The Safety and Efficacy of Vascular-Targeted Photodynamic Therapy in Low-Risk Prostate Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
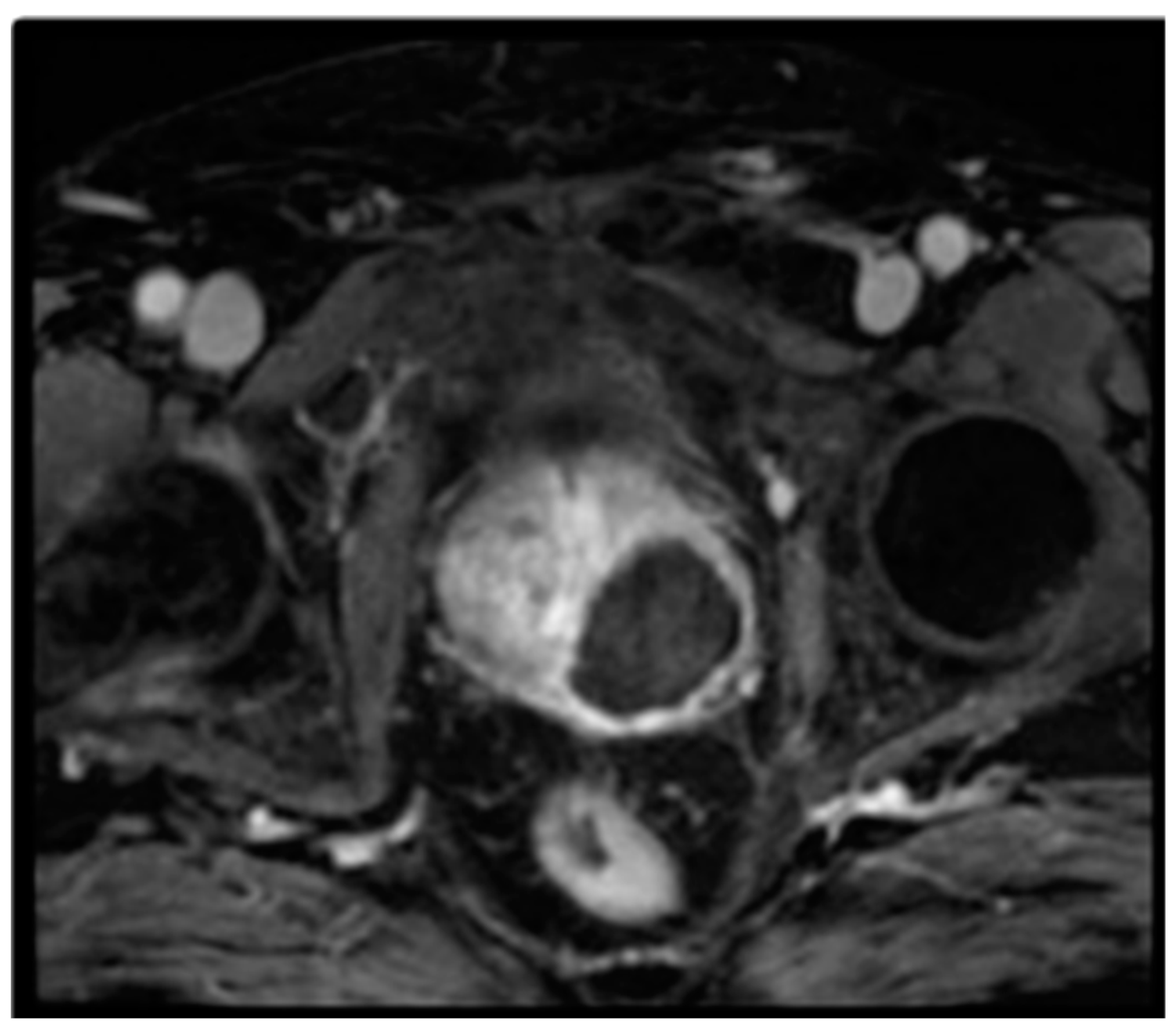
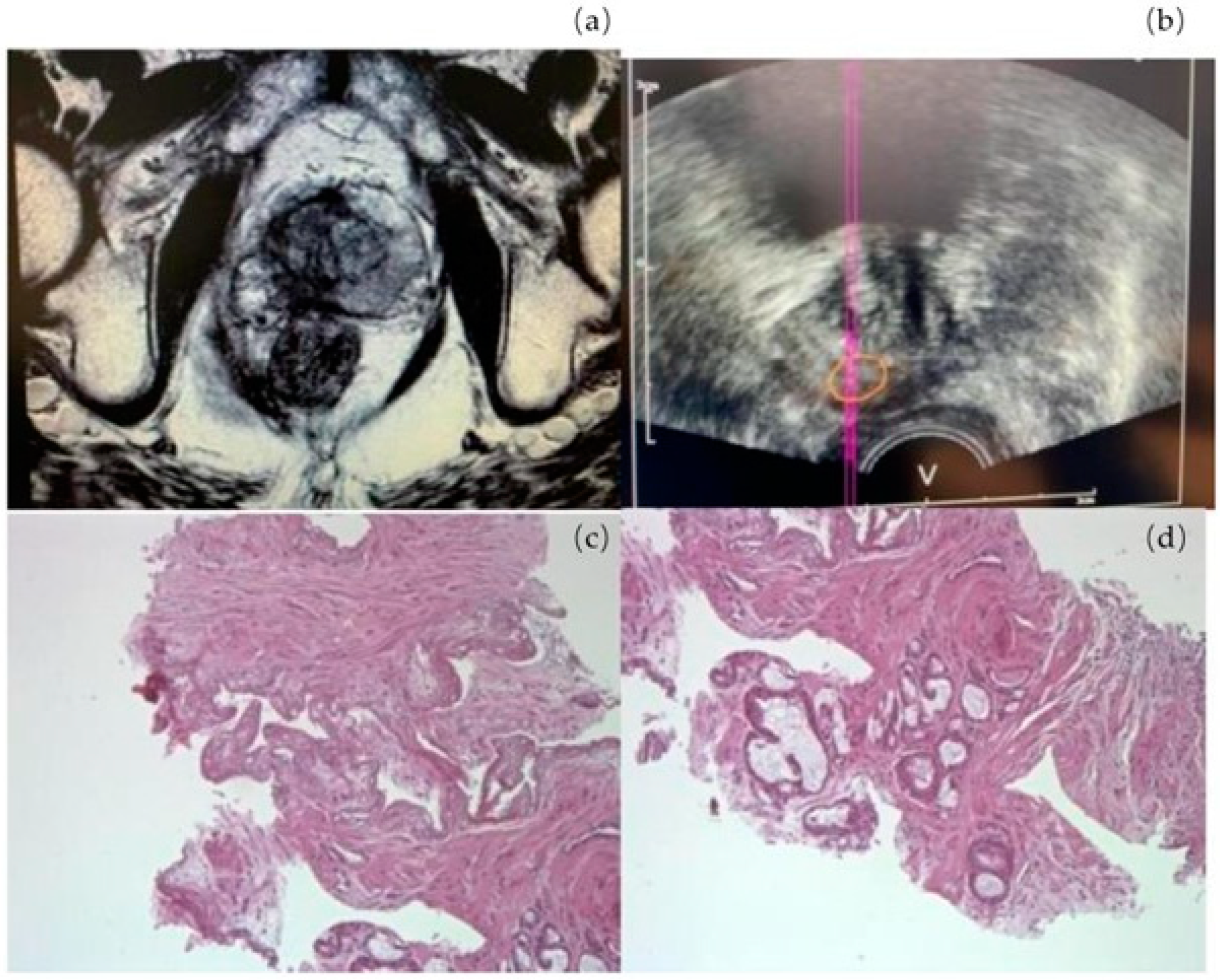
2.1. Padeliporfin VTP Procedure
| Padeliporfin VTP Procedure | Median (Range) |
| Operative time, median (range), minutes | 101.6 (70–135) |
| Number of fibers, median (range) | 13 (7–20) |
2.2. Oncological Outcome
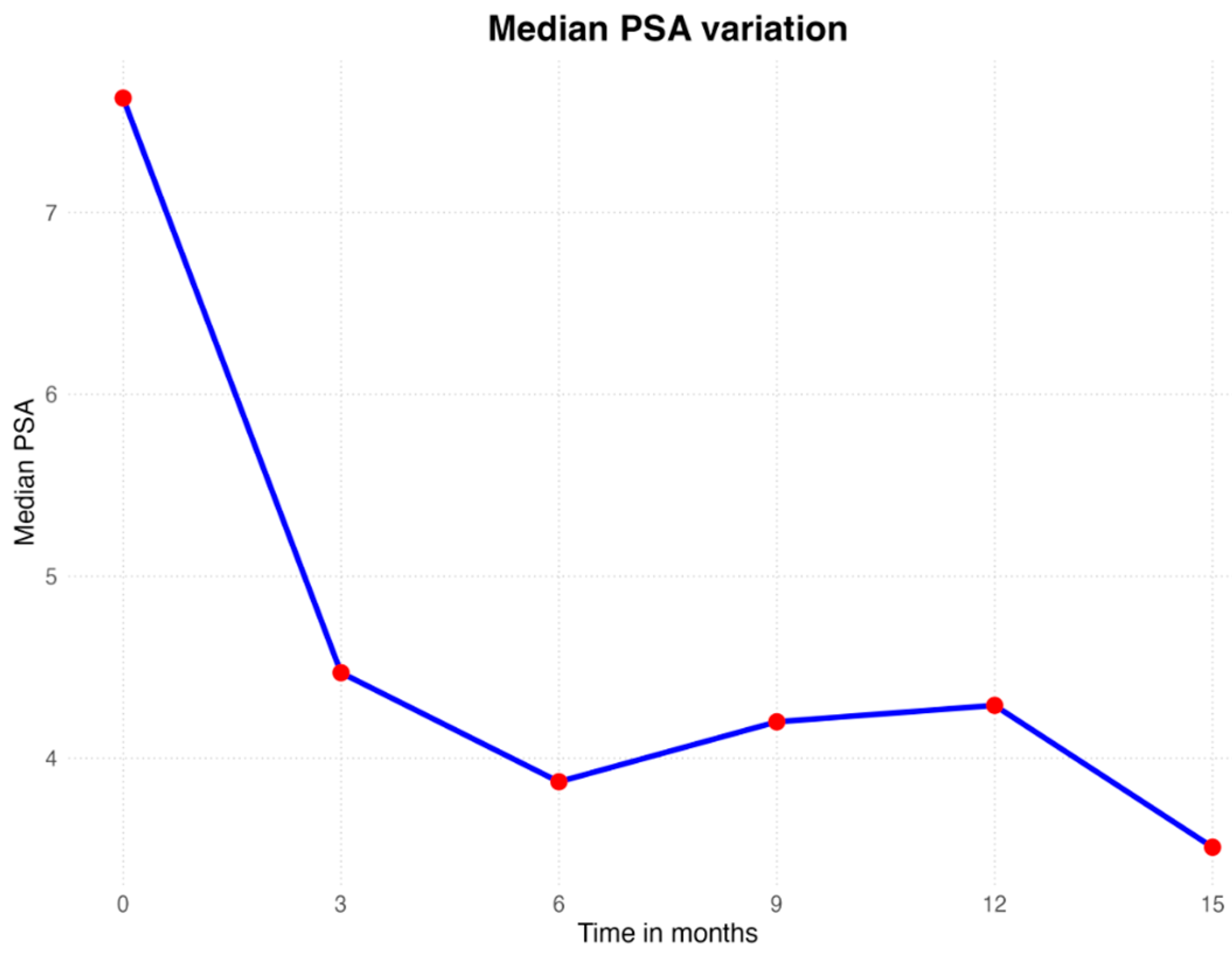
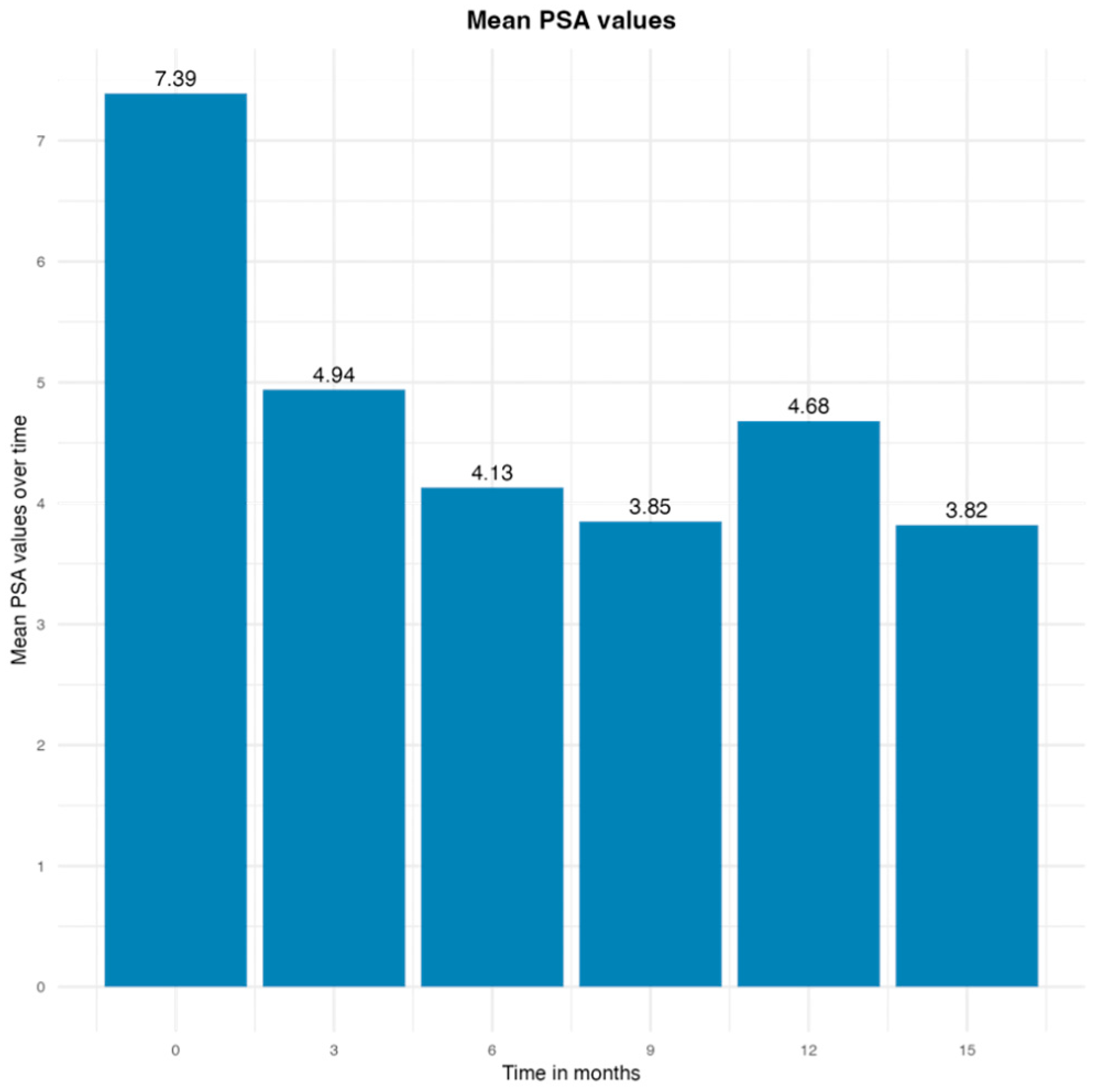
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries from 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Di Bello, F.; Scheipner, L.; Baudo, A.; de Angelis, M.; Jannello, L.M.I.; Siech, C.; Tian, Z.; Vitucci, K.; Goyal, J.A.; Collà Ruvolo, C.; et al. Cancer-specific mortality after radical prostatectomy versus radiotherapy in incidental prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Siech, C.; de Angelis, M.; Jannello, L.M.I.; Di Bello, F.; Rodriguez Peñaranda, N.; Goyal, J.A.; Tian, Z.; Saad, F.; Shariat, S.F.; Puliatti, S.; et al. Rare histological prostate cancer subtypes: Cancer-specific and other-cause mortality. Prostate Cancer Prostatic Dis. 2024, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; Brawer, M.K.; Jones, K.M.; Barry, M.J.; Aronson, W.J.; Fox, S.; Gingrich, J.R.; Wei, J.T.; Gilhooly, P.; Grob, B.M.; et al. Prostate Cancer Intervention versus Observation Trial (PIVOT) Study Group. Radical prostatectomy versus observation for localized prostate cancer. N. Engl. J. Med. 2012, 367, 203–213, Erratum in: N. Engl. J. Med. 2012, 367, 582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bill-Axelson, A.; Holmberg, L.; Ruutu, M.; Garmo, H.; Stark, J.R.; Busch, C.; Nordling, S.; Häggman, M.; Andersson, S.O.; Bratell, S.; et al. SPCG-4 Investigators. Radical prostatectomy versus watchful waiting in early prostate cancer. N. Engl. J. Med. 2011, 364, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. ProtecT Study Group. