Lycorine hydrochloride Suppresses the Proliferation and Invasion of Esophageal Cancer by Targeting TRIM22 and Inhibiting the JAK2/STAT3 and Erk Pathways
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Reagents, and Treatments
2.2. Normal Esophageal Epithelial Tissue, Patient, and Esophageal Squamous Cancer Samples
2.3. Immunohistochemistry
2.4. Cell Proliferation and Apoptosis Assay
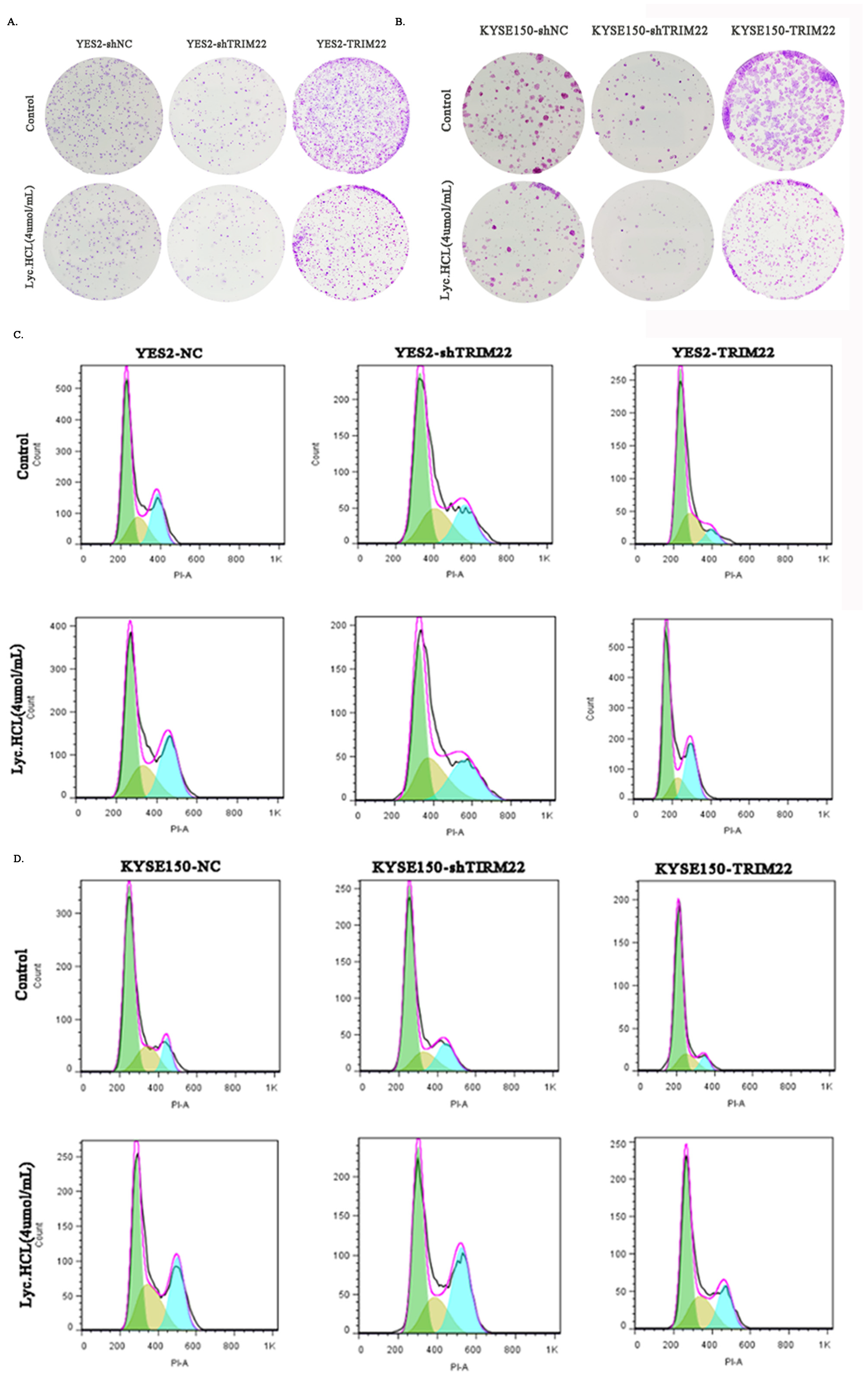
2.5. Colony Formation Assay
2.6. Cell Cycle Assay
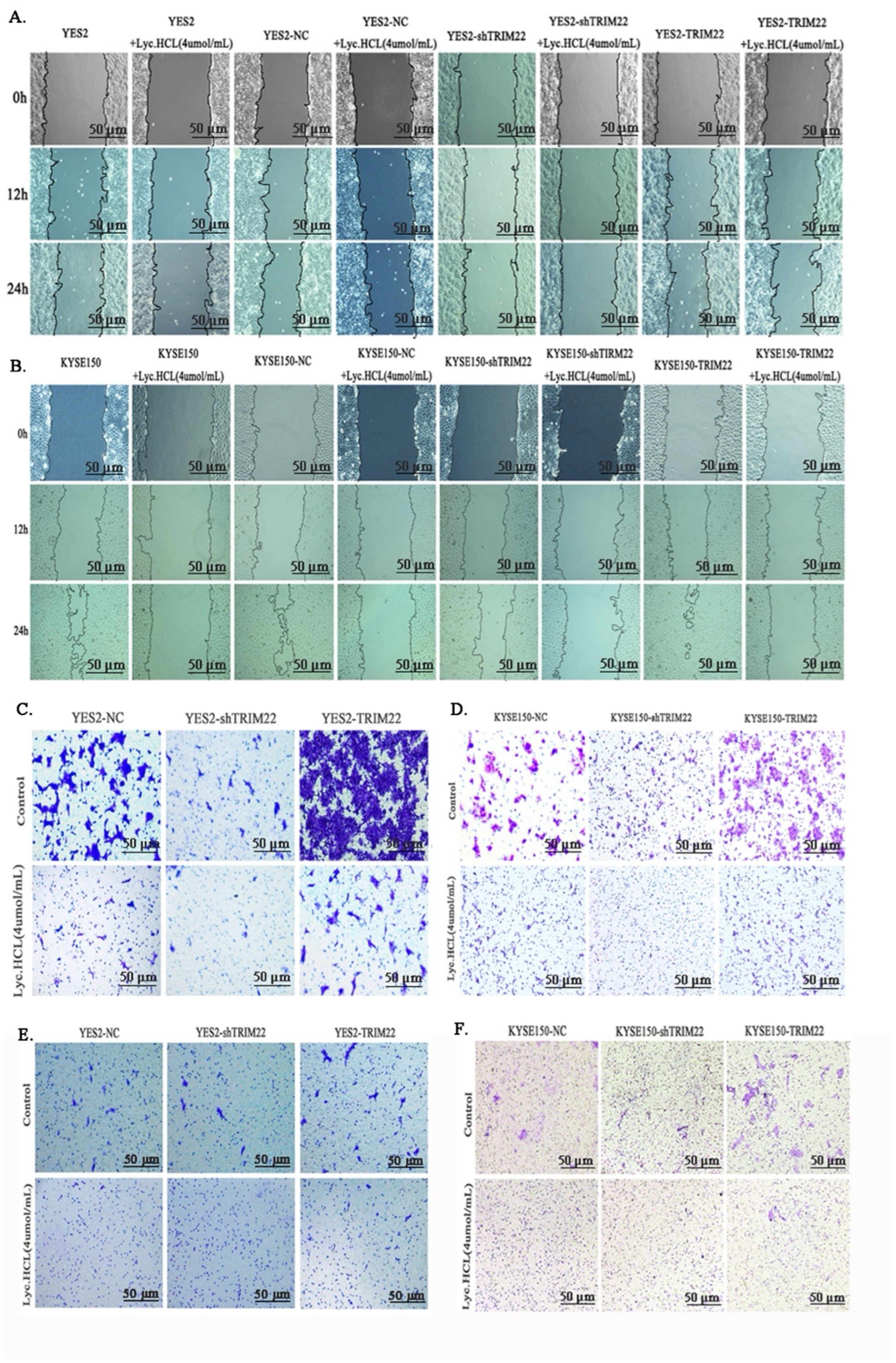
2.7. Cell Migration Assay
2.8. Transwell Migration Assays
2.9. Cell Invasion Assay
2.10. Lentiviral Infection, Transient Transfection, and Cas9/sgRNA KO
2.11. Docking Experiment
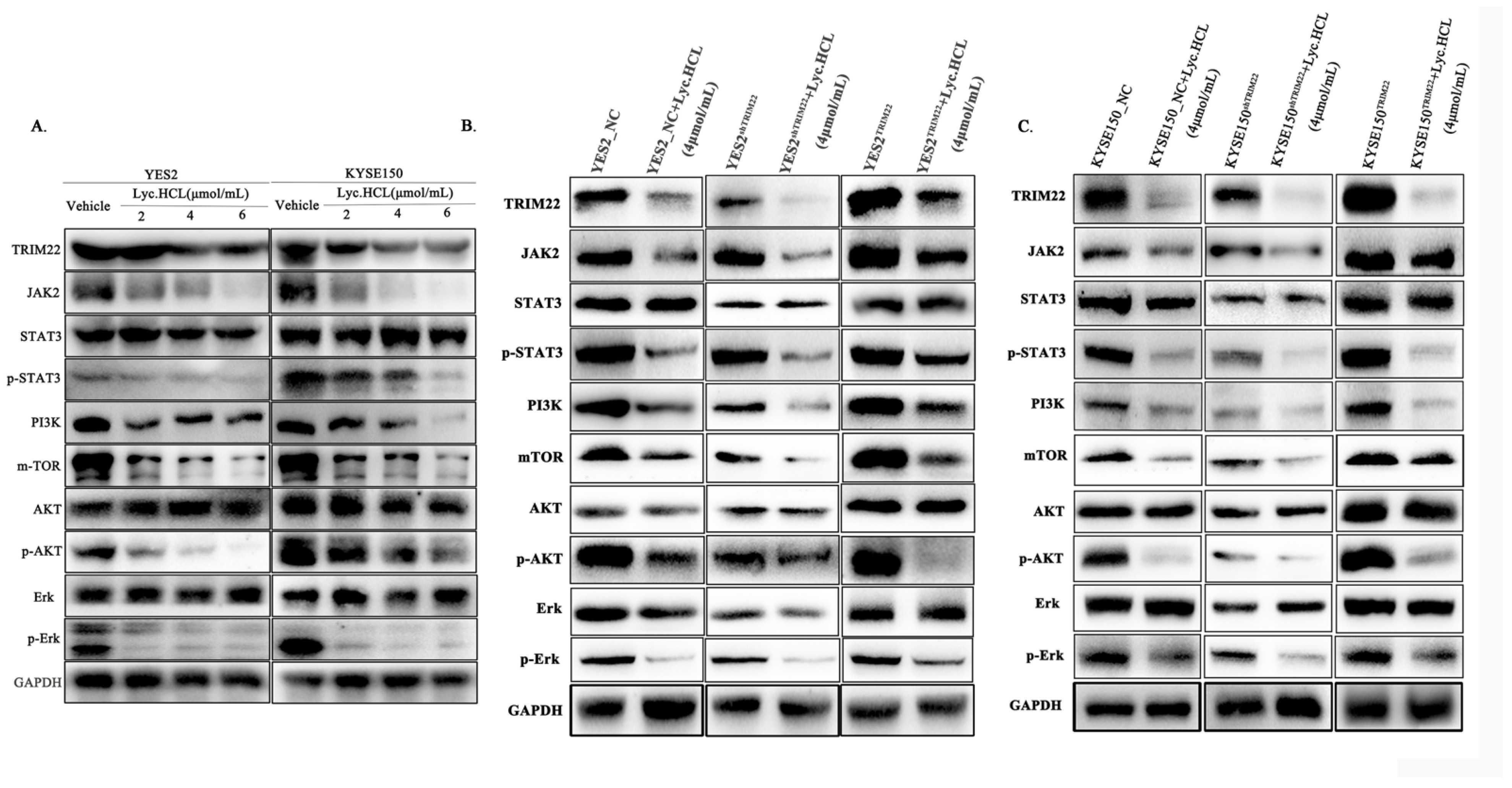
2.12. Western Blotting
2.13. Rescue Assays
2.14. Tumor Xenograft Experiments
2.15. Statistical Analysis
3. Results
3.1. TRIM22 Is Upregulated in ESCC Patient Tissues and Various Human ESCC Cell Lines (YES2, KYSE30, KYSE70, KYSE140, KYSE180, KYSE410, and KYSE450 Cells)
3.2. Lyc.HCL Inhibits the Proliferation of ESCC Cells
3.3. Lyc.HCL Induces Cell Cycle Arrest in ESCC Cells
3.4. Lyc.HCL Inhibits the Metastasis and Invasion of YES2 and KYSE150 Cells
3.5. Lyc.HCL Targets TRIM22 and Regulates Its Expression in YES2 and KYSE150 Cells
3.6. Lyc.HCL Exerts Its Anticancer Effect in ESCC Cells by Targeting TRIM22
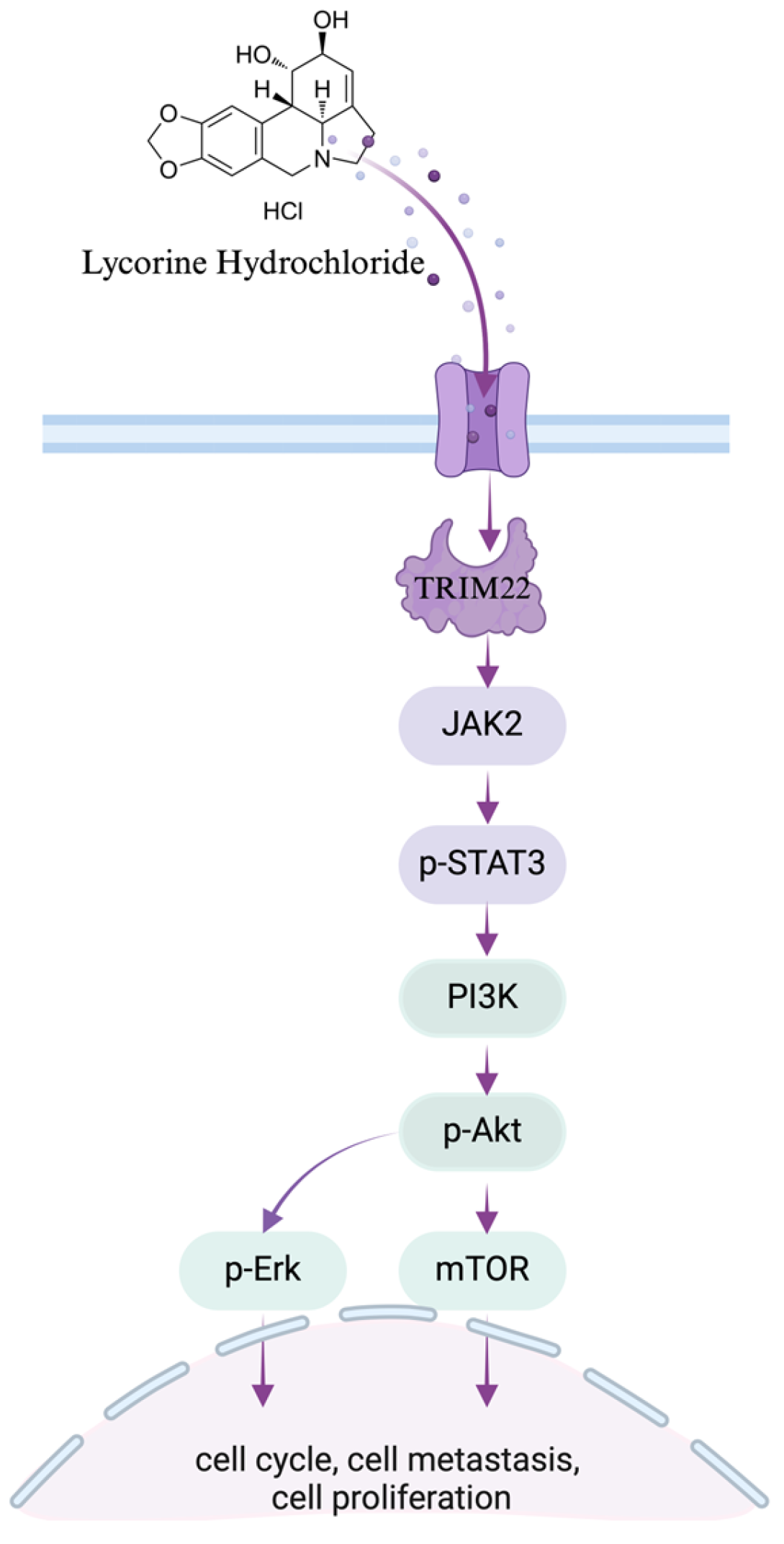
3.7. Lyc.HCL Targets TRIM22 and Has a Potential Anti-Esophageal Cancer Effect Through the Regulation of the JAK2/STAT3 and Erk Pathways
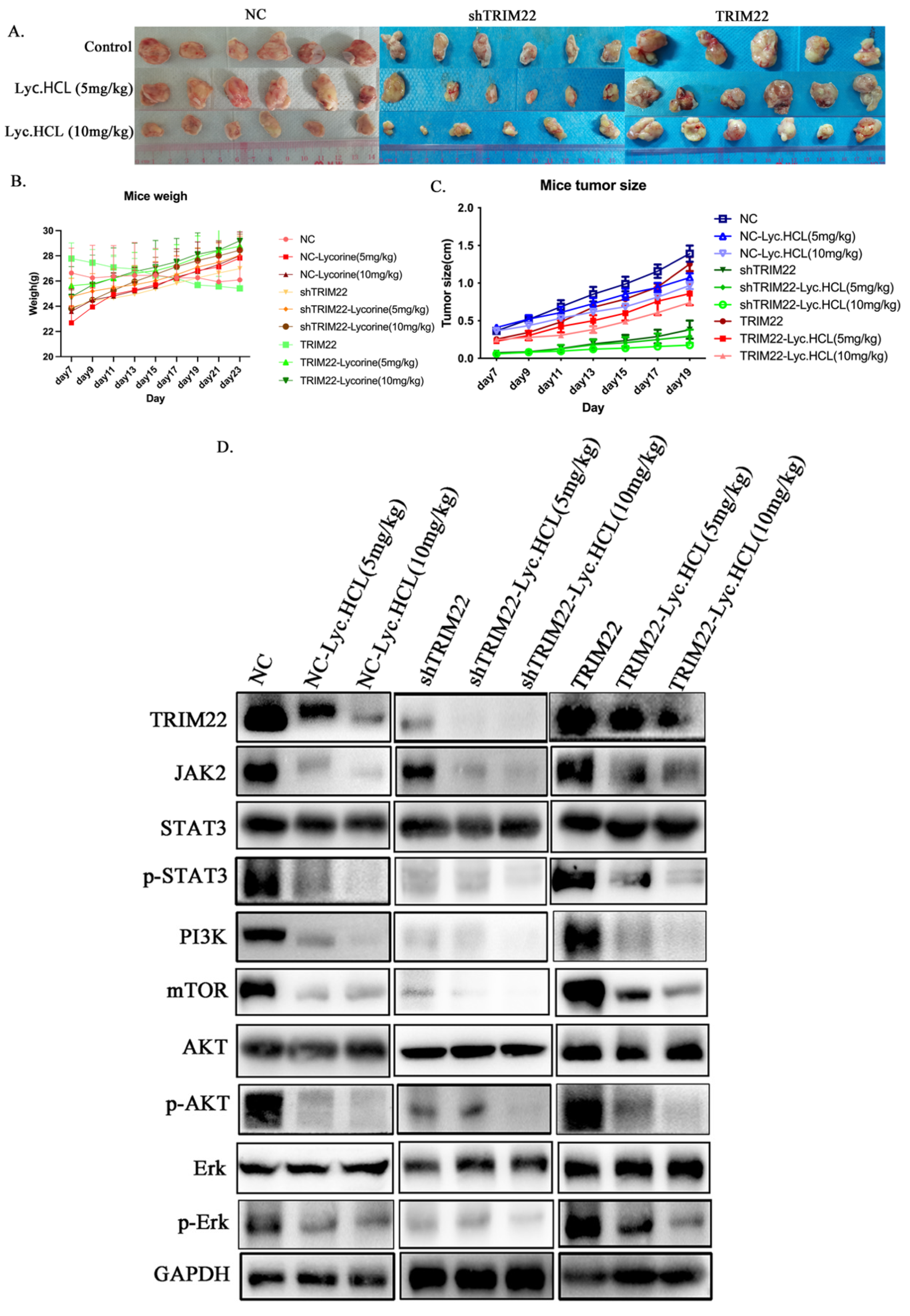
3.8. Lyc.HCL Alleviates Tumor Growth by Targeting TRIM22 In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESCC | esophageal squamous cell carcinoma |
| Lyc.HCL | lycorine hydrochloride |
| TRIM | tripartite motif-containing |
| TCM | traditional Chinese medicine |
| JAK2 | Janus kinase 2 |
| STAT3 | signal transducer and activator of transcription 3 |
| PI3K | phosphoinositide 3-kinase |
| AKT | protein kinase B |
| ERK | extracellular signal-regulated kinase |
| ECM | extracellular matrix |
