Behavior Changes for Smokers and Betel Quid Chewers Participating in the Organized Oral Mucosal Screening Between 2010 and 2021 in Taiwan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
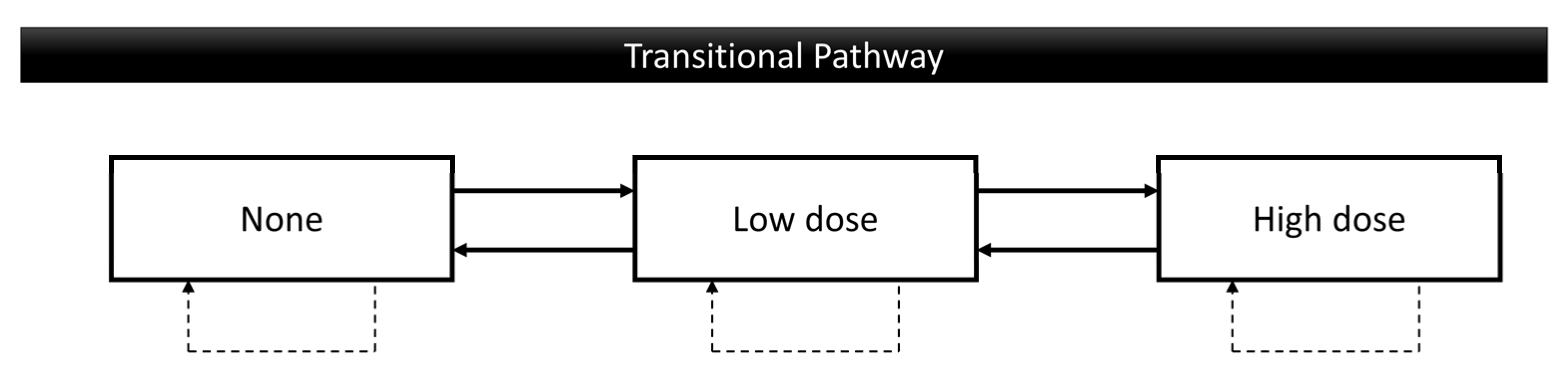
2.1. Study Design
2.2. Data Source and Collection
2.3. Sample Size and Variables
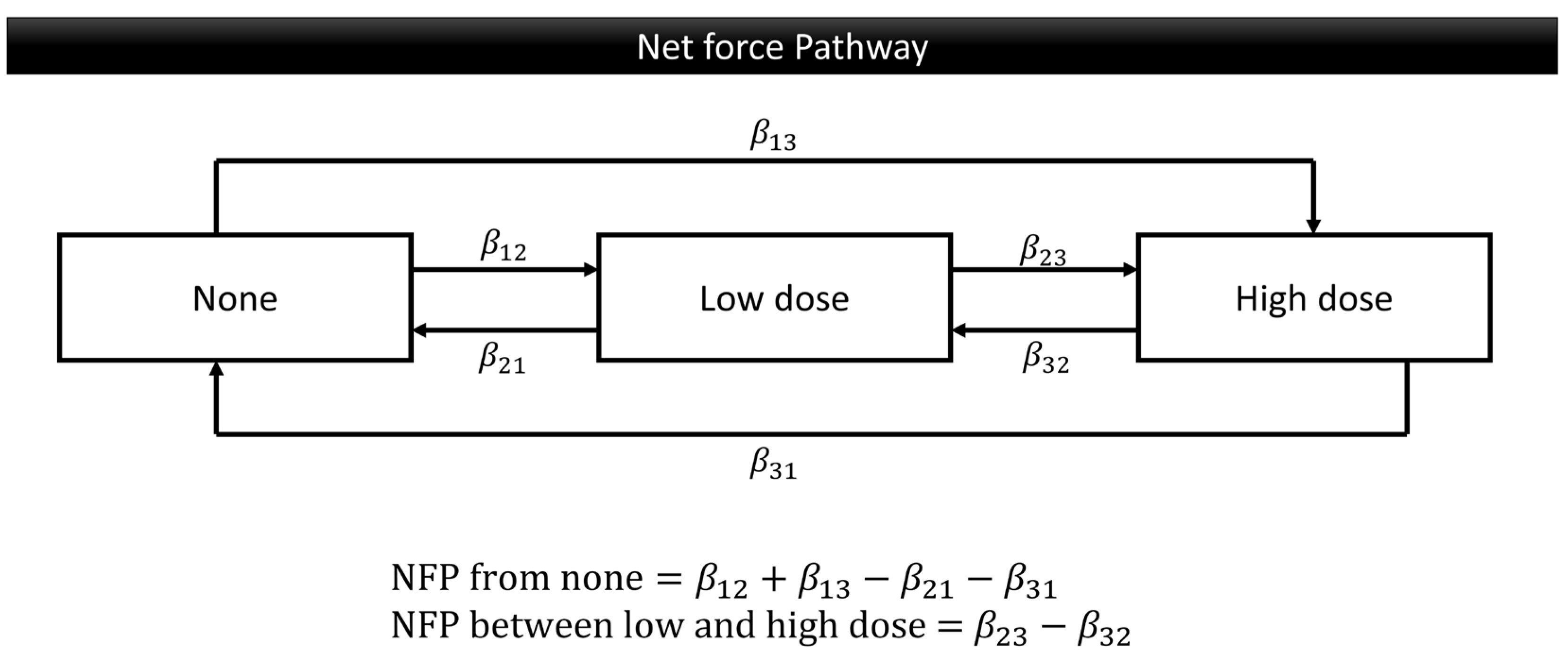
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Participants
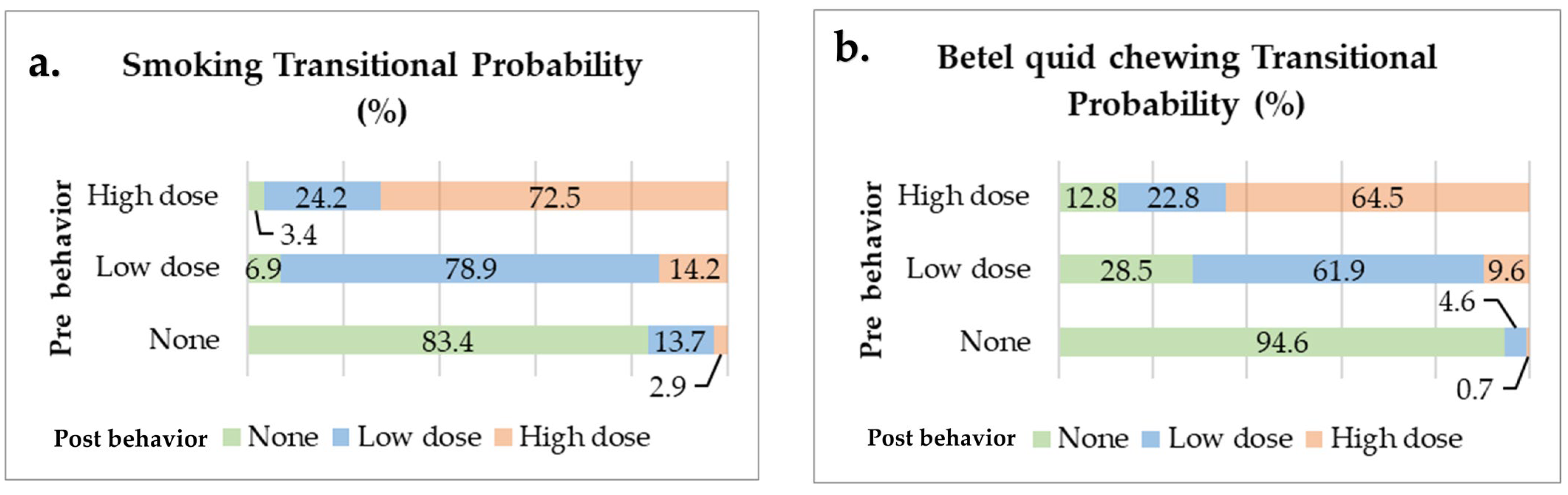
3.2. Transitional Probability of Oral Risk Behaviors
3.3. Effect of Risk Factors on Multiple Transitions for Smoking Behavior
3.4. Effect of Risk Factors on Multiple Transitions for Betel Quid Chewing Behavior
4. Discussion
4.1. Smoking
4.2. Betel Quid Chewing
4.3. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| OPMDs | Oral Potentially Malignant Disorders |
References
- World Cancer Research Fund International. Mouth and Oral Cancer Statistics; World Cancer Research Fund International: London, UK, 2022; Available online: https://www.wcrf.org/cancer-trends/mouth-and-oral-cancer-statistics/ (accessed on 18 March 2023).
