A Retrospective Analysis of the First Clinical 5DCT Workflow
Simple Summary
Abstract
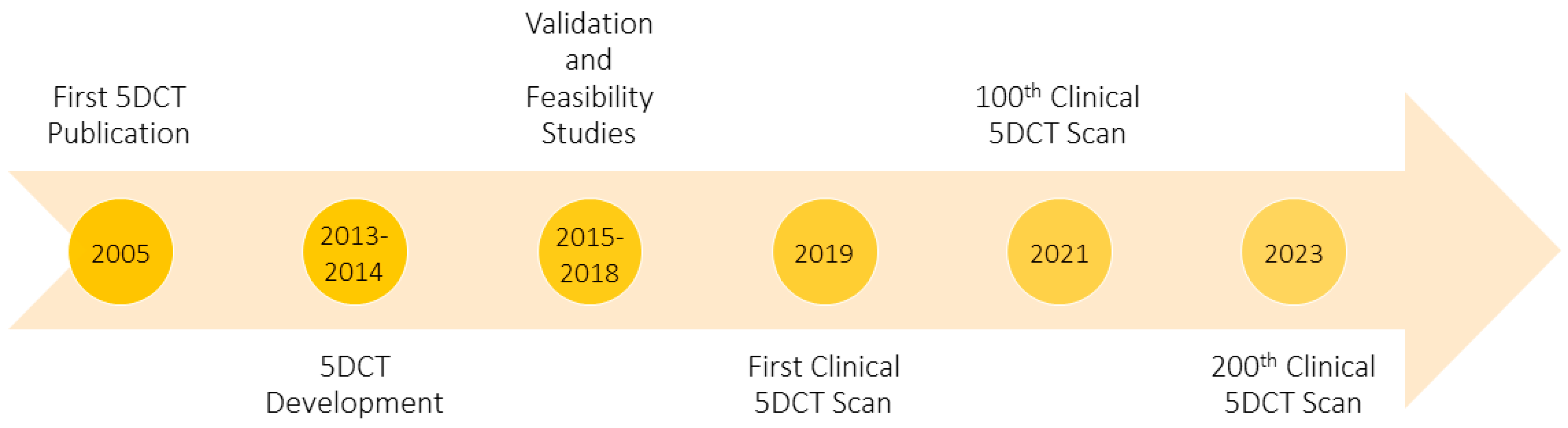
1. Introduction
2. Materials and Methods
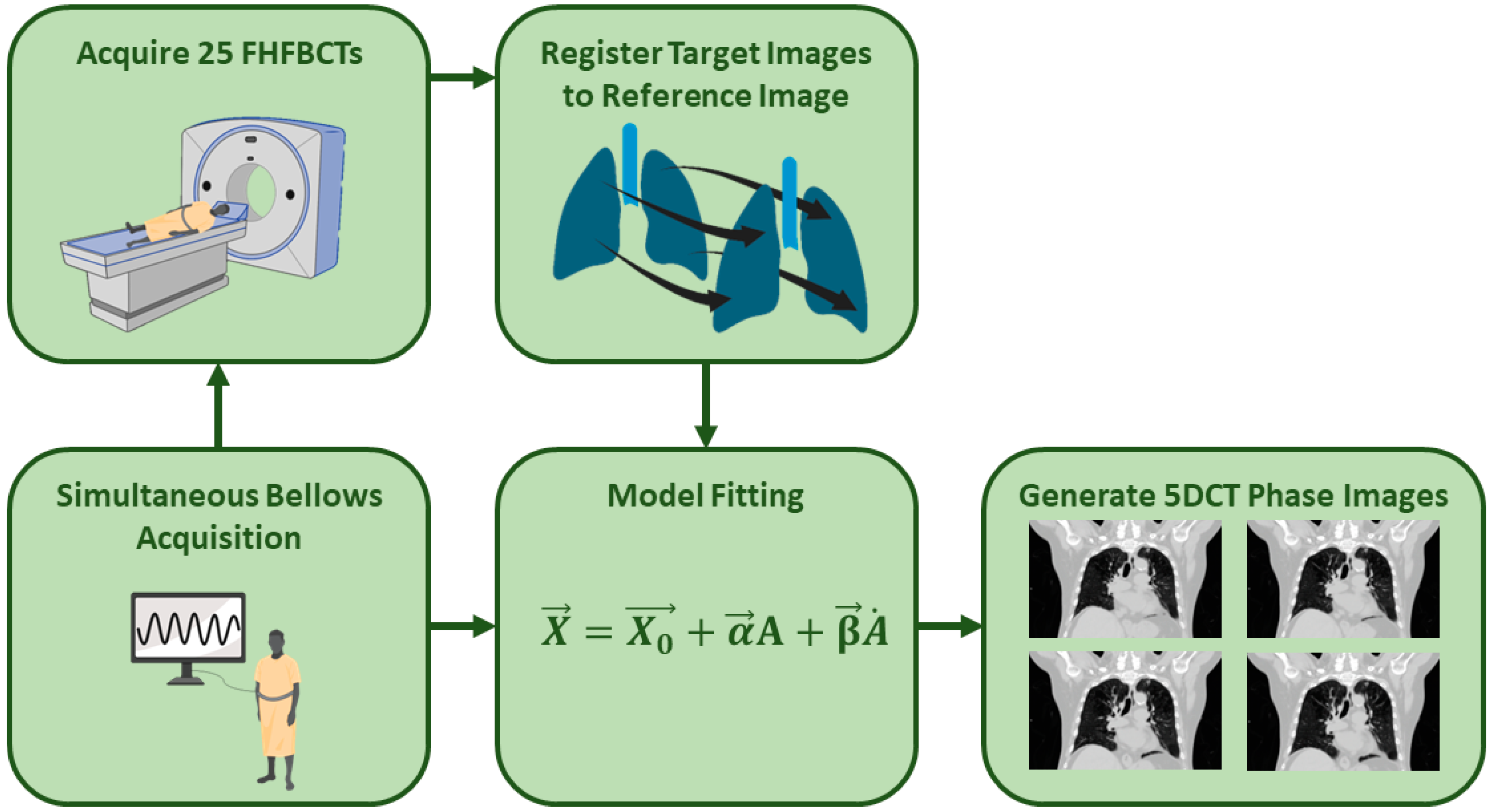
2.1. 5DCT Protocol
2.2. Clinical Workflow
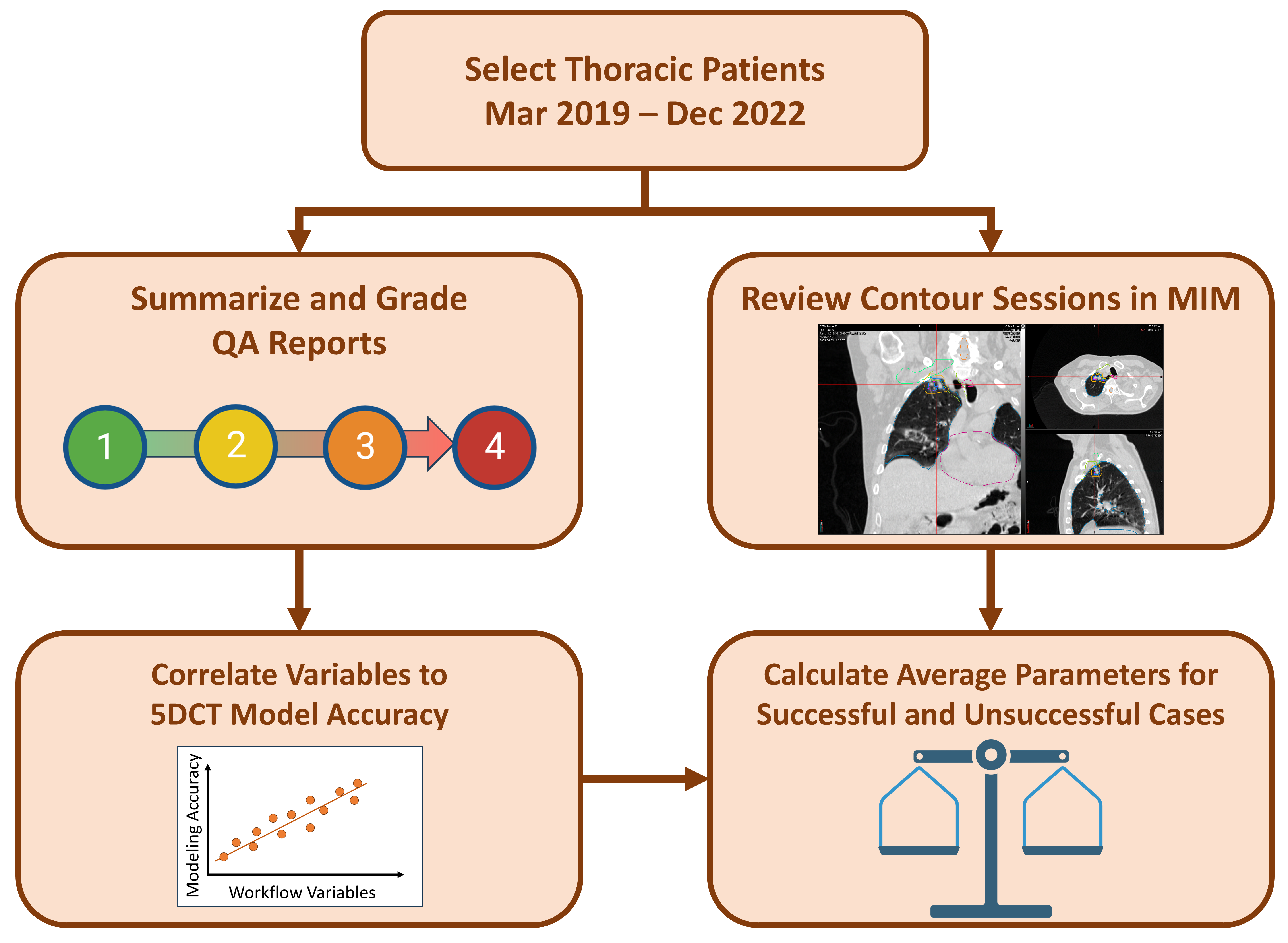
2.3. Quality Assurance (QA) Reports
2.4. Review and Grading of QA Reports
2.5. Determination of 5DCT Clinical Use
2.6. Statistical Analysis
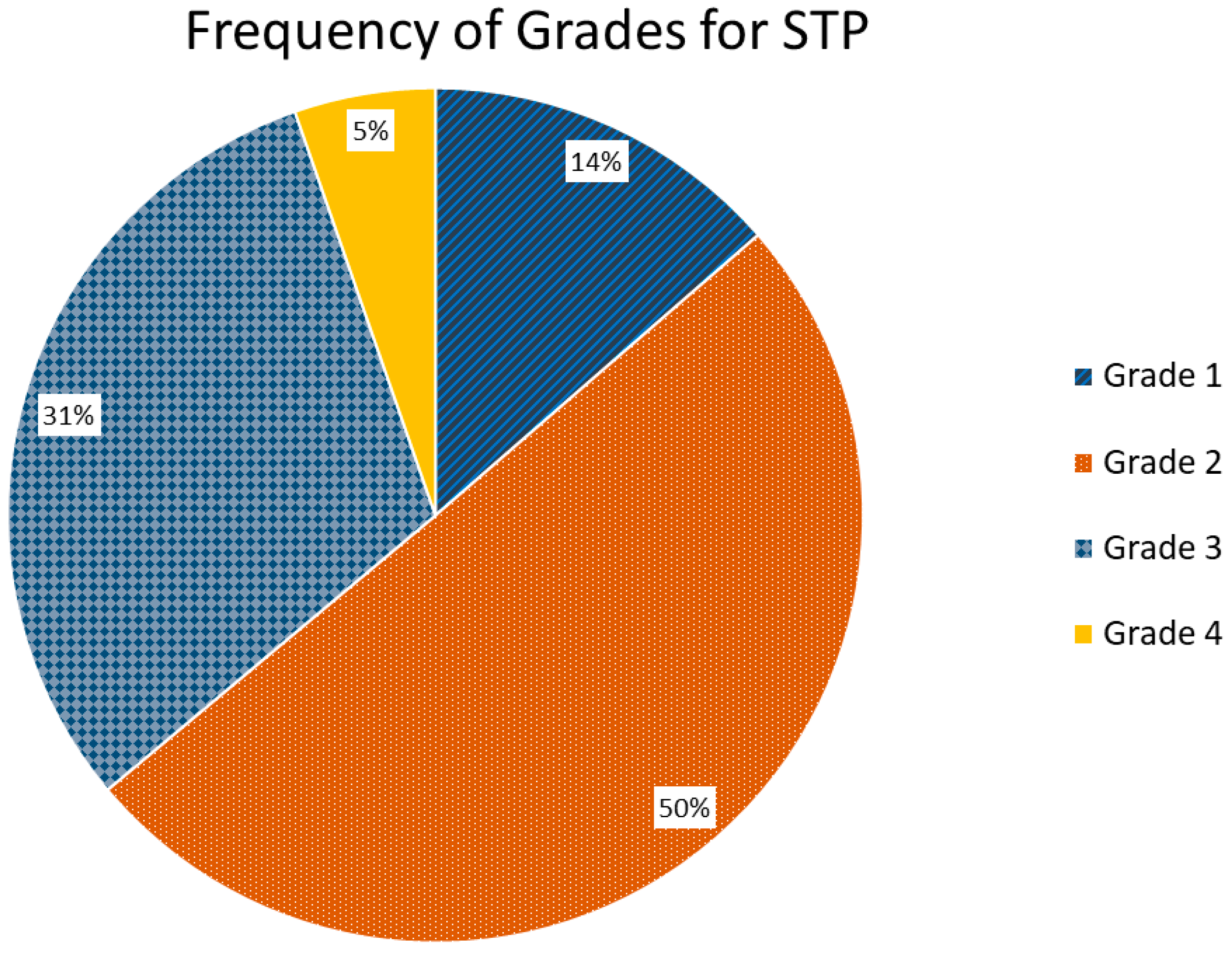
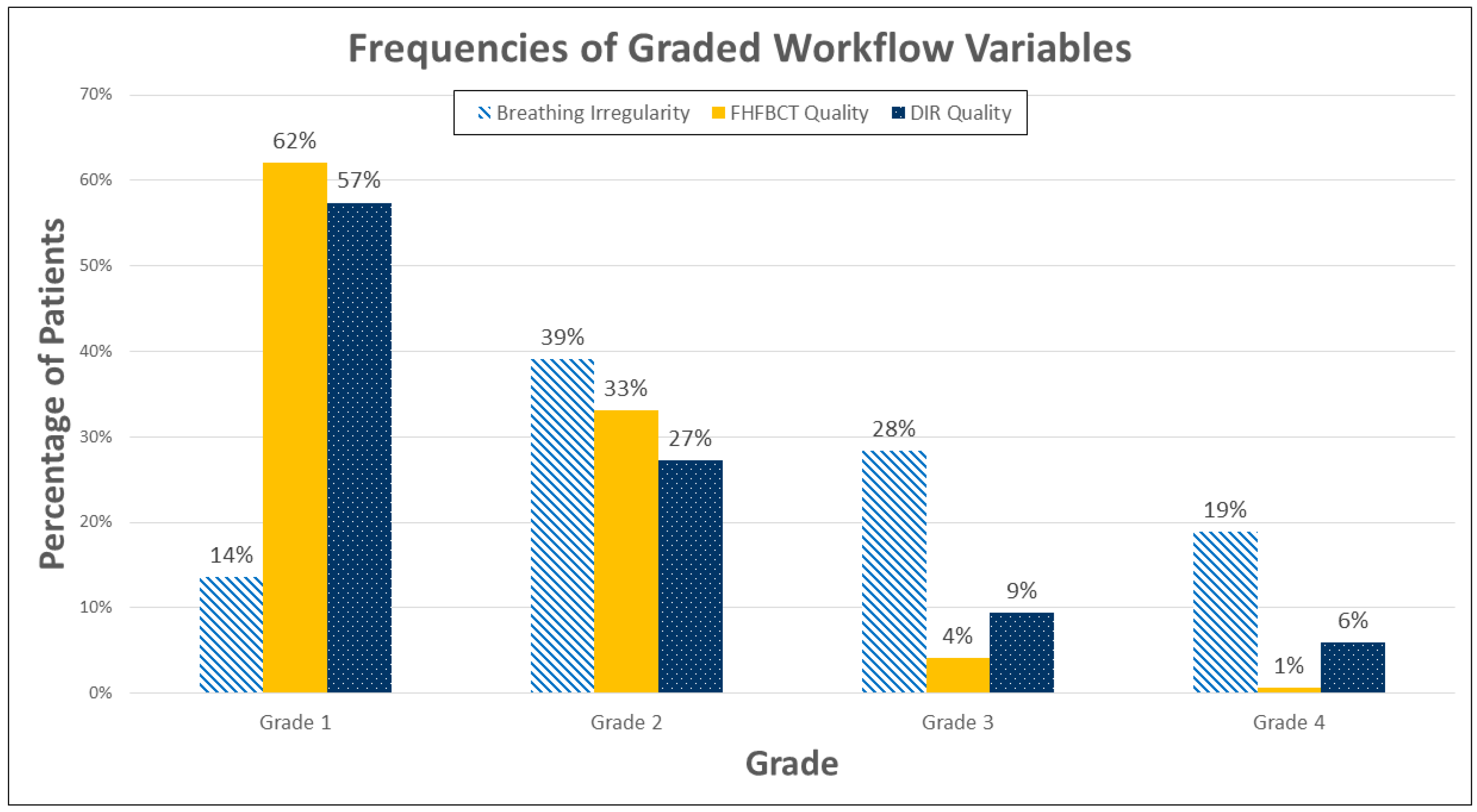
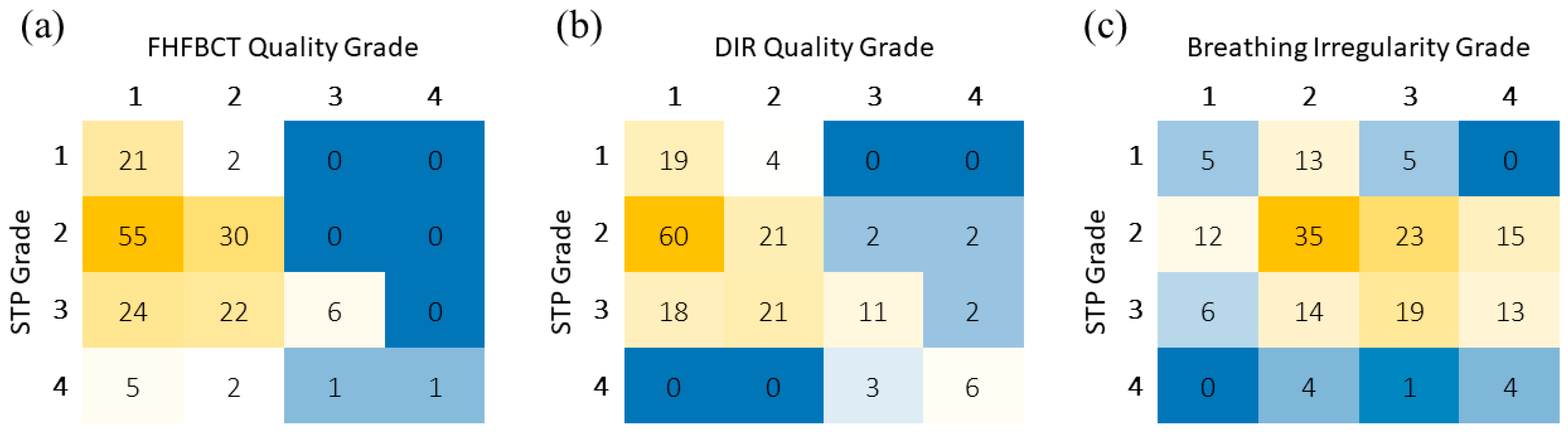
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bortfeld, T.; Jiang, S.B.; Rietzel, E. Effects of motion on the total dose distribution. In Seminars in Radiation Oncology; WB Saunders: Philadelphia, PA, USA, 2004; pp. 41–51. [Google Scholar]
- Low, D.A.; White, B.M.; Lee, P.P.; Thomas, D.H.; Gaudio, S.; Jani, S.S.; Wu, X.; Lamb, J.M. A novel CT acquisition and analysis technique for breathing motion modeling. Phys. Med. Biol. 2013, 58, L31. [Google Scholar] [CrossRef] [PubMed]
- Keall, P.J.; Mageras, G.S.; Balter, J.M.; Emery, R.S.; Forster, K.M.; Jiang, S.B.; Kapatoes, J.M.; Low, D.A.; Murphy, M.J.; Murray, B.R. The management of respiratory motion in radiation oncology report of AAPM Task Group 76 a. Med. Phys. 2006, 33, 3874–3900. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.; Mageras, G.; Yorke, E.; Rosenzweig, K.; Wagman, R.; Ling, C. Evaluation of respiratory movement during gated radiotherapy using film and electronic portal imaging. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.; Debois, M.M.; Mah, D.; Mageras, G.S.; Raben, A.; Rosenzweig, K.; Mychalczak, B.; Schwartz, L.H.; Gloeggler, P.J.; Lutz, W. Deep inspiration breath-hold technique for lung tumors: The potential value of target immobilization and reduced lung density in dose escalation. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 603–611. [Google Scholar] [CrossRef]
