The Impact of Progesterone Receptor Status on Survival Outcomes in Metastatic Breast Cancer Patients Treated with First-Line CDK4/6 Inhibitors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
2.3. Efficacy and Safety Measures
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
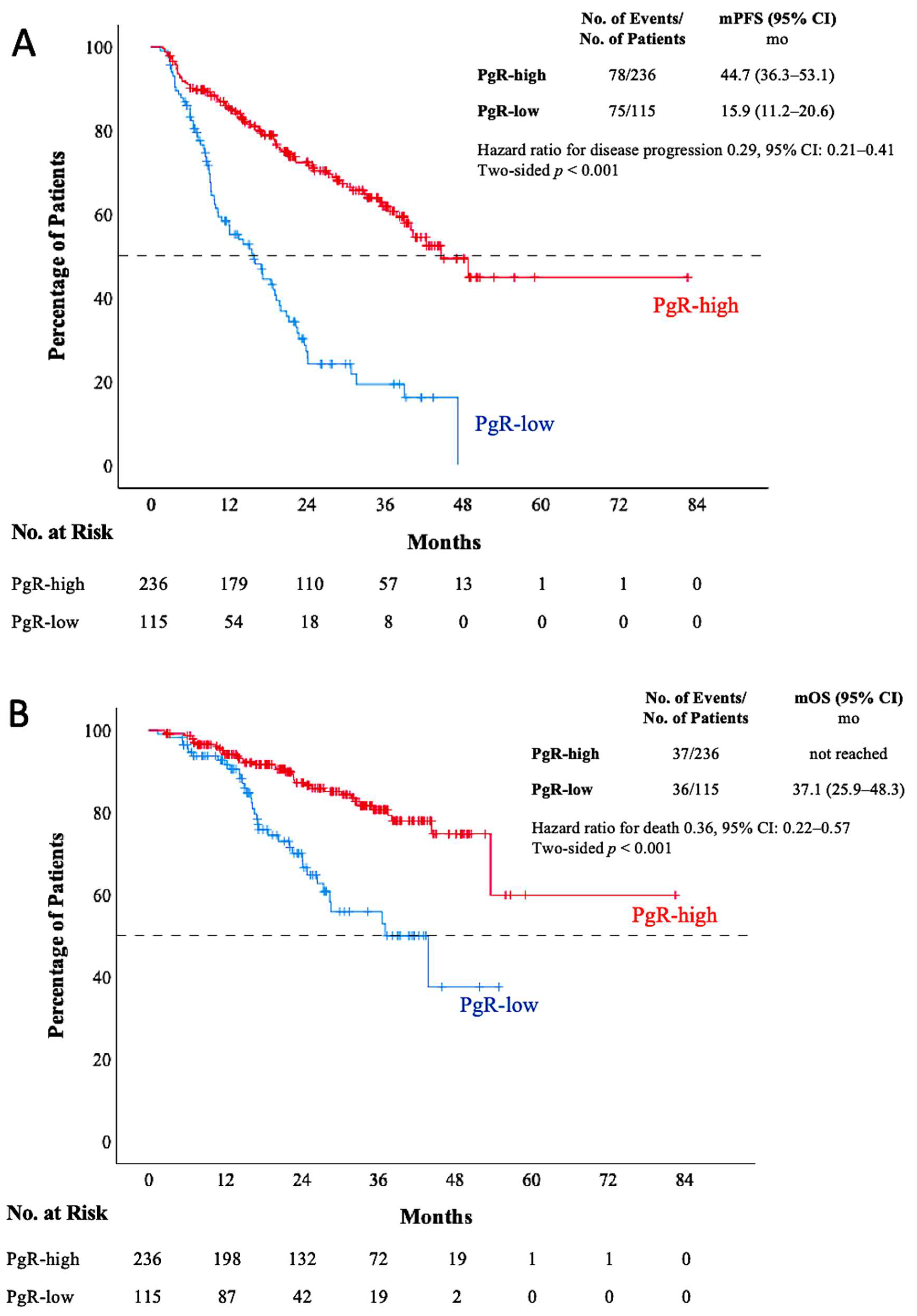
3.2. Survival Outcomes
3.3. Treatment Interventions
3.4. Treatment-Related Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Bailey, J.; Burstein, H.J.; et al. Breast Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.; Greene, H. Hormone Receptor-Positive, HER2-Negative Breast Cancer: Recent Advances and Best Practices. J. Adv. Pract. Oncol. 2020, 11, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of Chemotherapy and Hormonal Therapy for Early Breast Cancer on Recurrence and 15-Year Survival: An Overview of the Randomised Trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- Morrison, L.; Loibl, S.; Turner, N.C. The CDK4/6 Inhibitor Revolution—A Game-Changing Era for Breast Cancer Treatment. Nat. Rev. Clin. Oncol. 2024, 21, 89–105. [Google Scholar] [CrossRef]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.-A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 Final PFS: A Randomized Study of Abemaciclib as Initial Therapy for Advanced Breast Cancer. NPJ Breast Cancer 2019, 5, 5. [Google Scholar] [CrossRef]
- Rugo, H.S.; Finn, R.S.; Gelmon, K.; Joy, A.A.; Harbeck, N.; Castrellon, A.; Mukai, H.; Walshe, J.M.; Mori, A.; Gauthier, E.; et al. Progression-Free Survival Outcome Is Independent of Objective Response in Patients With Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer Treated With Palbociclib Plus Letrozole Compared With Letrozole: Analysis From PALOMA-2. Clin. Breast Cancer 2020, 20, e173–e180. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.-A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus Endocrine Therapy for Premenopausal Women with Hormone-Receptor-Positive, Advanced Breast Cancer (MONALEESA-7): A Randomised Phase 3 Trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus Palbociclib versus Fulvestrant plus Placebo for Treatment of Hormone-Receptor-Positive, HER2-Negative Metastatic Breast Cancer That Progressed on Previous Endocrine Therapy (PALOMA-3): Final Analysis of the Multicentre, Double-Blind, Phase 3 Randomised Controlled Trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Jerusalem, G.; De Laurentiis, M.; Im, S.; Petrakova, K.; Valeria Bianchi, G.; Martín, M.; Nusch, A.; et al. Ribociclib plus Fulvestrant for Postmenopausal Women with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer in the Phase III Randomized MONALEESA-3 Trial: Updated Overall Survival. Ann. Oncol. 2021, 32, 1015–1024. [Google Scholar] [CrossRef]
