Lymph Node Yield and Lymph Node Ratio for Prognosis of Long-Term Survival in Gastric Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
- -
- Histologically confirmed primary gastric adenocarcinoma;
- -
- Documented lymphadenectomy;
- -
- R0-resection.
- -
- UICC stage 0;
- -
- UICC stage IV;
- -
- Pre-existing infiltration of neighboring organs (invasion of the duodenum or esophagus alone did not lead to exclusion);
- -
- Postoperative mortality.
2.2. Statistical Analysis
3. Results
3.1. Lymph Node Yield Analysis
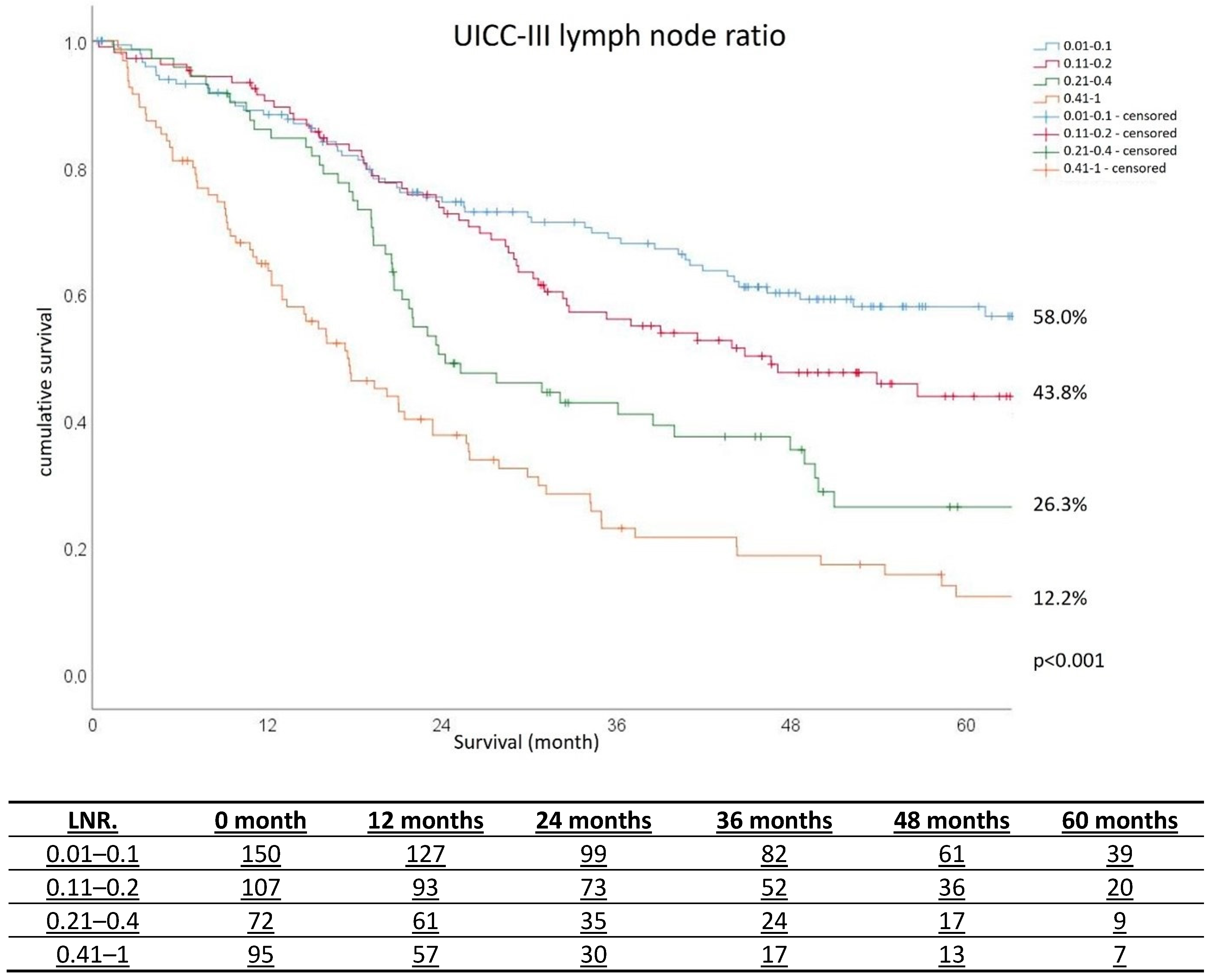
3.2. Long-Term Survival Analyses
4. Discussion
4.1. Lymph Node Yield
4.2. Lymph Node Ratio
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LNR | Lymph Node Ratio |
| TNM | Tumor Nodes Metastasis |
| LN | Lymph Node |
| BMI | Body Mass Index |
| UICC | Union for International Cancer Control |
| AJCC | American Joint Committee on Cancer |
| ASA | American Society of Anesthesiology |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Robert-Koch-Institute. Centre for Cancer Registry Data. 2020. Available online: www.rki.de/EN/Content/Health_Monitoring/Cancer_Registry/cancer_registry_node.html (accessed on 10 December 2023).