- Negoro, Y.; Nagata, Y.; Aoki, T.; Mizowaki, T.; Araki, N.; Takayama, K.; Kokubo, M.; Yano, S.; Koga, S.; Sasai, K. The effectiveness of an immobilization device in conformal radiotherapy for lung tumor: Reduction of respiratory tumor movement and evaluation of the daily setup accuracy. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Keall, P.; Kini, V.; Vedam, S.; Mohan, R. Motion adaptive x-ray therapy: A feasibility study. Phys. Med. Biol. 2001, 46, 1. [Google Scholar] [CrossRef]
- Lagerwaard, F.J.; de Koste, J.R.V.S.; Nijssen-Visser, M.R.; Schuchhard-Schipper, R.H.; Oei, S.S.; Munne, A.; Senan, S. Multiple “slow” CT scans for incorporating lung tumor mobility in radiotheraphy planning. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.C.; Mageras, G.; Yorke, E.; Ling, C. Respiration-correlated spiral CT: A method of measuring respiratory-induced anatomic motion for radiation treatment planning. Med. Phys. 2003, 30, 88–97. [Google Scholar] [CrossRef]
- Tryggestad, E.; Li, H.; Rong, Y. 4DCT is long overdue for improvement. J. Appl. Clin. Med. Phys. 2023, 24, e13933. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Parikh, P.J.; Hubenschmidt, J.P.; Bradley, J.D.; Low, D.A. A comparison between amplitude sorting and phase-angle sorting using external respiratory measurement for 4D CT. Med. Phys. 2006, 33, 2964–2974. [Google Scholar] [CrossRef]
- Abdelnour, A.; Nehmeh, S.; Pan, T.; Humm, J.; Vernon, P.; Schöder, H.; Rosenzweig, K.; Mageras, G.; Yorke, E.; Larson, S. Phase and amplitude binning for 4D-CT imaging. Phys. Med. Biol. 2007, 52, 3515. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Lee, T.Y.; Rietzel, E.; Chen, G.T. 4D-CT imaging of a volume influenced by respiratory motion on multi-slice CT. Med. Phys. 2004, 31, 333–340. [Google Scholar] [CrossRef]
- Antony, R.; Lonski, P.; Ungureanu, E.; Hardcastle, N.; Yeo, A.; Siva, S.; Kron, T. Independent review of 4DCT scans used for SABR treatment planning. J. Appl. Clin. Med. Phys. 2020, 21, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef] [PubMed]
- Ball, H.J.; Santanam, L.; Senan, S.; Tanyi, J.A.; van Herk, M.; Keall, P.J. Results from the AAPM Task Group 324 respiratory motion management in radiation oncology survey. J. Appl. Clin. Med. Phys. 2022, 23, e13810. [Google Scholar] [CrossRef]