- Mohammed, H.; Russell, I.A.; Stark, R.; Rueda, O.M.; Hickey, T.E.; Tarulli, G.A.; Serandour, A.A.; Birrell, S.N.; Bruna, A.; Saadi, A.; et al. Correction: Corrigendum: Progesterone Receptor Modulates ERα Action in Breast Cancer. Nature 2015, 526, 144. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, H.; Li, S.; Wu, P.; Mao, X. The Role of Progesterone Receptors in Breast Cancer. Drug Des. Devel Ther. 2022, 16, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Nordenskjöld, A.; Fohlin, H.; Fornander, T.; Löfdahl, B.; Skoog, L.; Stål, O. Progesterone Receptor Positivity Is a Predictor of Long-Term Benefit from Adjuvant Tamoxifen Treatment of Estrogen Receptor Positive Breast Cancer. Breast Cancer Res. Treat. 2016, 160, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Purdie, C.A.; Quinlan, P.; Jordan, L.B.; Ashfield, A.; Ogston, S.; Dewar, J.A.; Thompson, A.M. Progesterone Receptor Expression Is an Independent Prognostic Variable in Early Breast Cancer: A Population-Based Study. Br. J. Cancer 2014, 110, 565–572. [Google Scholar] [CrossRef]
- Stendahl, M.; Rydén, L.; Nordenskjöld, B.; Jönsson, P.E.; Landberg, G.; Jirström, K. High Progesterone Receptor Expression Correlates to the Effect of Adjuvant Tamoxifen in Premenopausal Breast Cancer Patients. Clin. Cancer Res. 2006, 12, 4614–4618. [Google Scholar] [CrossRef]
- Ahn, J.H.; Choi, S.B.; Park, J.M.; Kim, J.Y.; Park, H.S.; Kim, S.I.; Park, B.-W.; Park, S. Level of Combined Estrogen and Progesterone Receptor Expression Determines the Eligibility for Adjuvant Endocrine Therapy in Breast Cancer Patients. Cancers 2021, 13, 5007. [Google Scholar] [CrossRef]
- Anderson, H.; Hills, M.; Zabaglo, L.; A’Hern, R.; Leary, A.F.; Haynes, B.P.; Smith, I.E.; Dowsett, M. Relationship between Estrogen Receptor, Progesterone Receptor, HER-2 and Ki67 Expression and Efficacy of Aromatase Inhibitors in Advanced Breast Cancer. Ann. Oncol. 2011, 22, 1770–1776. [Google Scholar] [CrossRef]
- Rocca, A.; Farolfi, A.; Maltoni, R.; Carretta, E.; Melegari, E.; Ferrario, C.; Cecconetto, L.; Sarti, S.; Schirone, A.; Fedeli, A.; et al. Efficacy of Endocrine Therapy in Relation to Progesterone Receptor and Ki67 Expression in Advanced Breast Cancer. Breast Cancer Res. Treat. 2015, 152, 57–65. [Google Scholar] [CrossRef]
