The Role of the Gut Microbiota in Modulating Signaling Pathways and Oxidative Stress in Glioma Therapies
Simple Summary
Abstract
1. Introduction
2. CNS Cancers
3. Composition of Intestinal Microbiota Important in Maintaining CNS Homeostasis
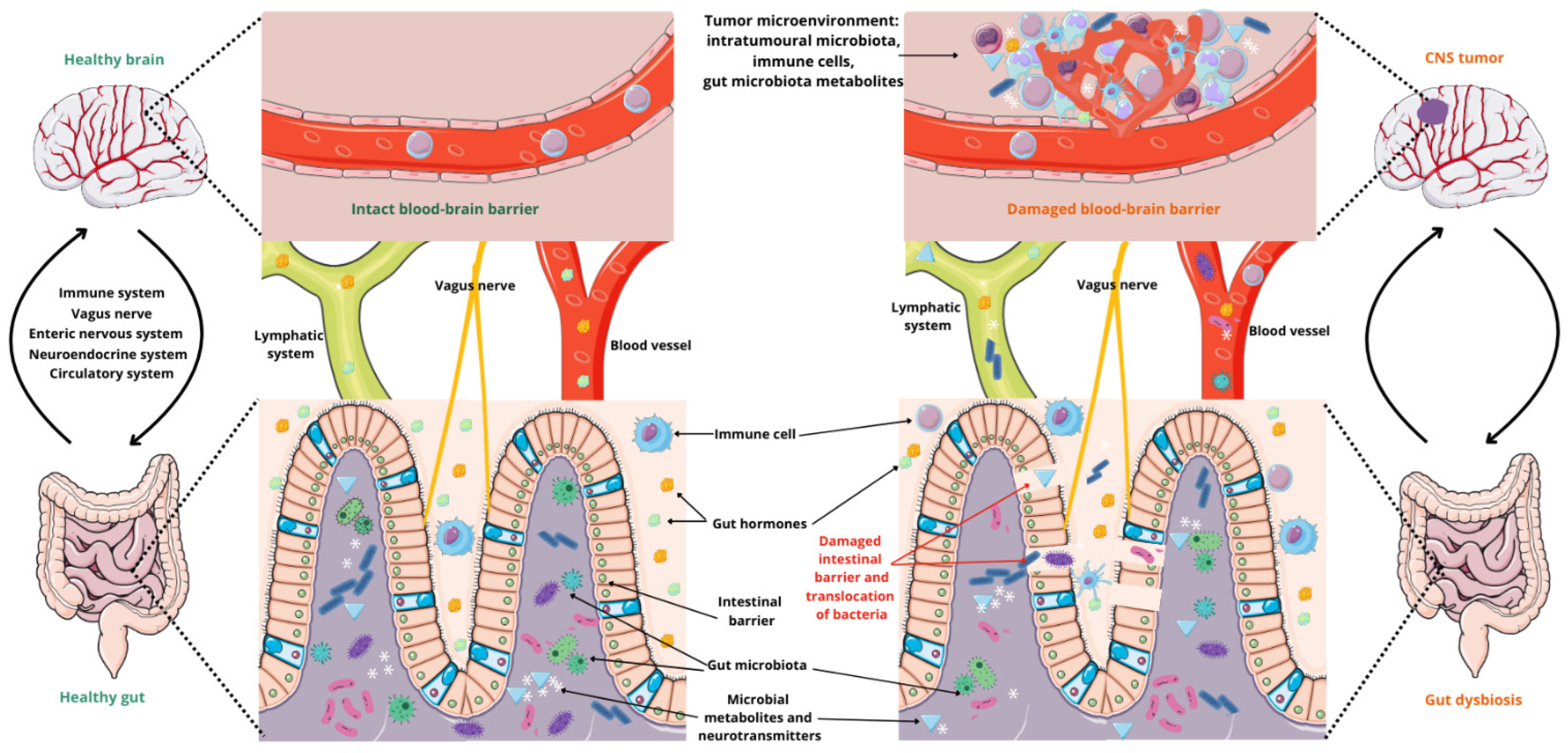
4. Microbiota–Gut–Brain Axis
4.1. The Ways of Communication
4.2. The Influence of Microbiota on Glioma Development
5. The Relationship Between Gut Microbiota, Oxidative Stress, and CNS Cancer
6. Main Signaling Pathways Associated with CNS Tumors
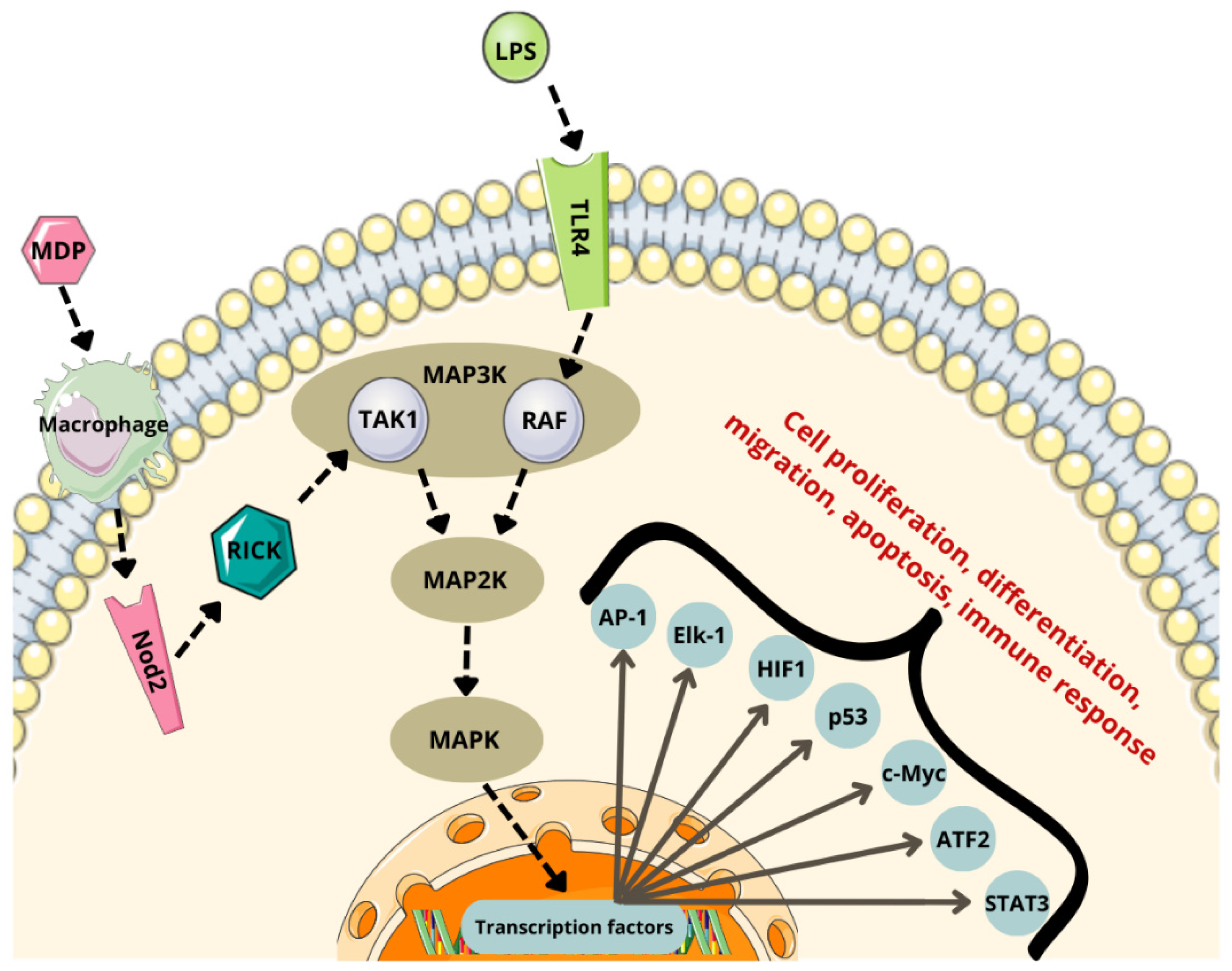
6.1. NF-κB Pathway
6.2. MAPK Pathway
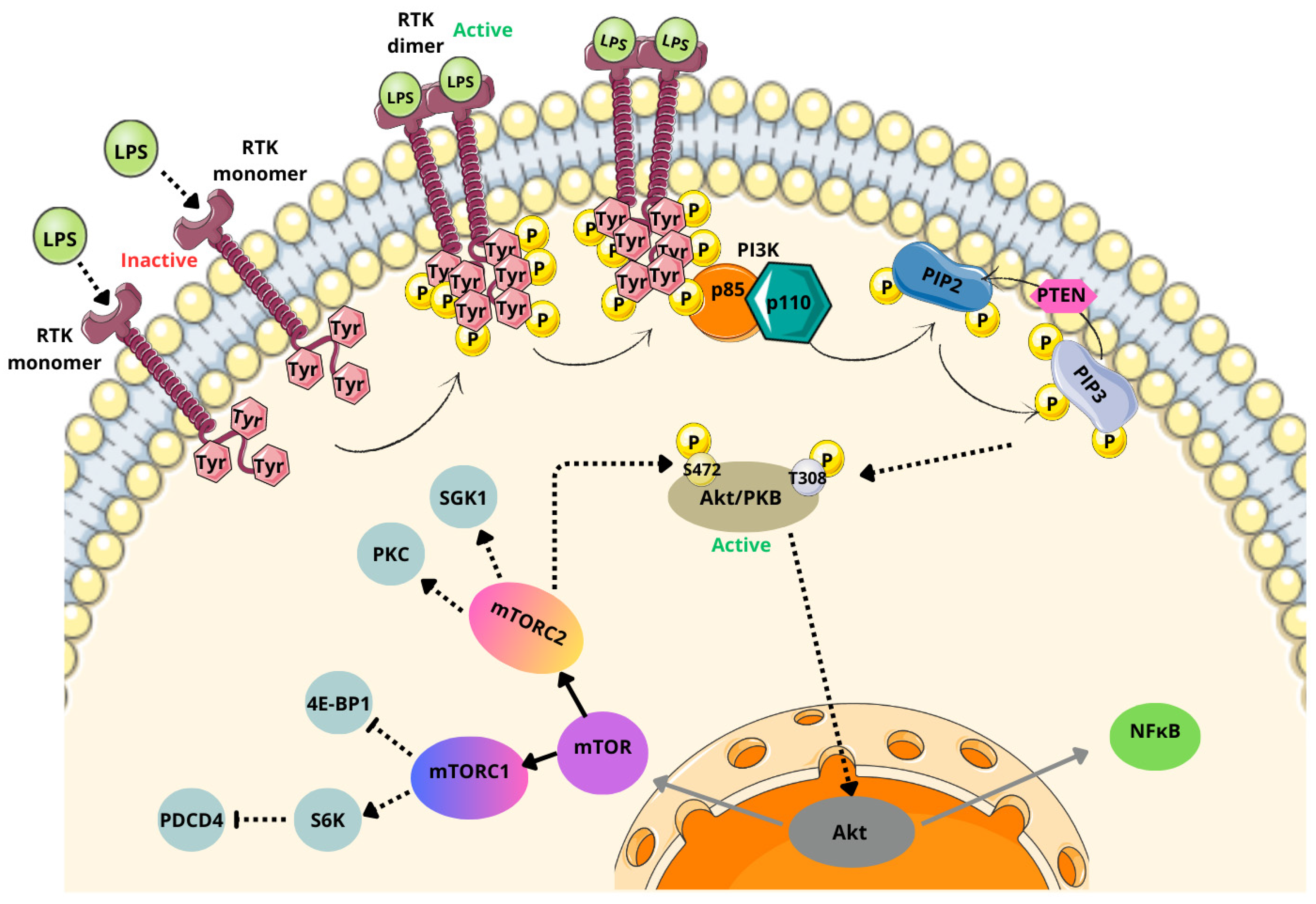
6.3. PI3K/Akt/mTOR Pathway
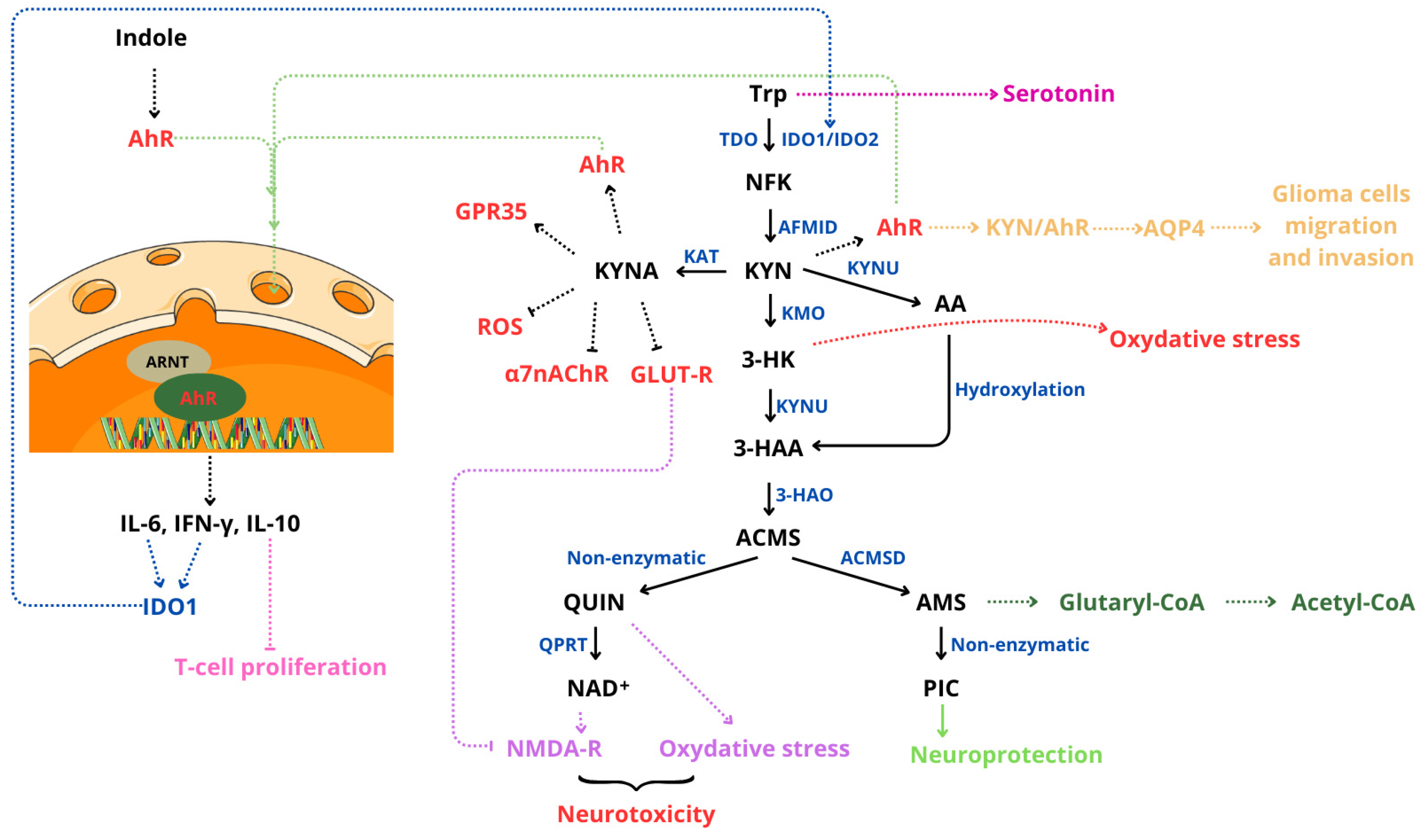
6.4. Kynurenine/Ahr Pathway
7. Therapeutic Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miranda-Filho, A.; Piñeros, M.; Soerjomataram, I.; Deltour, I.; Bray, F. Cancers of the Brain and CNS: Global Patterns and Trends in Incidence. Neuro-Oncology 2017, 19, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Hagemeyer, H.; Hellwinkel, O.J.C.; Plata-Bello, J. Zonulin as Gatekeeper in Gut–Brain Axis: Dysregulation in Glioblastoma. Biomedicines 2024, 12, 1649. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics Analysis Reveals Large Effects of Gut Microflora on Mammalian Blood Metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef]
- Whitfield, C.; Trent, M.S. Biosynthesis and Export of Bacterial Lipopolysaccharides. Annu. Rev. Biochem. 2014, 83, 99–128. [Google Scholar] [CrossRef]
- Gong, L.; Yang, S.; Huang, J.; Li, Y. Modulation of Gut Microbiota in Targeted Cancer Therapy: Insights on the EGFR/VEGF/KRAS Pathways. Cancer Biol. Med. 2024, 21, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Özoğul, F. Production of Biogenic Amines by Morganella morganii, Klebsiella pneumoniae and Hafnia alvei Using a Rapid HPLC Method. Eur. Food Res. Technol. 