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.L.; Hoffman, R.M.; Davis, K.M.; Luta, G.; Leimpeter, A.; Lobo, T.; Kelly, S.P.; Shan, J.; Aaronson, D.; Tomko, C.A.; et al. Treatment Preferences for Active Surveillance versus Active Treatment among Men with Low-Risk Prostate Cancer. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 1240–1250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ficarra, V.; Bartoletti, R.; Borghesi, M.; DENunzio, C.; Falagario, U.G.; Gandaglia, G.; Giannarini, G.; Minervini, A.; Mirone, V.; Porpiglia, F.; et al. Italian Society of Urology (SIU) panel. Prostate cancer diagnostic pathway in men with lower urinary tract symptoms or performing opportunistic screening: The Italian Society of Urology (SIU) position paper. Minerva Urol. Nephrol. 2024, 76, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Bartoletti, R.; Borghesi, M.; Caffo, O.; DENunzio, C.; Falagario, U.G.; Gandaglia, G.; Giannarini, G.; Minervini, A.; Mirone, V.; et al. Italian Society of Urology (SIU) panel. Organized prostate cancer screening program: A proposal from the Italian Society of Urology (SIU). Minerva Urol. Nephrol. 2024, 76, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Mouraviev, V.; Mayes, J.M.; Polascik, T.J. Pathologic basis of focal therapy for early-stage prostate cancer. Nat. Rev. Urol. 2009, 6, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, M.R.; Broering, J.M.; Kantoff, P.W.; Carroll, P.R. Contemporary trends in low risk prostate cancer: Risk assessment and treatment. J. Urol. 2007, 178 Pt 2, S14–S19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polascik, T.J.; Mayes, J.M.; Sun, L.; Madden, J.F.; Moul, J.W.; Mouraviev, V. Pathologic