Salivary Gland Cancers in the Era of Molecular Analysis: The Role of Tissue and Liquid Biomarkers
Simple Summary
Abstract
1. Introduction
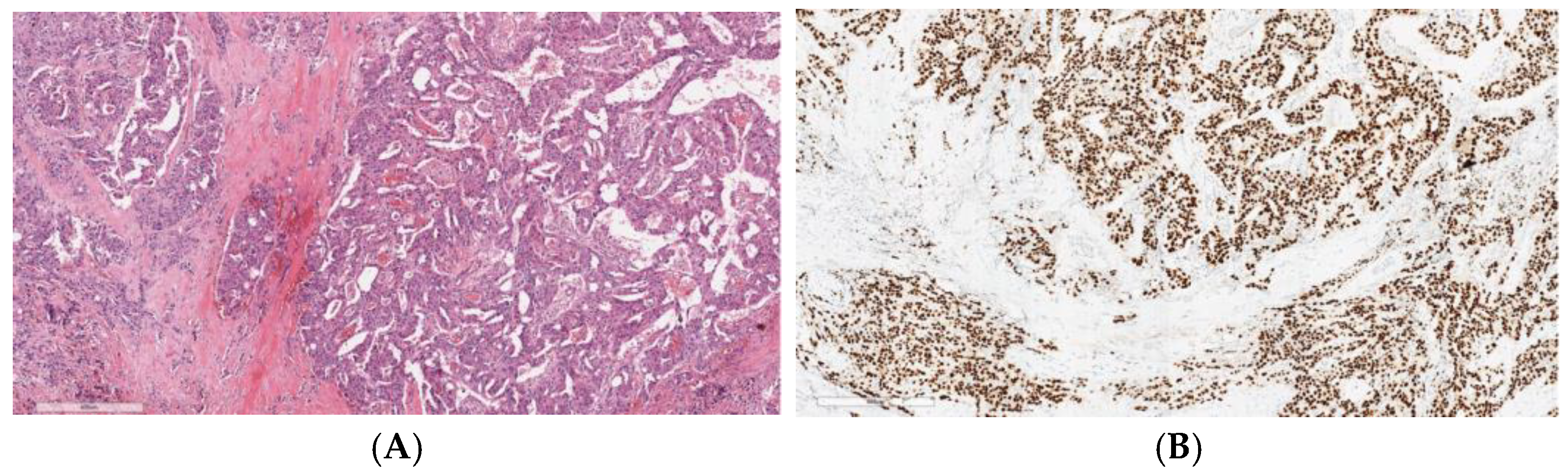
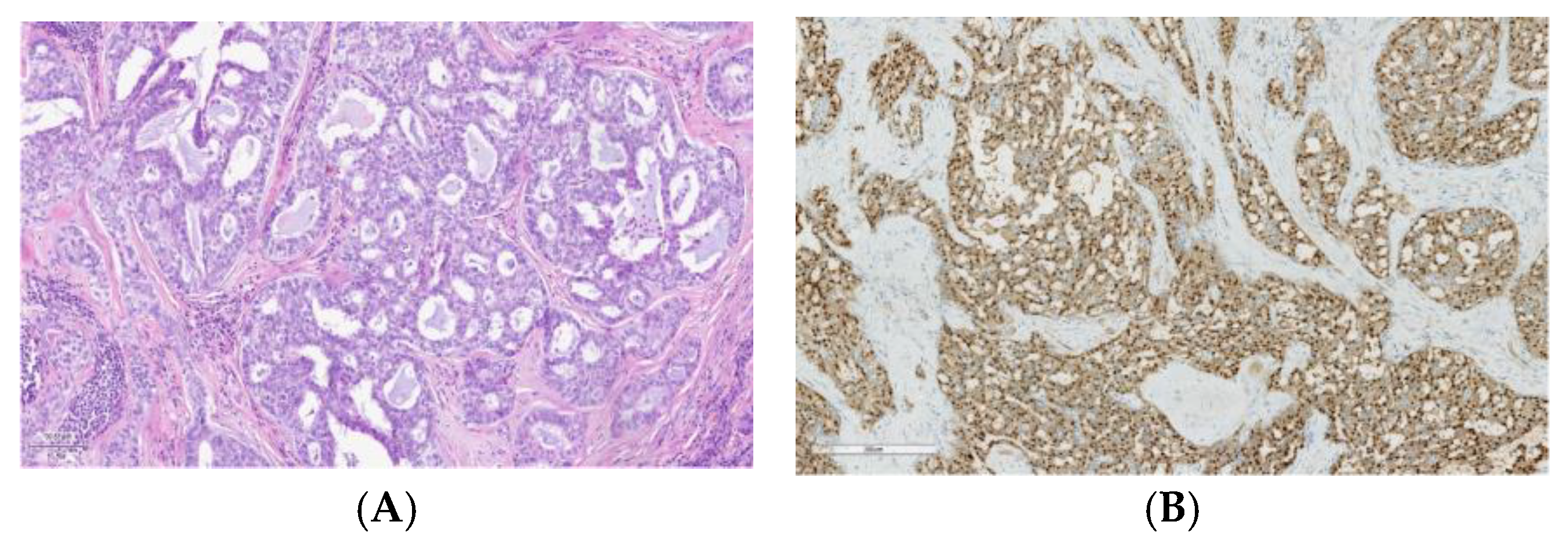
2. Tissue Biomarkers Across Various Salivary Gland Subtypes: Diagnostic and Prognostic Roles
2.1. Mucoepidermoid Carcinoma (MEC)
2.2. Adenoid Cystic Carcinoma
2.3. Acinic Cell Carcinoma
2.4. Salivary Duct Carcinoma
2.5. Other Subtypes
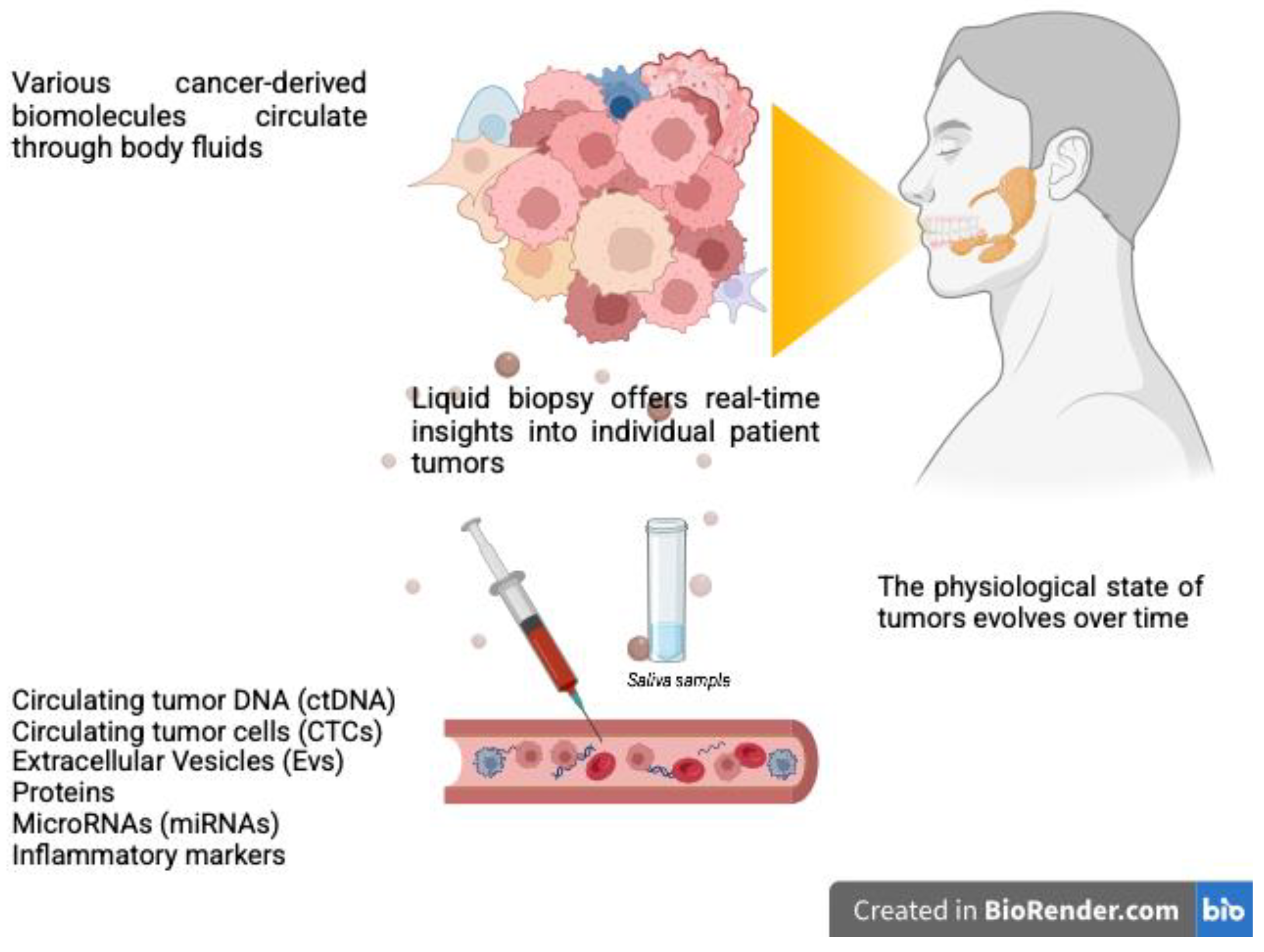
3. Liquid Biopsy for SGCs: Potential Clinical Applications and Future Perspectives
4. Precision Oncology in SGCs: A Brief Overview of Emerging Therapeutic Options
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today, Version 1.1; International Agency for Research on Cancer: Lyon, France, 2024. Available online: https://gco.iarc.who.int/today (accessed on 30 January 2025).
