Incisional Hernia in Cytoreductive Surgery for Advanced-Stage Ovarian Cancer: A Single-Center Retrospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preoperative Work-Up and Surgical Technique
2.2. Patient’s Follow-Up
2.3. Statistical Analysis
3. Results
3.1. Basal Characteristics
3.2. Surgical Technique
3.3. Postoperative Course
3.4. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fotopoulou, C.; Planchamp, F.; Aytulu, T.; Chiva, L.; Cina, A.; Ergönül, Ö.; Fagotti, A.; Haidopoulos, D.; Hasenburg, A.; Hughes, C.; et al. European Society of Gynaecological Oncology guidelines for the peri-operative management of advanced ovarian cancer patients undergoing debulking surgery. Int. J. Gynecol. Cancer 2021, 31, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M.; van der Burg, M.E.L.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Ferrandina, M.G.; Vizzielli, G.; Pasciuto, T.; Fanfani, F.; Gallotta, V.; Margariti, P.A.; Chiantera, V.; Costantini, B.; Alletti, S.G.; et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int. J. Gynecol. Cancer 2020, 30, 1657–1664. [Google Scholar] [CrossRef]
- Vergote, I.; Coens, C.; Nankivell, M.; Kristensen, G.B.; Parmar, M.K.B.; Ehlen, T.; Jayson, G.C.; Johnson, N.; Swart, A.M.; Verheijen, R.; et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: Pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018, 19, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef]
- Kurnit, K.C.; Fleming, G.F.; Lengyel, E. Updates and New Options in Advanced Epithelial Ovarian Cancer Treatment. Obstet. Gynecol. 2021, 137, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, C.; Hall, M.; Cruickshank, D.; Gabra, H.; Ganesan, R.; Hughes, C.; Kehoe, S.; Ledermann, J.; Morrison, J.; Naik, R.; et al. British Gynaecological Cancer Society (BGCS) epithelial ovarian/fallopian tube/primary peritoneal cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival Effect of Maximal Cytoreductive Surgery for Advanced Ovarian Carcinoma During the Platinum Era: A Meta-Analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Kroese, L.F.; Sneiders, D.; Kleinrensink, G.J.; Muysoms, F.; Lange, J.F. Comparing different modalities for the diagnosis of incisional hernia: A systematic review. Hernia 2018, 22, 229–242. [Google Scholar] [CrossRef]
- Du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzin. Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.C.; Kitchener, H.; Bacon, M.; Friedlander, M.; Ledermann, J.; Marth, C.; Thigpen, T.; Trimble, E. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: Report from the fourth ovarian cancer consensus conference. Int. J. Gynecol. Cancer 2011, 21, 750–755. [Google Scholar] [CrossRef]