- Gao, J.J.; Cheng, J.; Bloomquist, E.; Sanchez, J.; Wedam, S.B.; Singh, H.; Amiri-Kordestani, L.; Ibrahim, A.; Sridhara, R.; Goldberg, K.B.; et al. CDK4/6 Inhibitor Treatment for Patients with Hormone Receptor-Positive, HER2-Negative, Advanced or Metastatic Breast Cancer: A US Food and Drug Administration Pooled Analysis. Lancet Oncol. 2020, 21, 250–260. [Google Scholar] [CrossRef]
- Jia, L.; Peng, J.; Sun, N.; Chen, H.; Liu, Z.; Zhao, W.; Zhang, Q.; Li, L. Effect of PR Status on the Prognosis of Advanced ER-High HER2-Negative Breast Cancer Patients Receiving CDK4/6 Inhibitor Combined with Endocrine as First-Line Therapy. BMC Cancer 2024, 24, 850. [Google Scholar] [CrossRef]
- Shao, X.; Zheng, Y.; Cao, W.; Shen, X.; Li, G.; Chen, J.; Huang, Y.; Huang, P.; Shi, L.; Ye, W.; et al. Ki67 and Progesterone Receptor Status Predicts Sensitivity to Palbociclib: A Real-World Study. Ann. Transl. Med. 2021, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, L.N.; Teso, B.; Paco, P.F.; De la Haba Rodriguez, J.; Armenta, A.; Mora, F.; Alonso, B.R.; Estevez, C.M.; Quintela, I.P.; Mauriño, P.S.; et al. 432P Ki67 and Progesterone Receptor Status Could Be Predict Sensitivity to Cyclin-Dependent Kinase Inhibitor. Ann. Oncol. 2023, 34, S363–S364. [Google Scholar] [CrossRef]
- Palleschi, M.; Barzotti, E.; Melegari, E.; Manunta, S.; Mannozzi, F.; Vagheggini, A.; Maltoni, R.; Fedeli, A.; Sarti, S.; Cecconetto, L.; et al. Impact of Ki67 and Progesterone Receptor on PFS with Cyclin-Dependent Kinase 4/6 Inhibitors in HER2-Negative Advanced Breast Cancer: A Real World Mono-Institutional Experience. Eur. J. Cancer 2020, 138, S66–S67. [Google Scholar] [CrossRef]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; McShane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO–College of American Pathologists Guideline Update. J. Clin. Oncol. 2023, 41, 3867–3872. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the Treatment of Women with Early Breast Cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. CTCAE Versión 5.0. Evaluación de La Gravedad de Los Eventos Adversos Dermatológicos de Las Terapias Antineoplásicas. Actas Dermosifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef]
- Roberto, M.; Astone, A.; Botticelli, A.; Carbognin, L.; Cassano, A.; D’Auria, G.; Fabbri, A.; Fabi, A.; Gamucci, T.; Krasniqi, E.; et al. CDK4/6 Inhibitor Treatments in Patients with Hormone Receptor Positive, Her2 Negative Advanced Breast Cancer: Potential Molecular Mechanisms, Clinical Implications and Future Perspectives. Cancers 2021, 13, 332. [Google Scholar] [CrossRef]
