Long-Term Safety and Survival Outcomes of [225Ac]Ac-PSMA (Prostate-Specific Membrane Antigen) and [225Ac]Ac-/[177Lu]Lu-PSMA (TANDEM) Radioligand Therapy (PRLT) in Metastatic Castration-Resistant Prostate Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Cohort and PRLT Data
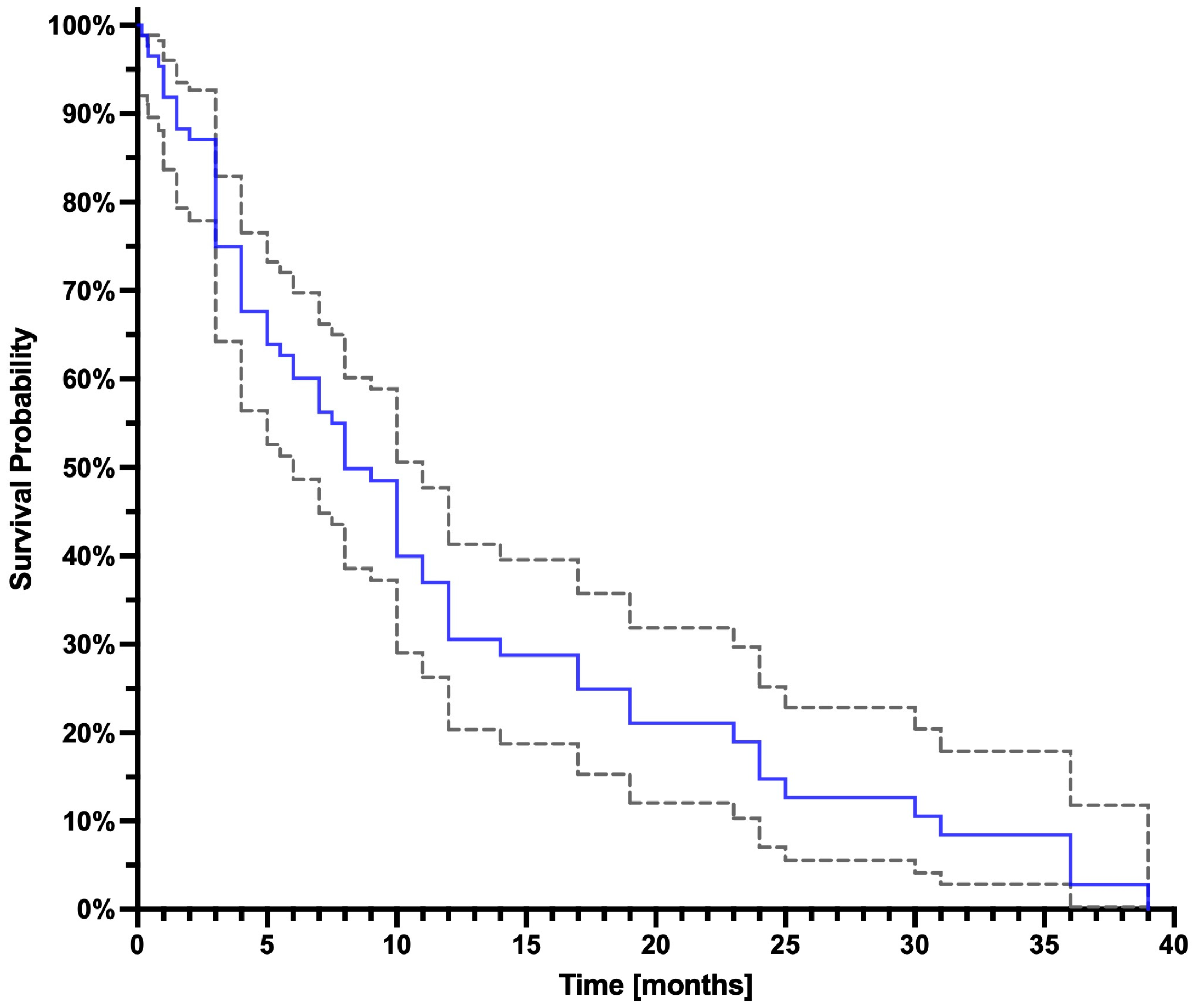
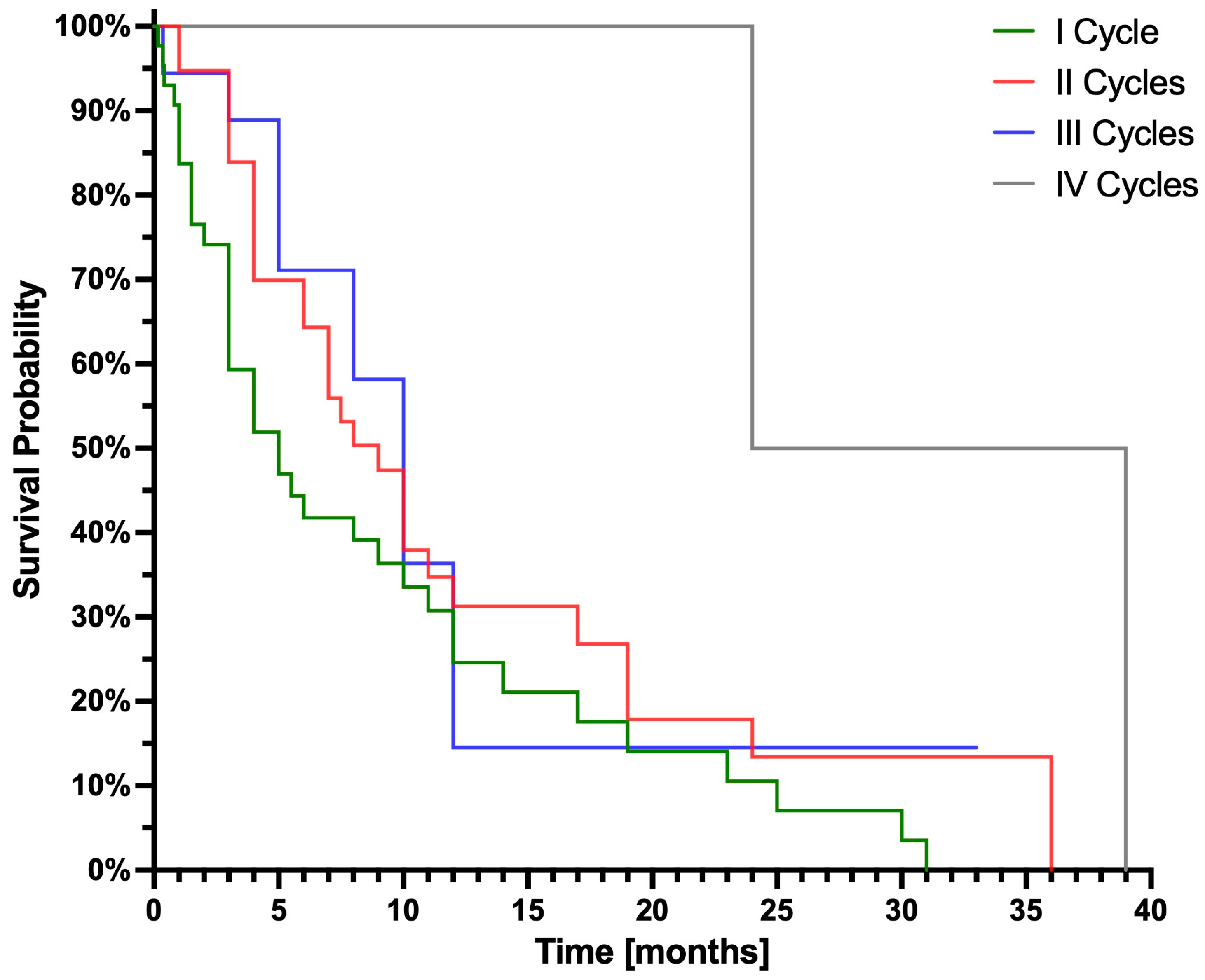
3.2. Survival Analysis
3.3. Tolerance After the Radiopharmaceutical Administration
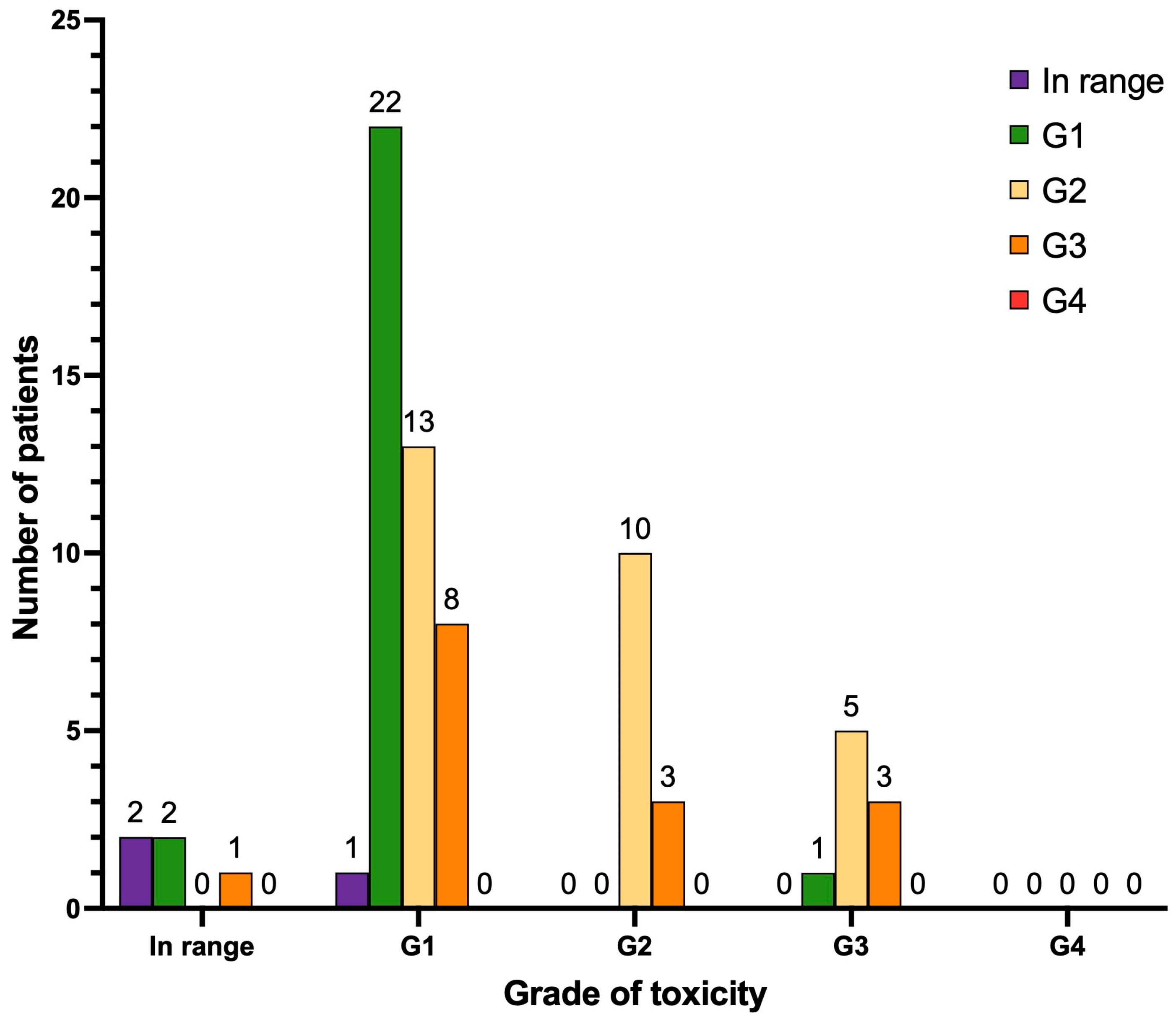
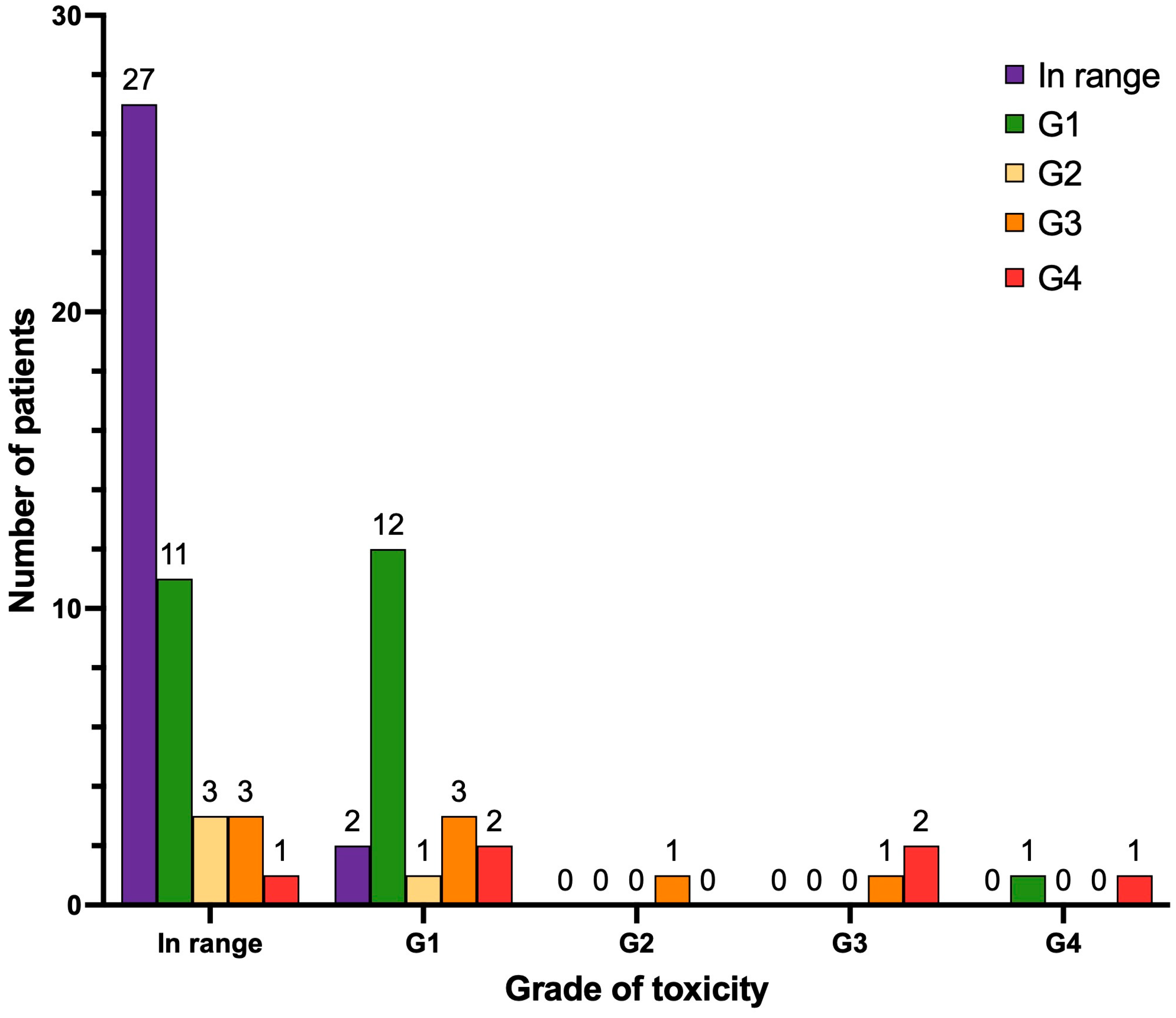
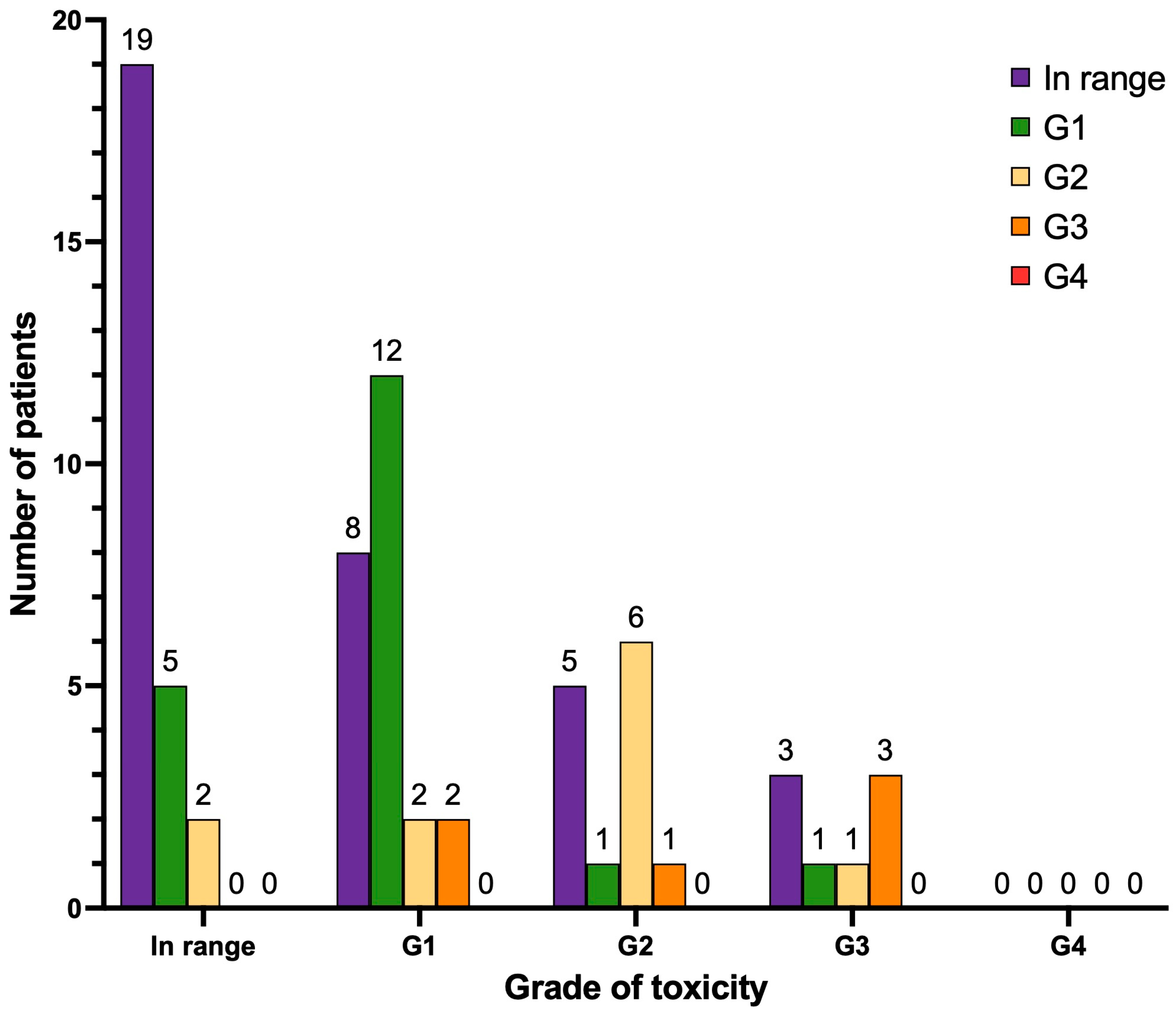
3.4. Hematological Toxicity
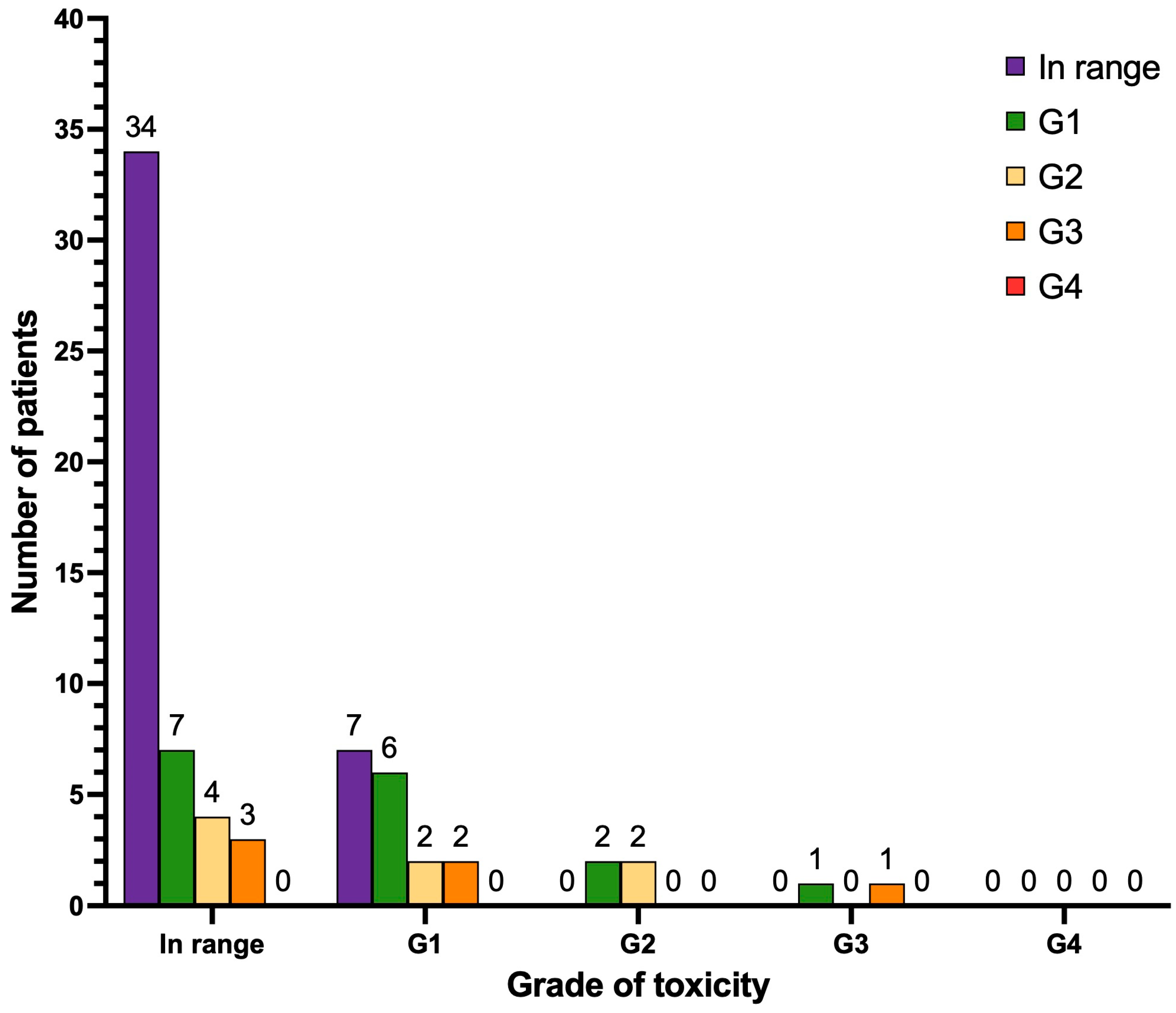
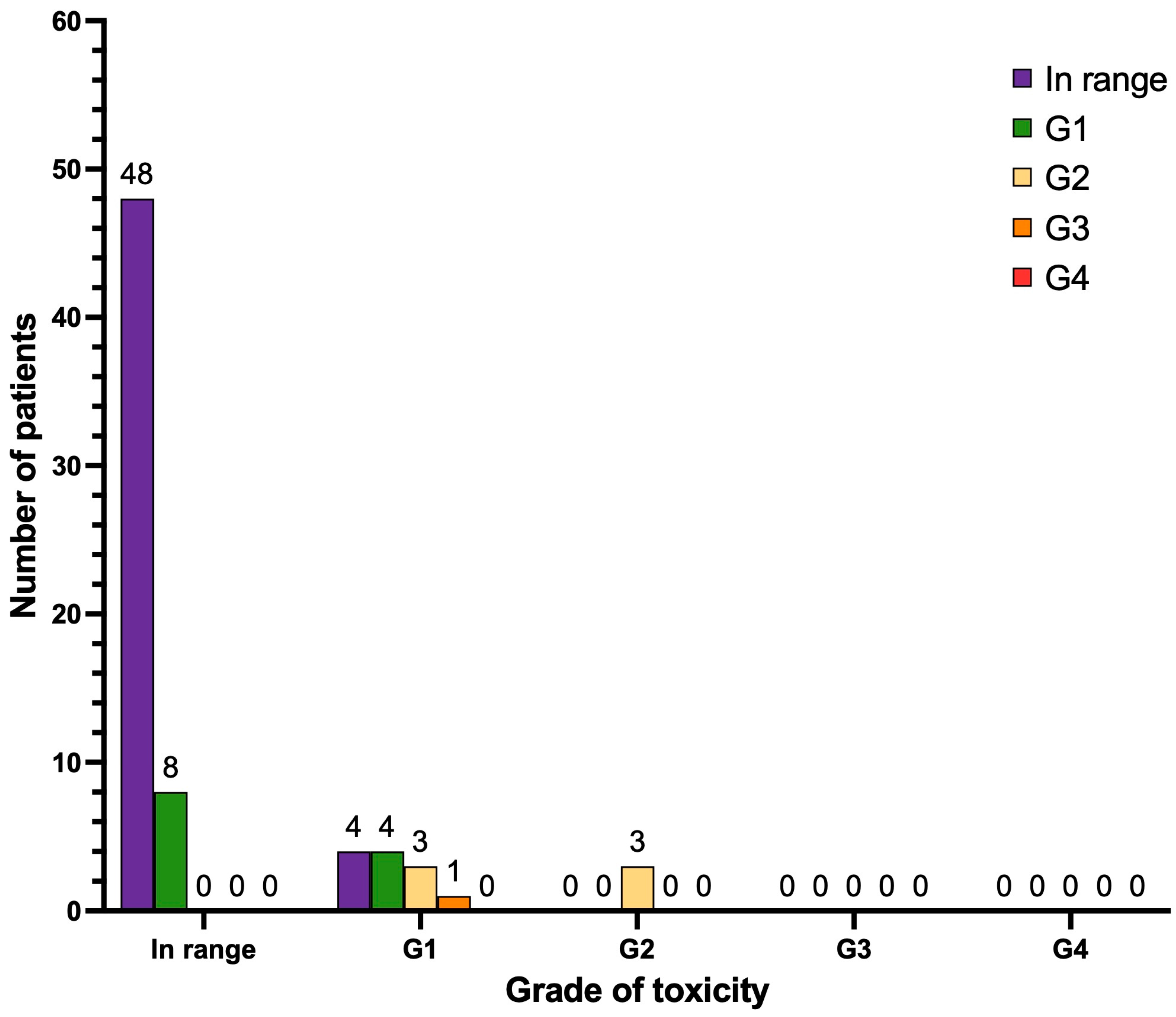
3.5. Nephrotoxicity and Hepatotoxicity
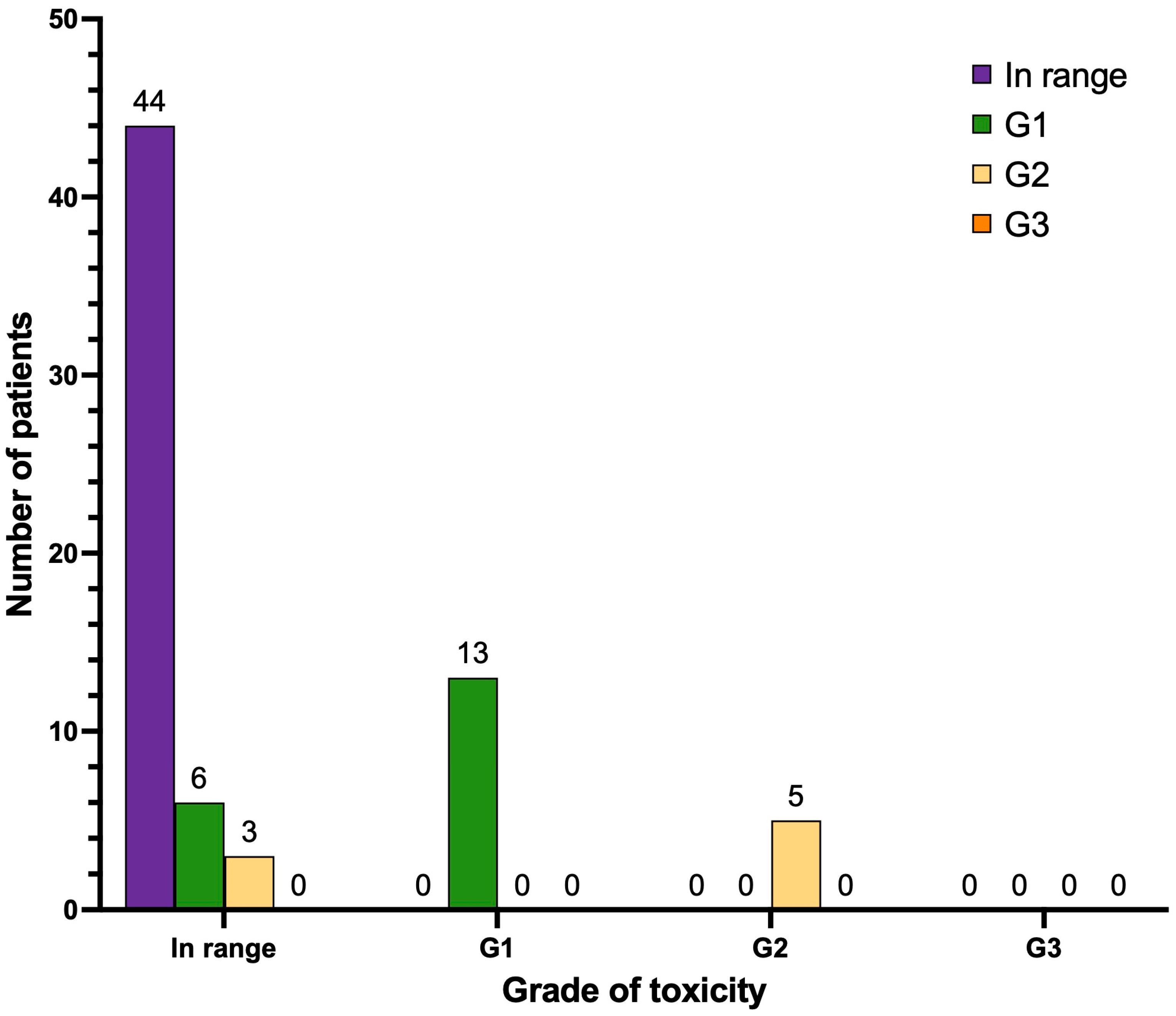