- Sanders, D.L.; Pawlak, M.M.; Simons, M.P.; Aufenacker, T.; Balla, A.; Berger, C.; Berrevoet, F.; de Beaux, A.C.; East, B.; Henriksen, N.A.; et al. Midline incisional hernia guidelines: The European Hernia Society. Br. J. Surg. 2023, 110, 1732–1768. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.V.; Paskar, D.D.; Nelson, R.L.; Vedula, S.S.; Steele, S.R. Closure methods for laparotomy incisions for preventing incisional hernias and other wound complications. Cochrane Database Syst. Rev. 2017, 2017, CD005661. [Google Scholar] [CrossRef]
- Deerenberg, E.B.; Henriksen, N.A.; Antoniou, G.A.; Antoniou, S.A.; Bramer, W.M.; Fischer, J.P.; Fortelny, R.H.; Gök, H.; Harris, H.W.; Hope, W.; et al. Updated guideline for closure of abdominal wall incisions from the European and American Hernia Societies. Br. J. Surg. 2022, 109, 1239–1250. [Google Scholar] [CrossRef]
- Spencer, R.J.; Hayes, K.D.; Rose, S.; Zhao, Q.; Rathouz, P.J.; Rice, L.W.; Al-Niaimi, A.N. Risk Factors for Early and Late-Occurring Incisional Hernias After Primary Laparotomy for Ovarian Cancer. J. Surg. Oncol. 2015, 125, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Maggioni, A.; Parma, G.; Scalambrino, S.; Milani, R. A randomized comparison of continuous versus interrupted mass closure of midline incisions in patients with gynecologic cancer. Obstet. Gynecol. 1997, 89, 684–689. [Google Scholar] [CrossRef]
- Long, K.C.; Levinson, K.L.; Diaz, J.P.; Gardner, G.J.; Chi, D.S.; Barakat, R.R.; Leitao, M.M. Ventral hernia following primary laparotomy for ovarian, fallopian tube, and primary peritoneal cancers. Gynecol. Oncol. 2011, 120, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Celiksoy, H.Y.; Sozen, H.; Canturk, M.M.; Baktiroglu, H.M.; Salihoglu, Y.; Topuz, S. Incisional hernia after ovarian debulking surgery. Ginekol. Polska 2023, 95, 190–194. [Google Scholar] [CrossRef]
- Straubhar, A.M.; Stroup, C.; Manorot, A.; McCool, K.; Rolston, A.; Reynolds, R.K.; McLean, K.; de Bear, O.; Siedel, J.; Uppal, S.; et al. Small bite fascial closure technique reduces incisional hernia rates in gynecologic oncology patients. Int. J. Gynecol. Cancer 2024, 34, 745–750. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, J.L.; Carbonell-Socias, M.; Pérez-Benavente, M.A.; Clua, S.M.; Manrique-Muñoz, S.; Gorriz, M.G.; Burgos-Peláez, R.; Gurrutxaga, H.S.; Serrano, M.P.; Gutiérrez-Barceló, M.D.P.; et al. PROFAST: A randomised trial implementing enhanced recovery after surgery for highcomplexity advanced ovarian cancer surgery. Eur. J. Cancer 2020, 136, 149–158. [Google Scholar] [CrossRef]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; Meyer, L.A.