- Simpson, D.R.; Lawson, J.D.; Nath, S.K.; Rose, B.S.; Mundt, A.J.; Mell, L.K. Utilization of advanced imaging technologies for target delineation in radiation oncology. J. Am. Coll. Radiol. 2009, 6, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Morton, N.; O’Brien, R.; Keall, P.; Reynolds, T. System requirements to improve adaptive 4-dimensional computed tomography (4D CT) imaging. Biomed. Phys. Eng. Express 2022, 8, 065017. [Google Scholar] [CrossRef]
- Watkins, W.T.; Li, R.; Lewis, J.; Park, J.C.; Sandhu, A.; Jiang, S.B.; Song, W.Y. Patient-specific motion artifacts in 4DCT. Med. Phys. 2010, 37, 2855–2861. [Google Scholar] [CrossRef]
- Yamamoto, T.; Langner, U.; Loo, B.W., Jr.; Shen, J.; Keall, P.J. Retrospective analysis of artifacts in four-dimensional CT images of 50 abdominal and thoracic radiotherapy patients. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1250–1258. [Google Scholar] [CrossRef]
- Low, D.A.; Parikh, P.J.; Lu, W.; Dempsey, J.F.; Wahab, S.H.; Hubenschmidt, J.P.; Nystrom, M.M.; Handoko, M.; Bradley, J.D. Novel breathing motion model for radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 921–929. [Google Scholar] [CrossRef]
- McClelland, J.R.; Hawkes, D.J.; Schaeffter, T.; King, A.P. Respiratory motion models: A review. Med. Image Anal. 2013, 17, 19–42. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Zhang, L.; Court, L.E.; Balter, P.A.; Dong, L. Modeling respiratory motion for reducing motion artifacts in 4D CT images. Med. Phys. 2013, 40, 041716. [Google Scholar] [CrossRef] [PubMed]
- Hoisak, J.D.; Sixel, K.E.; Tirona, R.; Cheung, P.C.; Pignol, J.-P. Correlation of lung tumor motion with external surrogate indicators of respiration. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Li, G. Advances and potential of optical surface imaging in radiotherapy. Phys. Med. Biol. 2022, 67, 16TR02. [Google Scholar] [CrossRef]
- Wikström, K.A.; Isacsson, U.M.; Pinto, M.C.; Nilsson, K.M.; Ahnesjö, A. Evaluation of irregular breathing effects on internal target volume definition for lung cancer radiotherapy. Med. Phys. 2021, 48, 2136–2144. [Google Scholar] [CrossRef]