- Dunnwald, L.K.; Rossing, M.A.; Li, C.I. Hormone Receptor Status, Tumor Characteristics, and Prognosis: A Prospective Cohort of Breast Cancer Patients. Breast Cancer Res. 2007, 9, R6. [Google Scholar] [CrossRef]
- Dauphine, C.; Moazzez, A.; Neal, J.C.; Chlebowski, R.T.; Ozao-Choy, J. Single Hormone Receptor-Positive Breast Cancers Have Distinct Characteristics and Survival. Ann. Surg. Oncol. 2020, 27, 4687–4694. [Google Scholar] [CrossRef] [PubMed]
- Kurozumi, S.; Matsumoto, H.; Hayashi, Y.; Tozuka, K.; Inoue, K.; Horiguchi, J.; Takeyoshi, I.; Oyama, T.; Kurosumi, M. Power of PgR Expression as a Prognostic Factor for ER-Positive/HER2-Negative Breast Cancer Patients at Intermediate Risk Classified by the Ki67 Labeling Index. BMC Cancer 2017, 17, 354. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.; Jang, S.Y.; Choi, J.Y.; Lee, H.; Shin, D.S.; Park, Y.H.; Kim, J.-Y.; Ahn, J.-S.; Chae, B.J.; Yu, J.; et al. Progesterone Receptor Expression Level Predicts Prognosis of Estrogen Receptor-Positive/HER2-Negative Young Breast Cancer: A Single-Center Prospective Cohort Study. Cancers 2023, 15, 3435. [Google Scholar] [CrossRef] [PubMed]
- Tashima, R.; Nishimura, R.; Arima, N.; Hujisue, M.; Nakano, M.; Okumura, Y.; Osako, T.; Toyozumi, Y. P260 Evaluation of PgR Expression as a Prognostic Factor in Luminal HER2-Negative Breast Cancer. Breast 2015, 24, S116. [Google Scholar] [CrossRef]
- Arima, N.; Nishimura, R.; Osako, T.; Okumura, Y.; Nakano, M.; Fujisue, M.; Nishiyama, Y.; Toyozumi, Y. Ki-67 Index Value and Progesterone Receptor Status Can Predict Prognosis and Suitable Treatment in Node-Negative Breast Cancer Patients with Estrogen Receptor-Positive and HER2-Negative Tumors. Oncol. Lett. 2019, 17, 616–622. [Google Scholar] [CrossRef]
- Prat, A.; Cheang, M.C.U.; Martín, M.; Parker, J.S.; Carrasco, E.; Caballero, R.; Tyldesley, S.; Gelmon, K.; Bernard, P.S.; Nielsen, T.O.; et al. Prognostic Significance of Progesterone Receptor-Positive Tumor Cells within Immunohistochemically Defined Luminal A Breast Cancer. J. Clin. Oncol. 2013, 31, 203–209. [Google Scholar] [CrossRef]
- Xiao, W.; Zheng, S.; Yang, A.; Zhang, X.; Zou, Y.; Tang, H.; Xie, X. Breast Cancer Subtypes and the Risk of Distant Metastasis at Initial Diagnosis: A Population-Based Study. Cancer Manag. Res. 2018, 10, 5329–5338. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, J.; Wu, Z.; Zhang, X.; Ming, J. Influence of Progesterone Receptor on Metastasis and Prognosis in Breast Cancer Patients with Negative HER-2. Gland. Surg. 2022, 11, 77–90. [Google Scholar] [CrossRef]