3.6. Xerostomia (Dry Mouth)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC; WHO. Data Visualization Tools for Exploring the Global Cancer Burden in 2020. Available online: https://gco.iarc.fr/today/home (accessed on 6 November 2024).
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Cornford, P.; Bergh, R.C.v.D.; Briers, E.; Broeck, T.V.D.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer—2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef]
- Tilki, D.; Bergh, R.C.v.D.; Briers, E.; Broeck, T.V.D.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. Part II—2024 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2024, 86, 164–182. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Sterzing, F.; Kratochwil, C.; Fiedler, H.; Katayama, S.; Habl, G.; Kopka, K.; Afshar-Oromieh, A.; Debus, J.; Haberkorn, U.; Giesel, F.L. 68Ga-PSMA-11 PET/CT: A new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur. J. Nucl. Med. 2015, 43, 34–41. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. New Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Fallah, J.; Agrawal, S.; Gittleman, H.; Fiero, M.H.; Subramaniam, S.; John, C.; Chen, W.; Ricks, T.K.; Niu, G.; Fotenos, A.; et al. FDA Approval Summary: Lutetium Lu 177 Vipivotide Tetraxetan for Patients with Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2022, 29, 1651–1657. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/pluvicto (accessed on 6 November 2024).
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef]
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Böning, G.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Schmidt-Hegemann, N.; Belka, C.; Kretschmer, A.; et al. First Clinical Results for PSMA-Targeted α-Therapy Using 225Ac-PSMA-I&T in Advanced-mCRPC Patients. J. Nucl. Med. 2020, 62, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Rosar, F.; Hau, F.; Bartholomä, M.; Maus, S.; Stemler, T.; Linxweiler, J.; Ezziddin, S.; Khreish, F. Molecular imaging and biochemical response assessment after a single cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in mCRPC patients who have progressed on [177Lu]Lu-PSMA-617 monotherapy. Theranostics 2021, 11, 4050–4060. [Google Scholar] [CrossRef]
- Sathekge, M.M.; O Lawal, I.; Bal, C.; Bruchertseifer, F.; Ballal, S.; Cardaci, G.; Davis, C.; Eiber, M.; Hekimsoy, T.; Knoesen, O.; et al. Actinium-225-PSMA radioligand therapy of metastatic castration-resistant prostate cancer (WARMTH Act): A multicentre, retrospective study. Lancet Oncol. 