- WHO. Oral Health; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 18 March 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Ramadas, K.; Thara, S.; Muwonge, R.; Thomas, G.; Anju, G.; Mathew, B. Long term effect of visual screening on oral cancer incidence and mortality in a randomized trial in Kerala, India. Oral Oncol. 2013, 49, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, P.; Anderson, B.O.; Basu, P.; Belinson, J.L.; Cruz, A.D.; Dhillon, P.K.; Gupta, P.; Jawahar, T.S.; Joshi, N.; Kailash, U.; et al. Recommendations for screening and early detection of common cancers in India. Lancet Oncol. 2015, 16, e352–e361. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.L.; Su, W.W.Y.; Chen, S.L.S.; Yen, A.M.F.; Wang, C.P.; Fann, J.C.Y.; Chiu, S.Y.H.; Lee, Y.C.; Chiu, H.M.; Chang, D.C.; et al. Population-based screening program for reducing oral cancer mortality in 2,334,299 Taiwanese cigarette smokers and/or betel quid chewers. Cancer 2017, 123, 1597–1609. [Google Scholar] [CrossRef]
- Anwar, N.; Pervez, S.; Chundriger, Q.; Awan, S.; Moatter, T.; Ali, T.S. Oral cancer: Clinicopathological features and associated risk factors in a high risk population presenting to a major tertiary care center in Pakistan. PLoS ONE 2020, 15, e0236359. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Z. Chapter 1—Oral Cancer. In Pharynx-Diagnosis and Treatment; Zhou, X., Zhang, Z., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Jose, M.; Rajagopal, V.; Thankam, F.G. Chapter 9—Oral Tissue Regeneration: Current Status and Future Perspectives. In Regenerated Organs; Sharma, C.P., Ed.; Academic Press: New York, NY, USA, 2021; pp. 169–187. Available online: https://www.sciencedirect.com/science/article/pii/B9780128210857000099 (accessed on 6 April 2023).
- Senevirathna, K.; Pradeep, R.; Jayasinghe, Y.A.; Jayawickrama, S.M.; Illeperuma, R.; Warnakulasuriya, S.; Jayasinghe, R.D. Carcinogenic Effects of Areca Nut and Its Metabolites: A Review of the Experimental Evidence. Clin. Pract. 2023, 13, 326–346. [Google Scholar] [CrossRef]
- Hübbers, C.U.; Akgül, B. HPV and cancer of the oral cavity. Virulence 2015, 6, 244–248. [Google Scholar] [CrossRef]
- American Dental Association. HPV and Oral Cancer; Mouthhelthy: Chicago, IL, USA, 2024; Available online: https://www.mouthhealthy.org/all-topics-a-z/hpv-and-oral-cancer (accessed on 29 October 2024).