- Arciero, C.A.; Guo, Y.; Jiang, R.; Behera, M.; O’Regan, R.; Peng, L.; Li, X. ER+/HER2+ Breast Cancer Has Different Metastatic Patterns and Better Survival Than ER−/HER2+ Breast Cancer. Clin. Breast Cancer 2019, 19, 236–245. [Google Scholar] [CrossRef]
- Cristofanilli, M.; DeMichele, A.; Giorgetti, C.; Turner, N.C.; Slamon, D.J.; Im, S.-A.; Masuda, N.; Verma, S.; Loi, S.; Colleoni, M.; et al. Predictors of Prolonged Benefit from Palbociclib plus Fulvestrant in Women with Endocrine-Resistant Hormone Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer in PALOMA-3. Eur. J. Cancer 2018, 104, 21–31. [Google Scholar] [CrossRef]
| Variables | All Patients (n = 351) | PgR-Low (n = 115) | PgR-High (n = 236) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | Median (range) | 57 (26–85) | 57 (26–84) | 57 (27–85) | 0.753 |
| ≥65, n (%) | 118 (33.6) | 37 (32.2) | 81 (34.3) | 0.689 | |
| Menopausal status, n (%) | Premenopausal | 99 (28.2) | 29 (25.2) | 70 (29.7) | 0.385 |
| Postmenopausal | 252 (71.8) | 86 (74.8) | 166 (70.3) | ||
| BMI (kg/m2) | Median (range) | 27.6 (15.4–54.5) | 27.6 (18.7–49.7) | 27.3 (15.4–54.5) | 0.442 |
| Histopathology, n (%) | IDC | 248 (70.7) | 79 (68.7) | 169 (71.6) | 0.731 |
| ILC | 38 (10.8) | 12 (10.4) | 26 (11) | ||
| Other | 65 (18.5) | 24 (20.9) | 41 (17.4) | ||
| Estrogen receptor level (%) | Median (range) | 95 (15–100) | 90 (30–100) | 95 (15–100) | 0.031 |
| Ki 67 (%) | Median (range) | 20 (1–90) | 30 (1–90) | 20 (1–80) | 0.002 |
| ≥20, n (%) | 208 (62.5) | 79 (71.8) | 129 (57.8) | 0.013 | |
| Tumor grade | Grade 1–2 Grade 3 | 187 (68.5) 86 (31.5) | 59 (66.3) 30 (33.7) | 128 (69.6) 56 (30.4) | 0.585 |
| HER-2 status, n (%) | Zero | 287 (81.8) | 93 (80.9) | 194 (82.2) | 0.761 |
| Low | 64 (18.2) | 22 (19.1) | 42 (17.8) | ||
| Metastatic disease, n (%) | De novo | 199 (56.7) | 55 (47.8) | 144 (61) | 0.019 |
| Recurrent | 152 (43.3) | 60 (52.2) | 92 (39) | ||
| Hormonal status, n (%) | Endocrine sensitive | 256 (72.9) | 77 (67) | 179 (75.8) | 0.078 |
| Endocrine resistance | 95 (27.1) | 38 (33) | 57 (24.2) | ||
| CDK 4/6 inhibitors, n (%) | Ribociclib | 245 (69.8) | 88 (76.5) | 157 (66.5) | 0.056 |
| Palbociclib | 106 (30.2) | 27 (23.5) | 79 (33.5) | ||
| Endocrine therapy, n (%) | Aromatase inhibitor | 268 (76.4) | 85 (73.9) | 183 (77.5) | 0.453 |
| Fulvestrant | 83 (23.6) | 30 (26.1) | 53 (22.5) | ||
| Bone lesion only, n (%) | 104 (29.6) | 28 (24.3) | 76 (32.2) | 0.130 | |
| Lymph node metastasis, n (%) | 138 (39.3) | 44 (38.3) | 94 (39.8) | 0.816 | |
| Visceral metastasis, n (%) | 155 (44.2) | 60 (52.2) | 95 (40.3) | 0.035 | |