2004, 219, 465–469. [Google Scholar] [CrossRef]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric Acid Production by Culturable Bacteria from the Human Intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Stanaszek, P.M.; Snell, J.F.; O’Neill, J.J. Isolation, Extraction, and Measurement of Acetylcholine from Lactobacillus Plantarum. Appl. Environ. Microbiol. 1977, 34, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; de las Rivas, B.; Marcobal, A.; Muñoz, R. Molecular Methods for the Detection of Biogenic Amine-Producing Bacteria on Foods. Int. J. Food Microbiol. 2007, 117, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Shishov, V.A.; Kirovskaya, T.A.; Kudrin, V.S.; Oleskin, A.V. Amine Neuromediators, Their Precursors, and Oxidation Products in the Culture of Escherichia Coli K-12. Appl. Biochem. Microbiol. 2009, 45, 494–497. [Google Scholar] [CrossRef]
- Komatsuzaki, N.; Shima, J.; Kawamoto, S.; Momose, H.; Kimura, T. Production of γ-Aminobutyric Acid (GABA) by Lactobacillus Paracasei Isolated from Traditional Fermented Foods. Food Microbiol. 2005, 22, 497–504. [Google Scholar] [CrossRef]
- Nwabo Kamdje, A.H.; Tagne Simo, R.; Fogang Dongmo, H.P.; Bidias, A.R.; Masumbe Netongo, P. Role of Signaling Pathways in the Interaction between Microbial, Inflammation and Cancer. Holist. Integr. Oncol. 2023, 2, 42. [Google Scholar] [CrossRef]
- Adams, S.; Braidy, N.; Bessesde, A.; Brew, B.J.; Grant, R.; Teo, C.; Guillemin, G.J. The Kynurenine Pathway in Brain Tumor Pathogenesis. Cancer Res. 2012, 72, 5649–5657. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J. Quinolinic Acid, the Inescapable Neurotoxin. FEBS J. 2012, 279, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Farmanfarma, K.h.K.; Mohammadian, M.; Shahabinia, Z.; Hassanipour, S.; Salehiniya, H. Brain Cancer in the World: An Epidemiological Review. World Cancer Res. J. 2019, 6, 5. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- CBTRUS. CBTRUS Fact Sheet 2024. Available online: https://cbtrus.org/cbtrus-fact-sheet/ (accessed on 8 December 2024).