| EMT | epithelial-to-mesenchymal transition |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bieerkehazhi, S.; Li, X.; Ma, L.; Yibulayin, W.; Ran, J. Survivin Regulates Bad Gene Expression by Binding to Its Promoter and Modulates Cell Cycle and Apoptosis in Esophageal Carcinoma Cell. J. Oncol. 2021, 2021, 1384289. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, W.; Wang, W.; Liang, Y.; Xue, Q.; Zhang, Z.; Yuan, J.; Fang, X. Demethylzeylasteral inhibits oxidative phosphorylation complex biogenesis by targeting LRPPRC in lung cancer. J. Cancer 2025, 16, 227–240. [Google Scholar] [CrossRef]
- Roy, M.; Liang, L.; Xiao, X.; Feng, P.; Ye, M.; Liu, J. Lycorine: A prospective natural lead for anticancer drug discovery. Biomed. Pharmacother. 2018, 107, 615–624. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Sun, X.; Zhang, Q.; Gong, T.; Zhang, Z. Mannosylated lipid nano-emulsions loaded with lycorine-oleic acid ionic complex for tumor cell-specific delivery. Theranostics 2012, 2, 1104–1114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.M.; Li, T.; Xu, C.C.; Qian, J.Y.; Guo, H.; Zhang, X.; Zhan, Z.J.; Lu, J.J. Uncover the anticancer potential of lycorine. Chin. Med. 2024, 19, 121. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Deng, C.; Pan, G.; Wang, X.; Zhang, K.; Dong, Z.; Zhao, G.; Tan, M.; Hu, X.; Shi, S.; et al. Lycorine hydrochloride inhibits cell proliferation and induces apoptosis through promoting FBXW7-MCL1 axis in gastric cancer. J. Exp. Clin. Cancer Res. CR 2020, 39, 230. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Qiu, Y.; Shao, Y.; Yin, S.; Wang, R.; Pang, X.; Ma, J.; Zhang, C.; Wu, B.; Koo, S.; et al. Lycorine Displays Potent Antitumor Efficacy in Colon Carcinoma by Targeting STAT3. Front. Pharmacol. 2018, 9, 881. [Google Scholar] [CrossRef]
- Zhao, F. Inhibition of SQLE or FDFT1 in RBE Cells by shRNA, or RBE Cells Treated with Lycorine Hydrochloride; NCBI Gene Expression Omnibus: Bethesda, MD, USA, 2023.