- Marrelli, D.; Piccioni, S.A.; Carbone, L.; Petrioli, R.; Costantini, M.; Malagnino, V.; Bagnacci, G.; Rizzoli, G.; Calomino, N.; Piagnerelli, R.; et al. Posterior and Para-Aortic (D2plus) Lymphadenectomy after Neoadjuvant/Conversion Therapy for Locally Advanced/Oligometastatic Gastric Cancer. Cancers 2024, 16, 1376. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Coburn, N.; Cosby, R.; Klein, L.; Knight, G.; Mamazza, J.; Mercer, C.D.; Ringash, J. Staging and surgical approaches in gastric cancer: A clinical practice guideline. Curr. Oncol. 2017, 24, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Dong, P.; Wu, X.; Li, M.; Ding, Q.; Zhang, L.; Yang, J.; Weng, H.; Ding, Q.; Tan, Z.; et al. Three-step method for systematic lymphadenectomy in gastric cancer surgery using the ‘curettage and aspiration dissection technique’ with Peng’s multifunctional operative dissector. World J. Surg. Oncol. 2014, 12, 322. [Google Scholar] [CrossRef][Green Version]
- Lordick, F.; Al-Batran, S.E.; Arnold, D.; Borner, M.; Bruns, C.J.; Eisterer, W.; Faber, G.; Gockel, I.; Köberle, D.; Lorenzen, S.; et al. In Kooperation Mit der AIO. Onkopedia Leitlinen—Magenkarzinom 2023. Available online: www.onkopedia.com/de/onkopedia/guidelines/magenkarzinom/ (accessed on 10 December 2023).
- Yu, J.; Yang, D.; Wei, F.; Sui, Y.; Li, H.; Shao, F.; Li, Y. The staging system of metastatic lymph node ratio in gastric cancer. Hepatogastroenterology 2008, 55, 2287–2290. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.; Baleanu, V.D.; Padureanu, V.; Radulescu, P.M.; Bordu, S.; Patrascu, S.; Socea, B.; Bacalbasa, N.; Surlin, M.V.; Georgescu, I.; et al. Neutrophil/Lymphocyte Ratio as Predictor of Anastomotic Leak after Gastric Cancer Surgery. Diagnostics 2020, 10, 799. [Google Scholar] [CrossRef]
- Lu, J.; Huang, C.M.; Zheng, C.H.; Li, P.; Xie, J.W.; Wang, J.B.; Lin, J.X. Consideration of tumor size improves the accuracy of TNM predictions in patients with gastric cancer after curative gastrectomy. Surg. Oncol. 2013, 22, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.F.; Sun, X.W.; Chen, Y.B.; Li, W.; Xu, D.Z.; Guan, X.X.; Huang, C.Y.; Zhan, Y.Q.; Zhou, Z.W. Prognosis of 980 patients with gastric cancer after surgical resection. Chin. J. Cancer 2010, 29, 923–930. [Google Scholar] [CrossRef]
- Díaz Del Arco, C.; Ortega Medina, L.; Estrada Muñoz, L.; García Gómez de Las Heras, L.; Fernández Aceñero, M.J. Pathologic Lymph Node Staging of Gastric Cancer. Am. J. Clin. Pathol. 2021, 156, 749–765. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Kim, H.O.; Son, B.H.; Shin, J.H.; Yoo, C.H. Prognostic significance of the metastatic lymph node ratio in patients with gastric cancer. World J. Surg. 2012, 36, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- A La-teng, B.L.; Li, Y.M.; Liu, C.G.; Wang, B.B.; Xu, H.M.; Chen, J.Q.; Wang, S.B.; Lu, P. Prognostic value of metastatic lymph node ratio in gastric cancer. Chin. J. Gastrointest. Surg. 2012, 15, 137–140. [Google Scholar]