- Cavalieri, S.; Filippini, D.M.; Ottini, A.; Bergamini, C.; Resteghini, C.; Colombo, E.; Lombardo, R.; Nuzzolese, I.; Alfieri, S.; Licitra, L.; et al. Immunotherapy in head and neck squamous cell carcinoma and rare head and neck malignancies. Explor. Target. Anti-tumor Ther. 2021, 2, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Filippini, D.M.; Carosi, F.; Querzoli, G.; Fermi, M.; Ricciotti, I.; Molteni, G.; Presutti, L.; Foschini, M.P.; Locati, L.D. Rare Head and Neck Cancers and Pathological Diagnosis Challenges: A Comprehensive Literature Review. Diagnostics 2024, 14, 2365. [Google Scholar] [CrossRef] [PubMed]
- Skálová, A.; Stenman, G.; Simpson, R.H.; Hellquist, H.; Slouka, D.; Svoboda, T.; Bishop, J.A.; Hunt, J.L.; Nibu, K.-I.; Rinaldo, A.; et al. The Role of Molecular Testing in the Differential Diagnosis of Salivary Gland Carcinomas. Am. J. Surg. Pathol. 2018, 42, e11–e27. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.A.; Tavares, M.R.; Dias, F.L.; Kligerman, J.; Nascimento, M.F.; Barbosa, M.M.; Cernea, C.R.; Soares, J.R.; Santos, I.C.; Salviano, S. Clinical prognostic factors in malignant parotid gland tumors. Otolaryngol. Head Neck Surg. 2005, 133, 702–708. [Google Scholar] [CrossRef]
- Walvekar, R.R.; Filho, P.A.A.; Seethala, R.R.; Gooding, W.E.; Heron, D.E.; Johnson, J.T.; Ferris, R.L. Clinicopathologic features as stronger prognostic factors than histology or grade in risk stratification of primary parotid malignancies. Head Neck 2011, 33, 225–231. [Google Scholar] [CrossRef]
- van Herpen, C.; Poorten, V.V.; Skalova, A.; Terhaard, C.; Maroldi, R.; van Engen, A.; Baujat, B.; Locati, L.; Jensen, A.; Smeele, L.; et al. Salivary gland cancer: ESMO–European Reference Network on Rare Adult Solid Cancers (EURACAN) Clinical Practice Guideline for diagnosis, treatment and follow-up. ESMO Open 2022, 7, 100602. [Google Scholar] [CrossRef]
- Nam, S.J.; Roh, J.-L.; Cho, K.-J.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Risk Factors and Survival Associated with Distant Metastasis in Patients with Carcinoma of the Salivary Gland. Ann. Surg. Oncol. 2016, 23, 4376–4383. [Google Scholar] [CrossRef] [PubMed]
- Lassche, G.; van Boxtel, W.; Ligtenberg, M.J.; van Engen-van Grunsven, A.C.H.; van Herpen, C.M. Advances and challenges in precision medicine in salivary gland cancer. Cancer Treat. Rev. 2019, 80, 101906. [Google Scholar] [CrossRef] [PubMed]
- Filippini, D.M.; Marret, G.; Bastien, E.; Sanchez, R.; Borcoman, E.; Le Tourneau, C. Phase I trials of single-agent new drugs in head and neck cancer: A scoping review. Chin. Clin. Oncol. 2024, 13, 73. [Google Scholar] [CrossRef]
- Pires, F.R.; Pringle, G.A.; de Almeida, O.P.; Chen, S.-Y. Intra-oral minor salivary gland tumors: A clinicopathological study of 546 cases. Oral Oncol. 2007, 43, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.A. Fusions in salivary gland neoplasms: A review of practical diagnostic applications. J. Clin. Pathol. 2024. [Google Scholar] [CrossRef]
- Sams, R.N.; Gnepp, D.R. P63 Expression can be used in differential diagnosis of salivary gland acinic cell and mucoepidermoid carcinomas. Head Neck Pathol. 2013, 7, 64–68. [Google Scholar] [CrossRef]
- Siyi, L.; Shengwen, L.; Min, R.; Wenjun, Y.; Lizheng, W.; Chenping, Z. Increased expression of MUC-1 has close relation with patient survivor in high-grade salivary gland mucoepidermoid carcinoma. J. Oral Pathol. Med. 2014, 43, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Honjo, K.; Hiraki, T.; Higashi, M.; Noguchi, H.; Nomoto, M.; Yoshimura, T.; Batra, S.K.; Yonezawa, S.; Semba, I.; Nakamura, N.; et al. Immunohistochemical expression profiles of mucin antigens in salivary gland mucoepidermoid carcinoma: MUC4-and MUC6-negative expression predicts a shortened survival in the early postoperative phase. Histol. Histopathol. 2018, 33, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Cros, J.; Sbidian, E.; Hans, S.; Roussel, H.; Scotte, F.; Tartour, E.; Brasnu, D.; Laurent-Puig, P.; Bruneval, P.; Blons, H.; et al. Expression and mutational status of treatment-relevant targets and key oncogenes in 123 malignant salivary gland tumours. Ann. Oncol. 2013, 24, 2624–2629. [Google Scholar] [CrossRef]
- Shinomiya, H.; Ito, Y.; Kubo, M.; Yonezawa, K.; Otsuki, N.; Iwae, S.; Inagaki, H.; Nibu, K.-I. Expression of amphiregulin in mucoepidermoid carcinoma of the major salivary glands: A molecular and clinicopathological study. Hum. Pathol. 2016, 57, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ramirez, C.; Zhang, Z.; Warner, K.A.; Herzog, A.E.; Mantesso, A.; Zhang, Z.; Yoon, E.; Wang, S.; Wicha, M.S.; Nör, J.E. p53 Inhibits Bmi-1-driven Self-Renewal and Defines Salivary Gland Cancer Stemness. Clin. Cancer Res. 2022, 28, 4757–4770. [Google Scholar] [CrossRef] [PubMed]
- Tirado, Y.; Williams, M.D.; Hanna, E.Y.; Kaye, F.J.; Batsakis, J.G.; El-Naggar, A.K. CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin’s tumors: Implications for histogenesis and biologic behavior. Genes Chromosom. Cancer 2007, 46, 708–715. [Google Scholar] [CrossRef]
- Skálová, A.; Hyrcza, M.D.; Leivo, I. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Salivary Glands. Head Neck Pathol. 2022, 16, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Mehta, S.; Vanik, S.; Trivedi, P.; Banerjee, N.; Dhar, H.; Datta, S.; Karanjai, S. The evolving role of molecular pathology in the diagnosis of salivary gland tumours with potential pitfalls. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 3769–3783. [Google Scholar] [CrossRef] [PubMed]
- García, J.J.; Hunt, J.L.; Weinreb, I.; McHugh, J.B.; Barnes, E.L.; Cieply, K.; Dacic, S.; Seethala, R.R. Fluorescence in situ hybridization for detection of MAML2 rearrangements in oncocytic mucoepidermoid carcinomas: Utility as a diagnostic test. Hum. Pathol. 2011, 42, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.D.; Ito, Y.D.; Masaki, A.; Fujii, K.D.; Beppu, S.; Sakakibara, T.C.; Takino, H.C.; Takase, H.B.; Ijichi, K.; Shimozato, K.D.; et al. Warthin-like Mucoepidermoid Carcinoma: A Combined Study of Fluorescence In Situ Hybridization and Whole-slide Imaging. Am. J. Surg. Pathol. 2015, 39, 1479–1487. [Google Scholar] [CrossRef]
- Schwarz, S.; Stiegler, C.; Müller, M.; Ettl, T.; Brockhoff, G.; Zenk, J.; Agaimy, A. Salivary gland mucoepidermoid carcinoma is a clinically, morphologically and genetically heterogeneous entity: A clinicopathological study of 40 cases with emphasis on grading, histological variants and presence of the t(11;19) translocation. Histopathology 2011, 58, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Murase, T.; Okumura, Y.; Ueda, K.; Sakamoto, Y.; Masaki, A.; Kawakita, D.; Tada, Y.; Nibu, K.; Shibuya, Y.; et al. Clinicopathological significance of EGFR pathway gene mutations and CRTC1/3–MAML2 fusions in salivary gland mucoepidermoid carcinoma. Histopathology 2020, 76, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.; Werenicz, S.; Trocchi, P.; Falk, M.; Friedrich, R.E.; Stammler, A.; Stang, A.; Oesterling, F.; Khil, L.; Stenman, G.; et al. Mucoepidermoid carcinoma of the salivary glands revisited with special reference to histologic grading and CRTC1/3-MAML2 genotyping. Virchows Arch. 2021, 479, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Anzick, S.L.; Chen, W.; Park, Y.; Meltzer, P.; Bell, D.; El-Naggar, A.K.; Kaye, F.J. Unfavorable prognosis of CRTC1-MAML2 positive mucoepidermoid tumors with CDKN2A deletions. Genes, Chromosom. Cancer 2010, 49, 59–69. [Google Scholar] [CrossRef]