- Hu, N.; Clifford, R.J.; Yang, H.H.; Wang, C.; Goldstein, A.M.; Ding, T.; Taylor, P.R.; Lee, M.P. Genome wide analysis of DNA copy number neutral loss of heterozygosity (CNNLOH) and its relation to gene expression in esophageal squamous cell carcinoma. BMC Genom. 2010, 11, 576. [Google Scholar] [CrossRef]
- Huang, N.; Sun, X.; Li, P.; Liu, X.; Zhang, X.; Chen, Q.; Xin, H. TRIM family contribute to tumorigenesis, cancer development, and drug resistance. Exp. Hematol. Oncol. 2022, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Offermann, A.; Kang, D.; Watermann, C.; Weingart, A.; Hupe, M.C.; Saraji, A.; Stegmann-Frehse, J.; Kruper, R.; Schüle, R.; Pantel, K.; et al. Analysis of tripartite motif (TRIM) family gene expression in prostate cancer bone metastases. Carcinogenesis 2021, 42, 1475–1484. [Google Scholar] [CrossRef]
- Yanagi, T.; Watanabe, M.; Hata, H.; Kitamura, S.; Imafuku, K.; Yanagi, H.; Homma, A.; Wang, L.; Takahashi, H.; Shimizu, H.; et al. Loss of TRIM29 Alters Keratin Distribution to Promote Cell Invasion in Squamous Cell Carcinoma. Cancer Res. 2018, 78, 6795–6806. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Min, W.; Zeng, Y.; Fan, N.; Qian, Q. Aberrant KAT2A accumulations render TRIM22-low melanoma sensitive to Notch1 inhibitors via epigenetic reprogramming. J. Transl. Med. 2023, 21, 443. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Watanabe, M.; Kondo, T.; Hirano, S.; Hatakeyama, S. TRIM22 negatively regulates MHC-II expression. Biochimica et biophysica acta. Mol. Cell Res. 2022, 1869, 119318. [Google Scholar] [CrossRef]
- Fei, X.; Wu, X.; Dou, Y.N.; Sun, K.; Guo, Q.; Zhang, L.; Li, S.; Wei, J.; Huan, Y.; He, X.; et al. TRIM22 orchestrates the proliferation of GBMs and the benefits of TMZ by coordinating the modification and degradation of RIG-I. Mol. Ther. Oncolytics 2022, 26, 413–428. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Y.; Wang, G.; Feng, S.; Ge, X.; Ye, W.; Wang, Z.; Zhu, Y.; Cai, W.; Bai, J.; et al. TRIM22 inhibits osteosarcoma progression through destabilizing NRF2 and thus activation of ROS/AMPK/mTOR/autophagy signaling. Redox Biol. 2022, 53, 102344. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zeng, T. Sporoderm-Removed Ganoderma lucidum Spore Powder May Suppress the Proliferation, Migration, and Invasion of Esophageal Squamous Cell Carcinoma Cells Through PI3K/AKT/mTOR and Erk Pathway. Integr. Cancer Ther. 2021, 20, 15347354211062157. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.N.; Woods, M.W.; Xhiku, S.; Barr, S.D. Ancient and recent adaptive evolution in the antiviral TRIM22 gene: Identification of a single-nucleotide polymorphism that impacts TRIM22 function. Hum. Mutat. 2014, 35, 1072–1081. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.M.; Yang, F.F.; Miao, Y.; Yin, Y.; Hu, X.J.; Hou, G.; Wang, Q.Y.; Kang, J. TRIM22 confers poor prognosis and promotes epithelial-mesenchymal transition through regulation of AKT/GSK3β/β-catenin signaling in non-small cell lung cancer. Oncotarget 2017, 8, 62069–62080. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qi, Y.; Ma, X.; Xiong, G.; Wang, L.; Bao, C. TRIM22 knockdown suppresses chronic myeloid leukemia via inhibiting PI3K/Akt/mTOR signaling pathway. Cell Biol. Int. 2018, 42, 1192–1199. [Google Scholar] [CrossRef]
- He, T.; Cui, J.; Wu, Y.; Sun, X.; Chen, N. Knockdown of TRIM66 inhibits cell proliferation, migration and invasion in colorectal cancer through JAK2/STAT3 pathway. Life Sci. 2019, 235, 116799. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Lin, Y.; Quan, Y.; Xiao, X.; Zhang, R. TRIM22 inhibits respiratory syncytial virus replication by targeting JAK-STAT1/2 signaling. J. Med. Virol. 2021, 93, 3412–3419. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Q.; Li, X.; Jin, Z.; Xu, P.; Xu, N.; Xu, A.; Xu, Y.; Zheng, S.; Zheng, J.; et al. Lycorine induces apoptosis of bladder cancer T24 cells by inhibiting phospho-Akt and activating the intrinsic apoptotic cascade. Biochem. Biophys. Res. Commun. 