- Sathish, N.; Wang, X.; Yuan, Y. Human Papillomavirus (HPV)-associated Oral Cancers and Treatment Strategies. J. Dent. Res. 2014, 93, 29S–36S. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Chuang, S.L.; Wang, C.P.; Chen, M.K.; Su, W.W.Y.; Su, C.W.; Chen, S.L.S.; Chiu, S.Y.H.; Fann, J.C.Y.; Yen, A.M.F. Malignant transformation to oral cancer by subtype of oral potentially malignant disorder: A prospective cohort study of Taiwanese nationwide oral cancer screening program. Oral Oncol. 2018, 87, 58–63. [Google Scholar] [CrossRef]
- Eum, Y.H.; Kim, H.J.; Bak, S.; Lee, S.H.; Kim, J.; Park, S.H.; Hwang, S.E.; Oh, B. Factors related to the success of smoking cessation: A retrospective cohort study in Korea. Tob. Induc. Dis. 2022, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Fagan, P.; Augustson, E.; Backinger, C.L.; O’connell, M.E.; Vollinger, R.E.; Kaufman, A.; Gibson, J.T. Quit attempts and intention to quit cigarette smoking among young adults in the United States. Am. J. Public Health 2007, 97, 1412–1420. [Google Scholar] [CrossRef]
- Feng, G.; Jiang, Y.; Li, Q.; Yong, H.-H.; Elton-Marshall, T.; Yang, J.; Li, L.; Sansone, N.; Fong, G.T. Individual-level factors associated with intentions to quit smoking among adult smokers in six cities of China: Findings from the ITC China Survey. Tob. Control 2010, 19 (Suppl. S2), i6–i11. [Google Scholar] [CrossRef] [PubMed]
- Yeom, H.; Lim, H.S.; Min, J.; Lee, S.; Park, Y.H. Factors affecting smoking cessation success of heavy smokers registered in the intensive care smoking cessation camp (data from the National Tobacco Control Center). Osong Public Health Res. Perspect. 2018, 9, 240–247. [Google Scholar] [CrossRef]
- Nadeem, M.; Malik, M.I.; Ullah, A.; Junaid, N. Smoking Dynamics: Factors Supplementing Tobacco Smoking in Pakistan. IEEE Trans. Comput. Soc. Syst. 2024, 11, 5367–5373. [Google Scholar] [CrossRef]
- Stead, L.F.; Buitrago, D.; Preciado, N.; Sanchez, G.; Hartmann-Boyce, J.; Lancaster, T. Physician advice for smoking cessation. Cochrane Database Syst. Rev. 2013, 2013, CD000165. [Google Scholar] [CrossRef]
- Carlebach, S.; Hamilton, S. Understanding the nurse’s role in smoking cessation. Br. J. Nurs. 2009, 18, 672–674,676. [Google Scholar] [CrossRef]
- Rice, V.H.; Hartmann-Boyce, J.; Stead, L.F. Nursing interventions for smoking cessation. Cochrane Database Syst. Rev. 2013, 2013, CD001188. [Google Scholar] [CrossRef]
- Luh, D.-L.; Chen, S.L.-S.; Yen, A.M.-F.; Chiu, S.Y.-H.; Fann, C.-Y.; Chen, H.-H. Effectiveness of advice from physician and nurse on smoking cessation stage in Taiwanese male smokers attending a community-based integrated screening program. Tob. Induc. Dis. 2016, 14, 15. [Google Scholar] [CrossRef]
- Tammemägi, M.C.; Berg, C.D.; Riley, T.L.; Cunningham, C.R.; Taylor, K.L. Impact of lung cancer screening results on smoking cessation. J. Natl. Cancer Inst. 2014, 106, dju084. [Google Scholar] [CrossRef]