- Chen, J.X.; Sun, J.W.; Wang, Y.; Pan, T.; Zhuang, L.P.; Lin, L.Z.; Lv, B.C. Lymph node ratio-based the ypTNrM staging system for gastric cancer after neoadjuvant therapy: A large population-based study. Surg. Today 2022, 52, 783–794. [Google Scholar] [CrossRef]
- Karaca, C.A.; Coker, A. Prognostic Value of Metastatic Lymph Node Ratio in Pancreatic Cancer. Indian J. Surg. Oncol. 2019, 10, 50–54. [Google Scholar] [CrossRef]
- Mroczkowski, P.; Kim Samuel Otto, R.; Lippert, H.; Zajdel, R.; Zajdel, K.; Merecz-Sadowska, A. Prognostic value of metastatic lymph node ratio and identification of factors influencing the lymph node yield in patients undergoing curative colon cancer resection. Cancers 2024, 16, 218. [Google Scholar] [CrossRef]
- German Cancer Society; German Cancer Aid; AWMF. Guideline Programme on Oncology: S3-Leitlinie Diagnostik und Therapie der Adenokarzinome des Magens und ösophagogastralen Übergangs (Version 2, 2019); AWMF Register Number: 032/009OL. Available online: www.leitlinienprogramm-onkologie.de/leitlinien/magenkarzinom/ (accessed on 10 December 2023).
- Ema, A.; Yamashita, K.; Sakuramoto, S.; Wang, G.; Mieno, H.; Nemoto, M.; Shibata, T.; Katada, N.; Kikuchi, S.; Watanabe, M. Lymph node ratio is a critical prognostic predictor in gastric cancer treated with S-1 chemotherapy. Gastric Cancer 2014, 17, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Masi, A.; Pinna, A.; Cohen, S.; Hatzaras, I.; Berman, R.; Pachter, L.H.; Newman, E. Does lymph node ratio affect prognosis in gastroesophageal cancer? Am. J. Surg. 2015, 210, 443–450. [Google Scholar] [CrossRef]
- Spychała, A.; Nowaczyk, P.; Murawa, D. Comparison of Lymphatic System Staging Classifications in Patients with Gastric Cancer. Pol. Przegl Chir. 2015, 87, 551–557. [Google Scholar] [CrossRef]
- Kutlu, O.C.; Watchell, M.; Dissanaike, S. Metastatic lymph node ratio successfully predicts prognosis in western gastric cancer patients. Surg. Oncol. 2015, 24, 84–88. [Google Scholar] [CrossRef]
- Wu, X.J.; Miao, R.L.; Li, Z.Y.; Bu, Z.D.; Zhang, L.H.; Wu, A.H.; Zong, X.L.; Li, S.X.; Shan, F.; Ji, X.; et al. Prognostic value of metastatic lymph node ratio as an additional tool to the TNM stage system in gastric cancer. Eur. J. Surg. Oncol. 2015, 41, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.M.; Lin, J.X.; Zheng, C.H.; Li, P.; Xie, J.W.; Lin, B.J.; Wang, J.B. Prognostic impact of metastatic lymph node ratio on gastric cancer after curative distal gastrectomy. World J. Gastroenterol. 2010, 16, 2055–2060. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.G.; Deng, J.Y.; Zhang, R.P.; Wu, L.L.; Wang, L.; Liu, H.G.; Hao, X.S.; Liang, H. Prognostic value of number of examined lymph nodes in patients with node-negative gastric cancer. World J. Gastroenterol. 2014, 20, 3640–3648. [Google Scholar] [CrossRef]
- Son, T.; Hyung, W.J.; Lee, J.H.; Kim, Y.M.; Kim, H.I.; An, J.Y.; Cheong, J.H.; Noh, S.H. Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer 2012, 118, 4687–4693. [Google Scholar] [CrossRef]
