Robotic Surgery in the Management of Renal Tumors During Pregnancy: A Narrative Review
Simple Summary
Abstract
1. Introduction
2. Study Design and Selection Criteria
3. Epidemiology and Etiology
4. Diagnosis and Imaging
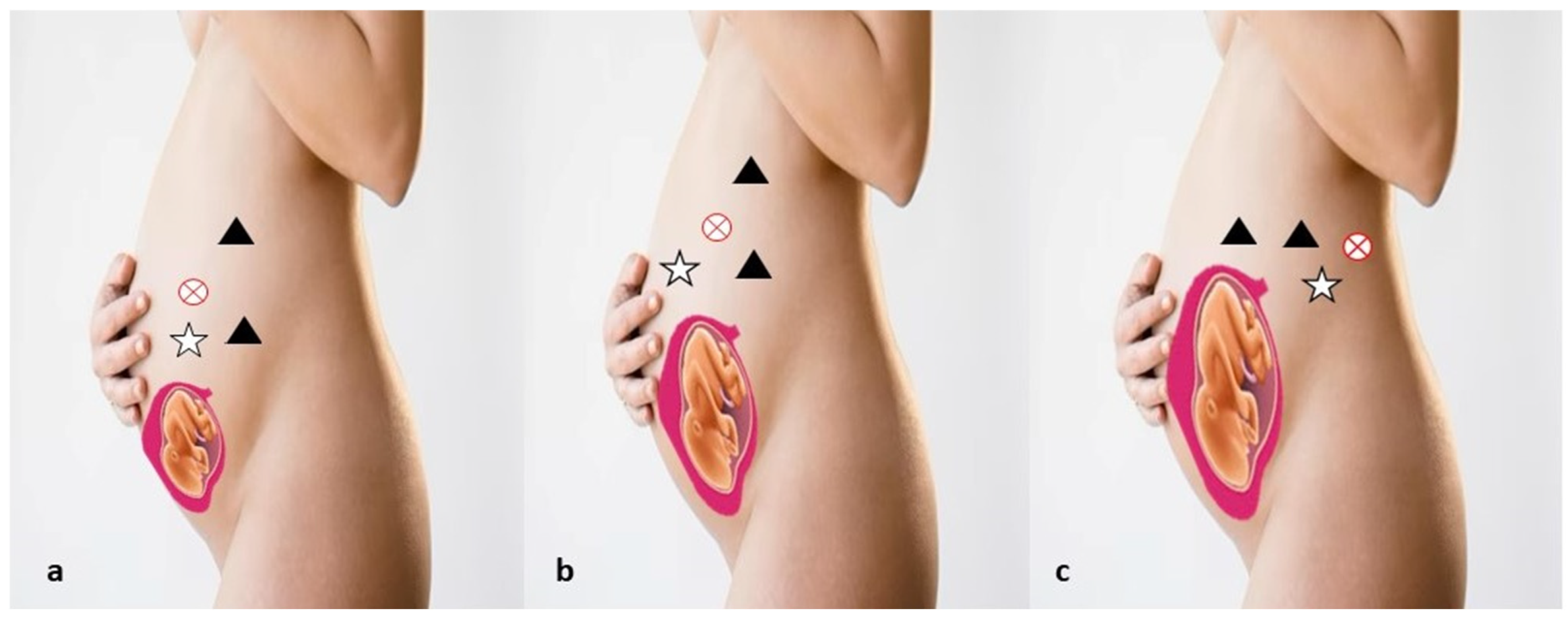
5. Robotic Management
6. Treatment Plan and Outcomes
Treatment of Metastatic Renal Tumors
7. Future Directions and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajeev, S.; Brendan, B. Robot-assisted radical nephrectomy for renal-cell carcinoma during twin pregnancy. Urol. Nephrol. Open Access J. 2023, 11, 111–113. [Google Scholar]
- Xu, H.; Tan, S. Diagnosis and treatment of renal cell carcinoma during pregnancy. Cancer Manag. Res. 2021, 13, 9423–9428. [Google Scholar] [CrossRef]
- Dell’Atti, L.; Borghi, C.; Galosi, A.B. Laparoscopic approach in management of renal cell carcinoma during pregnancy: State of the art. Clin. Genitourin. Cancer 2019, 17, e822–e830. [Google Scholar] [CrossRef]
- Palanivelu, C.; Rangarajan, M.; Senthilkumaran, S.; Parthasarathi, R. Safety and efficacy of laparoscopic surgery in pregnancy: Experience of a single institution. J. Laparoendosc. Adv. Surg. Tech. A 2017, 17, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Guerrero, E.; Claro, A.V.O.; Ledo Cepero, M.J.; Soto Delgado, M.; Álvarez-Ossorio Fernández, J.L. Robotic versus Laparoscopic Partial Nephrectomy in the New Era: Systematic Review. Cancers 2023, 15, 1793. [Google Scholar] [CrossRef]
- Gettman, M.T.; Blute, M.L.; Chow, G.K.; Neururer, R.; Bartsch, G.; Peschel, R. Robotic-assisted laparoscopic partial nephrectomy: Technique and initial clinical ex-perience with daVinci robotic system. Urology 2004, 64, 914–918. [Google Scholar] [CrossRef]
- Haseebuddin, M.; Benway, B.M.; Cabello, J.M.; Bhayani, S.B. Robot-Assisted partial nephrectomy: Evaluation of learning curve for an experienced renal surgeon. J. Endourol. 2010, 24, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Wolters, V.; Heimovaara, J.; Maggen, C.; Cardonick, E.; Boere, I.; Lenaerts, L.; Amant, F. Management of pregnancy in women with cancer. Int. J. Gynecol. Cancer 2021, 31, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Kalampokas, E.; Vlahos, N.; Kalampokas, T.; Gurumurthy, M. Common Malignancies During Pregnancy: A Comprehensive Review. Cancer Diagn. Progn. 2021, 1, 103–109. [Google Scholar] [CrossRef]
- Milosevic, B.; Likic Ladjevic, I.; Dotlic, J.; Beleslin, A.; Mihaljevic, O.; Pilic, I.; Kesic, V.; Gojnic, M.; Stefanovic, A.; Stefanovi, K. Cancer during pregnancy: Twenty-two years of experience from a tertiary referral center. Acta Obstet. Gynecol. Scand. 2024, 103, 716–728. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Peng, L.; Gou, X.; Fan, J. An update on recent developments in rupture of renal angiomyolipoma. Medicine 2018, 97, e0497. [Google Scholar] [CrossRef]