stage T2a and T2b prostate cancer in the recent prostate-specific antigen era: Implications for unilateral ablative therapy. Prostate 2008, 68, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Emberton, M.; Ahmed, H.U. Focal therapy for prostate cancer: Rationale and treatment opportunities. Clin. Oncol. (R. Coll. Radiol.) 2013, 25, 461–473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahmed, H.U.; Pendse, D.; Illing, R.; Allen, C.; van der Meulen, J.H.; Emberton, M. Will focal therapy become a standard of care for men with localized prostate cancer? Nat. Clin. Pract. Oncol. 2007, 4, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Eggener, S.E.; Scardino, P.T.; Carroll, P.R.; Zelefsky, M.J.; Sartor, O.; Hricak, H.; Wheeler, T.M.; Fine, S.W.; Trachtenberg, J.; Rubin, M.A.; et al. International Task Force on Prostate Cancer and the Focal Lesion Paradigm. Focal therapy for localized prostate cancer: A critical appraisal of rationale and modalities. J. Urol. 2007, 178, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Barqawi, A. Targeted focal therapy: A minimally invasive ablation technique for early prostate cancer. Oncology 2007, 21, 27–32, discussion 33–34, 39. [Google Scholar] [PubMed]
- Ayerra Perez, H.; Barba Abad, J.F.; Extramiana Cameno, J. An Update on Focal Therapy for Prostate Cancer. Clin. Genitourin. Cancer. 2023, 21, e1–e712. [Google Scholar] [CrossRef] [PubMed]
- Matin, S.F.; Tinkey, P.T.; Borne, A.T.; Stephens, L.C.; Sherz, A.; Swanson, D.A. A pilot trial of vascular targeted photodynamic therapy for renal tissue. J. Urol. 2008, 180, 338–342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Nogueira, L.; Tracey, A.T.; Alvim, R.; Reisz, P.; Scherz, A.; Coleman, J.A.; Kim, K. Developments in