- Thomas, D.; Lamb, J.; White, B.; Jani, S.; Gaudio, S.; Lee, P.; Ruan, D.; McNitt-Gray, M.; Low, D. A novel fast helical 4D-CT acquisition technique to generate low-noise sorting artifact–free images at user-selected breathing phases. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 191–198. [Google Scholar] [CrossRef]
- O’Connell, D.P.; Thomas, D.H.; Dou, T.H.; Lamb, J.M.; Feingold, F.; Low, D.A.; Fuld, M.K.; Sieren, J.P.; Sloan, C.M.; Shirk, M.A. Comparison of breathing gated CT images generated using a 5DCT technique and a commercial clinical protocol in a porcine model. Med. Phys. 2015, 42, 4033–4042. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, D.; Shaverdian, N.; Kishan, A.U.; Thomas, D.H.; Dou, T.H.; Lewis, J.H.; Lamb, J.M.; Cao, M.; Tenn, S.; Percy, L.P. Comparison of lung tumor motion measured using a model-based 4DCT technique and a commercial protocol. Pract. Radiat. Oncol. 2018, 8, e175–e183. [Google Scholar] [CrossRef]
- O’Connell, D.; Thomas, D.; Dou, T.; Aliotta, E.; Lewis, J.; Lamb, J.; Lee, P.; Low, D. Adaptive weighted median filtering for reduced blurring when fusing co-registered fast helical CT images. Biomed. Phys. Eng. Express 2017, 3, 067002. [Google Scholar] [CrossRef]
- Heinrich, M.P.; Jenkinson, M.; Bhushan, M.; Matin, T.; Gleeson, F.V.; Brady, M.; Schnabel, J.A. MIND: Modality independent neighbourhood descriptor for multi-modal deformable registration. Med. Image Anal. 2012, 16, 1423–1435. [Google Scholar] [CrossRef]
- Heinrich, M.P.; Jenkinson, M.; Papież, B.W.; Brady, M.; Schnabel, J.A. Towards realtime multimodal fusion for image-guided interventions using self-similarities. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Nagoya, Japan, 22–26 September 2013; pp. 187–194. [Google Scholar]
- White, B.; Zhao, T.; Lamb, J.; Wuenschel, S.; Bradley, J.; El Naqa, I.; Low, D. Distribution of lung tissue hysteresis during free breathing. Med. Phys. 2013, 40, 043501. [Google Scholar] [CrossRef] [PubMed]
- Doel, T. Pulmonary Toolkit. Available online: https://github.com/tomdoel/pulmonarytoolkit (accessed on 1 March 2019).