- Chu, J.S.; Wang, Z.J. Protocol optimization for renal mass detection and characterization. Radiol. Clin. N. Am. 2020, 58, 851–873. [Google Scholar] [CrossRef]
- Khazzaka, A.; Rassy, E.; Sleiman, Z.; Boussios, S.; Pavlidis, N. Systematic review of fetal and placental metastases among pregnant patients with cancer. Cancer Treat. Rev. 2022, 104, 102356. [Google Scholar] [CrossRef]
- Caglayan, A.; Rabbani, R.D.; Sanchez, E.; Choi, S.; Ismail, A.; Papadopoulos, V.; Adeleke, S.; Ghose, A.; Boussios, S. Gestational Renal Cell Cancer—An Update. Anticancer Res. 2023, 43, 3871–3880. [Google Scholar] [CrossRef] [PubMed]
- Lambe, M.; Lindblad, P.; Wuu, J.; Remler, R.; Hsieh, C.C. Pregnancy and risk of renal cell cancer: A population-based study in Sweden. Br. J. Cancer 2002, 86, 1425–1429. [Google Scholar] [CrossRef]
- Macleod, L.C.; Hotaling, J.M.; Wright, J.L.; Davenport, M.T.; Gore, J.L.; Harper, J.; White, E. Risk factors for renal cell carcinoma in the VITAL study. J. Urol. 2013, 190, 1657–1661. [Google Scholar] [CrossRef]
- Bergström, A.; Hsieh, C.C.; Lindblad, P.; Lu, C.M.; Cook, N.R.; Wolk, A. Obesity and renal cell cancer—A quantitative review. Br. J. Cancer 2001, 85, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Schouten, L.J.; Van De Pol, J.; Kviatkovsky, M.J.; Van Den Brandt, P.A. Reproductive and external hormonal factors and the risk of renal cell cancer in the Netherlands Cohort Study. Cancer Epidemiol. 2022, 79, 102171. [Google Scholar] [CrossRef]
- Aurilio, G.; Piva, F.; Santoni, M.; Cimadamore, A.; Sorgentoni, G.; Lopez-Beltran, A.; Cheng, L.; Battelli, N.; Nolè, F.; Montironi, R. The role of obesity in renal cell carcinoma patients: Clinical pathological implications. Int. J. Mol. Sci. 2019, 20, 5683. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of renal cell carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Deckers, I.A.; van Den Brandt, P.A.; van Engeland, M.; van Schooten, F.-J.; Godschalk, R.W.; Keszei, A.P.; Schouten, L.J. Polymorphisms in genes of the renin-angiotensin-aldosterone system and renal cell cancer risk: Interplay with hypertension and intakes of sodium, potassium and fluid. Int. J. Cancer 2015, 136, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Oguz, F.; Tuncay, G.; Melekoglu, R.; Beytur, A.; Coskun, E.I.; Gunes, A. Renal cell carcinoma diagnosed during pregnancy: A case report and literature review. J. Int. Med. Res. 2018, 46, 3422–3426. [Google Scholar] [CrossRef] [PubMed]
- Bettez, M.; Carmel, M.; Temmar, R.; Côté, A.M.; Sauvé, N.; Asselah, J.; Sabbagh, R. Fatal fast-growing renal cell carcinoma during pregnancy. J. Obstet. Gynaecol. Can. 2011, 33, 258–261. [Google Scholar] [CrossRef]