Vascular-Targeted Photodynamic Therapy for Urologic Malignancies. Molecules 2020, 25, 5417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azzouzi, A.R.; Barret, E.; Moore, C.M.; Villers, A.; Allen, C.; Scherz, A.; Muir, G.; de Wildt, M.; Barber, N.J.; Lebdai, S.; et al. TOOKAD(®) Soluble vascular-targeted photodynamic (VTP) therapy: Determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int. 2013, 112, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Flegar, L.; Baunacke, M.; Buerk, B.T.; Proschmann, R.; Zacharis, A.; Propping, S.; Huber, J.; Thomas, C.; Borkowetz, A. Decision Regret and Quality of Life after Focal Therapy with Vascular-Targeted Photodynamic Therapy (TOOKAD®) for Localized Prostate Cancer. Urol. Int. 2022, 106, 903–908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kok, E.T.; McDonnell, J.; Stolk, E.A.; Stoevelaar, H.J.; Busschbach, J.J. Triumph Research Group; Pan-European Expert Panel. The valuation of the International Prostate Symptom Score (IPSS) for use in economic evaluations. Eur. Urol. 2002, 42, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Rhoden, E.L.; Telöken, C.; Sogari, P.R.; Vargas Souto, C.A. The use of the simplified International Index of Erectile Function (IIEF-5) as a diagnostic tool to study the prevalence of erectile dysfunction. Int. J. Impot. Res. 2002, 14, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, D.; Mostafa, A.; Abdel-Fattah, M. A new validated score for detecting patient-reported success on postoperative ICIQ-SF: A novel two-stage analysis from two large RCT cohorts. Int. Urogynecol J. 2017, 28, 95–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siech, C.; de Angelis, M.; Jannello, L.M.I.; Di Bello, F.; Rodriguez Peñaranda, N.; Goyal, J.A.; Tian, Z.; Saad, F.; Shariat, S.F.; Puliatti, S.; et al. Life expectancy in rare histological prostate cancer subtypes. Int. J. Cancer 2024. [Google Scholar] [CrossRef] [PubMed]