2017, 483, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yu, D.; Fu, S.; Zhang, G.; Pan, Y.; Bao, M.; Tu, J.; Shang, B.; Guo, P.; Yang, P.; et al. Lycorine hydrochloride selectively inhibits human ovarian cancer cell proliferation and tumor neovascularization with very low toxicity. Toxicol. Lett. 2013, 218, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Soerjomataram, I.; Rumgay, H.; Coleman, H.G.; Thrift, A.P.; Vignat, J.; Laversanne, M.; Ferlay, J.; Arnold, M. The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates from GLOBOCAN 2020. Gastroenterology 2022, 163, 649–658.e2. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yang, P.; Zhou, Q. Multiple biological functions and pharmacological effects of lycorine. Sci. China Chem. 2013, 56, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Aljabban, J.; Syed, S.; Syed, S.; Rohr, M.; Weisleder, N.; McElhanon, K.E.; Hasan, L.; Safeer, L.; Hoffman, K.; Aljabban, N.; et al. Investigating genetic drivers of dermatomyositis pathogenesis using meta-analysis. Heliyon 2020, 6, e04866. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qian, C.; Zhang, W.; Shi, M.; Chen, X.; Wang, Y.; Lin, F. Ginkgetin suppresses ovarian cancer growth through inhibition of JAK2/STAT3 and MAPKs signaling pathways. Phytomed. Int. J. Phytother. Phytopharm. 2023, 116, 154846. [Google Scholar] [CrossRef] [PubMed]
- Hattlmann, C.J.; Kelly, J.N.; Barr, S.D. TRIM22: A Diverse and Dynamic Antiviral Protein. Mol. Biol. Int. 2012, 2012, 153415. [Google Scholar] [CrossRef]
- Ji, J.; Ding, K.; Luo, T.; Zhang, X.; Chen, A.; Zhang, D.; Li, G.; Thorsen, F.; Huang, B.; Li, X.; et al. TRIM22 activates NF-κB signaling in glioblastoma by accelerating the degradation of IκBα. Cell Death Differ. 2021, 28, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Peng, Y.; Wei, W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022, 32, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Xue, K.; Li, L.; Zhu, K.; Fu, R.; Xiong, Z. RNF122 promotes glioblastoma growth via the JAK2/STAT3/c-Myc signaling Axis. CNS Neurosci. Ther. 2024, 30, e70017. [Google Scholar] [CrossRef]