- Chen, C.Q.; Wu, X.J.; Yu, Z.; Bu, Z.D.; Zuo, K.Q.; Li, Z.Y.; Ji, J.F. Prognosis of patients with gastric cancer and solitary lymph node metastasis. World J. Gastroenterol. 2013, 19, 8611–8618. [Google Scholar] [CrossRef]
- Setälä, L.P.; Kosma, V.M.; Marin, S.; Lipponen, P.K.; Eskelinen, M.J.; Syrjänen, K.J.; Alhava, E.M. Prognostic factors in gastric cancer: The value of vascular invasion, mitotic rate and lymphoplasmacytic infiltration. Br. J. Cancer 1996, 74, 766–772. [Google Scholar] [CrossRef]
- Liang, Y.X.; Deng, J.Y.; Guo, H.H.; Ding, X.W.; Wang, X.N.; Wang, B.G.; Zhang, L.; Liang, H. Characteristics and prognosis of gastric cancer in patients aged ≥ 70 years. World J. Gastroenterol. 2013, 19, 6568–6578. [Google Scholar] [CrossRef] [PubMed]
- Mayol-Oltra, A.; Marti-Obiol, R.; López-Mozos, F.; Báguena-Requena, G.; Ortega-Serrano, J. The influence of advanced age on the morbi-mortality of gastric cancer after curative surgery. Rev. Esp. Enferm. Dig. 2013, 105, 194–199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eom, B.W.; Jung, K.W.; Won, Y.J.; Yang, H.; Kim, Y.W. Trends in Gastric Cancer Incidence According to the Clinicopathological Characteristics in Korea, 1999–2014. Cancer Res. Treat. 2018, 50, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, S.; Bu, Z.; Zhang, L.; Wu, X.; Shan, F.; Jia, Y.; Ji, X.; Ji, J. The effect of preoperative treatments on lymph node counts after total gastrectomy in esophagogastric adenocarcinoma. J. Surg. Oncol. 2018, 118, 657–663. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Gu, X.Z.; Yin, W.B.; Huang, G.J.; Zhang, D.W.; Zhang, R.G. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC)-report on 370 patients. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 929–934. [Google Scholar] [CrossRef]
- Tsuburaya, A.; Mizusawa, J.; Tanaka, Y.; Fukushima, N.; Nashimoto, A.; Sasako, M.; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br. J. Surg. 2014, 101, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Rha, S.Y.; Wyrwicz, L.S.; Oshima, T.; Karaseva, N.; Osipov, M.; Yasui, H.; Yabusaki, H.; Afanasyev, S.; Park, Y.K.; et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): An interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 2024, 25, 212–224. [Google Scholar] [CrossRef]
- Sun, X.; Lyu, J.; Yang, M.; Wu, K.; Liu, K.; Li, A.; Shuai, X.; Cai, K.; Wang, Z.; Wang, G.; et al. Two-Year Outcomes and Biomarker Analysis of Locally Advanced Gastric and Gastroesophageal Junction Adenocarcinoma After Neoadjuvant Chemotherapy and Immunotherapy from the Phase II WuhanUHGI001 Trial. Ann. Surg. Oncol. 2024, 31, 8157–8169. [Google Scholar] [CrossRef]