- Siewchaisakul, P.; Luh, D.-L.; Chiu, S.Y.H.; Yen, A.M.F.; Chen, C.-D.; Chen, H.-H. Smoking cessation advice from healthcare professionals helps those in the contemplation and preparation stage: An application with transtheoretical model underpinning in a community-based program. Tob. Induc. Dis. 2020, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-F.; Wang, J.-D.; Chen, P.-H.; Chang, S.-J.; Yang, Y.-H.; Ko, Y.-C. Predictors of betel quid chewing behavior and cessation patterns in Taiwan aborigines. BMC Public Health 2006, 6, 271. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.-F.; Ho, P.-S.; Kuo, H.-C.; Yang, Y.-H. Comparing factors affecting commencement and cessation of betel quid chewing behavior in Taiwanese adults. BMC Public Health 2008, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Flora, M.S.; Mascie-Taylor, N.; Rahman, M. Betel quid chewing and its risk factors in Bangladeshi adults. WHO South-East Asia J. Public Health 2012, 1, 169–181. [Google Scholar]
- Ghani, W.M.; Razak, I.A.; Yang, Y.-H.; Talib, N.A.; Ikeda, N.; Axell, T.; Gupta, P.C.; Handa, Y.; Abdullah, N.; Zain, R.B. Factors affecting commencement and cessation of betel quid chewing behaviour in Malaysian adults. BMC Public Health 2011, 11, 82. [Google Scholar] [CrossRef]
- Lai, C.-S.; Shieh, T.-Y.; Yang, Y.-H.C.; Chong, M.-Y.; Hung, H.-C.; Tsai, C.-C. Factors associated with quitting areca (betel) quid chewing. Community Dent. Oral Epidemiol. 2006, 34, 467–474. [Google Scholar] [CrossRef]


| Smoking | Betel Quid Chewing | Total (n) | |||||
|---|---|---|---|---|---|---|---|
| None (%) | Low Dose (%) | High Dose (%) | None (%) | Low Dose (%) | High Dose (%) | ||
| Gender | |||||||
| Male | 20.6 | 47.7 | 31.7 | 75.1 | 17.7 | 7.3 | 2,190,949 |
| Female | 32.7 | 52.5 | 14.8 | 85.7 | 10.9 | 3.4 | 387,296 |
| Age | |||||||
| ≤60 years | 16.6 | 51.8 | 31.6 | 74.3 | 18.2 | 7.5 | 1,877,590 |
| 61+ years | 38 | 39.2 | 22.8 | 83 | 12.5 | 4.5 | 700,655 |
| Education | |||||||
| Elementary and middle school (Low education) | 29.3 | 40.9 | 29.8 | 74.8 | 16.9 | 8.3 | 515,778 |
| High school and college (High education) | 17.5 | 54.5 | 28 | 79.9 | 15 | 5.1 | 860,054 |
| unknown | 22.9 | 47.2 | 29.8 | 75.2 | 17.7 | 7.1 | 1,202,413 |
| Living area | |||||||
| Urban | 18.3 | 52 | 29.7 | 80.8 | 14 | 5.2 | 1,609,524 |
| Rural | 29.2 | 42.5 | 28.3 | 69.9 | 21 | 9.1 | 968,718 |
| Screening place | |||||||
| Large hospital | 22.3 | 49.2 | 28.5 | 78.2 | 15.5 | 6.2 | 726,200 |
| Small hospital | 22.4 | 48.1 | 29.5 | 76.1 | 17.1 | 6.8 | 1,851,831 |
| Unknown | 9.3 | 65.9 | 24.8 | 82.2 | 15 | 2.8 | 214 |
| OPMD screening results | |||||||
| Positive | 15 | 42.2 | 42.8 | 59.8 | 23.9 | 16.3 | 193,256 |
| Negative | 23 | 48.9 | 28.1 | 78 | 16.1 | 5.9 | 2,384,989 |
| Overall | 22.4 | 48.4 | 29.2 | 76.7 | 16.7 | 6.7 | 2,578,245 |
| Net Force Progression from None Stage | Net Force Progression Between Low and High Dose | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95%CI | aRR | 95%CI | RR | 95%CI | aRR | 95%CI | |||||
| Gender | ||||||||||||
| Male | 1.94 | 1.85 | 2.06 | 2.4 | 2.26 | 2.57 | 2.51 | 2.48 | 2.55 | 2.53 | 2.49 | 2.56 |
| Female | ref | ref. | ref | ref | ||||||||
| Age | ||||||||||||
| Younger (≤60 years) | 13.74 | 13.42 | 14.06 | 10.85 | 10.53 | 11.17 | 1.22 | 1.21 | 1.23 | 1.43 | 1.42 | 1.45 |