- Patel, A.P.; Fisher, J.L.; Nichols, E.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Abraha, H.N.; Agius, D.; Alahdab, F.; Alam, T.; et al. Global, Regional, and National Burden of Brain and Other CNS Cancer, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Ilic, I.; Ilic, M. International Patterns and Trends in the Brain Cancer Incidence and Mortality: An Observational Study Based on the Global Burden of Disease. Heliyon 2023, 9, e18222. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wu, Z.; Zhang, H.; Zhang, N.; Wu, W.; Wang, Z.; Dai, Z.; Zhang, X.; Zhang, L.; Peng, Y.; et al. Glioma Targeted Therapy: Insight into Future of Molecular Approaches. Mol. Cancer 2022, 21, 39. [Google Scholar] [CrossRef]
- Esemen, Y.; Awan, M.; Parwez, R.; Baig, A.; Rahman, S.; Masala, I.; Franchini, S.; Giakoumettis, D. Molecular Pathogenesis of Glioblastoma in Adults and Future Perspectives: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 2607. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.J.; MacDonald, T.; Vezina, G. Central Nervous System Tumors. Hematol./Oncol. Clin. N. Am. 2010, 24, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Knight, R.; Leibel, R.L. The Gut Microbiota in Human Energy Homeostasis and Obesity. Trends Endocrinol. Metab. 2015, 26, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Altveş, S.; Yildiz, H.K.; Vural, H.C. Interaction of the Microbiota with the Human Body in Health and Diseases. Biosci. Microbiota Food Health 2019, 39, 23. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.H.M.P. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Lopetuso, L.R.; Ianiro, G.; Rienzo, T.D.; Gasbarrini, A.; Cammarota, G. Role of Microbiota and Innate Immunity in Recurrent Clostridium Difficile Infection. J. Immunol. Res. 2014, 2014, 462740. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Mu, C.; Yang, Y.; Zhu, W. Gut Microbiota: The Brain Peacekeeper. Front. Microbiol. 2016, 7, 345. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, S. Gut Microbiota and Central Nervous System Development. J. Infect. 2016, 73, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Żakowicz, J.; Bramorska, A.; Zarzycka, W.; Kovbasiuk, A.; Kuć, K.; Brzezicka, A. Wpływ mikrobiomu jelitowego na mózg i psychikę. Kosmos 2020, 69, 45–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The Role of Diet on Gut Microbiota Composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Houtman, T.A.; Eckermann, H.A.; Smidt, H.; de Weerth, C. Gut Microbiota and BMI throughout Childhood: The Role of Firmicutes, Bacteroidetes, and Short-Chain Fatty Acid Producers. Sci. Rep. 2022, 12, 3140. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A. Microbial Pathogenesis in Inflammatory Bowel Diseases. Microb. Pathog. 2022, 163, 105383. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Brook, I. Fusobacterial Head and Neck Infections in Children. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal Gut Microbiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Ryan, F.J.; Hoban, A.E.; Shanahan, F.; Clarke, G.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Microbes & Neurodevelopment–Absence of Microbiota during Early Life Increases Activity-Related Transcriptional Pathways in the Amygdala. Brain Behav. Immun. 2015, 50, 209–220. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Kabouridis, P.S.; Lasrado, R.; McCallum, S.; Chng, S.H.; Snippert, H.J.; Clevers, H.; Pettersson, S.; Pachnis, V. Microbiota Controls the Homeostasis of Glial Cells in the Gut Lamina Propria. Neuron 2015, 85, 289. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of Microbiota-Derived Short-Chain Fatty Acids in Nervous System Disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zeng, W.; Zhang, X.; Pei, Y.; Zhang, H.; Li, Y. The Role of Gut Microbiota in Patients with Benign and Malignant Brain Tumors: A Pilot Study. Bioengineered 2022, 13, 7846–7858. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Wang, X.; Liu, X.; Huang, Y.; Wang, Z.; Ma, Q.; Dong, L.; Qi, Y.; Zhang, H.; et al. Crosstalk Between the Gut and Brain: Importance of the Fecal Microbiota in Patient with Brain Tumors. Front. Cell. Infect. Microbiol. 2022, 12, 881071. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, B.; Luo, C. Gut Microbiota’s Role in Glioblastoma Risk, with a Focus on the Mediating Role of Metabolites. Front. Neurol. 2024, 15, 1386885. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yin, F.; Guo, Z.; Li, R.; Sun, W.; Wang, Y.; Geng, Y.; Sun, C.; Sun, D. Association between Gut Microbiota and Glioblastoma: A Mendelian Randomization Study. Front. Genet. 2024, 14, 1308263. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–Gut–Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Green, G.B.H.; Cox-Holmes, A.N.; Potier, A.C.E.; Marlow, G.H.; McFarland, B.C. Modulation of the Immune Environment in Glioblastoma by the Gut Microbiota. Biomedicines 2024, 12, 2429. [Google Scholar] [CrossRef] [PubMed]
- Larauche, M.; Mulak, A.; Taché, Y. Stress and visceral pain: From animal models to clinical therapies. Exp. Neurol. 2011, 233, 49. [Google Scholar] [CrossRef] [PubMed]
- Larauche, M.; Mulak, A.; Taché, Y. Stress-Related Alterations of Visceral Sensation: Animal Models for Irritable Bowel Syndrome Study. J. Neurogastroenterol. Motil. 2011, 17, 213. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis during Early Life Regulates the Hippocampal Serotonergic System in a Sex-Dependent Manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The Probiotic Bifidobacteria Infantis: An Assessment of Potential Antidepressant Properties in the Rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.; Rizza, S.; Federici, M. Microbiota-Gut-Brain Axis: Relationships among the Vagus Nerve, Gut Microbiota, Obesity, and Diabetes. Acta Diabetol. 2023, 60, 1007–1017. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal Microbial Colonization Programs the Hypothalamic–Pituitary–Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-B.; Powley, T.L. Vagal Innervation of Intestines: Afferent Pathways Mapped with New En Bloc Horseradish Peroxidase Adaptation. Cell Tissue Res. 2007, 329, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.-J. The Enteric Nervous System and Gastrointestinal Innervation: Integrated Local and Central Control. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Lyte, M., Cryan, J.F., Eds.; Springer: New York, NY, USA, 2014; pp. 39–71. ISBN 978-1-4939-0897-4. [Google Scholar]
- Furness, J.B. The Enteric Nervous System; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-1-4051-7344-5. [Google Scholar]
- Li, Y.; Hao, Y.; Zhu, J.; Owyang, C. Serotonin Released from Intestinal Enterochromaffin Cells Mediates Luminal Non–Cholecystokinin-Stimulated Pancreatic Secretion in Rats. Gastroenterology 2000, 118, 1197–1207. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2014, 29, 1395. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/Brain Axis and the Microbiota. J. Clin. Investig. 2015, 125, 926. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.W.; On, N.H.; Del Bigio, M.R.; Miller, D.W.; Hatch, G.M. Fatty Acid Transport Protein Expression in Human Brain and Potential Role in Fatty Acid Transport across Human Brain Microvessel Endothelial Cells. J. Neurochem. 2011, 117, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Vijay, N.; Morris, M.E. Role of Monocarboxylate Transporters in Drug Delivery to the Brain. Curr. Pharm. Des. 2014, 20, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host Microbiota Constantly Control Maturation and Function of Microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Nøhr, M.K.; Pedersen, M.H.; Gille, A.; Egerod, K.L.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.M.; Grunddal, K.V.; Seier Poulsen, S.; Han, S.; et al. GPR41/FFAR3 and GPR43/FFAR2 as Cosensors for Short-Chain Fatty Acids in Enteroendocrine Cells vs FFAR3 in Enteric Neurons and FFAR2 in Enteric Leukocytes. Endocrinology 2013, 154, 3552–3564. [Google Scholar] [CrossRef]
- Tibbs, T.N.; Lopez, L.R.; Arthur, J.C. The influence of the microbiota on immune development, chronic inflammation, and cancer in the context of aging. Microb. Cell 2019, 6, 324. [Google Scholar] [CrossRef] [PubMed]
- Zackular, J.P.; Baxter, N.T.; Iverson, K.D.; Sadler, W.D.; Petrosino, J.F.; Chen, G.Y.; Schloss, P.D. The Gut Microbiome Modulates Colon Tumorigenesis. mBio 2013, 4, e00692-13. [Google Scholar] [CrossRef]
- Tomkovich, S.; Dejea, C.M.; Winglee, K.; Drewes, J.L.; Chung, L.; Housseau, F.; Pope, J.L.; Gauthier, J.; Sun, X.; Mühlbauer, M.; et al. Human Colon Mucosal Biofilms from Healthy or Colon Cancer Hosts Are Carcinogenic. J. Clin. Investig. 2019, 129, 1699–1712. [Google Scholar] [CrossRef]