- Zhou, P.; Sun, X.; Zeng, L.; Zeng, X.; Xie, G.; Liu, X.; Tao, K.; Zhang, P. Lymph node ratio is a prognostic indicator for locally advanced gastric cancer after neoadjuvant immunochemotherapy. BMC Gastroenterol. 2024, 24, 371. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Li, C.C.; Jia, L.Y.; Chen, X.L.; Zhang, W.H.; Chen, X.Z.; Yang, K.; Liu, K.; Wang, Y.G.; Xue, L.; et al. Superiority of lymph node ratio-based staging system for prognostic prediction in 2575 patients with gastric cancer: Validation analysis in a large single center. Oncotarget 2016, 7, 51069–51081. [Google Scholar] [CrossRef] [PubMed]
- Tural, D.; Selçukbiricik, F.; Akar, E.; Serdengeçti, S.; Büyükünal, E. Gastric cancer: A case study in Turkey. J. Cancer Res. Ther. 2013, 9, 644–648. [Google Scholar] [PubMed]
- Wong, J.; Rahman, S.; Saeed, N.; Lin, H.Y.; Almhanna, K.; Shridhar, R.; Hoffe, S.; Meredith, K.L. Prognostic impact of lymph node retrieval and ratio in gastric cancer: A U.S. single center experience. J. Gastrointest. Surg. 2013, 17, 2059–2066. [Google Scholar] [CrossRef]
- Chen, J.-H.; Cai, S.-R.; Wu, H.; Chen, S.-L.; Xu, J.-B.; Zhai, E.-T.; Chen, C.-Q.; He, Y.-L. Prognostic value of three different lymph node staging systems in the survival of patients with gastric cancer following D2 lymphadenectomy. Tumour Biol. 2016, 37, 11105–11113. [Google Scholar]
- Zhu, J.; Xue, Z.; Zhang, S.; Guo, X.; Zhai, L.; Shang, S.; Zhang, Y.; Lu, H. Integrated analysis of the prognostic role of the lymph node ratio in node-positive gastric cancer: A meta-analysis. Int. J. Surg. 2018, 57, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rao, H.; Liu, J.; Geng, Q.; Guo, J.; Kong, P.; Li, S.; Liu, X.; Sun, X.; Zhan, Y.; et al. Lymph nodes ratio based nomogram predicts survival of resectable gastric cancer regardless of number of examined lymph nodes. Oncotarget 2017, 8, 45585–45596. [Google Scholar] [CrossRef]
- Topcu, R.; Şahiner, I.T.; Kendirci, M.; Erkent, M.; Sezikli, I.; Tutan, M.B. Does lymph node ratio (metastasis/total lymph node count) affect survival and prognosis in gastric cancer? Saudi Med. J. 2022, 43, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, Y.; Zhang, W.; Liu, H.; Wang, Z.; Zhang, Y. Modified Gastric Cancer AJCC Staging with a Classification Based on the Ratio of Regional Lymph Node Involvement: A Population-Based Cohort Study. Ann. Surg. Oncol. 2020, 27, 1480–1487. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, S. Does D2 plus para-aortic nodal dissection surgery offer a better survival outcome compared to D2 surgery only for gastric cancer consistently? A definite result based on a hospital population of nearly two decades. Scand. J. Surg. 2013, 102, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhang, R.; Wu, L.; Zhang, L.; Wang, X.; Liu, Y.; Hao, X.; Liang, H. Superiority of the ratio between negative and positive lymph nodes for predicting the prognosis for patients with gastric cancer. Ann. Surg. Oncol. 2015, 22, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
| Numbers of Lymph Node Stations | |||
|---|---|---|---|
| Type of Lymphadenectomy | Compartment I | Compartment II | Compartment III |
| D1 | 1, 2, 3, 4, 5, 6 | - | - |