- Casella, R.; Ferrier, C.; Giudici, G.; Dickenmann, M.; Giannini, O.; Hösli, I.; Bachmann, A.; Sulser, T. Surgical management of renal cell carcinoma during the second trimester of pregnancy. Urol. Int. 2006, 76, 180–181. [Google Scholar] [CrossRef] [PubMed]
- Bourgioti, C.; Konidari, M.; Gourtsoyianni, S.; Moulopoulos, L.A. Imaging during pregnancy: What the radiologist needs to know. Diagn. Interv. Imaging 2021, 102, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, E.; Thérasse, E.; Thomassin-Naggara, I.; Trop, I. Quality initiatives: Guidelines for use of medical imaging during pregnancy and lactation. Radiographics 2012, 32, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Gjelsteen, A.C.; Ching, B.H.; Meyermann, M.W.; Prager, D.P.; Murphy, T.F.; Berkey, B.D.; Mitchell, L.A. CT, MRI, PET, PET/CT, and ultrasound in the evaluation of obstetric and gynecologic patients. Surg. Clin. N. Am. 2008, 88, 361–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lee, R.J.; Tsodikov, A.; Smith, L.; Gaffney, D.K. Does radiotherapy around the time of pregnancy for Hodgkin’s disease modify the risk of breast cancer? Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Goldberg-Stein, S.; Liu, B.; Hahn, P.F.; Lee, S.I. Body CT during pregnancy: Utilization trends, examination indications, and fetal radiation doses. AJR Am. J. Roentgenol. 2011, 196, 146–151. [Google Scholar] [CrossRef]
- Kelaranta, A.; Mäkelä, T.; Kaasalainen, T.; Kortesniemi, M. Fetal radiation dose in three common CT examinations during pregnancy: Monte Carlo study. Phys. Med. 2017, 43, 199–206. [Google Scholar] [CrossRef]
- Angel, E.; Wellnitz, C.V.; Goodsitt, M.M.; Yaghmai, N.; DeMarco, J.J.; Cagnon, C.H.; Sayre, J.W.; Cody, D.D.; Stevens, D.M.; Primak, A.N.; et al. Radiation dose to the fetus for pregnant patients undergoing multidetector CT imaging: Monte Carlo simulations estimating fetal dose for a range of gestational age and patient size. Radiology 2008, 249, 220–227. [Google Scholar] [CrossRef]
- Han, S.N.; Amant, F.; Michielsen, K.; De Keyzer, F.; Fieuws, S.; Van Calsteren, K.; Dresen, R.C.; Gziri, M.M.; Vandecaveye, V. Feasibility of whole-body diffusion-weighted MRI for detection of primary tumour, nodal and distant metastases in women with cancer during pregnancy: A pilot study. Eur. Radiol. 2018, 28, 1862–1874. [Google Scholar] [CrossRef] [PubMed]
- Khochikar, M.V. Management of urological cancers during pregnancy. Nat. Rev. Urol. 2010, 7, 195–205. [Google Scholar] [CrossRef]
- Moguel González, B.; Garcia Nava, M.; Orozco Guillén, O.A.; Abraham, V.S.; Jimenez, E.V.; Iturbe, B.R.; Rovalo, M.M. Kidney biopsy during pregnancy: A difficult decision. A case series reporting on 20 patients from Mexico. J. Nephrol. 2022, 35, 2293–2300. [Google Scholar] [CrossRef]