- Blanchart, A.; Fernando, R.; Häring, M.; Assaife-Lopes, N.; Romanov, R.A.; Andäng, M.; Harkany, T.; Ernfors, P. Endogenous GABAA Receptor Activity Suppresses Glioma Growth. Oncogene 2017, 36, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Merzak, A.; Koochekpour, S.; Fillion, M.-P.; Fillion, G.; Pilkington, G.J. Expression of Serotonin Receptors in Human Fetal Astrocytes and Glioma Cell Lines: A Possible Role in Glioma Cell Proliferation and Migration. Mol. Brain Res. 1996, 41, 1–7. [Google Scholar] [CrossRef]
- Siddiqui, E.J.; Thompson, C.S.; Mikhailidis, D.P.; Mumtaz, F.H. The Role of Serotonin in Tumour Growth (Review). Oncol. Rep. 2005, 14, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Hisaoka, K.; Nishida, A.; Takebayashi, M.; Koda, T.; Yamawaki, S.; Nakata, Y. Serotonin Increases Glial Cell Line-Derived Neurotrophic Factor Release in Rat C6 Glioblastoma Cells. Brain Res. 2004, 1002, 167–170. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Leung, Y.-M.; Cheung, C.-W.; Chen, Y.-R.; Wong, K.-L. Glial Cell Line-Derived Neurotrophic Factor Induces Cell Migration and Matrix Metalloproteinase-13 Expression in Glioma Cells. Biochem. Pharmacol. 2010, 80, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Wiesenhofer, B.; Stockhammer, G.; Kostron, H.; Maier, H.; Hinterhuber, H.; Humpel, C. Glial Cell Line-Derived Neurotrophic Factor (GDNF) and Its Receptor (GFR-A1) Are Strongly Expressed in Human Gliomas. Acta Neuropathol. 2000, 99, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.S.L.; Klamt, F.; Thomé, C.C.; Worm, P.V.; Oliveira, D.L. de Metabotropic Glutamate Receptors as a New Therapeutic Target for Malignant Gliomas. Oncotarget 2017, 8, 22279. [Google Scholar] [CrossRef] [PubMed]
- Arcella, A.; Carpinelli, G.; Battaglia, G.; D’Onofrio, M.; Santoro, F.; Ngomba, R.T.; Bruno, V.; Casolini, P.; Giangaspero, F.; Nicoletti, F. Pharmacological Blockade of Group II Metabotropic Glutamate Receptors Reduces the Growth of Glioma Cells in Vivo. Neuro-Oncology 2005, 7, 236–245. [Google Scholar] [CrossRef]
- Takano, T.; Lin, J.H.-C.; Arcuino, G.; Gao, Q.; Yang, J.; Nedergaard, M. Glutamate Release Promotes Growth of Malignant Gliomas. Nat. Med. 2001, 7, 1010–1015. [Google Scholar] [CrossRef]
- de Groot, J.; Sontheimer, H. Glutamate and the biology of gliomas. Glia 2011, 59, 1181–1189. [Google Scholar] [CrossRef]
- Ishiuchi, S.; Tsuzuki, K.; Yoshida, Y.; Yamada, N.; Hagimura, N.; Okado, H.; Miwa, A.; Kurihara, H.; Nakazato, Y.; Tamura, M.; et al. Blockage of Ca2+-Permeable AMPA Receptors Suppresses Migration and Induces Apoptosis in Human Glioblastoma Cells. Nat. Med. 2002, 8, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Lu, L.; de Groot, J. AMPA Receptors Promote Perivascular Glioma Invasion via Β1 Integrin–Dependent Adhesion to the Extracellular Matrix. Neuro-Oncology 2009, 11, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.A.; Chung, W.J.; Weaver, A.K.; Ogunrinu, T.; Sontheimer, H. Autocrine Glutamate Signaling Promotes Glioma Cell Invasion. Cancer Res. 2007, 67, 9463–9471. [Google Scholar] [CrossRef]
- Li, J.; Zhu, S.; Kozono, D.; Ng, K.; Futalan, D.; Shen, Y.; Akers, J.C.; Steed, T.; Kushwaha, D.; Schlabach, M.; et al. Genome-Wide shRNA Screen Revealed Integrated Mitogenic Signaling between Dopamine Receptor D2 (DRD2) and Epidermal Growth Factor Receptor (EGFR) in Glioblastoma. Oncotarget 2014, 5, 882. [Google Scholar] [CrossRef] [PubMed]
- Weissenrieder, J.S.; Reed, J.L.; Green, M.V.; Moldovan, G.-L.; Koubek, E.J.; Neighbors, J.D.; Hohl, R.J. The Dopamine D2 Receptor Contributes to the Spheroid Formation Behavior of U87 Glioblastoma Cells. Pharmacology 2019, 105, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bartek, J.; Hodny, Z. Dopamine signaling: Target in glioblastoma. Oncotarget 2014, 5, 1116. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity via Interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Gramatzki, D.; Pantazis, G.; Schittenhelm, J.; Tabatabai, G.; Köhle, C.; Wick, W.; Schwarz, M.; Weller, M.; Tritschler, I. Aryl Hydrocarbon Receptor Inhibition Downregulates the TGF-Β/Smad Pathway in Human Glioblastoma Cells. Oncogene 2009, 28, 2593–2605. [Google Scholar] [CrossRef]
- Molfino, A.; Imbimbo, G.; Gallicchio, C.; Muscaritoli, M. Tryptophan metabolism and kynurenine metabolites in cancer: Systemic nutritional and metabolic implications. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 316. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.-C.; Rothstein, J.D.; Sontheimer, H. Compromised Glutamate Transport in Human Glioma Cells: Reduction–Mislocalization of Sodium-Dependent Glutamate Transporters and Enhanced Activity of Cystine–Glutamate Exchange. J. Neurosci. 1999, 19, 10767–10777. [Google Scholar] [CrossRef]
- Seltzer, M.J.; Bennett, B.D.; Joshi, A.D.; Gao, P.; Thomas, A.G.; Ferraris, D.V.; Tsukamoto, T.; Rojas, C.J.; Slusher, B.S.; Rabinowitz, J.D.; et al. Inhibition of Glutaminase Preferentially Slows Growth of Glioma Cells with Mutant IDH1. Cancer Res. 2010, 70, 8981–8987. [Google Scholar] [CrossRef]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-Associated IDH1 Mutations Produce 2-Hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef]
- Maus, A.; Peters, G.J. Glutamate and α-Ketoglutarate: Key Players in Glioma Metabolism. Amino Acids 2017, 49, 21–32. [Google Scholar] [CrossRef]
- Huuskonen, J.; Suuronen, T.; Nuutinen, T.; Kyrylenko, S.; Salminen, A. Regulation of Microglial Inflammatory Response by Sodium Butyrate and Short-Chain Fatty Acids. Br. J. Pharmacol. 2004, 141, 874–880. [Google Scholar] [CrossRef]
- Filippone, A.; Casili, G.; Scuderi, S.A.; Mannino, D.; Lanza, M.; Campolo, M.; Paterniti, I.; Capra, A.P.; Colarossi, C.; Bonasera, A.; et al. Sodium Propionate Contributes to Tumor Cell Growth Inhibition through PPAR-γ Signaling. Cancers 2023, 15, 217. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, T.; Pichumani, K.; Vemireddy, V.; Hatanpaa, K.J.; Singh, D.K.; Sirasanagandla, S.; Nannepaga, S.; Piccirillo, S.G.; Kovacs, Z.; Foong, C.; et al. Acetate Is a Bioenergetic Substrate for Human Glioblastoma and Brain Metastases. Cell 2014, 159, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Masui, K.; Tanaka, K.; Ikegami, S.; Villa, G.R.; Yang, H.; Yong, W.H.; Cloughesy, T.F.; Yamagata, K.; Arai, N.; Cavenee, W.K.; et al. Glucose-Dependent Acetylation of Rictor Promotes Targeted Cancer Therapy Resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 9406–9411. [Google Scholar] [CrossRef] [PubMed]
- Inan, M.S.; Rasoulpour, R.J.; Yin, L.; Hubbard, A.K.; Rosenberg, D.W.; Giardina, C. The Luminal Short-Chain Fatty Acid Butyrate Modulates NF-κB Activity in a Human Colonic Epithelial Cell Line. Gastroenterology 2000, 118, 724–734. [Google Scholar] [CrossRef]
- Zhu, G.; Huang, Q.; Zheng, W.; Huang, Y.; Hua, J.; Yang, S.; Zhuang, J.; Wang, J.; Chang, J.; Xu, J.; et al. LPS Upregulated VEGFR-3 Expression Promote Migration and Invasion in Colorectal Cancer via a Mechanism of Increased NF-κB Binding to the Promoter of VEGFR-3. Cell. Physiol. Biochem. 2016, 39, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, Y.; Guo, S.; Mei, Z.; Liao, H.; Dong, H.; Wu, K.; Ye, H.; Zhang, Y.; Zhu, Y.; et al. Tumor Microbiome Contributes to an Aggressive Phenotype in the Basal-like Subtype of Pancreatic Cancer. Commun. Biol. 2021, 4, 1019. [Google Scholar] [CrossRef]
- Dai, F.; Yu, W.; Song, J.; Li, Q.; Wang, C.; Xie, S. Extracellular polyamines-induced proliferation and migration of cancer cells by ODC, SSAT, and Akt1-mediated pathway. Anti-Cancer Drugs 2017, 28, 457. [Google Scholar] [CrossRef] [PubMed]
- Bonavida, B.; Garban, H. Nitric Oxide-Mediated Sensitization of Resistant Tumor Cells to Apoptosis by Chemo-Immunotherapeutics. Redox Biol. 2015, 6, 486–494. [Google Scholar] [CrossRef] [PubMed]
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-κB and the Link between Inflammation and Cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef]