2024, 25, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Beheshti, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur Urol. 2020, 79, 343–350. [Google Scholar] [CrossRef]
- Langbein, T.; Kulkarni, H.R.; Schuchardt, C.; Mueller, D.; Volk, G.F.; Baum, R.P. Salivary Gland Toxicity of PSMA-Targeted Radioligand Therapy with 177Lu-PSMA and Combined 225Ac- and 177Lu-Labeled PSMA Ligands (TANDEM-PRLT) in Advanced Prostate Cancer: A Single-Center Systematic Investigation. Diagnostics 2022, 12, 1926. [Google Scholar] [CrossRef]
- Kulkarni, H.R.; Zhang, J.; Langbein, T.; Schuchardt, C.; Singh, A.; Mueller, D.; Baum, R.P. Radioligand therapy using combination of Ac-225 and Lu-177 labelled PSMA ligands for progressive end-stage metastatic prostate cancer: Effective trade-off between response and toxicity. J. Nuc. Med. 2019, 60, 464. [Google Scholar]
- Fendler, W.P.; Kratochwil, C.; Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Schmidt, M.; Pfestroff, A.; Lützen, U.; Prasad, V.; Heinzel, A.; et al. 177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer. Nuklearmedizin 2016, 55, 123–128. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Hofman, M.S.; Emmett, L.; Calais, J.; Osborne, J.R.; Iravani, A.; Koo, P.; Lindenberg, L.; et al. Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). Eur. J. Nucl. Med. 2023, 50, 2830–2845. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v 5.0. 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 6 November 2024).