| D2 | 1, 2, 3, 4, 5, 6 | 7, 8, 9, 10,11 | - |
| D2-plus | 1, 2, 3, 4, 5, 6 | 7, 8, 9, 10,11 | 12, 13, 16 |
| D3 | 1, 2, 3, 4, 5, 6 | 7, 8, 9, 10,11 | 12, 13, 14, 15, 16 |
| Group 1 (n/%) | Group 2 (n/%) | p-Value * | ||
|---|---|---|---|---|
| Sex | Male | 213/68.6 | 868/60.9 | <0.001 |
| Female | 143/31.4 | 557/39.1 | ||
| ASA-classification | ASA I | 23/5.1 | 121/8.6 | <0.001 |
| ASA II | 199/44.3 | 767/54.5 | ||
| ASA III | 214/47.7 | 495/35.2 | ||
| ASA IV | 13/2.9 | 24/1.7 | ||
| Grading | G1 | 39/8.6 | 59/4.1 | <0.001 |
| G2 | 159/34.9 | 429/30.2 | ||
| G3 | 228/50.1 | 836/58.8 | ||
| G4 | 14/3.1 | 27/1.9 | ||
| Histology | Papillary adenocarcinoma | 30/6.6 | 97/6.8 | 0.874 |
| Tubular adenocarcinoma | 114/25.0 | 371/26.0 | 0.677 | |
| Mucinous adenocarcinoma | 56/12.3 | 128/9.0 | 0.038 | |
| Signet ring cell carcinoma | 89/19.5 | 351/24.6 | 0.026 | |
| Undifferentiated carcinoma | 25/5.5 | 84/5.9 | 0.750 | |
| Small cell carcinoma | 2/0.4 | 0/0.0 | - | |
| Squamous cell carcinoma | 4/0.9 | 7/0.5 | 0.345 | |
| Adenosquamous carcinoma | 0/0.0 | 3/0.2 | 0.327 | |
| Surgical approach | Laparotomy | 430/95.2 | 1411/99.1 | <0.001 |
| Laparoscopic | 18/4.0 | 7/0.5 | ||
| Surgical procedure | Proximal resection | 36/8.2 | 22/1.5 | <0.001 |
| Subtotal/distal gastrectomy | 166/37.9 | 364/25.6 | 0.112 | |
| Total gastrectomy | 160/36,4 | 827/58.1 | <0.001 | |
| Transhiatal extended gastrectomy | 30/6.8 | 144/10.1 | 0.024 | |
| Transthoracic extended gastrectomy | 17/3.9 | 33/2.3 | 0.101 | |
| Thoraco-abdominal esophagogastrectomy | 16/3.6 | 23/1.6 | 0.013 | |
| Other gastrectomy | 14/3.2 | 12/0.8 | <0.001 | |
| Neoadjuvant treatment | No | 363/80.3 | 1054/74.3 | 0.009 |
| Yes | 89/19.7 | 365/25.7 | ||
| Localization | Gastroesophageal junction | 125/27.4 | 280/19.6 | <0.001 |
| Fundus | 19/4.2 | 45/3.2 | 0.297 | |
| Corpus | 142/31.1 | 553/38.7 | 0.003 | |
| Antral/pyloric region | 181/39.7 | 601/41.5 | 0.366 | |
| Invasion depth | pT0 | 0/0.0 | 2/0.1 | <0.001 |
| pT1 | 200/44.0 | 460/32.3 | ||
| pT2 | 88/25.1 | 262/18.4 | ||
| pT3 | 167/36.7 | 699/49.1 | ||
| pN-stage | pN0 | 288/63.6 | 756/53.0 | <0.001 |
| pN1 | 81/17.9 | 239/16.8 | ||
| pN2 | 52/11.5 | 203/14.2 | ||
| pN3 | 32/7.1 | 228/16.0 | ||
| UICC-stage | I | 249/54.6 | 578/40.5 | <0.001 |
| II | 117/25.7 | 427/30.6 | ||
| III | 90/19.7 | 413/28.9 |
| LK-Quotient (Mean ± SD) | p-Value | ||
|---|---|---|---|
| Sex | Male | 0.11 ± 0.19 | 0.132 ** |
| Female | 0.10 ± 019 | ||
| Age groups <70, 70–80, >80 | <70 | 0.11 ± 0.19 | 0.213 * |
| 70–80 | 0.11 ± 0.19 | ||
| >80 | 0.12 ± 0.21 | ||
| BMI | <18.5 | 0.15 ± 0.23 | 0.682 * |
| 18.5–24.9 | 0.11 ± 0.20 | ||
| ≥25 | 0.10 ± 0.18 | ||
| Lauren classification | None | 0.11 ± 0.18 | <0.001 * |
| Intestinal | 0.08 ± 0.16 | ||
| Diffuse | 0.14 ± 0.23 | ||
| Mixed | 0.10 ± 0.17 | ||
| Localization | Fundus | 0,12 ± 0.20 | 0.889 ** |
| Gastroesophageal junction | 0.14 ± 0.20 | <0.001 ** | |
| Corpus | 0.11 ± 0.20 | 0.923 ** | |