| Lung metastasis, n (%) | 90 (25.6) | 30 (26.1) | 60 (25.4) | 0.894 | |
| Liver metastasis, n (%) | 68 (19.4) | 31 (27) | 37 (15.7) | 0.012 | |
| Brain metastasis, n (%) | 13 (3.7) | 8 (7) | 5 (2.1) | 0.024 | |
| Any grade TRAE, n (%) | 143 (40.7) | 42 (36.5) | 101 (42.8) | 0.261 | |
| Dose-reducing TRAE, n (%) | 107 (30.5) | 28 (24.3) | 79 (33.5) | 0.081 | |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age (≥65 vs. <65 years) | 0.88 | 0.62–1.24 | 0.456 | |||
| Menopausal status (pre vs. post) | 1.01 | 0.71–1.44 | 0.970 | |||
| BMI (≥30 vs. <30 kg/m2) | 0.64 | 0.45–0.92 | 0.017 | 0.71 | 0.46–1.08 | 0.109 |
| Ki-67 (≥20 vs. <20%) | 1.18 | 0.84–1.65 | 0.337 | |||
| Tumor grade (3 vs. 1–2) | 1.62 | 1.14–2.32 | 0.008 | 1.55 | 1.08–2.22 | 0.019 |
| HER-2 status (zero vs. low) | 1.21 | 0.82–1.79 | 0.342 | |||
| Progesterone receptor (high vs. low) | 0.29 | 0.21–0.41 | <0.001 | 0.41 | 0.28–0.61 | <0.001 |
| Metastatic disease status (recurrent vs. de novo) | 1.54 | 1.12–2.12 | 0.008 | 1.20 | 0.69–2.09 | 0.515 |
| Hormonal status (endocrine resistant vs. sensitive) | 1.96 | 1.41–2.72 | <0.001 | 1.60 | 1.10–2.31 | 0.013 |
| CDK 4/6 inhibitors (ribociclib vs. palbociclib) | 1.26 | 0.91–1.76 | 0.170 | |||
| Lymph node metastasis, n (%) | 1.26 | 0.91–1.74 | 0.156 | |||
| Liver metastasis (yes vs. no) | 2.87 | 2.03–4.06 | <0.001 | 2.06 | 1.34–3.17 | 0.001 |
| Lung metastasis (yes vs. no) | 1.20 | 0.84–1.75 | 0.312 | |||
| Bone lesion only (yes vs. no) | 0.48 | 0.33–0.72 | <0.001 | 0.66 | 0.42–1.05 | 0.077 |
| Brain metastasis (yes vs. no) | 3.54 | 1.95–6.40 | <0.001 | 2.28 | 1.18–4.44 | 0.015 |
| Any-grade TRAE (yes vs. no) | 1.04 | 0.75–1.43 | 0.826 | |||
| Dose-reducing TRAE (yes vs. no) | 0.92 | 0.65–1.31 | 0.649 | |||
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age (≥65 vs. <65 years) | 1.50 | 0.94–2.41 | 0.089 | |||
| Menopausal status (pre vs. post) | 1.88 | 1.03–3.42 | 0.039 | 2.35 | 1.18–4.67 | 0.015 |
| BMI (≥30 vs. <30 kg/m2) | 0.58 | 0.33–1.00 | 0.050 | |||
| Ki-67 (≥20 vs. <20%) | 1.13 | 0.69–1.85 | 0.619 | |||
| Tumor grade (3 vs. 1–2) | 1.86 | 1.13–3.08 | 0.015 | 1.83 | 1.10–3.02 | 0.019 |
| HER-2 status (zero vs. low) | 0.94 | 0.52–1.68 | 0.832 | |||
| Progesterone receptor (high vs. low) | 0.36 | 0.22–0.57 | <0.001 | 0.40 | 0.24–0.68 | 0.001 |
| Metastatic disease status (recurrent vs. de novo) | 0.99 | 0.62–1.57 | 0.961 | |||
| Hormonal status (endocrine resistant vs. sensitive) | 1.27 | 0.77–2.08 | 0.346 | |||
| CDK 4/6 inhibitors (ribociclib vs. palbociclib) | 1.19 | 0.74–1.91 | 0.479 | |||