- Zhu, M.; Peng, Y.; Qi, Q.; Zhang, Y.; Han, W.; Bao, Y.; Liu, Y. Mechanistic study of Nidus Vespae inhibiting gastric cancer in vitro through the JAK2/STAT3 signaling pathway. J. Ethnopharmacol. 2025, 338 Pt 1, 119027. [Google Scholar] [CrossRef] [PubMed]
- Steelman, L.S.; Pohnert, S.C.; Shelton, J.G.; Franklin, R.A.; Bertrand, F.E.; McCubrey, J.A. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia 2004, 18, 189–218. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, J.; Cai, Y.; Fu, S.; Zhang, N.; Fu, X.; Li, L. IFN-γ-mediated inhibition of lung cancer correlates with PD-L1 expression and is regulated by PI3K-AKT signaling. Int. J. Cancer 2018, 143, 931–943. [Google Scholar] [CrossRef]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells 2021, 10, 2509. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Yao, Y.; Xu, Y.; Zou, M.; Zhou, M.; Abudushalamu, G.; Chen, Y.; Cai, S.; Zhang, C.; Wu, G. Comprehensive evaluation of serum circHAS2 as a novel diagnostic and prognostic biomarker for gastric cancer. Mol. Carcinog. 2024, 63, 94–105. [Google Scholar] [CrossRef]
- Prajapati, K.S.; Kumar, S. Loss of miR-6844 alters stemness/self-renewal and cancer hallmark(s) markers through CD44-JAK2-STAT3 signaling axis in breast cancer stem-like cells. J. Cell. Biochem. 2023, 124, 1186–1202. [Google Scholar] [CrossRef] [PubMed]
- Limbu, K.R.; Chhetri, R.B.; Oh, Y.S.; Baek, D.J.; Park, E.Y. Mebendazole Impedes the Proliferation and Migration of Pancreatic Cancer Cells through SK1 Inhibition Dependent Pathway. Molecules 2022, 27, 8127. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, T.; Wu, C.; Shen, D.; Liu, L.; Chen, X.; Guan, Y.; Ding, H.; Tong, X. TRIM28 recruits E2F1 to regulate CBX8-mediated cell proliferation and tumor metastasis of ovarian cancer. Hum. Cell 2023, 36, 2113–2128. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, Y.; Wang, X.; Wu, Y.; Mao, J.; Li, Q.; Gong, S. THBS1-Mediated Degradation of Collagen via the PI3K/AKT Pathway Facilitates the Metastasis and Poor Prognosis of OSCC. Int. J. Mol. Sci. 2023, 24, 13312. [Google Scholar] [CrossRef]