- Boveiri, H.R.; Khayami, R.; Javidan, R.; Mehdizadeh, A. Medical image registration using deep neural networks: A comprehensive review. Comput. Electr. Eng. 2020, 87, 106767. [Google Scholar] [CrossRef]
- Nenoff, L.; Ribeiro, C.O.; Matter, M.; Hafner, L.; Josipovic, M.; Langendijk, J.A.; Persson, G.F.; Walser, M.; Weber, D.C.; Lomax, A.J. Deformable image registration uncertainty for inter-fractional dose accumulation of lung cancer proton therapy. Radiother. Oncol. 2020, 147, 178–185. [Google Scholar] [CrossRef]
- Heinrich, M.P.; Jenkinson, M.; Brady, M.; Schnabel, J.A. MRF-based deformable registration and ventilation estimation of lung CT. IEEE Trans. Med. Imaging 2013, 32, 1239–1248. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Wei, S.; Bian, Z.; Subramanian, S.; Carass, A.; Prince, J.L.; Du, Y. A survey on deep learning in medical image registration: New technologies, uncertainty, evaluation metrics, and beyond. Med. Image Anal. 2024, 100, 103385. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.; Naumann, L.; Lauria, M.; Miller, C.; Andosca, R.; Savjani, R.R.; O’Connel, D.; Moghanaki, D.; Barjaktarevic, I.; Goldin, J.; et al. Introducing a novel sub-millimeter lung CT image registration error quantitation tool. Med. Phys. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Wilms, M.; Werner, R.; Ehrhardt, J.; Schmidt-Richberg, A.; Schlemmer, H.; Handels, H. Multivariate regression approaches for surrogate-based diffeomorphic estimation of respiratory motion in radiation therapy. Phys. Med. Biol. 2014, 59, 1147. [Google Scholar] [CrossRef][Green Version]
- Castillo, E.; Castillo, R.; Vinogradskiy, Y.; Dougherty, M.; Solis, D.; Myziuk, N.; Thompson, A.; Guerra, R.; Nair, G.; Guerrero, T. Robust CT ventilation from the integral formulation of the Jacobian. Med. Phys. 2019, 46, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.; Castillo, E.; Martinez, J.; Guerrero, T. Ventilation from four-dimensional computed tomography: Density versus Jacobian methods. Phys. Med. Biol. 2010, 55, 4661. [Google Scholar] [CrossRef]
- Guerrero, T.; Sanders, K.; Noyola-Martinez, J.; Castillo, E.; Zhang, Y.; Tapia, R.; Guerra, R.; Borghero, Y.; Komaki, R. Quantification of regional ventilation from treatment planning CT. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 630–634. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kabus, S.; Lorenz, C.; Johnston, E.; Maxim, P.G.; Diehn, M.; Eclov, N.; Barquero, C.; Loo, B.W., Jr.; Keall, P.J. 4D CT lung ventilation images are affected by the 4D CT sorting method. Med. Phys. 2013, 40, 101907. [Google Scholar] [CrossRef] [PubMed]
- Vinogradskiy, Y.; Castillo, R.; Castillo, E.; Schubert, L.; Jones, B.L.; Faught, A.; Gaspar, L.E.; Kwak, J.; Bowles, D.W.; Waxweiler, T. Results of a multi-institutional phase 2 clinical trial for 4DCT-ventilation functional avoidance thoracic radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Vinogradskiy, Y.; Rusthoven, C.G.; Schubert, L.; Jones, B.; Faught, A.; Castillo, R.; Castillo, E.; Gaspar, L.E.; Kwak, J.; Waxweiler, T. Interim analysis of a two-institution, prospective clinical trial of 4DCT-ventilation-based functional avoidance radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Sonke, J.J.; Zijp, L.; Remeijer, P.; Van Herk, M. Respiratory correlated cone beam CT. Med. Phys. 2005, 32, 1176–1186. [Google Scholar] [CrossRef]