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations—2019 update. Int. J. Gynecol. Cancer 2019, 29, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Söderbäck, H.; Masood, A.; Leo, J.; Sandblom, G. Introduction of Small Stitch Small Bite technique: A retrospective long-term follow-up. Langenbeck’s Arch. Surg. 2022, 407, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Harlaar, J.J.; van Ramshorst, G.H.; Nieuwenhuizen, J.; Brinke, J.G.T.; Hop, W.C.; Kleinrensink, G.-J.; Jeekel, H.; Lange, J.F. Small stitches with small suture distances increase laparotomy closure strength. Am. J. Surg. 2009, 198, 392–395. [Google Scholar] [CrossRef]
- Caglià, P.; Tracia, A.; Borzì, L.; Amodeo, L.; Tracia, L.; Veroux, M.; Amodeo, C. Incisional hernia in the elderly: Risk factors and clinical considerations. Int. J. Surg. 2014, 12, S164–S169. [Google Scholar] [CrossRef] [PubMed]
- Walming, S.; Angenete, E.; Block, M.; Bock, D.; Gessler, B.; Haglind, E. Retrospective review of risk factors for surgical wound dehiscence and incisional hernia. BMC Surg. 2017, 17, 19. [Google Scholar] [CrossRef]
- Ge, L.-N.; Wang, F. Prognostic significance of preoperative serum albumin in epithelial ovarian cancer patients: A systematic review and dose–response meta-analysis of observational studies. Cancer Manag. Res. 2018, 10, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Van Rooijen, M.M.J.; Lange, J.F. Preventing incisional hernia: Closing the midline laparotomy. Tech. Coloproctol. 2018, 22, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Heger, P.; Feißt, M.; Krisam, J.; Klose, C.; Dörr-Harim, C.; Tenckhoff, S.; Büchler, M.W.; Diener, M.K.; Mihaljevic, A.L. Hernia reduction following laparotomy using small stitch abdominal wall closure with and without mesh augmentation (the HULC trial): Study protocol for a randomized controlled trial. Trials 2019, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.M.; Morton, M.; Lampert, E.; Yao, M.; Debernardo, R.; Rose, P.G.; Vargas, R. Use of prophylactic closed incision negative pressure therapy is associated with reduced surgical site infections in gynecologic oncology patients undergoing laparotomy. Am. J. Obstet. Gynecol. 2020, 223, 731.e1–731.e9. [Google Scholar] [CrossRef]
- Mithany, R.H.; Daniel, N.; Shahid, M.H.; Aslam, S.; Abdelmaseeh, M.; Gerges, F.; Gill, M.U.; Abdallah, S.B.; Hannan, A.; Saeed, M.T.; et al. Revolutionizing Surgical Care: The Power of Enhanced Recovery After Surgery (ERAS). Cureus 2023, 15, e48795. [Google Scholar] [CrossRef] [PubMed]
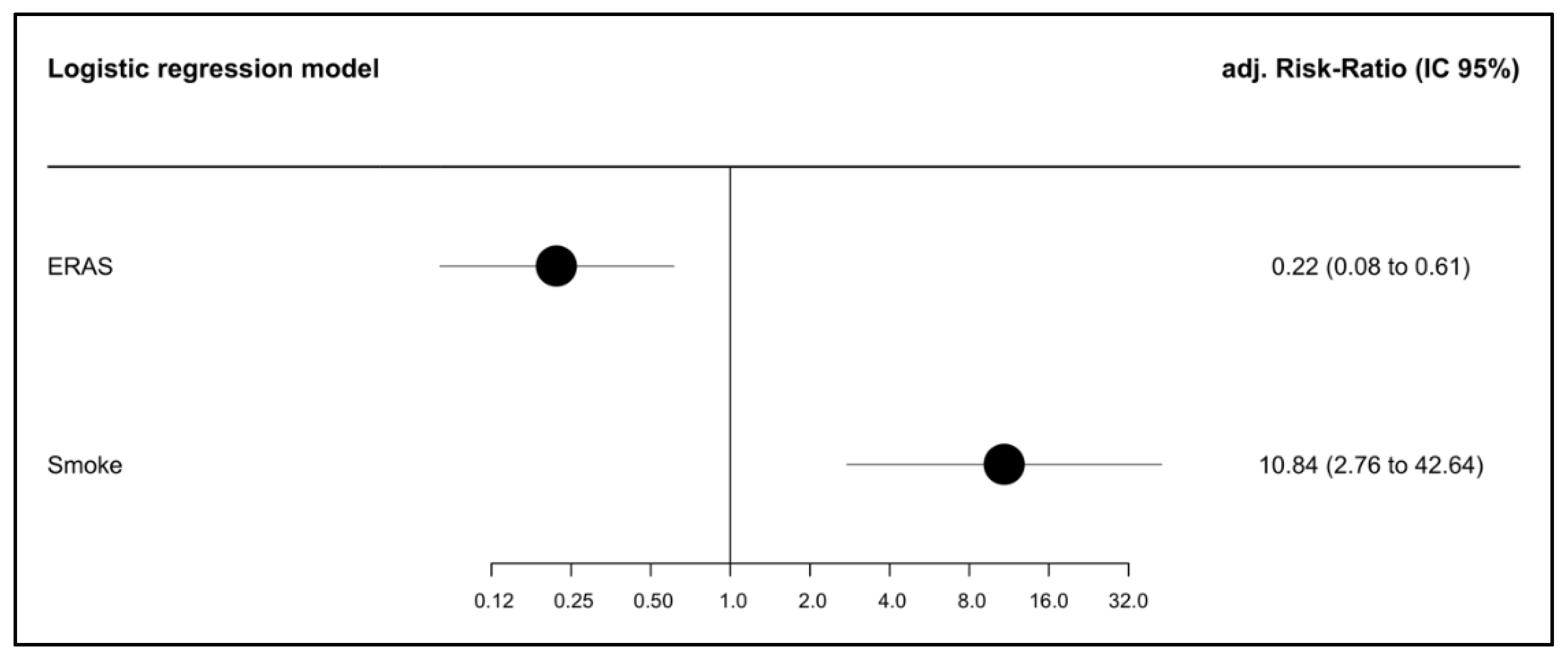
| Overall | No Hernia | Hernia | p-Value * | Missing (%) | ||