- Ghorbani, H.; Mottaghi, M.; Soltani, S. Renal Tumors in Pregnancy: A Case Report Focusing on the Timing of the Surgery and Patient Positioning. Case Rep. Obstet. Gynecol. 2022, 2022, 1143478. [Google Scholar] [CrossRef] [PubMed]
- Guerre, M.; Boehnlein, C.; Sohaey, R.; Seideman, C.A. Imaging of Prenatal and Neonatal Intra-abdominal Genitourinary Tumors: A Review of the Literature. Curr. Urol. Rep. 2022, 23, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, K. The management of urological malignancies during pregnancy. Br. J. Urol. 1995, 76, 639–644. [Google Scholar] [CrossRef]
- Akpayak, I.; Shuiabu, S.; Ofoha, C.; Dakum, N.; Ramyil, V. Renal cell carcinoma in pregnancy: Still a management challenge. Afr. J. Urol. 2015, 21, 167–170. [Google Scholar] [CrossRef][Green Version]
- Rose, T.L.; Kim, V.I. Renal cell carcinoma: A review. JAMA 2024, 332, 1001–1010. [Google Scholar] [CrossRef]
- Wiles, R.; Hankinson, B.; Benbow, E.; Sharp, A. Making decisions about radiological imaging in pregnancy. BMJ 2022, 377, e070486. [Google Scholar] [CrossRef] [PubMed]
- Capella, C.E.; Godovchik, J.; Chandrasekar, T.; Al-Kouatly, H.B. Nonobstetrical Robotic-Assisted Laparoscopic Surgery in Pregnancy: A Systematic Literature Review. Urology 2021, 151, 58–66. [Google Scholar] [CrossRef]
- Park, S.Y.; Ham, W.S.; Jung, H.J.; Jeong, W.; Kim, W.T.; Rha, K.H. Robot-assisted laparoscopic partial nephrectomy during pregnancy. J. Robot. Surg. 2008, 2, 193–195. [Google Scholar] [CrossRef]
- Ramirez, D.; Maurice, M.J.; Seager, C.; Haber, G.P. Robotic Partial Nephrectomy during Pregnancy: Case Report and Special Considerations. Urology 2016, 92, 1–5. [Google Scholar] [CrossRef]
- Völler, M.; Mahmud, W.; Vallo, S.; Grabbert, M.; John, P.; Khoder, W.Y. A 27-Year-Old Primigravida with a Right Renal Cell Carcinoma Removed at 30 Weeks of Gestation by Robot-Assisted Retroperitoneoscopic Partial Nephrectomy. Am. J. Case Rep. 2021, 22, e927164. [Google Scholar] [CrossRef]
- Tsutsui, K.; Miki, A.; Wakita, T.; Horibe, Y.; Tani, M.; Kakuta, Y.; Tsutahara, K.; Takao, T. A case of robot-assisted laparoscopic partial nephrectomy during pregnancy. IJU Case Rep. 2023, 6, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Jiang, W.; Chen, S.; Luo, S.; Ding, S.; Wang, D. Case report: Going through pregnancy safely after twice partial nephrectomy for bilateral kidneys with HLRCC-associated RCC. Front. Oncol. 2022, 12, 932996. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Collings, A.T.; Wunker, C.; Athanasiadis, D.I.; DeLong, C.G.; Hong, J.S.; Ansari, M.T.; Ahmed Abou-Setta, A.; Emily Oliver, E.; Berghella, V.; et al. SAGES guidelines for the use of laparoscopy during pregnancy. Surg. Endosc. 2024, 38, 2947–2963. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Z.; Xu, W.; Ji, Z.; Dong, J. Management of renal tumors during pregnancy: Case reports. BMC Nephrol. 2021, 22, 127. [Google Scholar] [CrossRef]