- Staub, D.; Docef, A.; Brock, R.S.; Vaman, C.; Murphy, M.J. 4D Cone-beam CT reconstruction using a motion model based on principal component analysis. Med. Phys. 2011, 38, 6697–6709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, Y.C.; Liu, F.; Goodman, K.; Rosenzweig, K.E.; Mageras, G.S. Correction of motion artifacts in cone-beam CT using a patient-specific respiratory motion model. Med. Phys. 2010, 37, 2901–2909. [Google Scholar] [CrossRef]
- Lauria, M.V.; Miller, C.; Singhrao, K.; Lewis, J.; Lin, W.; O’Connell, D.; Naumann, L.; Stiehl, B.; Santhanam, A.; Boyle, P. Motion compensated cone-beam CT reconstruction using an a Priori motion model from CT simulation: A pilot study. Phys. Med. Biol. 2024, 69, 075022. [Google Scholar] [CrossRef] [PubMed]
| Data Element | Description | Purpose |
|---|---|---|
| Image and Breathing Surrogate Acquisition | ||
| Scan Start/Stop Times | Binary signal of the CT on/off state, used to synchronize the scans to the bellows signal | This was used to assure that the noise level in the signal was not excessive, allowing for a clear determination of beam-on and beam-off times. |
| Scan Ranges | Plot of the slices available against the scan numbers, used to note if any slices or scans were missing from each scan | Some, especially early scan datasets, did not have common craniocaudal coverage due to CT sequence programming errors. These were rare, and if one of the 25 scans was too short, it was removed from further analysis by the physicist running the 5DCT protocol. |
| Corrected Shifts | Summary of any interpolations to correct offsets between head-first and foot-first scans | Even with careful programming, the scanner reconstructed the head-to-foot and foot-to-head with slightly different (sub-millimeter) couch positions. These were accounted for in the image processing, so this step was intended to assure that there were no >1 mm differences indicative of a CT programming error. |
| Bellows Signal/Abdominal Height | Summary figures of all detected abdomen heights and plot of the heights against the corresponding drift-corrected bellows signals, used to show the effectiveness of the drift correction and the correlation of the drift-corrected bellows signal and the abdomen heights. | This was used to assure that the correlation was high and did not have the appearance of randomness and to assure that the corresponding linear fit was a good representation of the data. |
| Respiratory Surrogate Analysis | ||
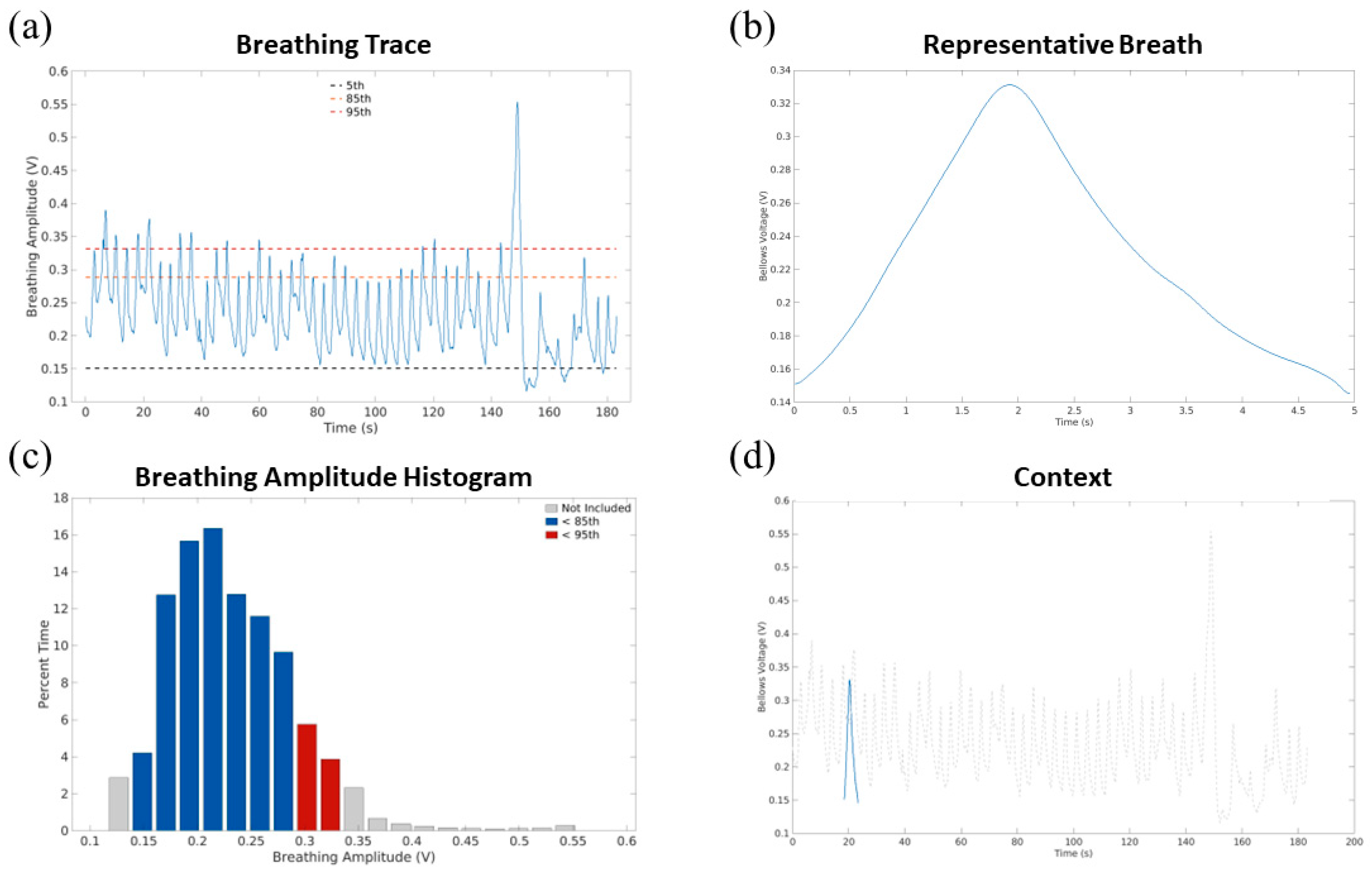
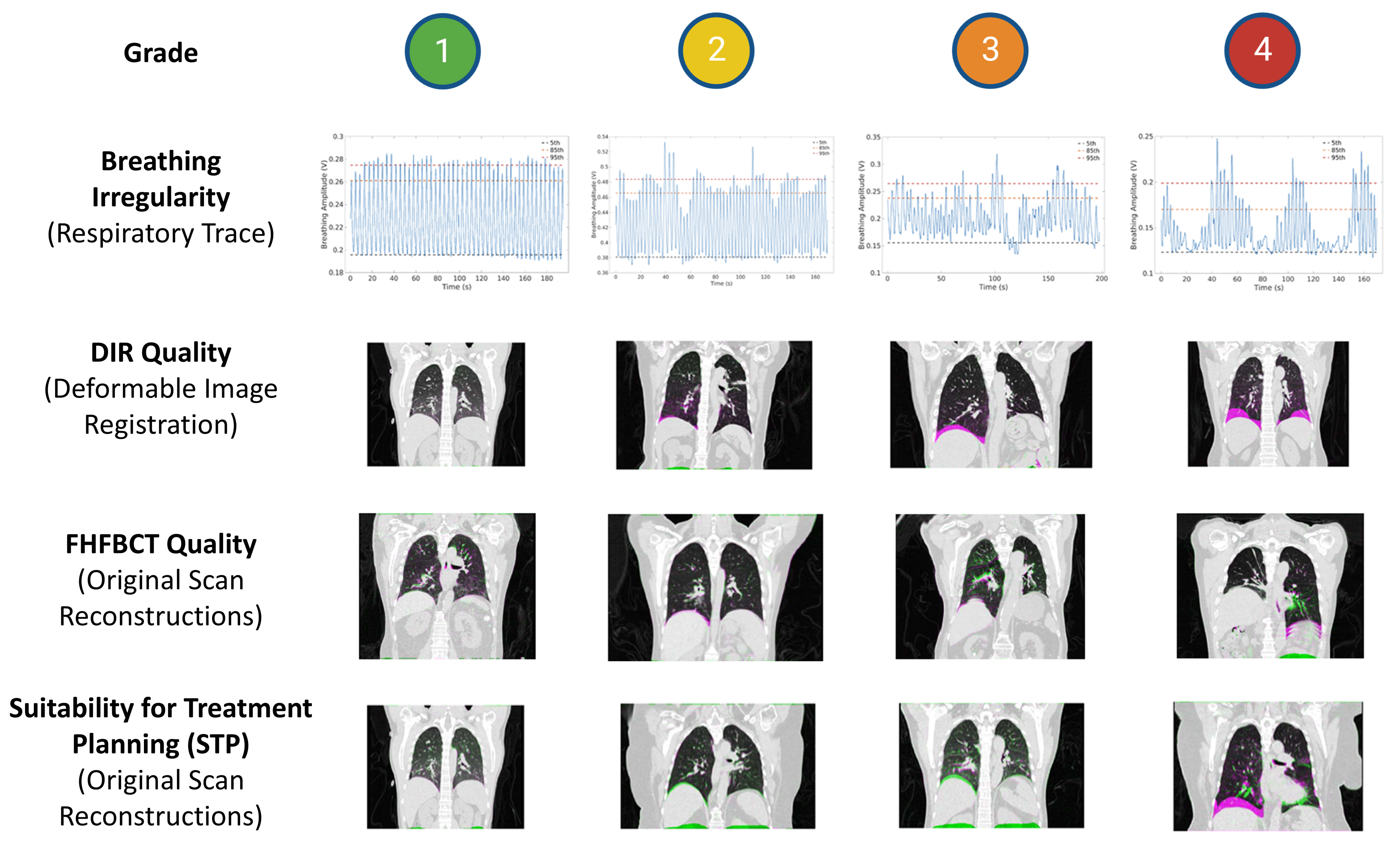
| Respiratory Trace | Plot of the bellows amplitude signal over time with 5th-, 85th-, and 95th-percentile amplitudes annotated. Used to evaluate breathing irregularity in this study. | Examine the breathing trace to evaluate for obvious anomalies such as discontinuities or severe uncompensated drift. |
| Respiratory Amplitude Histogram | Histogram of time while the signal was in each amplitude bin | Not evaluated. This histogram was provided for retrospective review. There were and are no evaluation criteria tied to these types of data, and the respiratory trace contained a more easily evaluable form of these data. |
| Waveform Segmentation | Plot of the bellows amplitude signal over time with each detected exhalation point annotated, used to show how breaths were segmented to determine the representative breath. | Examined the exhalation points, which were used in the process of creating the representative breath. |
| Representative Breath and Context | Plots of the representative breath, alone and superimposed over the breathing waveform at its appropriate amplitudes. | This was used to assure that the representative breath amplitudes (peak inhalation and exhalation) reflected the overall breathing pattern. |