- Xin, H.; Tang, Y.; Jin, Y.H.; Li, H.L.; Tian, Y.; Yu, C.; Zhao, Z.J.; Wu, M.S.; Pan, Y.F. Knockdown of LMNA inhibits Akt/β-catenin-mediated cell invasion and migration in clear cell renal cell carcinoma cells. Cell Adhes. Migr. 2023, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Hou, Y.; Fu, L.; Xi, L.; Yang, D.; Zhao, M.; Qin, Y.; Sun, K.; Teng, Y.; Liu, M. Cancer-associated fibroblast (CAF)-derived IL32 promotes breast cancer cell invasion and metastasis via integrin β3-p38 MAPK signaling. Cancer Lett. 2019, 442, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, Y.; Duan, L.; Hu, X.; Zhang, X.; Hu, J.; Huang, L.; He, R.; Hu, Z.; Luo, W.; et al. AKR1B10 promotes breast cancer cell migration and invasion via activation of ERK signaling. Oncotarget 2017, 8, 33694–33703. [Google Scholar] [CrossRef]
- Li, Q.T.; Feng, Y.M.; Ke, Z.H.; Qiu, M.J.; He, X.X.; Wang, M.M.; Li, Y.N.; Xu, J.; Shi, L.L.; Xiong, Z.F. KCNN4 promotes invasion and metastasis through the MAPK/ERK pathway in hepatocellular carcinoma. J. Investig. Med. 2020, 68, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, M.; Huang, J.; Zhang, F.; Lv, Z.; Jia, Y.; Cui, Y.Z.; Sun, L.Z.; Wang, Y.; Tang, Y.; et al. ORAI2 Promotes Gastric Cancer Tumorigenicity and Metastasis through PI3K/Akt Signaling and MAPK-Dependent Focal Adhesion Disassembly. Cancer Res. 2021, 81, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Fang, N.; Li, Y.; Guo, Z.; Jiang, W.; He, Y.; Ma, Z.; Chen, Y. Circular RNA LPAR3 sponge’s microRNA-198 to facilitate esophageal cancer migration, invasion, and metastasis. Cancer Sci. 2020, 111, 2824–2836. [Google Scholar] [CrossRef]
- Chen, L.; Guo, P.; He, Y.; Chen, Z.; Chen, L.; Luo, Y.; Qi, L.; Liu, Y.; Wu, Q.; Cui, Y.; et al. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signaling pathway. Cell Death Dis. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; Van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S.; et al. Identification of the tumour transition states occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef]
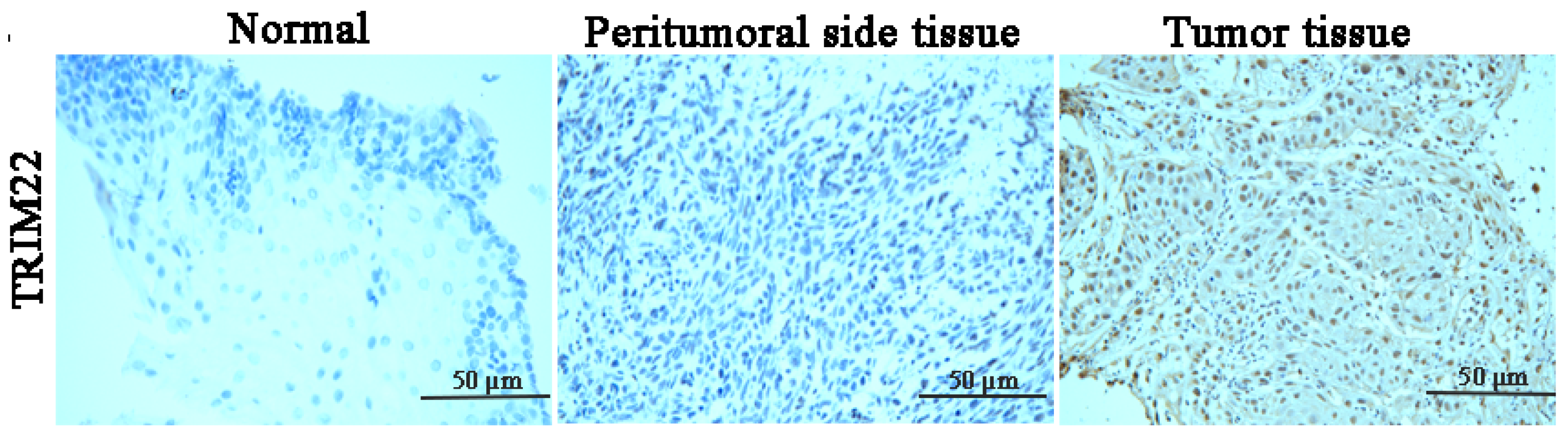
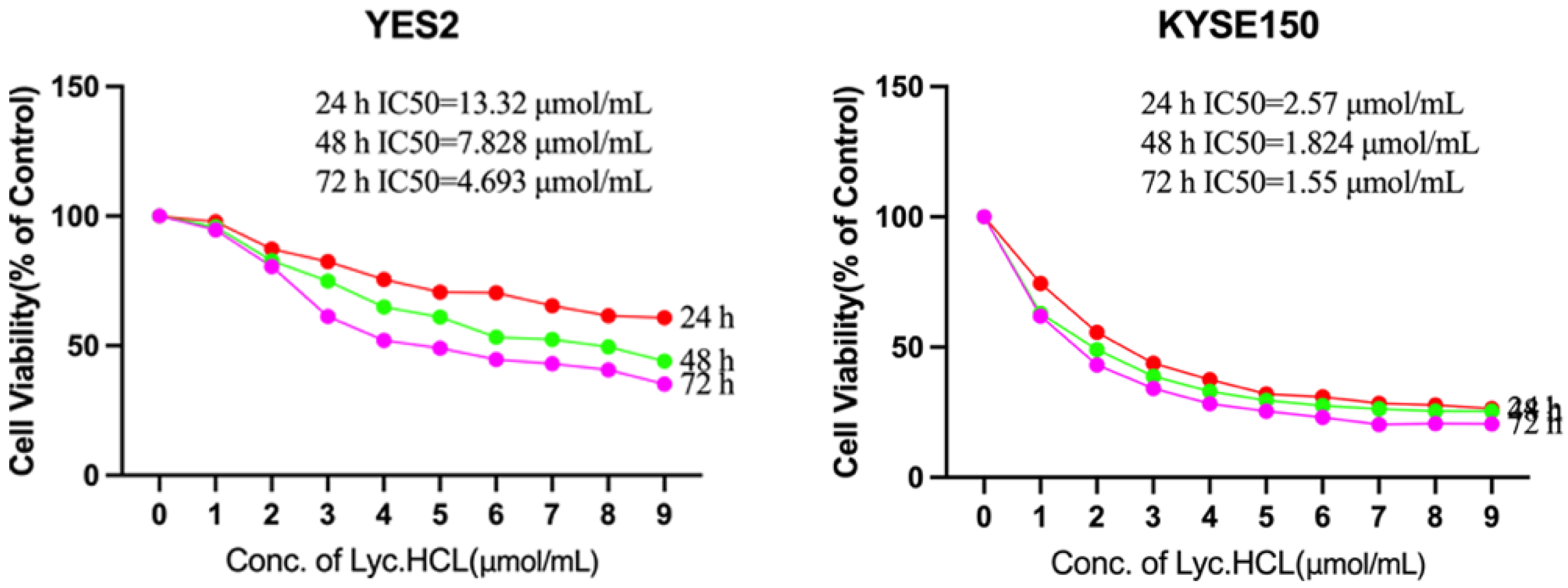