| Antrum/pylorum | 0.10 ± 0.20 | 0.030 ** | |
| ASA-classification | I | 0.10 ± 0.19 | 0.440 * |
| II | 0.11 ± 0.20 | ||
| III | 0.11 ± 0.19 | ||
| IV | 0.05 ± 0.10 | ||
| Neoadjuvant treatment | No | 0.10 ± 0.19 | <0.001 ** |
| Yes | 0.12 ± 0.19 | ||
| Surgical approach | Laparotomy | 0.11 ± 0.19 | 0.548 ** |
| Laparoscopic | 0.07 ± 0.12 | ||
| Grading | G1 | 0.01 ± 0.07 | <0.001 * |
| G2 | 0.08 ± 0.16 | ||
| G3 | 0.13 ± 0.21 | ||
| G4 | 0.11 ± 0.18 | ||
| pT-stage | pT0 | 0.06 ± 0.08 | <0.001 * |
| pT1 | 0.02 ± 0.08 | ||
| pT2 | 0.08 ±0.15 | ||
| pT3 | 0.17 ± 0.23 | ||
| pN-stage | pN0 | 0.01 ± 0.03 | <0.001 * |
| pN1 | 0.06 ± 0.05 | ||
| pN2 | 0.15 ± 0.07 | ||
| pN3 | 0.47 ± 0.22 | ||
| Lymph invasion | L0 | 0.03 ± 0.09 | <0.001 ** |
| L1 | 0.20 ± 0.23 | ||
| Venous invasion | V0 | 0.09 ± 0,17 | <0.001 ** |
| V1 | 0.24 ± 0.27 | ||
| UICC-stage | I | 0.003 ± 0.01 | <0.001 * |
| II | 0.05 ± 0.10 | ||
| III | 0.31 ± 0.24 |
| Odds Ratio (95% CI) | p-Value | ||
|---|---|---|---|
| Grading | G1 | 1 | |
| G2 | 1.982 (1.110–3.541) | 0.021 | |
| G3 | 2.154 (1.212–3.829) | 0.009 | |
| G4 | 0.739 (0.268–2.036) | 0.558 | |
| UICC-stage | I | 1 | |
| II | 1.441 (1.008–2.060) | 0.045 | |
| III | 1.707 (1.135–2.568) | 0.010 | |
| Age groups | >80 | 1 | |
| <70 | 1.818 (1.188–2.783) | 0.006 | |
| 70–80 | 1.358 (0.874–2.109) | 0.173 | |
| Sex | Men | 1 | |
| Women | 1.365 (1.000–1.863) | 0.050 | |
| Venous invasion | No (V0) | 1 | |
| Yes (V1) | 0.647 (0.411–1.016) | 0.059 |
| Hazard Ratio (95% CI) | p-Value | ||
|---|---|---|---|
| Lymph node ratio | 0.01–0.1 | 1 | |
| 0.11–0.2 | 1.207 (0.770–1.893) | 0.413 | |
| 0.21–0.4 | 1.652 (1.027–2.659) | 0.039 | |
| 0.41–1 | 2.746 (1.740–4.333) | <0.001 | |
| UICC-stage | I | 1 | |
| II | 0.485 (0.260–0.905) | 0.023 | |
| III | 0.849 (0.436–1.654) | 0.630 | |
| Age groups | <70 | 1 | |
| 70–80 | 1.374 (1.008–1.874) | 0.045 | |
| >80 | 1.806 (1.225–2.663) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jannasch, O.; Schwanz, M.; Otto, R.; Mik, M.; Lippert, H.; Mroczkowski, P. Lymph Node Yield and Lymph Node Ratio for Prognosis of Long-Term Survival in Gastric Carcinoma. Cancers 2025, 17, 414. https://doi.org/10.3390/cancers17030414
Jannasch O, Schwanz M, Otto R, Mik M, Lippert H, Mroczkowski P. Lymph Node Yield and Lymph Node Ratio for Prognosis of Long-Term Survival in Gastric Carcinoma. Cancers. 2025; 17(3):414. https://doi.org/10.3390/cancers17030414
Chicago/Turabian StyleJannasch, Olof, Martin Schwanz, Ronny Otto, Michal Mik, Hans Lippert, and Pawel Mroczkowski. 2025. "Lymph Node Yield and Lymph Node Ratio for Prognosis of Long-Term Survival in Gastric Carcinoma" Cancers 17, no. 3: 414. https://doi.org/10.3390/cancers17030414
APA StyleJannasch, O., Schwanz, M., Otto, R., Mik, M., Lippert, H., & Mroczkowski, P. (2025). Lymph Node Yield and Lymph Node Ratio for Prognosis of Long-Term Survival in Gastric Carcinoma. Cancers, 17(3), 414. https://doi.org/10.3390/cancers17030414