| Deformable Image Registration | ||
| Deformable Image Registration | A set of 24 coronal, right lung sagittal, and left lung sagittal images, showing a green/magenta overlay of the reference image and deformed target FHFBCT at the middle slice of each plane. Used to evaluate DIR quality in this study. | Examined to determine if the image registration failed. This was generally due to the DIR algorithm’s inability to register images with large differences in breathing amplitudes. |
| Motion Modeling | ||
| Summary | Summary table including number of scans, reference scan number, mean, standard deviation, and 95th percentile of the 5DCT model fit residuals. | Mean, standard deviation, and 95th percentile of the 5DCT model fit residuals were evaluated in conjunction with the 5DCT model fit residual histogram to determine if the 5DCT 8-phase images and 5DCT MIP should be used clinically. |
| Residual Histogram | Histogram of the 5DCT model residuals in 1 mm bins with frequency represented by percent of lung voxels | Used in conjunction with the mean, standard deviation, and 95th percentile of 5D model fit residuals to determine if the 5DCT 8-phase images and 5DCT MIP should be used clinically |
| 5DCT Model Residual AP/Lat MIPs (mm) | Coronal and sagittal maximum-intensity projections (MIPs) of the model residuals overlain with a projection of the anatomy. Residuals were shown on a green-to-red color wash. | These were used to determine the magnitude and rough locations of the 5DCT model residual error distribution. |
| Original Scan Reconstructions | Coronal, left lung sagittal, and right lung sagittal overlays of the 5DCT model-deformed reference image superimposed with each of the 25 FHFBCTs using the green/magenta color overlay. Used to evaluate image quality and STP in this study. | Used in conjunction with the 5DCT motion model residuals to determine overall 5DCT workflow quality and clinical usability. Since no quantitative values were assigned to these images, they were primarily used to verify that high or low residual values accurately reflected poor or good original scan reconstructions, respectively. |
| Variable | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Breathing Irregularity | Very regular | Regular | Irregular | Very irregular |
| FHFBCT Image Quality | No artifacts | Minor artifacts (Some blurring at the diaphragm) | Some artifacts (Slight doubling of the diaphragm) | Severe artifacts (Severe doubling of the diaphragm) |
| DIR Quality | Great alignment of the reference and target FHFBCTs | Good alignment of the reference and target FHFBCTs (Minor misalignments that would not significantly impact modeling) | Poor alignment of the reference and target FHFBCTs (Some images are misaligned by greater than 1 mm at the diaphragm or vessels) | Very poor alignment of the reference and target FHFBCTs (Many or all images are misaligned by much more than 1 mm at the diaphragm or vessels) |
| Suitability for Treatment Planning | Great alignment of the true FHFBCTs and the model-generated FHFBCTs | Good alignment of the true FHFBCTs and the model-generated FHFBCTs (minor misalignments that signify minor errors in modeling) | Poor alignment of the true FHFBCTs and the model-generated FHFBCTs (some images are misaligned by greater than 1 mm at the diaphragm or vessels that signify some meaningful errors in modeling) | Very poor alignment of the true FHFBCTs and the model-generated FHFBCTs (many or all images are misaligned by much more than 1 mm at the diaphragm or vessels that signify meaningful errors in modeling) |