- Mitani, Y.; Roberts, D.B.; Fatani, H.; Weber, R.S.; Kies, M.S.; Lippman, S.M.; El-Naggar, A.K. MicroRNA Profiling of Salivary Adenoid Cystic Carcinoma: Association of miR-17-92 Upregulation with Poor Outcome. PLoS ONE 2013, 8, e66778. [Google Scholar] [CrossRef]
- Naakka, E.; Barros-Filho, M.C.; Adnan-Awad, S.; Al-Samadi, A.; Marchi, F.A.; Kuasne, H.; Korelin, K.; Suleymanova, I.; Brown, A.L.; Scapulatempo-Neto, C.; et al. miR-22 and miR-205 Drive Tumor Aggressiveness of Mucoepidermoid Carcinomas of Salivary Glands. Front. Oncol. 2022, 11, 786150. [Google Scholar] [CrossRef] [PubMed]
- Kerche, L.E.; de Sousa, E.A.; Squarize, C.H.; Oliveira, K.K.; Marchi, F.A.; Bettim, B.B.; Kowalski, L.P.; Soares, F.A.; Lourenço, S.V.; Coutinho-Camillo, C.M. EMT in salivary gland tumors: The expression of microRNAs miR-155 and miR-200c is associated with clinical-pathological parameters. Mol. Biol. Rep. 2022, 49, 2157–2167. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Rodrigo, J.P.; Bradley, P.J.; Poorten, V.V.; Triantafyllou, A.; Hunt, J.L.; Strojan, P.; Rinaldo, A.; Haigentz, M., Jr.; Takes, R.P.; et al. Adenoid cystic carcinoma of the head and neck—An update. Oral Oncol. 2015, 51, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Cantù, G. Adenoid cystic carcinoma. An indolent but aggressive tumour. Part A: From aetiopathogenesis to diagnosis. Acta Otorhinolaryngol. Ital. 2021, 41, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Jaso, J.; Malhotra, R. Adenoid cystic carcinoma. Arch. Pathol. Lab. Med. 2011, 135, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-L.; Fan, Y.-L.; Jiang, J.; Li, K.-D.; Zheng, M.; Chen, W.; Ma, X.-R.; Geng, N.; Chen, Q.-M.; Chen, Y.; et al. C-kit induces epithelial-mesenchymal transition and contributes to salivary adenoid cystic cancer progression. Oncotarget 2014, 5, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Jia, D.; Yan, W.; Zhang, X.; Wang, C.; Li, Y.; Chen, H.; Huang, W.; Li, Z.; Zhang, X. KIT/PDGFRA/KDR amplification defines a novel molecular subtype of adenoid cystic carcinoma patients who may benefit from treatment with tyrosine kinase inhibitors. Transl. Cancer Res. 2020, 9, 4703–4714. [Google Scholar] [CrossRef] [PubMed]
- Swid, M.A.; Li, L.; Drahnak, E.M.; Idom, H.; Quinones, W. Updated Salivary Gland Immunohistochemistry: A Review. Arch. Pathol. Lab. Med. 2023, 147, 1383–1389. [Google Scholar] [CrossRef]
- Moore, A.; Bar, Y.; Maurice-Dror, C.; Ospovat, I.; Sarfaty, M.; Korzets, Y.; Goldvaser, H.; Gordon, N.; Billan, S.; Gutfeld, O.; et al. Next-generation sequencing in salivary gland carcinoma: Targetable alterations lead to a therapeutic advantage—Multicenter experience. Head Neck 2020, 42, 599–607. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Mitani, Y.; Diao, L.; Guijarro, I.; Wang, J.; Zweidler-McKay, P.; Bell, D.; William, W.N.; Glisson, B.S.; Wick, M.J.; et al. Activating NOTCH1 Mutations Define a Distinct Subgroup of Patients With Adenoid Cystic Carcinoma Who Have Poor Prognosis, Propensity to Bone and Liver Metastasis, and Potential Responsiveness to Notch1 Inhibitors. J. Clin. Oncol. 2017, 35, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Ochoa, A.; Jayakumaran, G.; Zehir, A.; Mayor, C.V.; Tepe, J.; Makarov, V.; Dalin, M.G.; He, J.; Bailey, M.; et al. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J. Clin. Investig. 2019, 129, 4276–4289. [Google Scholar] [CrossRef]
- Nordkvist, A.; Mark, J.; Gustafsson, H.; Bang, G.; Stenman, G. Non-random chromosome rearrangements in adenoid cystic carcinoma of the salivary glands. Genes, Chromosom. Cancer 1994, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.J.; Davies, H.R.; Mitani, Y.; Van Loo, P.; Shlien, A.; Tarpey, P.S.; Papaemmanuil, E.; Cheverton, A.; Bignell, G.R.; Butler, A.P.; et al. Whole exome sequencing of adenoid cystic carcinoma. J. Clin. Investig. 2013, 123, 2965–2968. [Google Scholar] [CrossRef]
- Mitani, Y.; Liu, B.; Rao, P.H.; Borra, V.J.; Zafereo, M.; Weber, R.S.; Kies, M.; Lozano, G.; Futreal, P.A.; Caulin, C.; et al. Novel MYBL1 Gene Rearrangements with Recurrent MYBL1–NFIB Fusions in Salivary Adenoid Cystic Carcinomas Lacking t(6;9) Translocations. Clin. Cancer Res. 2016, 22, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Roberts, D.; Karpowicz, M.; Hanna, E.Y.; Weber, R.S.; El-Naggar, A.K. Clinical significance of Myb protein and downstream target genes in salivary adenoid cystic carcinoma. Cancer Biol. Ther. 2011, 12, 569–573. [Google Scholar] [CrossRef]
- Andreasen, S.; Tan, Q.; Agander, T.K.; Hansen, T.V.O.; Steiner, P.; Bjørndal, K.; Høgdall, E.; Larsen, S.R.; Erentaite, D.; Olsen, C.H.; et al. MicroRNA dysregulation in adenoid cystic carcinoma of the salivary gland in relation to prognosis and gene fusion status: A cohort study. Virchows Arch. 2018, 473, 329–340. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, X.; Dong, Z.; Cao, G.; Zhang, S. Identification of microRNA profiles in salivary adenoid cystic carcinoma cells during metastatic progression. Oncol. Lett. 2014, 7, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-W.; Chen, B.; Lei, C.-B.; Liu, G.-X.; Wang, Y.-G.; Yi, C.; Wang, Y.-Y.; Zhang, S.-Y. miR-582-5p inhibits invasion and migration of salivary adenoid cystic carcinoma cells by targeting FOXC1. Jpn. J. Clin. Oncol. 2017, 47, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, V.; Straccia, P.; Tralongo, P.; Musarra, T.; Pierconti, F.; Martini, M.; Fadda, G.; Rossi, E.D.; Larocca, L.M. DOG1 as an Immunohistochemical Marker of Acinic Cell Carcinoma: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 9711. [Google Scholar] [CrossRef] [PubMed]
- Sheng, D.; Zhang, Y.; Xue, T.; Zhou, X.-Y.; Li, X.-Q. Identification of LMO2 as a new marker for acinic cell carcinoma of salivary gland. Diagn. Pathol. 2022, 17, 15. [Google Scholar] [CrossRef]
- Ross, J.S.; Gay, L.M.; Wang, K.; Vergilio, J.-A.; Suh, J.; Ramkissoon, S.; Somerset, H.; Johnson, J.M.; Russell, J.; Ali, S.; et al. Comprehensive genomic profiles of metastatic and relapsed salivary gland carcinomas are associated with tumor type and reveal new routes to targeted therapies. Ann. Oncol. 2017, 28, 2539–2546. [Google Scholar] [CrossRef]
- Dogan, S.; Xu, B.; Rana, S.; Chen, H.; Ghossein, R.A.; Berger, M.F.; Ho, A.L.; Katabi, N. Loss of CDKN2A/B is a Molecular Marker of High-grade Histology and is Associated with Aggressive Behavior in Acinic Cell Carcinoma. Mod. Pathol. 2023, 36, 100150. [Google Scholar] [CrossRef]
- Haller, F.; Bieg, M.; Will, R.; Körner, C.; Weichenhan, D.; Bott, A.; Ishaque, N.; Lutsik, P.; Moskalev, E.A.; Mueller, S.K.; et al. Enhancer hijacking activates oncogenic transcription factor NR4A3 in acinic cell carcinomas of the salivary glands. Nat. Commun. 2019, 10, 368. [Google Scholar] [CrossRef]
- Wong, K.S.; Mariño-Enriquez, A.; Hornick, J.L.; Jo, V.Y. NR4A3 Immunohistochemistry Reliably Discriminates Acinic Cell Carcinoma from Mimics. Head Neck Pathol. 2021, 15, 425–432. [Google Scholar] [CrossRef]
