.57) | - | 2.43 (1.17, 5.02) | - | 1.38 (0.64, 2.96) | - |
| Neo/adj radiation therapy | ||||||
| No | Reference | - | Reference | - | Reference | - |
| Yes | 0.93 (0.52, 1.67) | - | 1.38 (0.73, 2.60) | - | 1.66 (0.86, 3.20) | - |
| Neo/adj chemotherapy | ||||||
| No | Reference | Reference | Reference | - | Reference | Reference |
| Yes | 0.34 (0.18, 0.62) | 0.33 (0.17, 0.65) | 0.67 (0.36, 1.24) | - | 0.41 (0.21, 0.79) | 0.35 (0.17, 0.73) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, R.R.; Bishop, A.J.; Lazar, A.J.; Lin, P.P.; Benjamin, R.S.; Patel, S.R.; Ludwig, J.; Ravi, V.; Vaporciyan, A.A.; Araujo, D.M. Primary Intrathoracic Synovial Sarcoma: An Analysis of Outcomes of This Rare Disease. Cancers 2025, 17, 745. https://doi.org/10.3390/cancers17050745
Patel RR, Bishop AJ, Lazar AJ, Lin PP, Benjamin RS, Patel SR, Ludwig J, Ravi V, Vaporciyan AA, Araujo DM. Primary Intrathoracic Synovial Sarcoma: An Analysis of Outcomes of This Rare Disease. Cancers. 2025; 17(5):745. https://doi.org/10.3390/cancers17050745
Chicago/Turabian StylePatel, Riddhi R., Andrew J. Bishop, Alexander J. Lazar, Patrick P. Lin, Robert S. Benjamin, Shreyaskumar R. Patel, Joseph Ludwig, Vinod Ravi, Ara A. Vaporciyan, and Dejka M. Araujo. 2025. "Primary Intrathoracic Synovial Sarcoma: An Analysis of Outcomes of This Rare Disease" Cancers 17, no. 5: 745. https://doi.org/10.3390/cancers17050745
APA StylePatel, R. R., Bishop, A. J., Lazar, A. J., Lin, P. P., Benjamin, R. S., Patel, S. R., Ludwig, J., Ravi, V., Vaporciyan, A. A., & Araujo, D. M. (2025). Primary Intrathoracic Synovial Sarcoma: An Analysis of Outcomes of This Rare Disease. Cancers, 17(5), 745. https://doi.org/10.3390/cancers17050745