- Katsuyama, K.; Shichiri, M.; Marumo, F.; Hirata, Y. NO Inhibits Cytokine-Induced iNOS Expression and NF-κB Activation by Interfering with Phosphorylation and Degradation of IκB-α. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Rivoltini, L.; Carrabba, M.; Huber, V.; Castelli, C.; Novellino, L.; Dalerba, P.; Mortarini, R.; Arancia, G.; Anichini, A.; Fais, S.; et al. Immunity to Cancer: Attack and Escape in T Lymphocyte–Tumor Cell Interaction. Immunol. Rev. 2002, 188, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhang, X.; Zhao, F.; Wang, S.; Chen, A.; Huang, B.; Wang, J.; Li, X. Ursodeoxycholic Acid Inhibits Glioblastoma Progression via Endoplasmic Reticulum Stress Related Apoptosis and Synergizes with the Proteasome Inhibitor Bortezomib. ACS Chem. Neurosci. 2020, 11, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- McNulty, N.P.; Wu, M.; Erickson, A.R.; Pan, C.; Erickson, B.K.; Martens, E.C.; Pudlo, N.A.; Muegge, B.D.; Henrissat, B.; Hettich, R.L.; et al. Effects of Diet on Resource Utilization by a Model Human Gut Microbiota Containing Bacteroides Cellulosilyticus WH2, a Symbiont with an Extensive Glycobiome. PLoS Biol. 2013, 11, e1001637. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Chassard, C.; Lawson, P.A.; Bernalier-Donadille, A. Bacteroides cellulosilyticus Sp. Nov., a Cellulolytic Bacterium from the Human Gut Microbial Community. Int. J. Syst. Evol. Microbiol. 2007, 57, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Arnolds, K.L.; Yamada, E.; Neff, C.P.; Schneider, J.M.; Palmer, B.E.; Lozupone, C.A. Disruption of Genes Encoding Putative Zwitterionic Capsular Polysaccharides of Diverse Intestinal Bacteroides Reduces the Induction of Host Anti-Inflammatory Factors. Microb. Ecol. 2023, 85, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, S.; Kumar, S.; Kumar Jha, N.; Kumar Kesari, K.; Ruokolainen, J. Interplay of Gut Microbiota and Oxidative Stress: Perspective on Neurodegeneration and Neuroprotection. J. Adv. Res. 2022, 38, 223–244. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Popescu-Olaru, I.; Cozma, L.; Tulbă, D.; Hinescu, M.E.; Ceafalan, L.C.; Gherghiceanu, M.; Popescu, B.O. Oxidative Stress and the Microbiota-Gut-Brain Axis. Oxidative Med. Cell. Longev. 2018, 2018, 2406594. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Li, L.; Zhong, C.; Zhang, Y.; Yang, X.; Li, M.; Yang, C. The Role of Gut Microbiota in Intestinal Disease: From an Oxidative Stress Perspective. Front. Microbiol. 2024, 15, 1328324. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, C.; Chen, N.; Gu, H.; Yen, A.; Cao, L.; Wang, E.; Wang, L. PI3K/Akt/mTOR Signaling Pathway and Targeted Therapy for Glioblastoma. Oncotarget 2016, 7, 33440–33450. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tang, T. MAPK Signaling Pathway-Based Glioma Subtypes, Machine-Learning Risk Model, and Key Hub Proteins Identification. Sci. Rep. 2023, 13, 19055. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Oezen, I.; Opitz, C.A.; Radlwimmer, B.; von Deimling, A.; Ahrendt, T.; Adams, S.; Bode, H.B.; Guillemin, G.J.; Wick, W.; et al. The Endogenous Tryptophan Metabolite and NAD+ Precursor Quinolinic Acid Confers Resistance of Gliomas to Oxidative Stress. Cancer Res. 2013, 73, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z. Crosstalk of Reactive Oxygen Species and NF-κB Signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Zhang, Y.; Ma, Y.; Zhou, X.; Sun, L.; Jiang, C.; Zhang, Z.; Fu, W. Role of the Gut Microbiota and Its Metabolites in Tumorigenesis or Development of Colorectal Cancer. Adv. Sci. 2023, 10, 2205563. [Google Scholar] [CrossRef]
- Semenova, N.; Garashchenko, N.; Kolesnikov, S.; Darenskaya, M.; Kolesnikova, L. Gut Microbiome Interactions with Oxidative Stress: Mechanisms and Consequences for Health. Pathophysiology 2024, 31, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Olivier, C.; Oliver, L.; Lalier, L.; Vallette, F.M. Drug Resistance in Glioblastoma: The Two Faces of Oxidative Stress. Front. Mol. Biosci. 2021, 7, 620677. [Google Scholar] [CrossRef]
- Sharma, V.; Joseph, C.; Ghosh, S.; Agarwal, A.; Mishra, M.K.; Sen, E. Kaempferol Induces Apoptosis in Glioblastoma Cells through Oxidative Stress. Mol. Cancer Ther. 2007, 6, 2544–2553. [Google Scholar] [CrossRef]
- Khan, M.; Yi, F.; Rasul, A.; Li, T.; Wang, N.; Gao, H.; Gao, R.; Ma, T. Alantolactone Induces Apoptosis in Glioblastoma Cells via GSH Depletion, ROS Generation, and Mitochondrial Dysfunction. IUBMB Life 2012, 64, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Festa, M.; Capasso, A.; D’Acunto, C.W.; Masullo, M.; Rossi, A.G.; Pizza, C.; Piacente, S. Xanthohumol Induces Apoptosis in Human Malignant Glioblastoma Cells by Increasing Reactive Oxygen Species and Activating MAPK Pathways. J. Nat. Prod. 2011, 74, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Singer, E.; Judkins, J.; Salomonis, N.; Matlaf, L.; Soteropoulos, P.; McAllister, S.; Soroceanu, L. Reactive Oxygen Species-Mediated Therapeutic Response and Resistance in Glioblastoma. Cell Death Dis. 2015, 6, e1601. [Google Scholar] [CrossRef]
- Zheng, S.-X.; Chen, J.-P.; Liang, R.-S.; Zhuang, B.-B.; Wang, C.-H.; Zhang, G.-L.; Shi, S.-S.; Chen, J. Schizophyllum commune Fruiting Body Polysaccharides Inhibit Glioma by Mediating ARHI Regulation of PI3K/AKT Signalling Pathway. Int. J. Biol. Macromol. 2024, 279, 135326. [Google Scholar] [CrossRef] [PubMed]
- Zabolotneva, A.A.; Shatova, O.P.; Shegai, P.V.; Shestopalov, A.V. Immunosuppression in the Tumor Microenvironment Mediated by Metabolites Derived from the Gut Microbiota. Oncol. Adv. 2023, 1, 17–24. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Qi, S.; Gao, L.; Huang, G.; Ren, Z.; Li, K.; Peng, Y.; Yi, G.; Guo, J.; et al. Spliceosome-Regulated RSRP1-Dependent NF-κB Activation Promotes the Glioblastoma Mesenchymal Phenotype. Neuro-Oncology 2021, 23, 1693–1708. [Google Scholar] [CrossRef] [PubMed]
- Puliyappadamba, V.T.; Hatanpaa, K.J.; Chakraborty, S.; Habib, A.A. The Role of NF-κB in the Pathogenesis of Glioma. Mol. Cell. Oncol. 2014, 1, e963478. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.D.; Yeyeodu, S.; Cusack, J.C.; Baldwin, A.S.; Ewend, M.G. Potentiation of Chemotherapeutic Agents Following Antagonism of Nuclear Factor Kappa B in Human Gliomas. J. Neuro-Oncol. 2003, 61, 187–196. [Google Scholar] [CrossRef]
- Bonavia, R.; Inda, M.M.; Vandenberg, S.; Cheng, S.-Y.; Nagane, M.; Hadwiger, P.; Tan, P.; Sah, D.W.Y.; Cavenee, W.K.; Furnari, F.B. EGFRvIII Promotes Glioma Angiogenesis and Growth through the NF-κB, Interleukin-8 Pathway. Oncogene 2012, 31, 4054–4066. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Cusack, J.C.; Liu, R.; Baldwin, A.S. Control of Inducible Chemoresistance: Enhanced Anti-Tumor Therapy through Increased Apoptosis by Inhibition of NF-κB. Nat. Med. 1999, 5, 412–417. [Google Scholar] [CrossRef]
- Nagai, S.; Washiyama, K.; Kurimoto, M.; Takaku, A.; Endo, S.; Kumanishi, T. Aberrant nuclear factor-κB activity and its participation in the growth of human malignant astrocytoma. J. Neurosurg. 2002, 96, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, M.-A.; Zhou, S.; Nonnenmacher, L.; Karpel-Massler, G.; Jennewein, C.; Schneider, M.; Halatsch, M.-E.; Carragher, N.O.; Baumann, B.; Krause, A.; et al. Inhibition of NF-κB Signaling Ablates the Invasive Phenotype of Glioblastoma. Mol. Cancer Res. 2013, 11, 1611–1623. [Google Scholar] [CrossRef] [PubMed]
- Coupienne, I.; Bontems, S.; Dewaele, M.; Rubio, N.; Habraken, Y.; Fulda, S.; Agostinis, P.; Piette, J. NF-kappaB Inhibition Improves the Sensitivity of Human Glioblastoma Cells to 5-Aminolevulinic Acid-Based Photodynamic Therapy. Biochem. Pharmacol. 2011, 81, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhang, L.; Wang, J.; Zhou, H. Baicalein Induces the Apoptosis of U251 Glioblastoma Cell Lines via the NF-kB-P65-Mediated Mechanism. Anim. Cells Syst. 2016, 20, 296–302. [Google Scholar] [CrossRef]
- Avci, N.G.; Ebrahimzadeh-Pustchi, S.; Akay, Y.M.; Esquenazi, Y.; Tandon, N.; Zhu, J.-J.; Akay, M. NF-κB Inhibitor with Temozolomide Results in Significant Apoptosis in Glioblastoma via the NF-κB(P65) and Actin Cytoskeleton Regulatory Pathways. Sci. Rep. 2020, 10, 13352. [Google Scholar] [CrossRef] [PubMed]
- Spielbauer, J.; Glotfelty, E.J.; Sarlus, H.; Harris, R.A.; Diaz Heijtz, R.; Karlsson, T.E. Bacterial Peptidoglycan Signalling in Microglia: Activation by MDP via the NF-κB/MAPK Pathway. Brain Behav. Immun. 2024, 121, 43–55. [Google Scholar] [CrossRef]
- Krawczyk, A.; Stadler, S.M.; Strzalka-Mrozik, B. Nanomedicines for Dry Eye Syndrome: Targeting Oxidative Stress with Modern Nanomaterial Strategies. Molecules 2024, 29, 3732. [Google Scholar] [CrossRef]