- Azzouzi, A.R.; Barret, E.; Bennet, J.; Moore, C.; Taneja, S.; Muir, G.; Villers, A.; Coleman, J.; Allen, C.; Scherz, A.; et al. TOOKAD® Soluble focal therapy: Pooled analysis of three phase II studies assessing the minimally invasive ablation of localized prostate cancer. World J. Urol. 2015, 33, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Azzouzi, A.R.; Lebdai, S.; Benzaghou, F.; Stief, C. Vascular-targeted photodynamic therapy with TOOKAD® Soluble in localized prostate cancer: Standardization of the procedure. World J. Urol. 2015, 33, 937–944. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriguez-Rivera, J.A.; Rodriguez-Lay, R.; Zegarra-Montes, L.; Benzaghou, F.; Gaillac, B.; Azzouzi, A.R.; Reis, L.O.; Palma, P. Expanding indication of padeliporfin (WST11) vascular-targeted photodynamic therapy: Results of prostate cancer Latin-American multicenter study. Actas Urológicas Españolas 2018, 42, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Lebdai, S.; Bigot, P.; Leroux, P.A.; Berthelot, L.P.; Maulaz, P.; Azzouzi, A.R. Vascular Targeted Photodynamic Therapy with Padeliporfin for Low Risk Prostate Cancer Treatment: Midterm Oncologic Outcomes. J. Urol. 2017, 198, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Azzouzi, A.R.; Vincendeau, S.; Barret, E.; Cicco, A.; Kleinclauss, F.; van der Poel, H.G.; Stief, C.G.; Rassweiler, J.; Salomon, G.; Solsona, E.; et al. PCM301 Study Group. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): An open-label, phase 3, randomised controlled trial. Lancet Oncol. 