| Lymph node metastasis, n (%) | 0.91 | 0.57–1.48 | 0.713 | |||
| Liver metastasis (yes vs. no) | 2.41 | 1.46–3.97 | 0.001 | 2.37 | 1.33–4.21 | 0.003 |
| Lung metastasis (yes vs. no) | 1.30 | 0.78–2.16 | 0.307 | |||
| Bone lesion only (yes vs. no) | 0.69 | 0.40–1.17 | 0.169 | |||
| Brain metastasis (yes vs. no) | 3.43 | 1.57–7.51 | 0.002 | 2.06 | 0.82–5.20 | 0.127 |
| Any grade TRAE (yes vs. no) | 1.11 | 0.70–1.76 | 0.656 | |||
| Dose-reducing TRAE (yes vs. no) | 1.16 | 0.72–1.88 | 0.545 | |||
| Adverse Event | Any Grade | Dose-Reducing TRAEs |
|---|---|---|
| number of patients (percent) | ||
| Neutropenia | 122 (34.8%) | 89 (25.4%) |
| Thrombocytopenia | 10 (2.8%) | 8 (2.3%) |
| Anemia | 8 (2.3%) | 4 (1.1%) |
| Prolonged QTc | 7 (2%) | 7 (2%) |
| Increased alanine aminotransferase | 5 (1.4%) | 3 (0.8%) |
| Increased aspartate aminotransferase | 5 (1.4%) | 3 (0.8%) |
| Creatinine increased | 2 (0.6%) | 2 (0.6%) |
| Diarrhea | 2 (0.6%) | 2 (0.6%) |
| Oral mucositis | 1 (0.3%) | 1 (0.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guliyev, M.; Güren, A.K.; Özge, E.; Çolak, R.; Majidova, N.; Alkan Şen, G.; Safarov, S.; Günaltılı, M.; Fidan, M.C.; Gültürk, İ.; et al. The Impact of Progesterone Receptor Status on Survival Outcomes in Metastatic Breast Cancer Patients Treated with First-Line CDK4/6 Inhibitors. Cancers 2025, 17, 693. https://doi.org/10.3390/cancers17040693
Guliyev M, Güren AK, Özge E, Çolak R, Majidova N, Alkan Şen G, Safarov S, Günaltılı M, Fidan MC, Gültürk İ, et al. The Impact of Progesterone Receptor Status on Survival Outcomes in Metastatic Breast Cancer Patients Treated with First-Line CDK4/6 Inhibitors. Cancers. 2025; 17(4):693. https://doi.org/10.3390/cancers17040693
Chicago/Turabian StyleGuliyev, Murad, Ali Kaan Güren, Emre Özge, Rumeysa Çolak, Nargiz Majidova, Gülin Alkan Şen, Shamkhal Safarov, Murat Günaltılı, Mehmet Cem Fidan, İlkay Gültürk, and et al. 2025. "The Impact of Progesterone Receptor Status on Survival Outcomes in Metastatic Breast Cancer Patients Treated with First-Line CDK4/6 Inhibitors" Cancers 17, no. 4: 693. https://doi.org/10.3390/cancers17040693
APA StyleGuliyev, M., Güren, A. K., Özge, E., Çolak, R., Majidova, N., Alkan Şen, G., Safarov, S., Günaltılı, M., Fidan, M. C., Gültürk, İ., Yılmaz, M., Bayoğlu, İ. V., Demirci, N. S., & Alan, Ö. (2025). The Impact of Progesterone Receptor Status on Survival Outcomes in Metastatic Breast Cancer Patients Treated with First-Line CDK4/6 Inhibitors. Cancers, 17(4), 693. https://doi.org/10.3390/cancers17040693