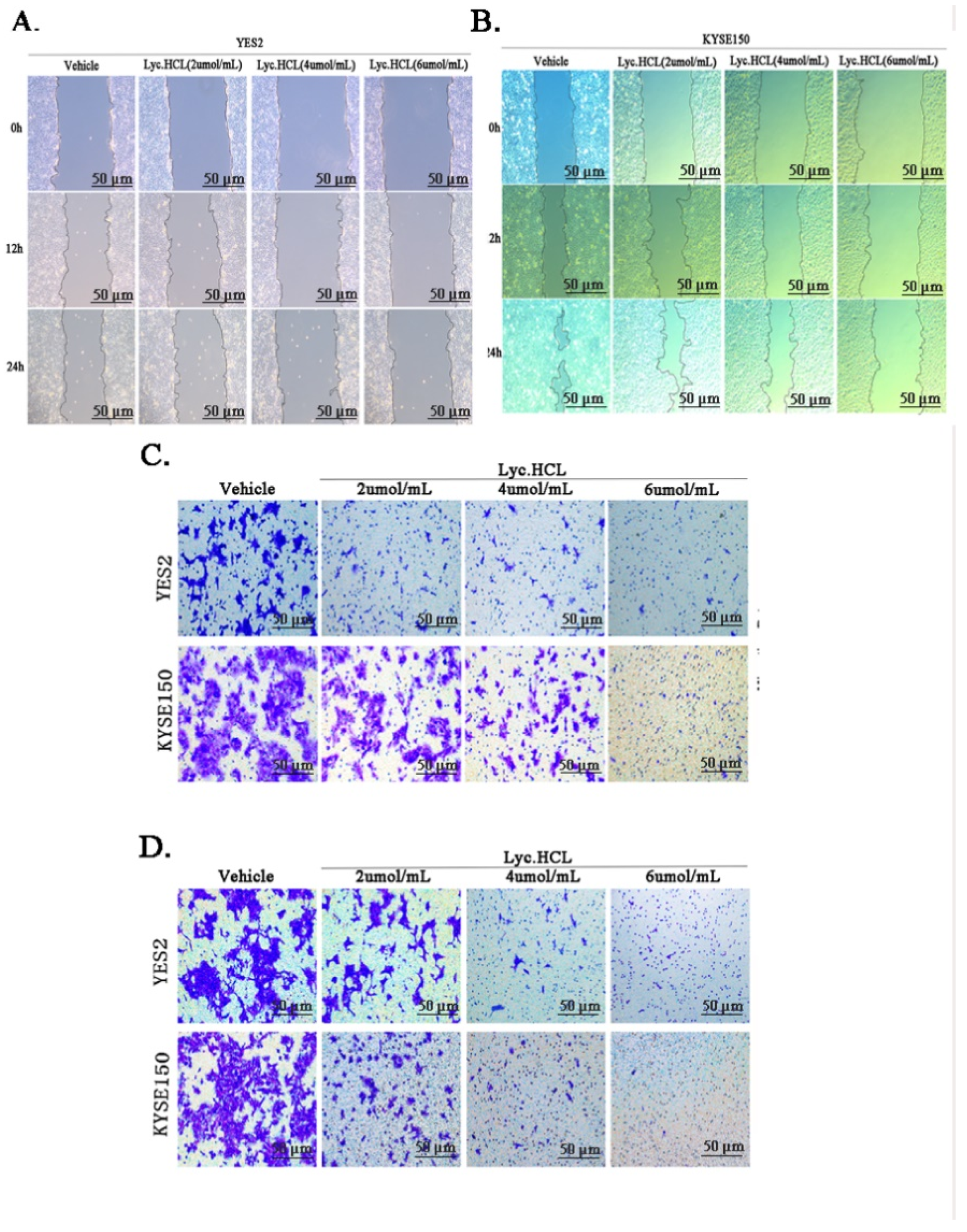
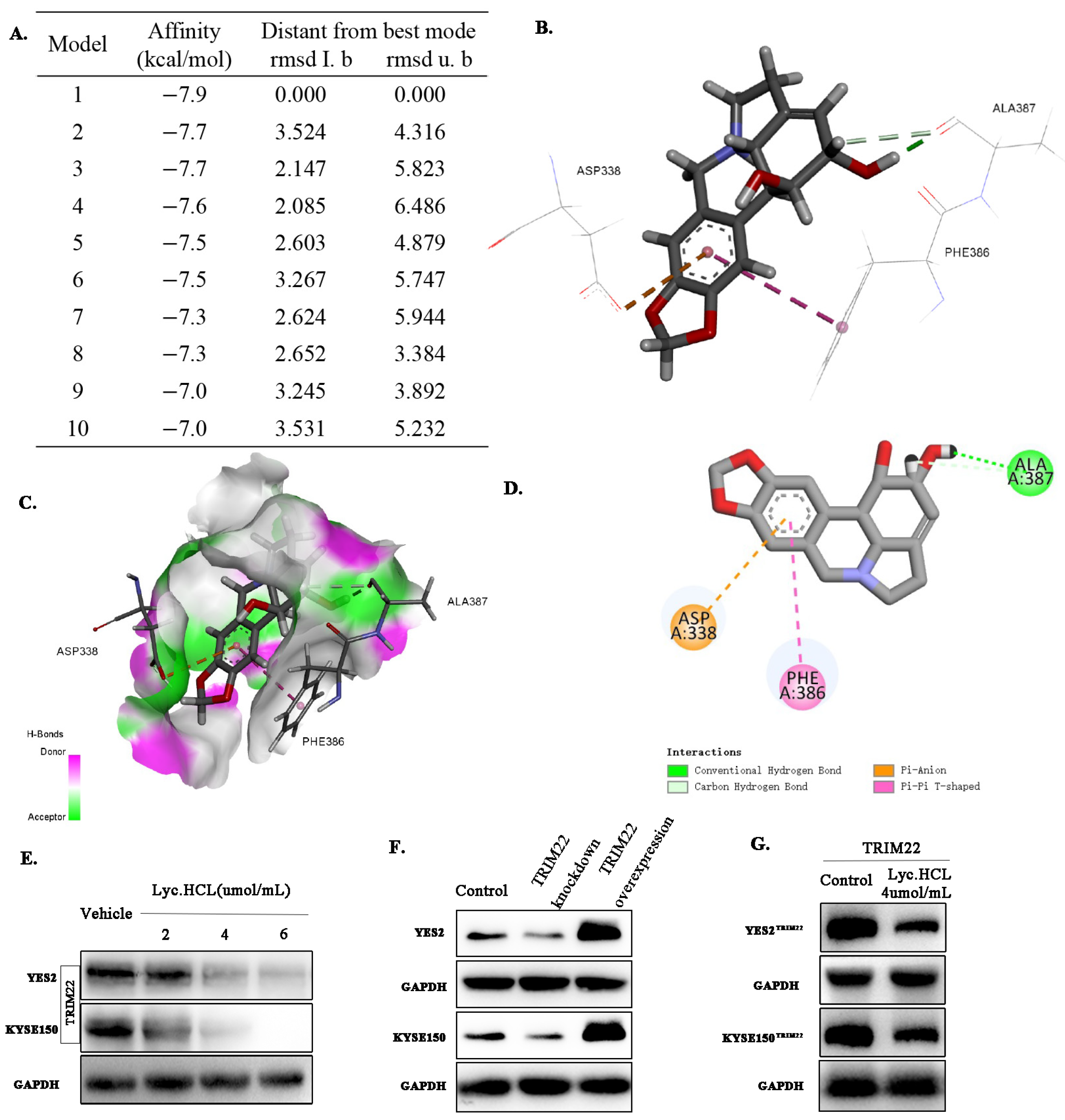

| Tissue No. | Normal Tissue | Peritumoral Tissue | ESCC Tissue |
|---|---|---|---|
| 1 | 0 | 0 | 1556 |
| 2 | 0 | 0 | 1507 |
| 3 | 0 | 0 | 1687 |
| 4 | 0 | 0 | 3657 |
| 5 | 0 | 0 | 3676 |
| 6 | 0 | 0 | 2031 |
| 7 | 0 | 0 | 2240 |
| 8 | 0 | 0 | 1786 |
| 9 | 0 | 0 | 1099 |
| 10 | 0 | 0 | 2345 |
| 11 | 0 | 0 | 1455 |
| 12 | 0 | 0 | 2345 |
| 13 | 0 | 0 | 1596 |
| 14 | 0 | 0 | 2329 |
| 15 | 0 | 0 | 2020 |
| 16 | 0 | 0 | 1945 |
| 17 | 0 | 0 | 1425 |
| 18 | 0 | 0 | 1709 |
| 19 | 0 | 0 | 2063 |
| 20 | 0 | 0 | 1113 |
| 21 | 0 | 0 | 1162 |
| 22 | 0 | 0 | 1092 |
| 23 | 0 | 0 | 1927 |
| 24 | 0 | 0 | 1634 |
| 25 | 0 | 0 | 1552 |
| 26 | 0 | 0 | 3374 |
| 27 | 0 | 0 | 3068 |
| 28 | 0 | 0 | 2813 |
| 29 | 0 | 0 | 1552 |
| 30 | 0 | 0 | 1142 |
| 31 | 0 | 0 | 1750 |
| 32 | 0 | 0 | 1414 |
| 33 | 0 | 9 | 1213 |
| 34 | 0 | 0 | 1709 |
| 35 | 0 | 0 | 1786 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Qiu, L.; Chen, J.; Zeng, T. Lycorine hydrochloride Suppresses the Proliferation and Invasion of Esophageal Cancer by Targeting TRIM22 and Inhibiting the JAK2/STAT3 and Erk Pathways. Cancers 2025, 17, 718. https://doi.org/10.3390/cancers17050718
Liu J, Qiu L, Chen J, Zeng T. Lycorine hydrochloride Suppresses the Proliferation and Invasion of Esophageal Cancer by Targeting TRIM22 and Inhibiting the JAK2/STAT3 and Erk Pathways. Cancers. 2025; 17(5):718. https://doi.org/10.3390/cancers17050718
Chicago/Turabian StyleLiu, Jingyan, Liangxian Qiu, Jialing Chen, and Tao Zeng. 2025. "Lycorine hydrochloride Suppresses the Proliferation and Invasion of Esophageal Cancer by Targeting TRIM22 and Inhibiting the JAK2/STAT3 and Erk Pathways" Cancers 17, no. 5: 718. https://doi.org/10.3390/cancers17050718
APA StyleLiu, J., Qiu, L., Chen, J., & Zeng, T. (2025). Lycorine hydrochloride Suppresses the Proliferation and Invasion of Esophageal Cancer by Targeting TRIM22 and Inhibiting the JAK2/STAT3 and Erk Pathways. Cancers, 17(5), 718. https://doi.org/10.3390/cancers17050718