- Ye, P.; Zhao, N.; Shu, J.; Shen, H.; Shen, H.; Wang, Y.; Chen, L.; Yan, X. Laparoscopy versus open surgery for adnexal masses in pregnancy: A meta-analytic review. Arch. Gynecol. Obstet. 2019, 299, 625–634. [Google Scholar] [CrossRef]
- Lee, D.; Abraham, N. Laparoscopic radical nephrectomy during pregnancy: Case report and review of the literature. J. Endourol. 2008, 22, 517–518. [Google Scholar] [CrossRef]
- Berczi, C.; Flasko, T. Renal Tumor in Pregnancy: A Case Report and Review of the Literature. Urol. Int. 2017, 99, 367–369. [Google Scholar] [CrossRef]
- Amant, F.; Berveiller, P.; Boere, I.A.; Boere, I.A.; Cardonick, E.; Fruscio, R.; Fumagalli, M.; Halaska, M.J.; Hasenburg, A.; Johansson, A.L.V.; et al. Gynecologic cancers in pregnancy: Guidelines based on a third international consensus meeting. Ann. Oncol. 2019, 30, 1601–1612. [Google Scholar] [CrossRef]
- Javit, M.C. Cancer in pregnancy: Overview and epidemiology. Abdom. Radiol. 2023, 48, 1559–1563. [Google Scholar] [CrossRef]
- Ohyama, T.; Shimbo, M.; Endo, F.; Kyono, Y.; Akitani, F.; Hayashi, T.; Komatsu, K.; Matsushita, K.; Suzuki, K.; Hattori, K. Renal cell carcinoma treatment during pregnancy: Histopathological findings suggestive of rapid tumor growth. IJU Case Rep. 2019, 2, 265–268. [Google Scholar] [CrossRef]
- Dey, S.; Pakrasi, R.; Saha, D.; Datta, S. Rapidly progressive growth of Renal Clear Cell Carcinoma and Gastrointestinal Stromal Tumor during the 3rd trimester of pregnancy: A clinical and diagnostic dilemma. Urol. Case Rep. 2021, 40, 101891. [Google Scholar] [CrossRef] [PubMed]
- Zambrana, F.; Barbancho, C.; Huelves, M.; de Santiago, B.G.; Martín, Y.; de Lengaria, M.M.; de Velasco, G. Successful Pregnancy and Cancer Outcomes with Ipilimumab and Nivolumab for Metastatic RenalCell Carcinoma: Case Report and Review of the Literature. J. Immunother. 2023, 46, 27–28. [Google Scholar] [CrossRef]
- Campbell, S.C.; Clark, P.E.; Karam, J.A.; Uzzo, R.G. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-up: AUA Guideline: Part II. J. Urol. 2021, 206, 209–218. [Google Scholar] [CrossRef]
- Goebell, P.J.; Ivanyi, P.; Bedke, J. Consensus paper: Current state of first- and second-line therapy in advanced clear-cell renal cell carcinoma. Future Oncol. 2020, 16, 2307–2328. [Google Scholar] [CrossRef]
- Yazigi, A.; Lecointe-Artzner, E.; Cesne, A.L.; Ray-Coquard, I.; Blay, J.-Y. Pregnancy in Women with Metastatic Sarcomas. Oncologist 2020, 25, e2010–e2012. [Google Scholar] [CrossRef]
- Khaled, H.; Lahloubi, N.A.; Rashad, N. Review on renal cell carcinoma and pregnancy: A challenging situation. J. Adv. Res. 2016, 7, 575–580. [Google Scholar] [CrossRef]