- Marina-García, N.; Franchi, L.; Kim, Y.-G.; Hu, Y.; Smith, D.E.; Boons, G.-J.; Núñez, G. Clathrin- and Dynamin-Dependent Endocytic Pathway Regulates Muramyl Dipeptide Internalization and NOD2 Activation1. J. Immunol. 2009, 182, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Omori, E.; Matsumoto, K.; Núñez, G.; Ninomiya-Tsuji, J. TAK1 Is a Central Mediator of NOD2 Signaling in Epidermal Cells. J. Biol. Chem. 2008, 283, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Strober, W.; Watanabe, T. NOD2, an Intracellular Innate Immune Sensor Involved in Host Defense and Crohn’s Disease. Mucosal Immunol. 2011, 4, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation Pathways: A General Review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Avery, T.Y.; Köhler, N.; Zeiser, R.; Brummer, T.; Ruess, D.A. Onco-Immunomodulatory Properties of Pharmacological Interference with RAS-RAF-MEK-ERK Pathway Hyperactivation. Front. Oncol. 2022, 12, 931774. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Tran, M.N.; Rivera, A.; Cheng, T.; Windsor, G.O.; Chabot, A.B.; Cavanaugh, J.E.; Collins-Burow, B.M.; Lee, S.B.; Drewry, D.H.; et al. MAP3K Family Review and Correlations with Patient Survival Outcomes in Various Cancer Types. Front. Biosci.-Landmark 2022, 27, 167. [Google Scholar] [CrossRef]
- Cuarental, L.; Sucunza-Sáenz, D.; Valiño-Rivas, L.; Fernandez-Fernandez, B.; Sanz, A.B.; Ortiz, A.; Vaquero, J.J.; Sanchez-Niño, M.D. MAP3K Kinases and Kidney Injury. Nefrología 2019, 39, 568–580. [Google Scholar] [CrossRef]
- Campbell, B.B.; Galati, M.A.; Stone, S.C.; Riemenschneider, A.N.; Edwards, M.; Sudhaman, S.; Siddaway, R.; Komosa, M.; Nunes, N.M.; Nobre, L.; et al. Mutations in the RAS/MAPK Pathway Drive Replication Repair–Deficient Hypermutated Tumors and Confer Sensitivity to MEK Inhibition. Cancer Discov. 2021, 11, 1454–1467. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.-J. Pathological Roles of MAPK Signaling Pathways in Human Diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Nigam, M.; Devi, K.; Coutinho, H.D.M.; Mishra, A.P. Exploration of Gut Microbiome and Inflammation: A Review on Key Signalling Pathways. Cell. Signal. 2024, 118, 111140. [Google Scholar] [CrossRef] [PubMed]
- Barona, I.; Fagundes, D.S.; Gonzalo, S.; Grasa, L.; Arruebo, M.P.; Plaza, M.Á.; Murillo, M.D. Role of TLR4 and MAPK in the Local Effect of LPS on Intestinal Contractility. J. Pharm. Pharmacol. 2011, 63, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Cantó, E.; Moga, E.; Ricart, E.; Garcia-Bosch, O.; Garcia-Planella, E.; Juarez, C.; Vidal, S. MDP-Induced Selective Tolerance to TLR4 Ligands: Impairment in NOD2 Mutant Crohn’s Disease Patients. Inflamm. Bowel Dis. 2009, 15, 1686–1696. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Gille, H.; Kortenjann, M.; Thomae, O.; Moomaw, C.; Slaughter, C.; Cobb, M.; Shaw, P. ERK Phosphorylation Potentiates Elk-1-mediated Ternary Complex Formation and Transactivation. EMBO J. 1995, 14, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Solís, C.; Castillo-Rodríguez, R.A.; Serrano-García, N.; Silva-Adaya, D.; Vargas-Cruz, S.; Chávez-Cortéz, E.G.; Gallardo-Pérez, J.C.; Zavala-Vega, S.; Cruz-Salgado, A.; Magaña-Maldonado, R. Metabolic Roles of HIF1, c-Myc, and P53 in Glioma Cells. Metabolites 2024, 14, 249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-S.; Hsu, J.-W.; Lin, H.-Y.; Lai, S.-W.; Huang, B.-R.; Tsai, C.-F.; Lu, D.-Y. Bradykinin B1 Receptor Contributes to Interleukin-8 Production and Glioblastoma Migration through Interaction of STAT3 and SP-1. Neuropharmacology 2019, 144, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Abou-Ghazal, M.; Yang, D.S.; Qiao, W.; Reina-Ortiz, C.; Wei, J.; Kong, L.-Y.; Fuller, G.N.; Hiraoka, N.; Priebe, W.; Sawaya, R.; et al. The Incidence, Correlation with Tumor-Infiltrating Inflammation, and Prognosis of Phosphorylated STAT3 Expression in Human Gliomas. Clin. Cancer Res. 2008, 14, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Jiang, X.; Qiao, H.; Zhai, B.; Zhang, L.; Zhang, Q.; Wu, Y.; Jiang, H.; Sun, X. STAT3 Interacts with Skp2/P27/P21 Pathway to Regulate the Motility and Invasion of Gastric Cancer Cells. Cell. Signal. 2013, 25, 931–938. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Lin, H.-Y.; Lai, S.-W.; Huang, C.-Y.; Huang, B.-R.; Chen, P.-Y.; Wei, K.-C.; Lu, D.-Y. MiR-181b Modulates EGFR-Dependent VCAM-1 Expression and Monocyte Adhesion in Glioblastoma. Oncogene 2017, 36, 5006–5022. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, R.; Madjid Ansari, A.; Forouzesh, F.; Shahriari, F.; Shariatpanahi, S.P.; Salaritabar, A.; Javidi, M.A. P53 Status, and G2/M Cell Cycle Arrest, Are Determining Factors in Cell-Death Induction Mediated by ELF-EMF in Glioblastoma. Sci. Rep. 2023, 13, 10845. [Google Scholar] [CrossRef]
- Watson, S.A.; McStay, G.P. Functions of Cytochrome c Oxidase Assembly Factors. Int. J. Mol. Sci. 2020, 21, 7254. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Ryan, K.M. P53 and Metabolism. Nat. Rev. Cancer 2009, 9, 691–700. [Google Scholar] [CrossRef]
- Kawauchi, K.; Araki, K.; Tobiume, K.; Tanaka, N. P53 Regulates Glucose Metabolism through an IKK-NF-κB Pathway and Inhibits Cell Transformation. Nat. Cell Biol. 2008, 10, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, W.; Chen, B.; Jiang, T.; Wang, Z. Prognostic and Predictive Value of P53 in Low MGMT Expressing Glioblastoma Treated with Surgery, Radiation and Adjuvant Temozolomide Chemotherapy. Neurol. Res. 2010, 32, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Yang, H.; Chen, L. Metabolic Regulation on the Immune Environment of Glioma through Gut Microbiota. Semin. Cancer Biol. 2022, 86, 990–997. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The Emerging Mechanisms of Isoform-Specific PI3K Signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz-Gil, J. Inhibitory szlaku PI3K-Akt/PKB-mTOR w leczeniu glejaków. Postępy Biol. Komórki 2009, 36, 189–201. [Google Scholar]
- Fingar, D.C.; Richardson, C.J.; Tee, A.R.; Cheatham, L.; Tsou, C.; Blenis, J. mTOR Controls Cell Cycle Progression through Its Cell Growth Effectors S6K1 and 4E-BP1/Eukaryotic Translation Initiation Factor 4E. Mol. Cell. Biol. 2004, 24, 200–216. [Google Scholar] [CrossRef]
- Yang, M.; Lu, Y.; Piao, W.; Jin, H. The Translational Regulation in mTOR Pathway. Biomolecules 2022, 12, 802. [Google Scholar] [CrossRef] [PubMed]
- Rabanal-Ruiz, Y.; Otten, E.G.; Korolchuk, V.I. mTORC1 as the Main Gateway to Autophagy. Essays Biochem. 2017, 61, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Goel-Bhattacharya, S.; Sengupta, S.; Cochran, B.H. A Large-Scale RNAi Screen Identifies SGK1 as a Key Survival Kinase for GBM Stem Cells. Mol. Cancer Res. 2018, 16, 103–114. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, A.; Balça-Silva, J.; Matias, D.; Lopes, M.C. PKC Signaling in Glioblastoma. Cancer Biol. Ther. 2013, 14, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Heng, B.; Guillemin, G.J. The Gut Microbiota, Kynurenine Pathway, and Immune System Interaction in the Development of Brain Cancer. Front. Cell Dev. Biol. 2020, 8, 562812. [Google Scholar] [CrossRef] [PubMed]
- Grishanova, A.Y.; Perepechaeva, M.L. Kynurenic Acid/AhR Signaling at the Junction of Inflammation and Cardiovascular Diseases. Int. J. Mol. Sci. 2024, 25, 6933. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Cullen, K.M.; Lim, C.K.; Smythe, G.A.; Garner, B.; Kapoor, V.; Takikawa, O.; Brew, B.J. Characterization of the Kynurenine Pathway in Human Neurons. J. Neurosci. 2007, 27, 12884–12892. [Google Scholar] [CrossRef] [PubMed]
- Reyes Ocampo, J.; Lugo Huitrón, R.; González-Esquivel, D.; Ugalde-Muñiz, P.; Jiménez-Anguiano, A.; Pineda, B.; Pedraza-Chaverri, J.; Ríos, C.; Pérez de la Cruz, V. Kynurenines with Neuroactive and Redox Properties: Relevance to Aging and Brain Diseases. Oxidative Med. Cell. Longev. 2014, 2014, 646909. [Google Scholar] [CrossRef] [PubMed]
- Beninger, R.J.; Colton, A.M.; Ingles, J.L.; Jhamandas, K.; Boegman, R.J. Picolinic Acid Blocks the Neurotoxic but Not the Neuroexcitant Properties of Quinolinic Acid in the Rat Brain: Evidence from Turning Behaviour and Tyrosine Hydroxylase Immunohistochemistry. Neuroscience 1994, 61, 603–612. [Google Scholar] [CrossRef]
- Sun, T.; Xie, R.; He, H.; Xie, Q.; Zhao, X.; Kang, G.; Cheng, C.; Yin, W.; Cong, J.; Li, J.; et al. Kynurenic Acid Ameliorates NLRP3 Inflammasome Activation by Blocking Calcium Mobilization via GPR35. Front. Immunol. 2022, 13, 1019365. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Xing, Z.; Tao, B.; Li, T.; Yang, D.; Li, W.; Zheng, Y.; Kuang, C.; Yang, Q. Both IDO1 and TDO Contribute to the Malignancy of Gliomas via the Kyn–AhR–AQP4 Signaling Pathway. Signal Transduct. Target. Ther. 2020, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, H.M.; Yasin, R.; Mohammad, I.S.; Fan, Y.; Li, H.; Shahzad, M.; Xu, J. The Gut-Brain-Axis: A Positive Relationship between Gut Microbial Dysbiosis and Glioblastoma Brain Tumour. Heliyon 2024, 10, e30494. [Google Scholar] [CrossRef]