- Haller, F.; Skálová, A.; Ihrler, S.; Märkl, B.; Bieg, M.; Moskalev, E.A.; Erber, R.; Blank, S.; Winkelmann, C.; Hebele, S.; et al. Nuclear NR4A3 Immunostaining Is a Specific and Sensitive Novel Marker for Acinic Cell Carcinoma of the Salivary Glands. Am. J. Surg. Pathol. 2019, 43, 1264–1272. [Google Scholar] [CrossRef]
- Andreasen, S.; Varma, S.; Barasch, N.; Thompson, L.D.; Miettinen, M.; Rooper, L.; Stelow, E.B.; Agander, T.K.; Seethala, R.R.; Chiosea, S.I.; et al. The HTN3-MSANTD3 Fusion Gene Defines a Subset of Acinic Cell Carcinoma of the Salivary Gland. Am. J. Surg. Pathol. 2019, 43, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Sun, L.; Zhang, Y.; Liu, X.; Li, X.M.; Zhou, Z.; Cui, Y.M.; Zhou, C.-X.; Li, T.-J. PON3::LCN1 and HTN3::MSANTD3 Gene Fusions With NR4A3/NR4A2 Expression in Salivary Acinic Cell Carcinoma. Am. J. Surg. Pathol. 2024, 48, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Nakaguro, M.; Tada, Y.; Faquin, W.C.; Sadow, P.M.; Wirth, L.J.; Nagao, T. Salivary duct carcinoma: Updates in histology, cytology, molecular biology, and treatment. Cancer Cytopathol. 2020, 128, 693–703. [Google Scholar] [CrossRef]
- Santana, T.; Pavel, A.; Martinek, P.; Steiner, P.; Grossmann, P.; Baněčková, M.; Skálová, A. Biomarker immunoprofile and molecular characteristics in salivary duct carcinoma: Clinicopathological and prognostic implications. Hum. Pathol. 2019, 93, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.H.W. Salivary duct carcinoma: New developments—Morphological variants including pure in situ high grade lesions; Proposed molecular classification. Head Neck Pathol. 2013, 7, S48–S58. [Google Scholar] [CrossRef] [PubMed]
- Filippini, D.M.; Pagani, R.; Tober, N.; Lorini, L.; Riefolo, M.; Molinari, G.; Burato, A.; Alfieri, S.; Bossi, P.; Presutti, L. HER2-targeted therapies for salivary gland cancers. Oral Oncol. 2024, 148, 106612. [Google Scholar] [CrossRef] [PubMed]
- Glisson, B.; Colevas, A.D.; Haddad, R.; Krane, J.; El-Naggar, A.; Kies, M.; Costello, R.; Summey, C.; Arquette, M.; Langer, C.; et al. HER2 expression in salivary gland carcinomas: Dependence on histological subtype. Clin. Cancer Res. 2004, 10, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.A.; Gauthier, M.-E.A.; Blackburn, J.; Grady, J.P.; Kraitsek, S.; Hajdu, E.; Dettmer, M.S.; Dahlstrom, J.E.; Lee, C.S.; Luk, P.P.; et al. Molecular patterns in salivary duct carcinoma identify prognostic subgroups. Mod. Pathol. 2020, 33, 1896–1909. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, S.; Tada, Y.; Ando, M.; Nakaguro, M.; Shirai, Y.; Ueno, T.; Kojima, S.; Hirai, H.; Saigusa, N.; Kano, S.; et al. Identification of novel prognostic and predictive biomarkers in salivary duct carcinoma via comprehensive molecular profiling. NPJ Precis. Oncol. 2022, 6, 82. [Google Scholar] [CrossRef]
- Dalin, M.G.; Desrichard, A.; Katabi, N.; Makarov, V.; Walsh, L.A.; Lee, K.-W.; Wang, Q.; Armenia, J.; West, L.; Dogan, S.; et al. Comprehensive Molecular Characterization of Salivary Duct Carcinoma Reveals Actionable Targets and Similarity to Apocrine Breast Cancer. Clin. Cancer Res. 2016, 22, 4623–4633. [Google Scholar] [CrossRef] [PubMed]
- Skálová, A.; Vanecek, T.; Sima, R.; Laco, J.; Weinreb, I.; Perez-Ordonez, B.; Starek, I.; Geierova, M.; Simpson, R.H.; Passador-Santos, F.; et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: A hitherto undescribed salivary gland tumor entity. Am. J. Surg. Pathol. 2010, 34, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.T.; Fowler, E.; Krings, G.; Chen, Y.-Y.; Bean, G.R.; Vincent-Salomon, A.; Fuhrmann, L.; Barnick, S.E.; Chen, B.; Hosfield, E.M.; et al. Pan-TRK Immunohistochemistry: A Useful Diagnostic Adjunct For Secretory Carcinoma of the Breast. Am. J. Surg. Pathol. 2019, 43, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Guilmette, J.; Dias-Santagata, D.; Nosé, V.; Lennerz, J.K.; Sadow, P.M. Novel gene fusions in secretory carcinoma of the salivary glands: Enlarging the ETV6 family. Hum. Pathol. 2019, 83, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Rooper, L.M.; Karantanos, T.; Ning, Y.; Bishop, J.A.; Gordon, S.W.; Kang, H. Salivary Secretory Carcinoma With a Novel ETV6-MET Fusion: Expanding the Molecular Spectrum of a Recently Described Entity. Am. J. Surg. Pathol. 2018, 42, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Black, M.; Liu, C.Z.; Onozato, M.; Iafrate, A.J.; Darvishian, F.; Jour, G.; Cotzia, P. Concurrent Identification of Novel EGFR–SEPT14 Fusion and ETV6–RET Fusion in Secretory Carcinoma of the Salivary Gland. Head Neck Pathol. 2020, 14, 817–821. [Google Scholar] [CrossRef]
- Drilon, A. TRK inhibitors in TRK fusion-positive cancers. Ann. Oncol. 2019, 30, viii23–viii30. [Google Scholar] [CrossRef]
- Rooper, L.; Sharma, R.; Bishop, J.A. Polymorphous low grade adenocarcinoma has a consistent p63+/p40− immunophenotype that helps distinguish it from adenoid cystic carcinoma and cellular pleomorphic adenoma. Head Neck Pathol. 2015, 9, 79–84. [Google Scholar] [CrossRef]
- de Andrade, E.P.; Teixeira, L.N.; Montalli, V.A.M.; Garcia, F.d.M.; Passador-Santos, F.; Soares, A.B.; de Araújo, V.C. Epithelial membrane antigen and DOG1 expression in minor salivary gland tumours. Ann. Diagn. Pathol. 2019, 43, 151408. [Google Scholar] [CrossRef] [PubMed]
- Katabi, N.; Xu, B. Polymorphous Adenocarcinoma. Surg. Pathol. Clin. 2021, 14, 127–136. [Google Scholar] [CrossRef]
- Hahn, E.; Xu, B.; Katabi, N.; Dogan, S.; Smith, S.M.; Perez-Ordonez, B.; Patel, P.B.; MacMillan, C.; Lubin, D.J.; Gagan, J.; et al. Comprehensive Molecular Characterization of Polymorphous Adenocarcinoma, Cribriform Subtype: Identifying Novel Fusions and Fusion Partners. Mod. Pathol. 2023, 36, 100305. [Google Scholar] [CrossRef] [PubMed]
- de Jager, V.D.; de Visscher, S.A.H.J.; Schuuring, E.; Doff, J.J.; van Kempen, L.C. A novel PPP2R2A::PRKD1 fusion in a cribriform adenocarcinoma of salivary gland. Genes, Chromosom. Cancer 2023, 62, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Owosho, A.A.; Baker, E.; Wood, C.B.; Jain, R. A novel STRN3::PRKD1 fusion in a cribriform adenocarcinoma of salivary gland with high-grade transformation. Genes Chromosom. Cancer 2023, 62, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Freiberger, S.N.; Brada, M.; Fritz, C.; Höller, S.; Vogetseder, A.; Horcic, M.; Bihl, M.; Michal, M.; Lanzer, M.; Wartenberg, M.; et al. SalvGlandDx—A comprehensive salivary gland neoplasm specific next generation sequencing panel to facilitate diagnosis and identify therapeutic targets. Neoplasia 2021, 23, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Barbieri, A.L.; Bishop, J.A.; Chiosea, S.I.; Dogan, S.; Di Palma, S.; Faquin, W.C.; Ghossein, R.; Hyrcza, M.; Jo, V.Y.; et al. Histologic Classification and Molecular Signature of Polymorphous Adenocarcinoma (PAC) and Cribriform Adenocarcinoma of Salivary Gland (CASG): An International Interobserver Study. Am. J. Surg. Pathol. 2020, 44, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Milchgrub, S.; Gnepp, D.R.; Vuitch, F.; Delgado, R.; Albores-Saavedra, J. Hyalinizing clear cell carcinoma of salivary gland. Am. J. Surg. Pathol. 1994, 18, 74–82. [Google Scholar] [CrossRef]
- Antonescu, C.R.; Katabi, N.; Zhang, L.; Sung, Y.S.; Seethala, R.R.; Jordan, R.C.; Perez-Ordoñez, B.; Have, C.; Asa, S.L.; Leong, I.T.; et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosom. Cancer 2011, 50, 559–570. [Google Scholar] [CrossRef]