| Category | Description |
|---|---|
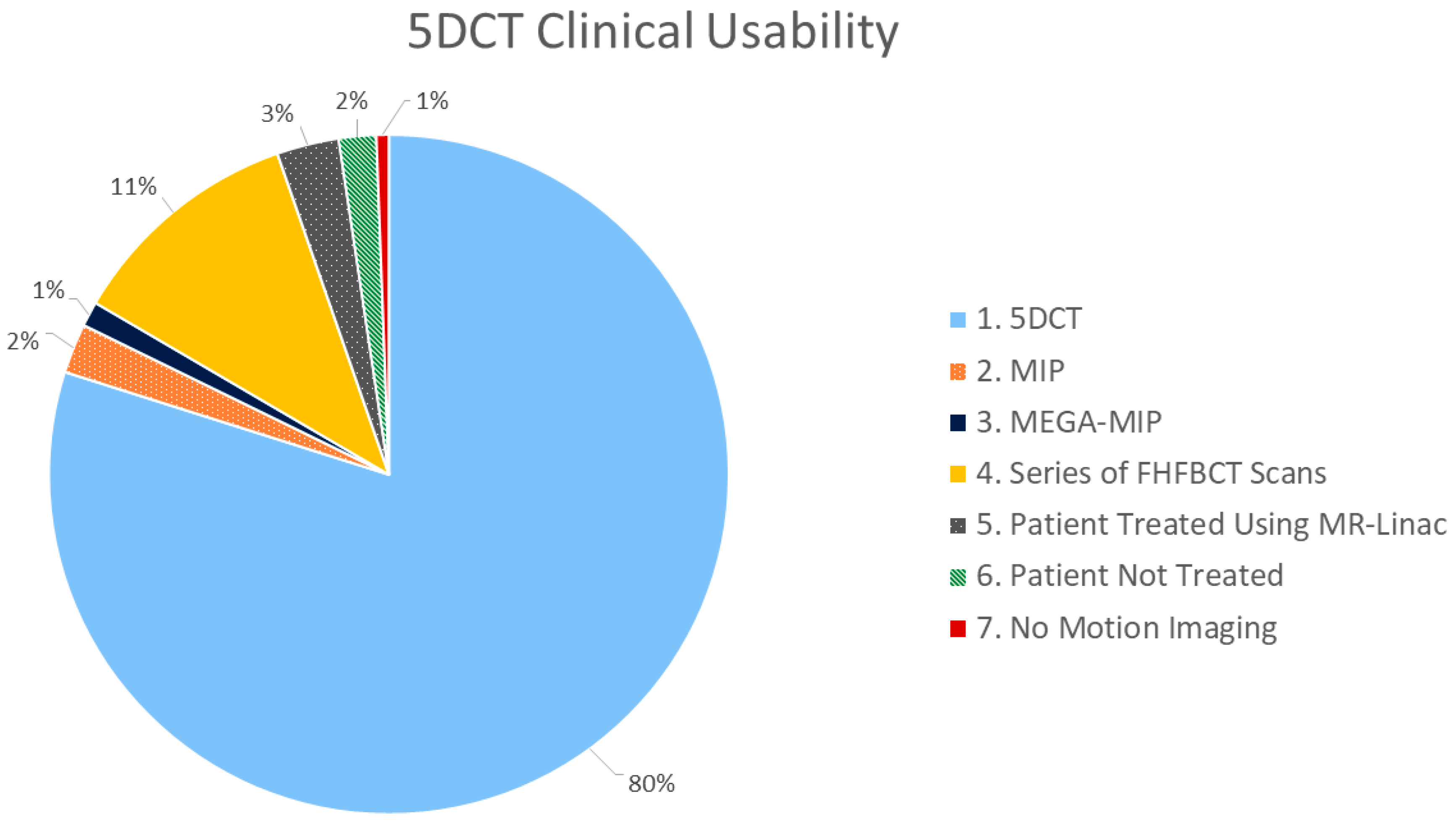
| 5DCT Used for ITV Contouring (N = 118) | |
| 1 | 5DCT phase images reconstructions used for ITV contouring |
| 2 | MIP generated from 5DCT phase images reconstructions used for ITV contouring |
| Backup Protocols Used for ITV Contouring (N = 21) | |
| 3 | MEGA-MIP used for ITV contouring |
| 4 | Series of FHFBCT scans in sequence used for contouring |
| Other (No ITV Identified) (N = 30) | |
| 5 | Patient treated using an MR-linac (unrelated to 5DCT quality) |
| 6 | Patient not treated (unrelated to 5DCT quality) |
| 7 | No motion imaging used in contour session (due to lack of evidence of motion) |
| Correlation | Spearman Coefficient | Spearman Confidence Interval |
|---|---|---|
| Breathing irregularity Grade vs. Suitability for Treatment Planning Grade | 0.260 (p < 0.001) | [0.109, 0.399] |
| Imaging FHFBCT Quality Grade vs. Suitability for Treatment Planning Grade | 0.301 (p < 0.001) | [0.153, 0.436] |
| DIR Quality Grade vs. Suitability for Treatment Planning Grade | 0.500 (p < 0.001) | [0.374, 0.608] |
| Criteria | Unstandardized Coefficient (B) | 95% Confidence Interval for B | Standardized Coefficient (β) |
|---|---|---|---|
| Constant | 0.996 (p < 0.001) | [0.541, 1.451] | - |
| Breathing Irregularity Grade | 0.133 (p = 0.008) | [0.034, 0.231] | 0.165 (p = 0.008) |
| FHFBCT Quality Grade | 0.290 (p < 0.001) | [0.130, 0.449] | 0.230 (p < 0.001) |
| DIR Quality Grade | 0.404 (p < 0.001) | [0.292, 0.517] | 0.467 (p <0.001) |
| Criteria | Unstandardized Coefficient (B) | 95% Confidence Interval for B | Standardized Coefficient (β) |
|---|---|---|---|
| (Constant) | 0.571 (p < 0.001) | [0.253, 0.888] | - |
| Breathing irregularity Grade | 0.214 (p < 0.001) | [0.145, 0.282] | 0.407 (p < 0.001) |
| FHFBCT Quality grade | 0.147 (p = 0.010) | [0.035, 0.258] | 0.177 (p = 0.010) |
| DIR Quality Grade | 0.102 (p = 0.011) | [0.024, 0.181] | 0.181 (p = 0.011) |
| Criteria | Unstandardized Coefficient (B) | 95% Confidence Interval for B | Standardized Coefficient (β) |
|---|---|---|---|
| (Constant) | 0.758 (p = 0.012) | [0.170, 1.346] | - |
| Breathing irregularity Grade | 0.279 (p < 0.001) | [0.152, 0.406] | 0.281 (p < 0.001) |
| FHFBCT Quality grade | 0.248 (p = 0.019) | [0.042, 0.455] | 0.159 (p = 0.019) |
| DIR Quality Grade | 0.412 (p < 0.001) | [0.266, 0.557] | 0.384 (p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauria, M.; Kim, M.; O’Connell, D.; Lao, Y.; Miller, C.R.; Naumann, L.; Boyle, P.; Raldow, A.; Lee, A.; Savjani, R.R.; et al. A Retrospective Analysis of the First Clinical 5DCT Workflow. Cancers 2025, 17, 531. https://doi.org/10.3390/cancers17030531
Lauria M, Kim M, O’Connell D, Lao Y, Miller CR, Naumann L, Boyle P, Raldow A, Lee A, Savjani RR, et al. A Retrospective Analysis of the First Clinical 5DCT Workflow. Cancers. 2025; 17(3):531. https://doi.org/10.3390/cancers17030531
Chicago/Turabian StyleLauria, Michael, Minji Kim, Dylan O’Connell, Yi Lao, Claudia R. Miller, Louise Naumann, Peter Boyle, Ann Raldow, Alan Lee, Ricky R. Savjani, and et al. 2025. "A Retrospective Analysis of the First Clinical 5DCT Workflow" Cancers 17, no. 3: 531. https://doi.org/10.3390/cancers17030531
APA StyleLauria, M., Kim, M., O’Connell, D., Lao, Y., Miller, C. R., Naumann, L., Boyle, P., Raldow, A., Lee, A., Savjani, R. R., Moghanaki, D., & Low, D. A. (2025). A Retrospective Analysis of the First Clinical 5DCT Workflow. Cancers, 17(3), 531. https://doi.org/10.3390/cancers17030531