- Ting, N.L.-N.; Lau, H.C.-H.; Yu, J. Cancer Pharmacomicrobiomics: Targeting Microbiota to Optimise Cancer Therapy Outcomes. Gut 2022, 71, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Villéger, R.; Lopès, A.; Carrier, G.; Veziant, J.; Billard, E.; Barnich, N.; Gagnière, J.; Vazeille, E.; Bonnet, M. Intestinal Microbiota: A Novel Target to Improve Anti-Tumor Treatment? Int. J. Mol. Sci. 2019, 20, 4584. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, L.; Ren, Q.; Zhu, C.; Du, H.; Wang, Z.; Qi, Y.; Xian, X.; Chen, D. Gut Microbiota as a Biomarker and Modulator of Anti-Tumor Immunotherapy Outcomes. Front. Immunol. 2024, 15, 1471273. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Fan, H.; Han, M.; Xie, J.; Du, J.; Peng, F. Bifidobacterium lactis Combined with Lactobacillus plantarum Inhibit Glioma Growth in Mice through Modulating PI3K/AKT Pathway and Gut Microbiota. Front. Microbiol. 2022, 13, 986837. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, Y.; Han, M.; Wang, L.; Li, X.; Kuang, X.; Du, J.; Peng, F. Multi-Omics-Based Investigation of Bifidobacterium’s Inhibitory Effect on Glioma: Regulation of Tumor and Gut Microbiota, and MEK/ERK Cascade. Front. Microbiol. 2024, 15, 1344284. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, L.; Biassoni, V.; Schiavello, E.; Carenzo, A.; Iannò, M.F.; Licata, A.; Marra, M.; Carollo, M.; Boschetti, L.; Massimino, M. DIPG-36. The Brain-Gut-Microbiota Axis to Predict Outcome in Pediatric Diffuse Intrinsic Pontine Glioma. Neuro-Oncology 2022, 24, i26. [Google Scholar] [CrossRef]
- Fan, Y.; Su, Q.; Chen, J.; Wang, Y.; He, S. Gut Microbiome Alterations Affect Glioma Development and Foxp3 Expression in Tumor Microenvironment in Mice. Front. Oncol. 2022, 12, 836953. [Google Scholar] [CrossRef]
- Wang, W.; Ou, Z.; Huang, X.; Wang, J.; Li, Q.; Wen, M.; Zheng, L. Microbiota and Glioma: A New Perspective from Association to Clinical Translation. Gut Microbes 2024, 16, 2394166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hong, Y.; Wu, T.; Ben, E.; Li, S.; Hu, L.; Xie, T. Role of Gut Microbiota in Regulating Immune Checkpoint Inhibitor Therapy for Glioblastoma. Front. Immunol. 2024, 15, 1401967. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.; Idbaih, A.; Vieito, M.; Tabatabai, G.; Stradella, A.; Ghiringhelli, F.; Burger, M.; Mildenberger, I.; Herrlinger, U.; González, M.; et al. 642 EO2401 Microbiome Derived Therapeutic Vaccine + Nivolumab, with/without Standard Continuous, or Low-Dose Symptom Directed, Bevacizumab, in Recurrent Glioblastoma: Phase 1–2 EOGBM1–18/ROSALIE Study. J. Immunother. Cancer 2022, 10. [Google Scholar] [CrossRef]
- Dono, A.; Patrizz, A.; McCormack, R.M.; Putluri, N.; Ganesh, B.P.; Kaur, B.; McCullough, L.D.; Ballester, Y.L.; Esquenazi, Y. Glioma Induced Alterations in Fecal Short-Chain Fatty Acids and Neurotransmitters. CNS Oncol. 2020, 9, CNS57. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, G.; Antonangeli, F.; Marrocco, F.; Porzia, A.; Lauro, C.; Santoni, A.; Limatola, C. Gut Microbiota Alterations Affect Glioma Growth and Innate Immune Cells Involved in Tumor Immunosurveillance in Mice. Eur. J. Immunol. 2020, 50, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Zhang, A.; Yu, W.; Lei, Q.; Xiao, B.; Luo, Z. Investigational Microbiological Therapy for Glioma. Cancers 2022, 14, 5977. [Google Scholar] [CrossRef] [PubMed]
- Vannini, E.; Maltese, F.; Olimpico, F.; Fabbri, A.; Costa, M.; Caleo, M.; Baroncelli, L. Progression of Motor Deficits in Glioma-Bearing Mice: Impact of CNF1 Therapy at Symptomatic Stages. Oncotarget 2017, 8, 23539–23550. [Google Scholar] [CrossRef] [PubMed]
- Vannini, E.; Panighini, A.; Cerri, C.; Fabbri, A.; Lisi, S.; Pracucci, E.; Benedetto, N.; Vannozzi, R.; Fiorentini, C.; Caleo, M.; et al. The Bacterial Protein Toxin, Cytotoxic Necrotizing Factor 1 (CNF1) Provides Long-Term Survival in a Murine Glioma Model. BMC Cancer 2014, 14, 449. [Google Scholar] [CrossRef]
- Naghavian, R.; Faigle, W.; Oldrati, P.; Wang, J.; Toussaint, N.C.; Qiu, Y.; Medici, G.; Wacker, M.; Freudenmann, L.K.; Bonté, P.-E.; et al. Microbial Peptides Activate Tumour-Infiltrating Lymphocytes in Glioblastoma. Nature 2023, 617, 807–817. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, F.; Gao, Y.; Kang, S.; Li, J.; Guo, J. Microbiome in Radiotherapy: An Emerging Approach to Enhance Treatment Efficacy and Reduce Tissue Injury. Mol. Med. 2024, 30, 105. [Google Scholar] [CrossRef] [PubMed]
- Çakır, T.; Güven, M.; Taşpınar, M.; Denizler, F.N.; Kartal, B. The Effect of Sodium Butyrate on Radiosensitivity in Glioblastoma Cell. Van Med. J. 2019, 26, 550–556. [Google Scholar] [CrossRef]
- Entin-Meer, M.; Rephaeli, A.; Yang, X.; Nudelman, A.; VandenBerg, S.R.; Haas-Kogan, D.A. Butyric Acid Prodrugs Are Histone Deacetylase Inhibitors That Show Antineoplastic Activity and Radiosensitizing Capacity in the Treatment of Malignant Gliomas. Mol. Cancer Ther. 2005, 4, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Sawa, H.; Nagane, M.; Noguchi, A.; Hara, M.; Saito, I. Inhibitory Effects of Sodium Butyrate on Proliferation and Invasiveness of Human Glioma Cells. Neurosurgery 2001, 49, 430. [Google Scholar] [PubMed]
- Okumura, S.; Konishi, Y.; Narukawa, M.; Sugiura, Y.; Yoshimoto, S.; Arai, Y.; Sato, S.; Yoshida, Y.; Tsuji, S.; Uemura, K.; et al. Gut Bacteria Identified in Colorectal Cancer Patients Promote Tumourigenesis via Butyrate Secretion. Nat. Commun. 2021, 12, 5674. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-M.; Chieng, W.-W.; Huang, S.-W.; Hsu, L.-J.; Jan, M.-S. The Synergistic Tumor Growth-Inhibitory Effect of Probiotic Lactobacillus on Transgenic Mouse Model of Pancreatic Cancer Treated with Gemcitabine. Sci. Rep. 2020, 10, 20319. [Google Scholar] [CrossRef]
- Ni, Y.; Li, R.; Shen, X.; Yi, D.; Ren, Y.; Wang, F.; Geng, Y.; You, Q. Diaphorobacter nitroreducens Synergize with Oxaliplatin to Reduce Tumor Burden in Mice with Lung Adenocarcinoma. mSystems 2024, 9, e01323-23. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Du, H.; Deng, Y.; Wang, H.; Liu, J.; Qiao, J.; Liu, W.; Shu, X.; Sun, B.; Liu, Y. Gut Microbiota Mediated the Individualized Efficacy of Temozolomide via Immunomodulation in Glioma. J. Transl. Med. 2023, 21, 198. [Google Scholar] [CrossRef]
- Meléndez-Vázquez, N.M.; Nguyen, T.T.; Fan, X.; López-Rivas, A.R.; Fueyo, J.; Gomez-Manzano, C.; Godoy-Vitorino, F. Gut Microbiota Composition Is Associated with the Efficacy of Delta-24-RGDOX in Malignant Gliomas. Mol. Ther. Oncol. 2024, 32, 200787. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, M.; Lu, J.; Chu, B.; Yang, Y.; Song, B.; Wang, H.; He, Y. Bacteria Loaded with Glucose Polymer and Photosensitive ICG Silicon-Nanoparticles for Glioblastoma Photothermal Immunotherapy. Nat. Commun. 2022, 13, 5127. [Google Scholar] [CrossRef]
- Hao, R.; Liu, Q.; Wang, L.; Jian, W.; Cheng, Y.; Zhang, Q.; Hayer, K.; Kamarudin Raja Idris, R.; Zhang, Y.; Lu, O.; et al. Anti-Inflammatory Effect of Lactiplantibacillus Plantarum T1 Cell-Free Supernatants through Suppression of Oxidative Stress and NF-κB- and MAPK-Signaling Pathways. Appl. Environ. Microbiol. 2023, 89, e00608-23. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, N.-R.; Gim, M.G.; Lee, J.M.; Lee, S.Y.; Ko, M.Y.; Kim, J.Y.; Han, S.H.; Chung, D.K. Lipoteichoic Acid Isolated from Lactobacillus Plantarum Inhibits Lipopolysaccharide-Induced TNF-α Production in THP-1 Cells and Endotoxin Shock in Mice1. J. Immunol. 2008, 180, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, P.P.; Sordillo, L.A.; Helson, L. The Kynurenine Pathway: A Primary Resistance Mechanism in Patients with Glioblastoma. Anticancer Res. 2017, 37, 2159–2171. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Si, Q.; Qi, R.; Liu, W.; Li, M.; Guo, M.; Wei, L.; Yao, Z. Indoleamine 2,3-Dioxygenase 1: A Promising Therapeutic Target in Malignant Tumor. Front. Immunol. 2021, 12, 800630. [Google Scholar] [CrossRef]
- Zhai, L.; Lauing, K.L.; Chang, A.L.; Dey, M.; Qian, J.; Cheng, Y.; Lesniak, M.S.; Wainwright, D.A. The Role of IDO in Brain Tumor Immunotherapy. J. Neurooncol. 2015, 123, 395–403. [Google Scholar] [CrossRef]
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolušić, E.; Frédérick, R.; De Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.; et al. Reversal of Tumoral Immune Resistance by Inhibition of Tryptophan 2,3-Dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodado, V.; Dowdy, T.; Lita, A.; Kramp, T.; Zhang, M.; Jung, J.; Dios-Esponera, A.; Zhang, L.; Herold-Mende, C.C.; Camphausen, K.; et al. Cysteine Is a Limiting Factor for Glioma Proliferation and Survival. Mol. Oncol. 2022, 16, 1777–1794. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, W.; Wang, K.; Wang, X.; Yin, F.; Li, C.; Wang, C.; Zhao, B.; Zhong, C.; Zhang, J.; et al. Methionine and Cystine Double Deprivation Stress Suppresses Glioma Proliferation via Inducing ROS/Autophagy. Toxicol. Lett. 2015, 232, 349–355. [Google Scholar] [CrossRef]