- Chapman, E.; Skalova, A.; Ptakova, N.; Martinek, P.; Goytain, A.; Tucker, T.; Xiong, W.; Leader, M.; Kudlow, B.A.; Haimes, J.D.; et al. Molecular Profiling of Hyalinizing Clear Cell Carcinomas Revealed a Subset of Tumors Harboring a Novel EWSR1-CREM Fusion: Report of 3 Cases. Am. J. Surg. Pathol. 2018, 42, 1182–1189. [Google Scholar] [CrossRef]
- Bishop, J.A. IDK what’s next for IDC: The unfolding saga of intraductal carcinoma of salivary glands. Cancer Cytopathol. 2021, 129, 926–927. [Google Scholar] [CrossRef]
- Bishop, J.A. Proceedings of the North American Society of Head and Neck Pathology, Los Angeles, CA, March 20, 2022: Emerging Entities in Salivary Gland Tumor Pathology. Head Neck Pathol. 2022, 16, 179–189. [Google Scholar] [CrossRef]
- Thompson, L.D.R.; Bishop, J.A. Salivary Gland Intraductal Carcinoma: How Do 183 Reported Cases Fit Into a Developing Classification. Adv. Anat. Pathol. 2023, 30, 112–129. [Google Scholar] [CrossRef] [PubMed]
- Skálová, A.; Vanecek, T.; Uro-Coste, E.; Bishop, J.A.; Weinreb, I.; Thompson, L.D.; de Sanctis, S.; Schiavo-Lena, M.; Laco, J.; Badoual, C.; et al. Molecular Profiling of Salivary Gland Intraductal Carcinoma Revealed a Subset of Tumors Harboring NCOA4-RET and Novel TRIM27-RET Fusions: A Report of 17 cases. Am. J. Surg. Pathol. 2018, 42, 1445–1455. [Google Scholar] [CrossRef]
- Robiony, M.; Politi, M.; Avellini, C.; Orsaria, M. Epithelial-myoepithelial carcinoma of the parotid gland: Clinicopathological aspect, diagnosis and surgical consideration. Ann. Maxillofac. Surg. 2014, 4, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Chiosea, S.I.; Miller, M.; Seethala, R.R. HRAS Mutations in epithelial–myoepithelial carcinoma. Head Neck Pathol. 2014, 8, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Nakaguro, M.; Tanigawa, M.D.; Hirai, H.; Yamamoto, Y.M.; Urano, M.; Takahashi, R.H.; Sukeda, A.; Okumura, Y.; Honda, S.; Tasaki, K.; et al. The Diagnostic Utility of RAS Q61R Mutation-specific Immunohistochemistry in Epithelial-Myoepithelial Carcinoma. Am. J. Surg. Pathol. 2021, 45, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, I.; Vollbrecht, C.; Meinrath, J.; Meyer, M.F.; Heukamp, L.C.; Drebber, U.; Quaas, A.; Beutner, D.; Hüttenbrink, K.-B.; Wardelmann, E.; et al. Targeted next generation sequencing of parotid gland cancer uncovers genetic heterogeneity. Oncotarget 2015, 6, 18224–18237. [Google Scholar] [CrossRef] [PubMed]
- Hellquist, H.; Paiva-Correia, A.; Poorten, V.V.; Quer, M.; Hernandez-Prera, J.C.; Andreasen, S.; Zbären, P.; Skalova, A.; Rinaldo, A.; Ferlito, A. Analysis of the Clinical Relevance of Histological Classification of Benign Epithelial Salivary Gland Tumours. Adv. Ther. 2019, 36, 1950–1974. [Google Scholar] [CrossRef]
- Stenman, G. Fusion oncogenes in salivary gland tumors: Molecular and Clinical consequences. Head Neck Pathol. 2013, 7, 12–19. [Google Scholar] [CrossRef]
- de Lima-Souza, R.A.D.; Altemani, A.; Michal, M.; Mariano, F.V.D.; Leivo, I.; Skálová, A. Expanding the Molecular Spectrum of Carcinoma Ex Pleomorphic Adenoma: An Analysis of 84 Cases With a Novel HMGA2::LINC02389 Fusion. Am. J. Surg. Pathol. 2024, 48, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Myers, J.N.; Rao, P.H.; El-Naggar, A.K. t(3;8) as the sole chromosomal abnormality in a myoepithelial carcinoma ex pleomorphic adenoma: A putative progression event. Head Neck 2013, 35, E181–E183. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Mneimneh, W.; Torrence, D.E.; Higgins, K.; Klimstra, D.; Ghossein, R.; Katabi, N. Misinterpreted Myoepithelial Carcinoma of Salivary Gland: A Challenging and Potentially Significant Pitfall. Am. J. Surg. Pathol. 2019, 43, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Dalin, M.G.; Katabi, N.; Persson, M.; Lee, K.-W.; Makarov, V.; Desrichard, A.; Walsh, L.A.; West, L.; Nadeem, Z.; Ramaswami, D.; et al. Multi-dimensional genomic analysis of myoepithelial carcinoma identifies prevalent oncogenic gene fusions. Nat. Commun. 2017, 8, 1197. [Google Scholar] [CrossRef]
- Skálová, A.M.; Weinreb, I.; Hyrcza, M.M.; Simpson, R.H.; Laco, J.M.; Agaimy, A.; Vazmitel, M.M.; Majewska, H.M.; Vanecek, T.R.; Talarčik, P.; et al. Clear cell myoepithelial carcinoma of salivary glands showing EWSR1 rearrangement: Molecular analysis of 94 salivary gland car-cinomas with prominent clear cell component. Am. J. Surg. Pathol. 2015, 39, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.; Freitas, C.; Fernandes, M.G.; Sousa, C.; Reboredo, C.; Cruz-Martins, N.; Mosquera, J.; Hespanhol, V.; Campelo, R. Liquid biopsy: The value of different bodily fluids. Biomarkers Med. 2022, 16, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Kuligina, E.S.; Yanus, G.A.; Imyanitov, E.N. Diversity of the Circulating Tumor Markers: Perspectives of a Multimodal Liquid Biopsy. Biochemistry 2024, 89, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Mouliere, F.; Chandrananda, D.; Piskorz, A.M.; Moore, E.K.; Morris, J.; Ahlborn, L.B.; Mair, R.; Goranova, T.; Marass, F.; Heider, K.; et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci. Transl. Med. 2018, 10, eaat4921. [Google Scholar] [CrossRef]
- Weijers, J.A.; Weijers, J.A.; Weijers, J.A.; de Bitter, T.J.; de Bitter, T.J.; de Bitter, T.J.; Verhaegh, G.W.; Verhaegh, G.W.; Verhaegh, G.W.; van Boxtel, W.; et al. Exploring the potential of circulating tumour DNA to monitor treatment response in salivary duct carcinoma patients of the CABO-ASAP trial. Oral Oncol. 2023, 147, 106620. [Google Scholar] [CrossRef] [PubMed]
- Visal, T.H.; Hollander, P.D.; Cristofanilli, M.; Mani, S.A. Circulating tumour cells in the -omics era: How far are we from achieving the ‘singularity’? Br. J. Cancer 2022, 127, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Vorobyeva, A.; Luan, Q.; Papautsky, I. Single Cell Analysis of Inertial Migration by Circulating Tumor Cells and Clusters. Micromachines 2023, 14, 787. [Google Scholar] [CrossRef]
- De Renzi, G.; De Marco, G.; De Meo, M.; Del Rosso, E.; Gazzaniga, P.; Nicolazzo, C. In vitro cultures of circulating tumor cells: A potential tool to unravel drug sensitivity. Cancer Drug Resist. 2022, 5, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Kahounová, Z.; Pícková, M.; Drápela, S.; Bouchal, J.; Szczyrbová, E.; Navrátil, J.; Souček, K. Circulating tumor cell-derived preclinical models: Current status and future perspectives. Cell Death Dis. 2023, 14, 530. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Tiwari, R.; Nauta, J.P.J.; Van der Waal, I.; Snow, G.B. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer 1993, 71, 452–456. [Google Scholar] [CrossRef]
- Weller, P.; Nel, I.; Hassenkamp, P.; Gauler, T.; Schlueter, A.; Lang, S.; Dountsop, P.; Hoffmann, A.-C.; Lehnerdt, G. Detection of circulating tumor cell subpopulations in patients with head and neck squamous cell carcinoma (HNSCC). PLoS ONE 2014, 9, e113706. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Tang, K.; Warkiani, M.; Punyadeera, C.; Batstone, M.D. A pilot study for presence of circulating tumour cells in adenoid cystic carcinoma. Int. J. Oral Maxillofac. Surg. 2021, 50, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Gužvić, N.S.; Lüke, F.; Treitschke, S.; Coluccio, A.; Hoffmann, M.; Feliciello, G.; Varadarajan, A.R.; Lu, X.; Weidele, K.; Botteron, C.; et al. Cellular liquid biopsy provides unique chances for disease monitoring, preclinical model generation and therapy adjustment in rare salivary gland cancer patients. Mol. Oncol. 2024. [Google Scholar] [CrossRef]