|---|---|---|---|---|---|---|
| n | 156 | 126 | 30 | |||
| Age (mean (SD)) | 59.1 (12.4) | 59.2 (12.9) | 58.7 (10.4) | 0.864 | 0.0 | |
| Type of surgery (%) | PDS | 91 (58.3) | 76 (60.3) | 15 (50.0) | 0.410 | 0.0 |
| IDS | 65 (41.7) | 50 (39.7) | 15 (50.0) | |||
| Smoker (%) | no | 143 (91.7) | 120 (95.2) | 23 (76.7) | 0.003 | 0.0 |
| yes | 13 (8.3) | 6 (4.8) | 7 (23.3) | |||
| BMI (median [IQR]) | 24.2 [21.95, 27.30] | 23.9 [21.50, 27.30] | 25.3 [23.42, 26.85] | 0.133 | 0.0 | |
| Definitive histology (%) | clear-cell carcinoma | 12 (7.7) | 11 (8.7) | 1 (3.3) | 0.343 | 0.0 |
| dedifferentiated carcinoma | 1 (0.6) | 1 (0.8) | 0 (0.0) | |||
| high-grade serous carcinoma | 120 (76.9) | 96 (76.2) | 24 (80.0) | |||
| low-grade serous carcinoma | 6 (3.8) | 6 (4.8) | 0 (0.0) | |||
| carcinosarcoma | 1 (0.6) | 1 (0.8) | 0 (0.0) | |||
| endometrioid | 11 (7.1) | 7 (5.6) | 4 (13.3) | |||
| squamous | 1 (0.6) | 1 (0.8) | 0 (0.0) | |||
| mucinous | 1 (0.6) | 1 (0.8) | 0 (0.0) | |||
| serous borderline | 2 (1.3) | 2 (1.6) | 0 (0.0) | |||
| yolk sac | 1 (0.6) | 0 (0.0) | 1 (3.3) | |||
| DM (%) | no | 147 (94.2) | 117 (92.9) | 30 (100.0) | 0.284 | 0.0 |
| yes | 9 (5.8) | 9 (7.1) | 0 (0.0) | |||
| COPD (%) | no | 149 (95.5) | 121 (96.0) | 28 (93.3) | 0.880 | 0.0 |
| yes | 7 (4.5) | 5 (4.0) | 2 (6.7) | |||
| Corticosteroids treatment (%) | no | 154 (98.7) | 125 (99.2) | 29 (96.7) | 0.835 | 0.0 |
| yes | 2 (1.3) | 1 (0.8) | 1 (3.3) | |||
| IS (%) | no | 154 (98.7) | 124 (98.4) | 30 (100.0) | 1.000 | 0.0 |
| yes | 2 (1.3) | 2 (1.6) | 0 (0.0) | |||
| MDL (%) | LMA | 22 (14.1) | 17 (13.5) | 5 (16.7) | 0.269 | 0.0 |
| no | 124 (79.5) | 99 (78.6) | 25 (83.3) | |||
| Pfannestiel | 10 (6.4) | 10 (7.9) | 0 (0.0) | |||
| Alb preqx (median [IQR]) | 4.10 [3.80, 4.30] | 4.10 [3.80, 4.30] | 4.10 [3.90, 4.20] | 0.747 | 7.1 | |
| Prot preqx (median [IQR]) | 7.00 [6.60, 7.30] | 7.00 [6.60, 7.40] | 6.95 [6.60, 7.23] | 0.791 | 5.8 | |
| Hb preqx (median [IQR]) | 11.90 [11.00, 13.20] | 12.00 [11.05, 13.25] | 11.65 [10.83, 13.00] | 0.559 | 1.9 | |
| Ca 125 (median [IQR]) | 123.05 [31.10, 369.28] | 128.10 [42.20, 394.20] | 53.10 [25.00, 242.10] | 0.210 | 1.3 | |
| Ascitis | 59 (38.3) | 52 (41.9) | 7 (23.3) | |||
| Vasc (median [IQR]) | 600.00 [200.00, 2350.00] | 700.00 [200.00, 3000.00] | 200.00 [200.00, 950.00] | 0.279 | 1.3 |
| Overall | No Hernia | Hernia | p-Value * | Missing | ||
|---|---|---|---|---|---|---|
| n | 156 | 126 | 30 | |||
| Definitive FIGO Stage (%) | IIA | 3 (1.9) | 2 (1.6) | 1 (3.3) | 0.653 | 0.0 |
| IIB | 9 (5.8) | 6 (4.8) | 3 (10.0) | |||
| IIIA | 13 (8.3) | 12 (9.5) | 1 (3.3) | |||