| Elder (60+ years) | ref | ref | ref | ref | ||||||||
| Education | ||||||||||||
| Low education | ref | ref | ref | ref | ||||||||
| High education | 5.31 | 5.12 | 5.5 | 1.8 | 1.74 | 1.86 | 0.68 | 0.67 | 0.69 | 0.63 | 0.62 | 0.63 |
| Living area | ||||||||||||
| Municipality | 3.51 | 3.42 | 3.6 | 2.77 | 2.7 | 2.84 | 0.78 | 0.78 | 0.79 | 0.83 | 0.82 | 0.84 |
| County | ref | ref | ref | ref | ||||||||
| Hospital | ||||||||||||
| Small hospital | ref | ref | ref | ref | ||||||||
| Large hospital | 2.06 | 2.01 | 2.12 | 1.86 | 1.8 | 1.91 | 0.88 | 0.87 | 0.89 | 0.87 | 0.86 | 0.88 |
| OPMD result | ||||||||||||
| Positive | 1.67 | 1.6 | 1.74 | 1.46 | 1.4 | 1.53 | 1.74 | 1.72 | 1.77 | 1.61 | 1.59 | 1.63 |
| Negative | ref | ref | ref | ref | ||||||||
| Net Force Progression from None Stage | Net Force Progression Between Low and High Dose | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95%CI | aRR | 95%CI | RR | 95%CI | aRR | 95%CI | |||||
| Gender | ||||||||||||
| Male | 2.34 | 2.19 | 2.46 | 2.21 | 2.09 | 2.37 | 1.54 | 1.5 | 1.59 | 1.57 | 1.53 | 1.62 |
| Female | ref | ref | ref | ref | ||||||||
| Age | ||||||||||||
| Younger (≤60 years) | 4.4 | 4.26 | 4.56 | 7.93 | 7.66 | 8.2 | 1.48 | 1.44 | 1.51 | 1.58 | 1.54 | 1.62 |
| Elder (60+ years) | ref | ref | ref | ref | ||||||||
| Education | ||||||||||||
| Low education | ref | ref | ref | ref | ||||||||
| High education | 0.49 | 0.48 | 0.51 | 0.35 | 0.34 | 0.36 | 0.74 | 0.72 | 0.76 | 0.62 | 0.61 | 0.64 |
| Living area | ||||||||||||
| Municipality | 0.26 | 0.25 | 0.26 | 0.28 | 0.27 | 0.29 | 0.92 | 0.9 | 0.94 | 0.85 | 0.83 | 0.87 |
| County | ref | ref | ref | ref | ||||||||
| Hospital | ||||||||||||
| Small hospital | ref | ref | ref | ref | ||||||||
| Large hospital | 0.61 | 0.59 | 0.63 | 0.62 | 0.6 | 0.64 | 1 | 0.98 | 1.02 | 0.98 | 0.95 | 1 |
| OPMD result | ||||||||||||
| Positive | 3.24 | 3.11 | 3.37 | 2.87 | 2.76 | 2.99 | 1.73 | 1.68 | 1.77 | 1.68 | 1.63 | 1.72 |
| Negative | ref | ref | ref | ref | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munpolsri, P.; Su, C.-W.; Chen, S.L.-S.; Yen, A.M.-F. Behavior Changes for Smokers and Betel Quid Chewers Participating in the Organized Oral Mucosal Screening Between 2010 and 2021 in Taiwan. Cancers 2025, 17, 397. https://doi.org/10.3390/cancers17030397
Munpolsri P, Su C-W, Chen SL-S, Yen AM-F. Behavior Changes for Smokers and Betel Quid Chewers Participating in the Organized Oral Mucosal Screening Between 2010 and 2021 in Taiwan. Cancers. 2025; 17(3):397. https://doi.org/10.3390/cancers17030397
Chicago/Turabian StyleMunpolsri, Pattaranan, Chiu-Wen Su, Sam Li-Sheng Chen, and Amy Ming-Fang Yen. 2025. "Behavior Changes for Smokers and Betel Quid Chewers Participating in the Organized Oral Mucosal Screening Between 2010 and 2021 in Taiwan" Cancers 17, no. 3: 397. https://doi.org/10.3390/cancers17030397
APA StyleMunpolsri, P., Su, C.-W., Chen, S. L.-S., & Yen, A. M.-F. (2025). Behavior Changes for Smokers and Betel Quid Chewers Participating in the Organized Oral Mucosal Screening Between 2010 and 2021 in Taiwan. Cancers, 17(3), 397. https://doi.org/10.3390/cancers17030397