- Vaiaki, E.M.; Falasca, M. Comparative analysis of the minimal information for studies of extracellular vesicles guidelines: Advancements and implications for extracellular vesicle research. Semin. Cancer Biol. 2024, 101, 12–24. [Google Scholar] [CrossRef]
- Goberdhan, D.C.I. Large tumour-derived extracellular vesicles as prognostic indicators of metastatic cancer patient survival. Br. J. Cancer 2023, 128, 471–473. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, J.; Zhang, Z.; Qian, Y.; Wang, G.; Duan, M.; Zhao, H.; Yang, Z.; Jiang, X. Mesenchymal stem cell-derived exosome: A tumor regulator and carrier for targeted tumor therapy. Cancer Lett. 2022, 526, 29–40. [Google Scholar] [CrossRef]
- Cocks, A.; Martinez-Rodriguez, V.; Del Vecchio, F.; Schukking, M.; Broseghini, E.; Giannakopoulos, S.; Fabbri, M. Diverse roles of EV-RNA in cancer progression. Semin. Cancer Biol. 2021, 75, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, F.; Martinez-Rodriguez, V.; Schukking, M.; Cocks, A.; Broseghini, E.; Fabbri, M. Professional killers: The role of extracellular vesicles in the reciprocal interactions between natural killer, CD8+ cytotoxic T-cells and tumour cells. J. Extracell. Vesicles 2021, 10, e12075. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.; Dittmar, R.L.; Liu, Y.; Liang, M.; et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom. 2013, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.; Mariscal, J.; Kim, M.; Guerra, G.; Victor, B.; Qian, C.; Broseghini, E.; Posadas, E.; Freeman, M.R.; Sharma, S.; et al. miR-1227 Targets SEC23A to Regulate the Shedding of Large Extracellular Vesicles. Cancers 2021, 13, 5850. [Google Scholar] [CrossRef]
- Durante, G.; Broseghini, E.; Comito, F.; Naddeo, M.; Milani, M.; Salamon, I.; Campione, E.; Dika, E.; Ferracin, M. Circulating microRNA biomarkers in melanoma and non-melanoma skin cancer. Expert Rev. Mol. Diagn. 2022, 22, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Nieszporek, A.; Wierzbicka, M.; Labedz, N.; Zajac, W.; Cybinska, J.; Gazinska, P. Role of Exosomes in Salivary Gland Tumors and Technological Advances in Their Assessment. Cancers 2024, 16, 3298. [Google Scholar] [CrossRef]
- Pawel, S.; Maciej, M.; Maciej, Z.; Bogdan, M.; Monika, A.-S. Novel interleukin-33 and its soluble ST2 receptor as potential serum biomarkers in parotid gland tumors. Exp. Biol. Med. 2018, 243, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Dyckhoff, G.; Warta, R.; Gonnermann, A.; Plinkert, P.K.; Flechtenmacher, C.; Volkmann, M. Carbohydrate Antigen 19-9 in Saliva: Possible Preoperative Marker of Malignancy in Parotid Tumors. Otolaryngol--head. Otolaryngol. Neck Surg. 2011, 145, 772–777. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, G.; Zhou, L.; Liu, Y. A joint detection of CEA and CA-50 levels in saliva and serum of patients with tumors in oral region and salivary gland. J. Cancer Res. Clin. Oncol. 2009, 135, 1315–1321. [Google Scholar] [CrossRef]
- Summerer, I.; Unger, K.; Braselmann, H.; Schuettrumpf, L.; Maihoefer, C.; Baumeister, P.; Kirchner, T.; Niyazi, M.; Sage, E.; Specht, H.M.; et al. Circulating microRNAs as prognostic therapy biomarkers in head and neck cancer patients. Br. J. Cancer 2015, 113, 76–82. [Google Scholar] [CrossRef]
- Broseghini, E.; Filippini, D.M.; Fabbri, L.; Leonardi, R.; Abeshi, A.; Molin, D.D.; Fermi, M.; Ferracin, M.; Fernandez, I.J. Diagnostic and Prognostic Value of microRNAs in Patients with Laryngeal Cancer: A Systematic Review. Non-Coding RNA 2023, 9, 9. [Google Scholar] [CrossRef]
- Kabzinski, J.; Maczynska, M.; Majsterek, I. MicroRNA as a Novel Biomarker in the Diagnosis of Head and Neck Cancer. Biomolecules 2021, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Dharmawardana, N.; Ooi, E.H.; Woods, C.; Hussey, D. Circulating microRNAs in head and neck cancer: A scoping review of methods. Clin. Exp. Metastasis 2019, 36, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.R.; Thachil, T.; Gee, H.; Milic, N. Circulating MicroRNAs as Prognostic Molecular Biomarkers in Human Head and Neck Cancer: A Systematic Review and Meta-Analysis. Dis. Markers 2019, 2019, 8632018. [Google Scholar] [CrossRef]
- Filippini, D.M.; Broseghini, E.; Carosi, F.; Molin, D.D.; Riefolo, M.; Fabbri, L.; Abeshi, A.; Fernandez, I.J.; Ferracin, M. A Systematic Review of Diagnostic and Prognostic Biomarkers for Head and Neck Cancer of Unknown Primary: An Unmet Clinical Need. Diagnostics 2023, 13, 1492. [Google Scholar] [CrossRef] [PubMed]
- Cinpolat, O.; Unal, Z.N.; Ismi, O.; Gorur, A.; Unal, M. Comparison of microRNA profiles between benign and malignant salivary gland tumors in tissue, blood and saliva samples: A prospective, case-control study. Braz. J. Otorhinolaryngol. 2017, 83, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.S.; Normando, A.G.C.; Scarini, J.F.; Crescencio, L.R.; de Lima-Souza, R.A.; Mariano, F.V.; Leme, A.F.P. Diagnostic and prognostic value of miRNAs on salivary gland tumors: A systematic review and meta-analysis. Oral Maxillofac. Surg. 2021, 25, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Matse, J.H.; Yoshizawa, J.; Wang, X.; Elashoff, D.; Bolscher, J.G.M.; Veerman, E.C.I.; Bloemena, E.; Wong, D.T.W. Discovery and prevalidation of salivary extracellular microRNA biomarkers panel for the noninvasive detection of benign and malignant parotid gland tumors. Clin. Cancer Res. 2013, 19, 3032–3038. [Google Scholar] [CrossRef]
- Matse, J.H.; Yoshizawa, J.; Wang, X.; Elashoff, D.; Bolscher, J.G.M.; Veerman, E.C.I.; Leemans, C.R.; Pegtel, M.D.; Wong, D.T.W.; Bloemena, E. Human Salivary Micro-RNA in Patients with Parotid Salivary Gland Neoplasms. PLoS ONE 2015, 10, e0142264. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, M.; Palisi, A.; Rossi, G.; Stillitano, I.; Faiella, F.; Montoro, P.; Rodriquez, M.; Palladino, R.; D’ursi, A.M.; Romano, R. Saliva of patients affected by salivary gland tumour: An NMR metabolomics analysis. J. Pharm. Biomed. Anal. 2018, 160, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Tada, Y.; Saotome, T.; Akazawa, K.; Ojiri, H.; Fushimi, C.; Masubuchi, T.; Matsuki, T.; Tani, K.; Osamura, R.Y.; et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2–Positive salivary duct carcinoma. J. Clin. Oncol. 2019, 37, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Shen, R.; Buonocore, D.; Olah, Z.T.; Ni, A.; Ginsberg, M.S.; Ulaner, G.A.; Offin, M.; Feldman, D.; Hembrough, T.; et al. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J. Clin. Oncol. 2018, 36, 2532–2537. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Lassche, G.; Tada, Y.; van Herpen, C.M.L.; Jonker, M.A.; Nagao, T.; Saotome, T.; Hirai, H.; Saigusa, N.; Takahashi, H.; Ojiri, H.; et al. Predictive and Prognostic Biomarker Identification in a Large Cohort of Androgen Receptor-Positive Salivary Duct Carcinoma Patients Scheduled for Combined Androgen Blockade. Cancers 2021, 13, 3527. [Google Scholar] [CrossRef]