| IIIB | 14 (9.0) | 10 (7.9) | 4 (13.3) | |||
| IIIC | 63 (40.4) | 51 (40.5) | 12 (40.0) | |||
| IVA | 11 (7.1) | 10 (7.9) | 1 (3.3) | |||
| IVB | 43 (27.6) | 35 (27.8) | 8 (26.7) | |||
| Bowel resection (%) | 0 | 55 (35.5) | 46 (36.8) | 9 (30.0) | 0.415 | 0.6 |
| 1 | 65 (41.9) | 52 (41.6) | 13 (43.3) | |||
| 2 | 28 (18.1) | 23 (18.4) | 5 (16.7) | |||
| 3 | 7 (4.5) | 4 (3.2) | 3 (10.0) | |||
| Type of bowel resection (%) | anastomosis | 96 (93.2) | 76 (93.8) | 20 (90.9) | 0.996 | 34.0 |
| ostomy | 7 (6.8) | 5 (6.2) | 2 (9.1) | |||
| Blood loss (median [IQR]) | 400.00 [250.00, 750.00] | 400.00 [237.50, 712.50] | 425.00 [287.50, 762.50] | 0.750 | 10.3 | |
| Surgical time (min) (median [IQR]) | 300.00 [240.00, 352.50] | 300.00 [240.00, 342.50] | 300.00 [255.00, 360.00] | 0.419 | 7.7 | |
| Type of fascia suture (%) | monofilament | 122 (98.4) | 97 (98.0) | 25 (100.0) | 1000 | 20.5 |
| multifilament | 2 (1.6) | 2 (2.0) | 0 (0.0) | |||
| Type of fascia suture (%) | no resorbable | 23 (18.7) | 15 (15.0) | 8 (34.8) | 0.058 | 21.2 |
| absorbable | 100 (81.3) | 85 (85.0) | 15 (65.2) | |||
| Size of suture: number of 0 (mean (SD)) | 0 | 3 (3.0) | 3 (3.7) | 0 (0.0) | 0.669 | 35.3 |
| 1 | 39 (38.6) | 32 (39.0) | 7 (36.8) | |||
| 2 | 59 (58.4) | 47 (57.3) | 12 (63.2) | |||
| Fascia technique suturing (%) | continuous | 59 (92.2) | 51 (92.7) | 8 (88.9) | 1000 | 59.9 |
| single | 5 (7.8) | 4 (7.3) | 1 (11.1) | |||
| Type of cutaneous suture (%) | staples | 54 (45.0) | 39 (40.2) | 15 (65.2) | 0.053 | 23.1 |
| intradermic | 66 (55.0) | 58 (59.8) | 8 (34.8) | |||
| Subcutaneous drainage (%) | no | 149 (96.1) | 120 (96.0) | 29 (96.7) | 1000 | 0.6 |
| yes | 6 (3.9) | 5 (4.0) | 1 (3.3) | |||
| Abdominal drainage (%) | no | 83 (53.5) | 67 (53.6) | 16 (53.3) | 1000 | 0.6 |
| yes | 72 (46.5) | 58 (46.4) | 14 (46.7) | |||
| Negative pressure therapy (%) | no | 75 (48.4) | 55 (44.0) | 20 (66.7) | 0.043 | 0.6 |
| yes | 80 (51.6) | 70 (56.0) | 10 (33.3) |
| Overall | No Hernia | Hernia | p-Value * | Missing | ||
|---|---|---|---|---|---|---|
| n | 156 | 126 | 30 | |||
| Days of hospitalization (median [IQR]) | 6.00 [4.00, 9.00] | 6.00 [4.00, 9.00] | 7.00 [6.00, 10.00] | 0.358 | 2.6 | |
| ERAS (%) | no | 66 (45.5) | 47 (39.8) | 19 (70.4) | 0.008 | 7.1 |
| yes | 79 (54.5) | 71 (60.2) | 8 (29.6) | |||
| Surgical infection (<30 d) (%) | no | 122 (79.7) | 101 (81.5) | 21 (72.4) | 0.405 | 1.9 |
| yes | 31 (20.3) | 23 (18.5) | 8 (27.6) | |||
| Infection localization (%) | abdominal abscess | 11 (35.5) | 10 (43.5) | 1 (12.5) | 0.144 | 80.1 |
| lymphocele | 2 (6.5) | 2 (8.7) | 0 (0.0) | |||
| ascites | 2 (6.5) | 2 (8.7) | 0 (0.0) | |||