- Mueller, J.; Langbein, T.; Mishra, A.; Baum, R.P. Safety of High-Dose Botulinum Toxin Injections for Parotid and Submandibular Gland Radioprotection. Toxins 2022, 14, 64. [Google Scholar] [CrossRef]
- Kohler, P.F.; Winter, M.E. A quantitative test for xerostomia. The saxon test, an oral equivalent of the schirmer test. Arthritis Rheum. 1985, 28, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mueller, J.; Fan, X.; Zhao, T.; Jakobsson, V.; Baum, R.P. Botulinum toxin plus transdermal scopolamine reduce salivary gland uptake of 225Ac/177Lu-PSMA ligands. In Proceedings of the European Association of Nuclear Medicine, Hamburg, Germany, 22 October 2024. [Google Scholar]
- Paganelli, G.; Sarnelli, A.; Severi, S.; Sansovini, M.; Belli, M.L.; Monti, M.; Foca, F.; Celli, M.; Nicolini, S.; Tardelli, E.; et al. Dosimetry and safety of 177Lu PSMA-617 along with polyglutamate parotid gland protector: Preliminary results in metastatic castration-resistant prostate cancer patients. Eur. J. Nucl. Med. 2020, 47, 3008–3017. [Google Scholar] [CrossRef] [PubMed]
- Bidkar, A.P.; Zerefa, L.; Yadav, S.; VanBrocklin, H.F.; Flavell, R.R. Actinium-225 targeted alpha particle therapy for prostate cancer. Theranostics 2024, 14, 2969–2992. [Google Scholar] [CrossRef]
- Ling, S.W.; de Blois, E.; Hooijman, E.; van der Veldt, A.; Brabander, T. Advances in 177Lu-PSMA and 225Ac-PSMA Radionuclide Therapy for Metastatic Castration-Resistant Prostate Cancer. Pharmaceutics 2022, 14, 2166. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.O.; Mokoala, K.; Reed, J.; Maseremule, L.; Ndlovu, H.; Hlongwa, K.; Maes, A.; et al. 225Ac-PSMA-617 radioligand therapy of de novo metastatic hormone-sensitive prostate carcinoma (mHSPC): Preliminary clinical findings. Eur. J. Nucl. Med. 2023, 50, 2210–2218. [Google Scholar] [CrossRef]
- Tönnesmann, R.; Meyer, P.T.; Eder, M.; Baranski, A.-C. [177Lu]Lu-PSMA-617 Salivary Gland Uptake Characterized by Quantitative In Vitro Autoradiography. Pharmaceuticals 2019, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Rupp, N.J.; Umbricht, C.A.; Pizzuto, D.A.; Lenggenhager, D.; Töpfer, A.; Müller, J.; Muehlematter, U.J.; Ferraro, D.A.; Messerli, M.; Morand, G.B.; et al. First Clinicopathologic Evidence of a Non–PSMA-Related Uptake Mechanism for 68Ga-PSMA-11 in Salivary Glands. J. Nucl. Med. 2019, 60, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- van Kalmthout, L.W.M.; Lam, M.G.E.H.; de Keizer, B.; Krijger, G.C.; Ververs, T.F.T.; de Roos, R.; Braat, A.J.A.T. Impact of external cooling with icepacks on 68Ga-PSMA uptake in salivary glands. EJNMMI Res. 2018, 8, 56. [Google Scholar] [CrossRef]
- Taïeb, D.; Foletti, J.-M.; Bardiès, M.; Rocchi, P.; Hicks, R.J.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy and Salivary Gland Toxicity: Why Does It Matter? J. Nucl. Med. 2018, 59, 747–748. [Google Scholar] [CrossRef]
- Baum, R.P.; Langbein, T.; Singh, A.; Shahinfar, M.; Schuchardt, C.; Volk, G.F.; Kulkarni, H. Injection of Botulinum Toxin for Preventing Salivary Gland Toxicity after PSMA Radioligand Therapy: An Empirical Proof of a Promising Concept. Nucl. Med. Mol. Imaging 2018, 52, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Pepin, A.; Lee, V.; O’Brien, S.; Mulugeta, P.; Taunk, N.K. Management of Dry Eye Toxicity After Treatment With 177Lu-PSMA-617 Radioligand Therapy. Pr. Radiat. Oncol. 2024, 14, 301–304. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Tripathi, M.; Seth, A.; Bal, C. Efficacy and safety of 225Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant Prostate Cancer patients. Theranostics 2020, 10, 9364–9377. [Google Scholar] [CrossRef] [PubMed]
- Khreish, F.; Ebert, N.; Ries, M.; Maus, S.; Rosar, F.; Bohnenberger, H.; Stemler, T.; Saar, M.; Bartholomä, M.; Ezziddin, S. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: Pilot experience. Eur. J. Nucl. Med. 2019, 47, 721–728. [Google Scholar] [CrossRef]
- Rathke, H.; Winter, E.; Bruchertseifer, F.; Röhrich, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A.; Kratochwil, C. Deescalated225Ac-PSMA-617 Versus177Lu/225Ac-PSMA-617 Cocktail Therapy: A Single-Center Retrospective Analysis of 233 Patients. J. Nucl. Med. 2024, 65, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.C.; Amghar, M.; Wacker, A.S.; Bakos, G.; Taş, H.; Roscher, M.; Kelly, J.M.; Benešová-Schäfer, M. Future Treatment Strategies for Cancer Patients Combining Targeted Alpha Therapy with Pillars of Cancer Treatment: External Beam Radiation Therapy, Checkpoint Inhibition Immunotherapy, Cytostatic Chemotherapy, and Brachytherapy. Pharmaceuticals. 2024, 17, 1031. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Heussel, C.P.; Kazdal, D.; Endris, V.; Nientiedt, C.; Bruchertseifer, F.; Kippenberger, M.; Rathke, H.; Leichsenring, J.; et al. Patients resistant against PSMA-targeting alpha-radiation therapy often harbor mutations in DNA-repair associated genes. J. Nucl. Med. 2019, 61, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Falzone, N.; Pearson, M.; Pook, D.; Sivaratnam, D. 177Lu-PSMA With Olaparib Radiosensitization Potentiates Response and Toxicity in Extensive Castration-Resistant Metastatic Prostate cancer. Clin. Nucl. Med. 2024, 49, 966–967. [Google Scholar] [CrossRef]