- Saigusa, N.; Hirai, H.; Tada, Y.; Kawakita, D.; Nakaguro, M.; Tsukahara, K.; Kano, S.; Ozawa, H.; Kondo, T.; Okami, K.; et al. The Role of the EZH2 and H3K27me3 Expression as a Predictor of Clinical Outcomes in Salivary Duct Carcinoma Patients: A Large-Series Study With Emphasis on the Relevance to the Combined Androgen Blockade and HER2-Targeted Therapy. Front. Oncol. 2021, 11, 779882. [Google Scholar] [CrossRef]
- Fushimi, C.; Tada, Y.; Takahashi, H.; Nagao, T.; Ojiri, H.; Masubuchi, T.; Matsuki, T.; Miura, K.; Kawakita, D.; Hirai, H.; et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann. Oncol. 2018, 29, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Cavalieri, S.; Bergamini, C.; Resteghini, C.; Colombo, E.; Calareso, G.; Mariani, L.; Quattrone, P.; Alfieri, S.; Bossi, P.; et al. Abiraterone Acetate in Patients With Castration-Resistant, Androgen Receptor–Expressing Salivary Gland Cancer: A Phase II Trial. J. Clin. Oncol. 2021, 39, 4061–4068. [Google Scholar] [CrossRef] [PubMed]
- van Boxtel, W.; Locati, L.; van Engen-van Grunsven, A.C.H.; Bergamini, C.; Jonker, M.; Fiets, E.; Cavalieri, S.; Tooten, S.; Bos, E.; Quattrone, P.; et al. Adjuvant androgen deprivation therapy for poor-risk, androgen receptor–positive salivary duct carcinoma. Eur. J. Cancer 2019, 110, 62–70. [Google Scholar] [CrossRef]
- Weijers, J.A.M.; Verhaegh, G.W.; Lassche, G.; van Engen-van Grunsven, A.C.H.; Driessen, C.M.L.; van Erp, N.P.; Jonker, M.A.; Schalken, J.A.; van Herpen, C.M.L. A randomized phase II trial on the addition of dutasteride to combined androgen blockade therapy versus combined androgen blockade therapy alone in patients with advanced or metastatic salivary duct carcinoma—The DUCT study protocol. BMC Cancer 2024, 24, 1174. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum, J.; Shestopalov, I.A.; Henderson, R.E.; Chau, N.G.; Knoechel, B.; Wick, M.J.; Zon, L.I. Zebrafish blastomere screen identifies retinoic acid suppression of MYB in adenoid cystic carcinoma. J. Exp. Med. 2018, 215, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Ferrarotto, R.; Eckhardt, G.; Patnaik, A.; LoRusso, P.; Faoro, L.; Heymach, J.; Kapoun, A.; Xu, L.; Munster, P. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann. Oncol. 2018, 29, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Stathis, A.; Lopez-Miranda, E.; Racca, F.; Quon, D.; Leyvraz, S.; Hess, D.; Keam, B.; Rodon, J.; Ahn, M.-J.; et al. A Phase I Study of the Pan-Notch Inhibitor CB-103 for Patients with Advanced Adenoid Cystic Carcinoma and Other Tumors. Cancer Res. Commun. 2023, 3, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.; Bossi, P.; Perrone, F.; Potepan, P.; Crippa, F.; Mariani, L.; Casieri, P.; Orsenigo, M.; Losa, M.; Bergamini, C.; et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: A phase II study. Oral Oncol. 2009, 45, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Amoroso, V.; Buglione, M.; Rosati, A.; Gatta, R.; Pizzocaro, C.; Ferrari, V.D.; Marini, G. Cetuximab in the treatment of metastatic mucoepidermoid carcinoma of the salivary glands: A case report and review of literature. J. Med. Case Rep. 2008, 2, 320. [Google Scholar] [CrossRef]
- Kawahara, K.; Hiraki, A.; Yoshida, R.; Arita, H.; Matsuoka, Y.; Yamashita, T.; Koga, K.-I.; Nagata, M.; Hirosue, A.; Fukuma, D.; et al. Salivary duct carcinoma treated with cetuximab-based targeted therapy: A case report. Mol. Clin. Oncol. 2017, 6, 886–892. [Google Scholar] [CrossRef]
- Lin, V.T.; Nabell, L.M.; Spencer, S.A.; Carroll, W.R.; Harada, S.; Yang, E.S. First-Line Treatment of Widely Metastatic BRAF-Mutated Salivary Duct Carcinoma With Combined BRAF and MEK Inhibition. J. Natl. Compr. Cancer Netw. 2018, 16, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G.; Kang, S.; Vogt, P.K. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Sheth, H.; Kumar, P.; Shreenivas, A.; Sambath, J.; Pragya, R.; Madre, C.; Athikari, N.; Khandare, H.; Peshattiwar, V.; Datar, R.; et al. Excellent Response With Alpelisib and Bicalutamide for Advanced Salivary Duct Carcinoma With PIK3CA Mutation and High Androgen Receptor Expression—A Case Report. JCO Precis. Oncol. 2021, 5, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Yamaki, H.; Komatsuda, H.; Wakisaka, R.; Inoue, T.; Kumai, T.; Takahara, M. Exploring Immunological Effects and Novel Immune Adjuvants in Immunotherapy for Salivary Gland Cancers. Cancers 2024, 16, 1205. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Kumai, T.; Ishida, Y.; Yuasa, R.; Kubota, A.; Wakisaka, R.; Komatsuda, H.; Yamaki, H.; Wada, T.; Harabuchi, Y. The efficacy of PD-1 inhibitors in patients with salivary gland carcinoma: A retrospective observational study. Laryngoscope Investig. Otolaryngol. 2022, 7, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, U.; Bang, A.; Chen, Y.-H.; Mak, R.H.; Lorch, J.H.; Hanna, G.J.; Nishino, M.; Manuszak, C.; Thrash, E.M.; Severgnini, M.; et al. A Randomized Phase 2 Study of Pembrolizumab With or Without Radiation in Patients With Recurrent or Metastatic Adenoid Cystic Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, S.; Tasoulas, J.; Masaoutis, C.; Kokkali, S.; Klijanienko, J. Salivary gland cancer in the era of immunotherapy: Can we exploit tumor microenvironment? Expert Opin. Ther. Targets 2020, 24, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Vos, J.L.; Burman, B.; Jain, S.; Fitzgerald, C.W.R.; Sherman, E.J.; Dunn, L.A.; Fetten, J.V.; Michel, L.S.; Kriplani, A.; Ng, K.K.; et al. Nivolumab plus ipilimumab in advanced salivary gland cancer: A phase 2 trial. Nat. Med. 2023, 29, 3077–3089. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Baik, C.; Bauman, J.; Gilbert, J.; Brose, M.S.; Grilley-Olson, J.E.; Patil, T.; McDermott, R.; Raez, L.E.; Johnson, J.M.; et al. Larotrectinib treatment for patients With TRK fusion-positive salivary gland cancers. Oncol. 2022, 29, e779–e788. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Li, G.; Dogan, S.; Gounder, M.; Shen, R.; Arcila, M.; Wang, L.; Hyman, D.M.; Hechtman, J.; Wei, G.; et al. What hides behind the MASC: Clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann. Oncol. 2016, 27, 920–926. [Google Scholar] [CrossRef]
- Hyman, D.; Kummar, S.; Farago, A.; Geoerger, B.; Mau-Sorensen, M.; Taylor, M.; Garralda, E.; Nagasubramanian, R.; Natheson, M.; Song, L.; et al. Abstract CT127: Phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi). Cancer Res. 2019, 79, CT127. [Google Scholar] [CrossRef]
- Drilon, A.; Ou, S.-H.I.; Cho, B.C.; Kim, D.-W.; Lee, J.; Lin, J.J.; Zhu, V.W.; Ahn, M.-J.; Camidge, D.R.; Nguyen, J.; et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov. 2018, 8, 1227–1236. [Google Scholar] [CrossRef]
- Rodriguez, C.P.; Wu, Q.; Voutsinas, J.; Fromm, J.R.; Jiang, X.; Pillarisetty, V.G.; Lee, S.M.; Santana-Davila, R.; Goulart, B.; Baik, C.S.; et al. A Phase II Trial of Pembrolizumab and Vorinostat in Recurrent Metastatic Head and Neck Squamous Cell Carcinomas and Salivary Gland Cancer. Clin. Cancer Res. 2020, 26, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Filippini, D.M.; Le Tourneau, C. The potential roles of antibody-drug conjugates in head and neck squamous cell carcinoma. Curr. Opin. Oncol. 2024, 36, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.M.; Mitani, Y.; Marques-Piubelli, M.; Hoff, C.; Bonini, F.; Purushothaman, A.; McGrail, D.; El-Naggar, A.; Ferrarotto, R. 909P TROP2 expression in salivary gland adenoid cystic carcinoma (ACC): A new potential therapeutic target for the non-solid subtype. Ann. Oncol. 2023, 34, S578. [Google Scholar] [CrossRef]