2017, 18, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Gill, I.S.; Azzouzi, A.R.; Emberton, M.; Coleman, J.A.; Coeytaux, E.; Scherz, A.; Scardino, P.T. PCM301 Study Group. Randomized Trial of Partial Gland Ablation with Vascular Targeted Phototherapy versus Active Surveillance for Low Risk Prostate Cancer: Extended Followup and Analyses of Effectiveness. J. Urol. 2018, 200, 786–793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barret, E.; Ganzer, R.; Salomon, G.; Fielder, M.; Celia, A.; Enikeev, D.; Martínez-Salamanca, J.I.; Liatsikos, E.; Gómez Rivas, J. Future of focal therapy for the treatment of prostate cancer- european section of urotechnology (ESUT) position. Arch. Esp. Urol. 2019, 72, 167–173. [Google Scholar] [PubMed]
- Pierrard, V.; Lebdai, S.; Kleinclauss, F.; Azzouzi, A.R.; Terrier, J.E.; Fortier, E.; Joniau, S.; Van Der Poel, H.; Salomon, G.; Casanova, J.; et al. Radical Prostatectomy after Vascular Targeted Photodynamic Therapy with Padeliporfin: Feasibility, and Early and Intermediate Results. J. Urol. 2019, 201, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Clery, R.; Grande, P.; Seisen, T.; Gobert, A.; Duquesne, I.; Villers, A.; Olivier, J.; Bernhard, J.C.; Robert, G.; Beauval, J.B.; et al. Outcomes after salvage radical prostatectomy and first-line radiation therapy or HIFU for recurrent localized prostate cancer: Results from a multicenter study. World J. Urol. 2019, 37, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Median (Range) or n° (%) |
|---|---|
| Age, median (range), years | 63.4 (50–77) |
| Weight, median (range), kg | 74 (60–108) |
| Prostate volume, median (range), mL | 53.53 (35–70) |
| PSA, median, (range), ng/ml | 7.38 (2.7–9.8) |
| mpMRI (PIRADS) | 3 (7) 4 (4) 5 (2) |
| Number of positive cores, median (range) | 1.4 (1–2) |
| Median total cancer core length (range), mm | 2.8 (0.5–5.1) |
| Gleason score | 3 + 3 (13) |
| T-stage | T1c 7 T2a 6 |
| IPSS, median, (range) | 15.4 (8–30) |