- Kramer, C.S.; Zhang, J.; Baum, R.P. Extraordinary therapeutic effect of PSMA radioligand therapy in treatment-refractory progressive metastatic prostate cancer with the transketolase inhibitor benfo-oxythiamine as a radiosensitizer—A case report. Front. Med. 2024, 11, 1462234. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Urso, L.; Bianconi, F.; Palumbo, B.; Marzola, M.C.; Evangelista, L.; Schillaci, O. Radiomics and theranostics with molecular and metabolic probes in prostate cancer: Toward a personalized approach. Expert Rev. Mol. Diagn. 2023, 23, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R. Is212Pb Really Happening? The Post-177Lu/225Ac Blockbuster? J. Nucl. Med. 2024, 65, 176–177. [Google Scholar] [CrossRef]

| Before Treatment | After Treatment | |||
|---|---|---|---|---|
| Anemia (Grading) | Number (n) | Percent (%) | Number (n) | Percent (%) |
| In range | 5 | 7.0 | 3 | 4.2 |
| G1 | 44 | 62.0 | 25 | 35.2 |
| G2 | 13 | 18.3 | 28 | 39.4 |
| G3 | 9 | 12.7 | 15 | 21.1 |
| G4 | 0 | 0 | 0 | 0 |
| Leukocytopenia (Grading) | Number (n) | Percent (%) | Number (n) | Percent (%) |
| In range | 48 | 67.6 | 41 | 57.7 |
| G1 | 17 | 23.9 | 16 | 22.5 |
| G2 | 4 | 5.6 | 8 | 11.3 |
| G3 | 2 | 2.8 | 6 | 8.4 |
| G4 | 0 | 0 | 0 | 0 |
| Thrombocytopenia (Grading) | Number (n) | Percent (%) | Number (n) | Percent (%) |
| In range | 45 | 63.4 | 29 | 40.8 |
| G1 | 20 | 28.2 | 24 | 33.8 |
| G2 | 1 | 1.4 | 4 | 5.6 |
| G3 | 3 | 4.2 | 8 | 11.3 |
| G4 | 2 | 2.8 | 6 | 8.4 |
| Before Treatment | After Treatment | |||
|---|---|---|---|---|
| Renal function (Grading) | Number (n) | Percent (%) | Number (n) | Percent (%) |
| In range | 56 | 78.9 | 52 | 73.2 |
| G1 | 12 | 16.9 | 12 | 16.9 |
| G2 | 3 | 4.2 | 6 | 8.4 |
| G3 | 0 | 0 | 1 | 1.4 |
| G4 | 0 | 0 | 0 | 0 |
| Hepatic function (Grading) | Number (n) | Percent (%) | Number (n) | Percent (%) |
| In range | 26 | 36.6 | 35 | 49.3 |
| G1 | 24 | 33.8 | 19 | 26.8 |
| G2 | 13 | 18.3 | 11 | 15.5 |
| G3 | 8 | 11.3 | 6 | 8.4 |
| G4 | 0 | 0 | 0 | 0 |
| Before Treatment | After Treatment | |||
|---|---|---|---|---|
| Xerostomia (Grading) | Number (n) | Percent (%) | Number (n) | Percent (%) |
| In range | 53 | 74.6 | 44 | 62.0 |
| G1 | 13 | 18.3 | 19 | 26.8 |
| G2 | 5 | 7.0 | 8 | 11.3 |
| G3 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrone, E.; Giordano, A.; Calcagni, M.L.; Leccisotti, L.; Moretti, R.; Eismant, A.; Ghai, K.; Parkar, T.; Mishra, A.; Heidenreich, A.; et al. Long-Term Safety and Survival Outcomes of [225Ac]Ac-PSMA (Prostate-Specific Membrane Antigen) and [225Ac]Ac-/[177Lu]Lu-PSMA (TANDEM) Radioligand Therapy (PRLT) in Metastatic Castration-Resistant Prostate Cancer. Cancers 2025, 17, 405. https://doi.org/10.3390/cancers17030405
Perrone E, Giordano A, Calcagni ML, Leccisotti L, Moretti R, Eismant A, Ghai K, Parkar T, Mishra A, Heidenreich A, et al. Long-Term Safety and Survival Outcomes of [225Ac]Ac-PSMA (Prostate-Specific Membrane Antigen) and [225Ac]Ac-/[177Lu]Lu-PSMA (TANDEM) Radioligand Therapy (PRLT) in Metastatic Castration-Resistant Prostate Cancer. Cancers. 2025; 17(3):405. https://doi.org/10.3390/cancers17030405
Chicago/Turabian StylePerrone, Elisabetta, Alessandro Giordano, Maria Lucia Calcagni, Lucia Leccisotti, Roberto Moretti, Aleksandr Eismant, Kriti Ghai, Tanay Parkar, Aditi Mishra, Axel Heidenreich, and et al. 2025. "Long-Term Safety and Survival Outcomes of [225Ac]Ac-PSMA (Prostate-Specific Membrane Antigen) and [225Ac]Ac-/[177Lu]Lu-PSMA (TANDEM) Radioligand Therapy (PRLT) in Metastatic Castration-Resistant Prostate Cancer" Cancers 17, no. 3: 405. https://doi.org/10.3390/cancers17030405
APA StylePerrone, E., Giordano, A., Calcagni, M. L., Leccisotti, L., Moretti, R., Eismant, A., Ghai, K., Parkar, T., Mishra, A., Heidenreich, A., Wirtz, R. M., Müller, J., Greifenstein, L., & Baum, R. P. (2025). Long-Term Safety and Survival Outcomes of [225Ac]Ac-PSMA (Prostate-Specific Membrane Antigen) and [225Ac]Ac-/[177Lu]Lu-PSMA (TANDEM) Radioligand Therapy (PRLT) in Metastatic Castration-Resistant Prostate Cancer. Cancers, 17(3), 405. https://doi.org/10.3390/cancers17030405