| Histological Subtype | Histopathological and Immunohistochemical Markers | Detectable Mutations | Molecular Alterations |
|---|---|---|---|
| Mucoepidermoid carcinoma (MEC) | Positive for p63 or p40 with no detection of S100 or SOX10; however, a subset of solid/trabecular MECs may be completely negative for p63 and p40. | CDKN2A TP53 PIK3CA HRAS POUF6F2 | CRTC1::MAML2 CRTC3::MAML2 EWSR1::POU5F1 |
| Adenoid cystic carcinoma (ACC) | Pancytokeratin strong positivity in ductal cells and weaker staining in myoepithelial cells. CK7 and c-KIT (CD117) are generally expressed in ductal cells, while p63, p40, calponin, and α-SMA are associated with myoepithelial cells. MYB protein overexpression. | PIK3CA TP53 NOTCH TERT | MYB::NFIB MYBL1::NFIB MYB::PDCD1LG2 MYB::EFFR3A MYBL1::RAD51B MYBL1::YTHDF3 NIB::AIG1 |
| Acinic cell carcinoma (AciCC) | Positive for CK7, SOX10, DOG1 and NR4A3 (NOR1) and negative for p40/p63, mammaglobin, and S100. | PTEN TP53 CDKN2A/B BRAF NF1 | SCPP::NR4A3 HTN3::MSANTD3 PON3-LCN1 |
| Salivary duct carcinoma (SDC) | CK7 shows consistent positivity, while S100 and SOX10 are negative. Staining for p63 can assist in identifying the intraductal component by highlighting the basal/myoepithelial cells around the neoplastic cells. AR-positive. Strong and diffuse immunoreactivity for HER2 in approximately one-third of SDCs. | TP53 PIK3CA PTEN HRAS | AR gene alterations ERBB2 amplifications NF1 KMT2C EGFR ALK CDKN2A NOTCH1 KDM5C NRAS BRAF AKT ETV6 NTRK3 |
| Secretory carcinoma (SC) | CK7, S100, SOX10, vimentin, mammaglobin positive; p63, p40, NR4A3, and DOG1 negative. Pan-TRK positive. | - | ETV6-NTRK3 fusion ETV6-RET ETV6-MAML3 ETV6-MET |
| Polymorphous adenocarcinoma (PAC) | CK-7, S100, CEA, GFAP, SOX10 positive, patchy expression of p63; p40 typically negative. | PRKD1 PTEN FGFR1 TSC2 | ARID1A::PRKD1 ARID1A::DDX3X PRKD2, PRKD3 |
| Hyalinizing clear cell carcinoma (HCCC) | p40 and CK5/6 diffuse positivity; mucin positive; p16 positive with no transcriptionally active HPV. Myoepithelial markers such as S100, SMA, and calponin are negative. | - | EWSR1::ATF1 fusion EWSR1::CREM |
| Intraductal carcinoma (IDC) | Intercalated ducts and oncocytic IDCs exhibit positivity for S100, SOX10, and mammaglobin, but do not express AR or GCDFP-15. In contrast, apocrine IDCs display an inverse staining pattern. Mixed IDCs present a combined immunoprofile. Myoepithelial cells within IDCs consistently stain positive for p40/p63, CK14, SMA, and calponin. | PIK3CA HRAS BRAF p.V600E TP53 | NCOA4::RET TRIM33::RET TRIM27::RET TUT1::ETV5, KIAA1217::RET STRN::ALK fusion |
| Epithelial–myoepithelial carcinoma (EMC) | Luminal cells typically express CK7, while abluminal cells are generally positive for SMA, calponin, and p63/p40. | HRAS PLAG1 HMGA2 | CTNNB1 AKT1 PIK3CA FBXW7 |
| Carcinoma ex-pleomorphic adenoma (CA ex PA) | PLAG1 and HMGA2 for the identification of the PA component. AR positivity could be indicative of salivary duct CA ex PA. | - | PLAG1/HMGA2 rearrangements |
| Myoepithelial carcinoma (MECA) de novo, MECA ex PA | Positive for SOX10, S100, and myoepithelial markers such as SMA, calponin, and p63/p40. | - | PLAG1::FGFR1 PLAG1::TGFBR3 EWSR1 rearrangement EWSR1-ATF1 |
| Liquid Biomarker | Description | Origin | Function | References |
|---|---|---|---|---|
| Circulating tumor DNA (ctDNA) | Fragments of tumor-derived DNA released into bodily fluids; enriched in fragments of 90–150 bp length. | Plasma, saliva | Early diagnostic biomarker, molecular characterization (ctDNA mutation), real-time efficacy monitoring biomarker. | [99,100] |
| Circulating tumor cells (CTCs) | Malignant cells detached from tumors, traveling in bodily fluids; low abundance in blood (1–10 cells/10 mL). | Peripheral blood | Insights into metastasis, treatment response and surveillance. | [102,103,107,108] |
| Extracellular vesicles (EVs) | Membrane-bound vesicles (exosomes, microvesicles, apoptotic bodies) containing RNA, proteins, and other biomolecules. | Peripheral blood, saliva | Diagnostic and prognostic potential; modulation of tumor microenvironment and immune response. | [109,110,115,116] |
| Exosomes | A subtype of EVs (40–150 nm) containing RNAs, miRNA, lncRNA, snoRNA, and proteins. | Peripheral blood, saliva | Diagnostic potential; implicated in tumor progression, modulation of tumor microenvironment; therapeutic targeting. | [118] |
| Inflammatory markers | Elevated IL-33 and its sST2 receptor levels in parotid gland tumors. | Serum | Differentiation between tumors and healthy controls. | [119] |
| CEA, CA-50, CA 19-9 | Tumor-associated antigens; higher levels in saliva of patients with malignant tumors compared to benign and controls. | Saliva | Salivary antigens levels more sensitive than serum measurements for tumor detection; differentiation between malignant and benign parotid gland tumors. | [120,121] |
| Circulating miRNAs | Dysregulated miRNAs with specific profiles for benign and malignant tumors; notable miRNAs include miR-21, miR-23a, miR-30e, and others. | Peripheral blood, saliva | Diagnostic and prognostic markers; potential for differentiation between malignant and benign tumors. | [122,128,130,131] |
| Metabolomic biomarkers | Distinct metabolomic profiles in patients with parotid tumors; altered alanine and leucine levels. | Saliva | Indication of metabolic disruptions; potential diagnostic tool: differentiation between tumors and healthy controls. | [132] |
| Biomarker | Targeted Therapy | Cancer Type | References |
|---|---|---|---|
| HER2 overexpression/amplification | Trastuzumab, pertuzumab, T-DM1, T-DXd | SDC | [133,134,135] |
| AR overexpression | Androgen deprivation therapy (ADT) | SDC | [138,139,140,141] |
| NOTCH mutations | Gamma-secretase inhibitors | ACC | [143,144] |
| BRAF V600E mutation | BRAF + MEK inhibitors | SDC | [148] |
| NTRK fusions | Larotrectinib, entrectinib | Secretory carcinoma | [156,157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broseghini, E.; Carosi, F.; Berti, M.; Compagno, S.; Ghelardini, A.; Fermi, M.; Querzoli, G.; Filippini, D.M. Salivary Gland Cancers in the Era of Molecular Analysis: The Role of Tissue and Liquid Biomarkers. Cancers 2025, 17, 660. https://doi.org/10.3390/cancers17040660
Broseghini E, Carosi F, Berti M, Compagno S, Ghelardini A, Fermi M, Querzoli G, Filippini DM. Salivary Gland Cancers in the Era of Molecular Analysis: The Role of Tissue and Liquid Biomarkers. Cancers. 2025; 17(4):660. https://doi.org/10.3390/cancers17040660
Chicago/Turabian StyleBroseghini, Elisabetta, Francesca Carosi, Mirea Berti, Samuele Compagno, Anna Ghelardini, Matteo Fermi, Giulia Querzoli, and Daria Maria Filippini. 2025. "Salivary Gland Cancers in the Era of Molecular Analysis: The Role of Tissue and Liquid Biomarkers" Cancers 17, no. 4: 660. https://doi.org/10.3390/cancers17040660
APA StyleBroseghini, E., Carosi, F., Berti, M., Compagno, S., Ghelardini, A., Fermi, M., Querzoli, G., & Filippini, D. M. (2025). Salivary Gland Cancers in the Era of Molecular Analysis: The Role of Tissue and Liquid Biomarkers. Cancers, 17(4), 660. https://doi.org/10.3390/cancers17040660