- Chys, B.; Dumont, S.; Van Calsteren, K.; Albersen, M.; Joniau, S. Renal neoplasm during pregnancy: A single center experience. Clin. Genitourin. Cancer 2018, 16, e501–e507. [Google Scholar] [CrossRef] [PubMed]
- Boere, I.; Lok, C.; Vandenbroucke, T.; Amant, F. Cancer in pregnancy: Safety and efficacy of systemic therapies. Curr. Opin. Oncol. 2017, 29, 328–334. [Google Scholar] [CrossRef]
- Xu, Z.; Khokhlova, T.D.; Cho, C.S.; Khokhlova, V.A. Histotripsy: A Method for Mechanical Tissue Ablation with Ultrasound. Annu. Rev. Biomed. Eng. 2024, 26, 141–167. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, M.E.; Ahrar, K. Ablation of Small Renal Masses. Tech. Vasc. Interv. Radiol. 2020, 23, 100674. [Google Scholar] [CrossRef] [PubMed]
- De Maio, A.; Alfieri, G.; Mattone, M.; Ghanouni, P.; Napoli, A. High-Intensity Focused Ultrasound Surgery for Tumor Ablation: A Review of Current Applications. Radiol. Imaging Cancer 2024, 6, e230074. [Google Scholar] [CrossRef]

| Study | Park SY et al. [42] | Ramirez D et al. [43] | Völler M et al. [44] | Tsutsui K et al. [47] | Rajeev S et al. [1] | Dai K et al. [46] |
|---|---|---|---|---|---|---|
| Patients, n. | 1 | 1 | 1 | 1 | 1 | 1 |
| Age, y | 36 | 35 | 27 | 36 | 23 | 25 |
| Age of gestation at diagnosis | 14th w | 20th w | - | 5th w | 8th w | 6th w |
| Age of gestation at treatment | 16th w | 21th w | 30th w | 15th w | 13th w | 8 and 20th w |
| Staging Imaging | MRI | MRI | MRI | CT | CT | CT |
| Kidney Side | Left | Right | Right | Left | Left | Bilateral |
| Kidney Localization | Middle portion | Upper pole | Lower pole | Upper pole | Lower pole | Lower pole |
| Renal biopsy | No | No | No | No | Yes | No |
| Pathologic stage | T1a | T1b | T1b | T1a | T2a | T3a |
| Treatment | PN | PN | PN | PN | RN | PN bilateral |
| Surgical approach | T | T | R | T | T | T |
| Histologic findings | RCC | RCC | CRCC | RCC | CRCC | HLRCC RCC |
| Adjuvant treatments | No | No | No | No | No | Ipilimumab |
| Pregnancy outcome | live-birth | live-birth | live-birth | live-birth | live-birth | live-birth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Atti, L.; Slyusar, V. Robotic Surgery in the Management of Renal Tumors During Pregnancy: A Narrative Review. Cancers 2025, 17, 574. https://doi.org/10.3390/cancers17040574
Dell’Atti L, Slyusar V. Robotic Surgery in the Management of Renal Tumors During Pregnancy: A Narrative Review. Cancers. 2025; 17(4):574. https://doi.org/10.3390/cancers17040574
Chicago/Turabian StyleDell’Atti, Lucio, and Viktoria Slyusar. 2025. "Robotic Surgery in the Management of Renal Tumors During Pregnancy: A Narrative Review" Cancers 17, no. 4: 574. https://doi.org/10.3390/cancers17040574
APA StyleDell’Atti, L., & Slyusar, V. (2025). Robotic Surgery in the Management of Renal Tumors During Pregnancy: A Narrative Review. Cancers, 17(4), 574. https://doi.org/10.3390/cancers17040574