| Uroflowmetry (Qmax), median, (range) | 12.3 (9.5–18) |
| Quality of life (QoL) score, median, (range) | 2.5 (1–4) |
| IIEF-5 score, median, (range) | 17.2 (3–24) |
| Adverse Event | n (%) |
|---|---|
| Any adverse event | 18 (86%) |
| Hematuria | 5 (27.7%) |
| Perineal pain | 5 (27.7%) |
| Transient dysuria | 3 (16.6%) |
| Micturition urgency | 2 (11.1%) |
| Hematospermia | 1 (5.5%) |
| Glans erythema | 1 (5.5%) |
| Urinary retention | 1 (5.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saldutto, P.; Cavacece, F.; Rocca, R.L.; Di Mauro, E.; Verratti, V.; Sangiorgi, G.M.; Vena, W.; Patelli, G.; Iacono, F.; Di Bello, F.; et al. The Safety and Efficacy of Vascular-Targeted Photodynamic Therapy in Low-Risk Prostate Cancer. Cancers 2025, 17, 661. https://doi.org/10.3390/cancers17040661
Saldutto P, Cavacece F, Rocca RL, Di Mauro E, Verratti V, Sangiorgi GM, Vena W, Patelli G, Iacono F, Di Bello F, et al. The Safety and Efficacy of Vascular-Targeted Photodynamic Therapy in Low-Risk Prostate Cancer. Cancers. 2025; 17(4):661. https://doi.org/10.3390/cancers17040661
Chicago/Turabian StyleSaldutto, Pietro, Fernando Cavacece, Roberto La Rocca, Ernesto Di Mauro, Vittore Verratti, Giuseppe Massimo Sangiorgi, Walter Vena, Gianluigi Patelli, Fabrizio Iacono, Francesco Di Bello, and et al. 2025. "The Safety and Efficacy of Vascular-Targeted Photodynamic Therapy in Low-Risk Prostate Cancer" Cancers 17, no. 4: 661. https://doi.org/10.3390/cancers17040661
APA StyleSaldutto, P., Cavacece, F., Rocca, R. L., Di Mauro, E., Verratti, V., Sangiorgi, G. M., Vena, W., Patelli, G., Iacono, F., Di Bello, F., Napolitano, L., & Altieri, V. M. (2025). The Safety and Efficacy of Vascular-Targeted Photodynamic Therapy in Low-Risk Prostate Cancer. Cancers, 17(4), 661. https://doi.org/10.3390/cancers17040661