| peritonitis | 9 (29.0) | 6 (26.1) | 3 (37.5) | |||
| wound (skin and subcutaneous tissue) | 7 (22.6) | 3 (13.0) | 4 (50.0) | |||
| Wound dehiscence (%) | no | 120 (78.9) | 102 (82.9) | 18 (62.1) | 0.026 | 2.6 |
| yes | 32 (21.1) | 21 (17.1) | 11 (37.9) | |||
| Postoperative noradrenaline (%) | no | 119 (76.8) | 98 (78.4) | 21 (70.0) | 0.461 | 0.6 |
| yes | 36 (23.2) | 27 (21.6) | 9 (30.0) | |||
| ICU admission | no | 146 (94.2) | 119 (95.2) | 27 (90.0) | 0.510 | |
| yes | 9 (5.8) | 6 (4.8) | 3 (10.0) | |||
| Days in ICU (median [IQR]) | 10.00 [3.00, 21.00] | 10.50 [4.75, 25.25] | 3.00 [2.50, 12.00] | 0.291 | 0.6 | |
| Blood transfusion (%) | no | 67 (43.2) | 56 (44.8) | 11 (36.7) | 0.547 | 0.6 |
| yes | 88 (56.8) | 69 (55.2) | 19 (63.3) | |||
| Nº concentrates (%) | 0 | 67 (43.2) | 56 (44.8) | 11 (36.7) | 0.349 | 0.6 |
| 1 | 29 (18.7) | 20 (16.0) | 9 (30.0) | |||
| 2 | 36 (23.2) | 30 (24.0) | 6 (20.0) | |||
| 3 | 10 (6.5) | 9 (7.2) | 1 (3.3) | |||
| 4 | 6 (3.9) | 5 (4.0) | 1 (3.3) | |||
| 5 | 2 (1.3) | 2 (1.6) | 0 (0.0) | |||
| 6 | 1 (0.6) | 0 (0.0) | 1 (3.3) | |||
| 7 | 1 (0.6) | 1 (0.8) | 0 (0.0) | |||
| 8 | 3 (1.9) | 2 (1.6) | 1 (3.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Míguez Medina, M.; Luzarraga, A.; Catalán, S.; Acosta, Ú.; Hernández-Fleury, A.; Bebia, V.; Monreal-Clua, S.; Angeles, M.A.; Bonaldo, G.; Gil-Moreno, A.; et al. Incisional Hernia in Cytoreductive Surgery for Advanced-Stage Ovarian Cancer: A Single-Center Retrospective Study. Cancers 2025, 17, 418. https://doi.org/10.3390/cancers17030418
Míguez Medina M, Luzarraga A, Catalán S, Acosta Ú, Hernández-Fleury A, Bebia V, Monreal-Clua S, Angeles MA, Bonaldo G, Gil-Moreno A, et al. Incisional Hernia in Cytoreductive Surgery for Advanced-Stage Ovarian Cancer: A Single-Center Retrospective Study. Cancers. 2025; 17(3):418. https://doi.org/10.3390/cancers17030418
Chicago/Turabian StyleMíguez Medina, Marta, Ana Luzarraga, Sara Catalán, Úrsula Acosta, Alina Hernández-Fleury, Vicente Bebia, Sonia Monreal-Clua, Martina Aida Angeles, Giulio Bonaldo, Antonio Gil-Moreno, and et al. 2025. "Incisional Hernia in Cytoreductive Surgery for Advanced-Stage Ovarian Cancer: A Single-Center Retrospective Study" Cancers 17, no. 3: 418. https://doi.org/10.3390/cancers17030418
APA StyleMíguez Medina, M., Luzarraga, A., Catalán, S., Acosta, Ú., Hernández-Fleury, A., Bebia, V., Monreal-Clua, S., Angeles, M. A., Bonaldo, G., Gil-Moreno, A., Pérez-Benavente, A., & Sánchez-Iglesias, J. L. (2025). Incisional Hernia in Cytoreductive Surgery for Advanced-Stage Ovarian Cancer: A Single-Center Retrospective Study. Cancers, 17(3), 418. https://doi.org/10.3390/cancers17030418