- Silver, D.J.; Lathia, J.D.; Hine, C. Hydrogen Sulfide Operates as a Glioblastoma Suppressor and Is Lost under High Fat Diet. Mol. Cell. Oncol. 2021, 8, 1973312. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.G.; Fenton, K.E.; Preul, M.C.; Rho, J.M.; Lynch, A.; Stafford, P.; Scheck, A.C. The Ketogenic Diet Is an Effective Adjuvant to Radiation Therapy for the Treatment of Malignant Glioma. PLoS ONE 2012, 7, e36197. [Google Scholar] [CrossRef]
- Martuscello, R.T.; Vedam-Mai, V.; McCarthy, D.J.; Schmoll, M.E.; Jundi, M.A.; Louviere, C.D.; Griffith, B.G.; Skinner, C.L.; Suslov, O.; Deleyrolle, L.P.; et al. A Supplemented High-Fat Low-Carbohydrate Diet for the Treatment of Glioblastoma. Clin. Cancer Res. 2016, 22, 2482–2495. [Google Scholar] [CrossRef] [PubMed]

| Phylum | % Microbiome | Genus | Relevant Function | References |
|---|---|---|---|---|
| Firmicutes | 60–65% | Bacillus Clostridium Dialister Enterococcus Faecalibacterium Lactobacillus Roseburia Ruminicoccus Staphylococcus | Some are involved in the production of short-chain fatty acids (SCFAs) | [24,27,28,29,30,31,33,34] |
| Bacteroidetes | 20–25% | Alistipes Bacteroides Parabacteroides Prevotella Sphingobacterium Tannerella | Some are involved in the production of SCFAs | [24,27,28,29,30,31,33,34] |
| Proteobacteria | 5–10% | Bilophila Desulfovibrio Escherichia Helicobacter Shigella | Signal microbial dysbiosis—for a healthy person this makes up a small part of the intestinal microbiota | [24,25,26,27,28,29,30,31,33,35] |
| Actinobacteria | 3% | Atopobium Bifidobacterium Corynebacterium | Some are involved in the de novo synthesis of essential vitamins for the host, including vit. B12 | [27,28,29,31,33,34,36] |
| Fusobacteria | <1% | Fusobacterium | β-lactamase production | [24,27,29,31,37] |
| Verrucomicrobia | <1% | Akkermansia | Some are involved in the production of SCFAs | [24,27,29,31,34] |
| What Effect Does It Have? | References | |
|---|---|---|
| Neurotransmitters | ||
| GABA | Activation of the GABAA-R receptor induces cell depolarization through the efflux of chloride ions, thereby suppressing glioma cell proliferation and promoting cellular quiescence. | [72] |
| Serotonin | Activation of 5-HT1 and 5-HT2 receptors enhances cell proliferation, differentiation, migration, and gene expression in glioma cells. Specifically, activation of 5-HT2 receptors increases the expression of glial cell line-derived neurotrophic factor (GDNF) mRNA and the secretion of GDNF by C6 cells, which supports the survival, proliferation, and activation of glioma cells. | [73,74,75,76,77] |
| Glutamate | High levels of metabotropic glutamate receptor class II (GluR1/GluR4) contribute to increased cell proliferation and migration, as well as heightened activation of the MAPK and PI3K pathways. The release of glutamate further promotes the growth of malignant gliomas. Overexpression of calcium-permeable AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate) receptors facilitates tumor cell migration and proliferation by activating the PI3K/AKT signaling pathway. Additionally, AMPA receptor activation promotes perivascular invasion through β1-integrin-dependent adhesion to the extracellular matrix, both in vitro and in vivo. | [78,79,80,81,82,83,84] |
| Dopamine | It regulates cell survival and proliferation. The activity of the D2 receptor, in conjunction with the epidermal growth factor receptor (EGFR), is linked to increased proliferation of spheroids enriched with cancer stem cells. | [85,86,87] |
| Norepinephrine | Activation of the β2-adrenergic receptor inhibits the proliferation of astrocytoma 1321N1 cells. Norepinephrine suppresses MMP-11, thereby inhibiting the migration and invasion of glioblastoma cells. However, other studies have shown that β2-adrenergic receptor activation induces phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK1/2), which can increase the expression of matrix metalloproteinases (MMPs) and promote the proliferation of U251 glioblastoma cell lines. | [87,88,89] |
| Gut microbiota metabolites | ||
| Tryptophan | Activates the aryl hydrocarbon receptor (AhR), which modulates the immune response and supports glioma cell survival. It also participates in the kynurenine pathway, contributing to immunosuppression and nicotinamide adenine dinucleotide (NAD+) metabolism, thereby promoting glioma growth. | [14,88,89,90] |
| Glutamine, glutamate | Increases neurotoxicity and supports glioma growth. Glutamate is metabolized into α-ketoglutarate (α-KG), linking it to the tricarboxylic acid (TCA) cycle. In gliomas, α-KG fuels the TCA cycle, maintains redox balance, and regulates epigenetic modifications essential for tumor proliferation and survival. | [91,92,93,94] |
| SCFA | Short-chain fatty acids (SCFAs) regulate inflammation and epigenetic pathways, shaping the glioma microenvironment and influencing its aggressiveness. They modulate the inflammatory cascade by inhibiting NF-κB and histone deacetylase pathways. A reduction in circulating SCFAs leads to a state of chronic stress, which influences tumor development through stress-related pathways. Butyrate induces Treg differentiation, while propionate inhibits glioma development and progression by promoting apoptosis and autophagy via peroxisome proliferator-activated receptor gamma (PPAR-γ) signaling, thereby counteracting tumorigenesis and slowing tumor growth. Both butyrate and propionate reduce VEGF levels and downregulate the PI3K/Akt/mTOR signaling pathway. In contrast, acetate affects acetyl-CoA production in glioma cells, leading to Rictor acetylation and the activation of mTORC2, which drives tumor proliferation. | [6,95,96,97,98,99] |
| LPSs | Lipopolysaccharides (LPSs) promote the migration and invasion of tumor cells by inducing the activation of the PI3K/Akt/mTOR pathway. LPSs can also over-activate Kirsten rat sarcoma virus (KRAS), contributing to carcinogenesis. Additionally, LPSs upregulate the expression of VEGFR by enhancing NF-κB activity, thereby promoting tumor angiogenesis. | [100,101] |
| Arginine | Arginine-derived metabolites in the body include polyamines and nitric oxide (NO). Polyamines promote the expression of ornithine decarboxylase, spermidine, spermine acetyltransferase, and serine/threonine kinase 1 (Akt1), driving tumor proliferation and metastasis. NO exhibits dual effects. It can induce tumor apoptosis through DNA and mitochondrial damage. However, elevated levels of NO inhibit NF-κB activity, which promotes angiogenesis and glioma growth. Additionally, NO acts as a factor that induces T cell apoptosis, contributing to immune suppression. | [102,103,104,105,106] |
| Bile acids (DCA, LCA, UDCA) | Deoxycholic acid (DCA) increases the activity of the VEGF and EGF pathways by activating their receptors and also stimulates the PI3K/Akt pathway. In contrast, ursodeoxycholic acid (UDCA) acts in the opposite way, partially inhibiting EGFR and promoting cancer cell apoptosis. UDCA contributes to decreased mitochondrial membrane potential, overproduction of reactive oxygen species (ROS), and endoplasmic reticulum stress. Lithocholic acid (LCA) activates the NF-κB pathway and induces ROS production, leading to oxidative DNA damage and inflammatory reactions. | [6,107] |
| TMAO | Trimethylamine N-oxide (TMAO) activates the NF-κB pathway and increases the production and secretion of VEGF from tumor cells, promoting angiogenesis and enhancing CD8+ T cell-dependent antitumor immunity. | [6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczyk, A.; Sladowska, G.E.; Strzalka-Mrozik, B. The Role of the Gut Microbiota in Modulating Signaling Pathways and Oxidative Stress in Glioma Therapies. Cancers 2025, 17, 719. https://doi.org/10.3390/cancers17050719
Krawczyk A, Sladowska GE, Strzalka-Mrozik B. The Role of the Gut Microbiota in Modulating Signaling Pathways and Oxidative Stress in Glioma Therapies. Cancers. 2025; 17(5):719. https://doi.org/10.3390/cancers17050719
Chicago/Turabian StyleKrawczyk, Aleksandra, Gabriela Elzbieta Sladowska, and Barbara Strzalka-Mrozik. 2025. "The Role of the Gut Microbiota in Modulating Signaling Pathways and Oxidative Stress in Glioma Therapies" Cancers 17, no. 5: 719. https://doi.org/10.3390/cancers17050719
APA StyleKrawczyk, A., Sladowska, G. E., & Strzalka-Mrozik, B. (2025). The Role of the Gut Microbiota in Modulating Signaling Pathways and Oxidative Stress in Glioma Therapies. Cancers, 17(5), 719. https://doi.org/10.3390/cancers17050719






