Characteristics and Outcomes of T1a Renal Cell Carcinoma Presenting with Metastasis
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patient Population and Study Design
2.2. Data Collection
2.3. Statistical Analysis
3. Results
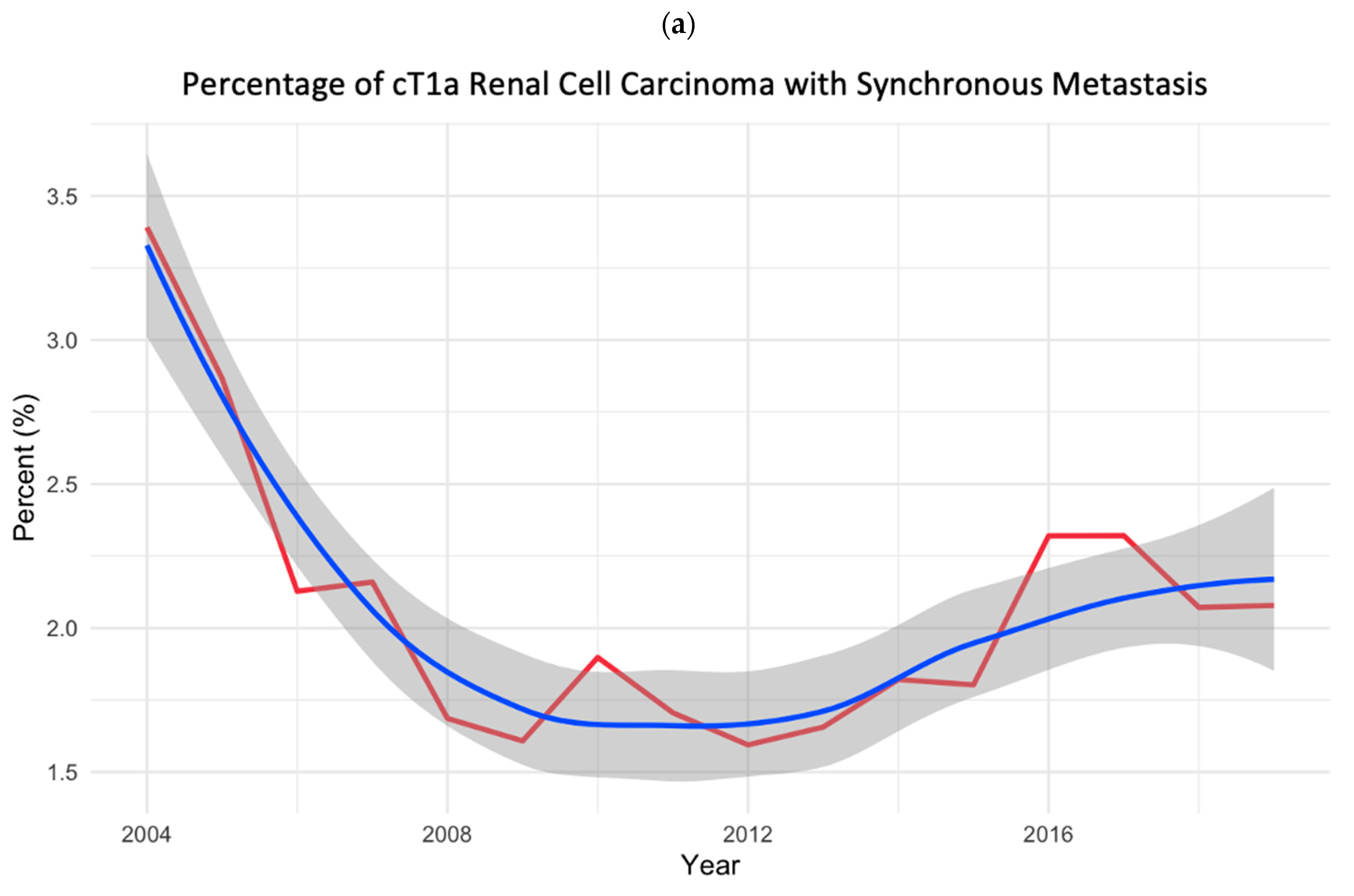
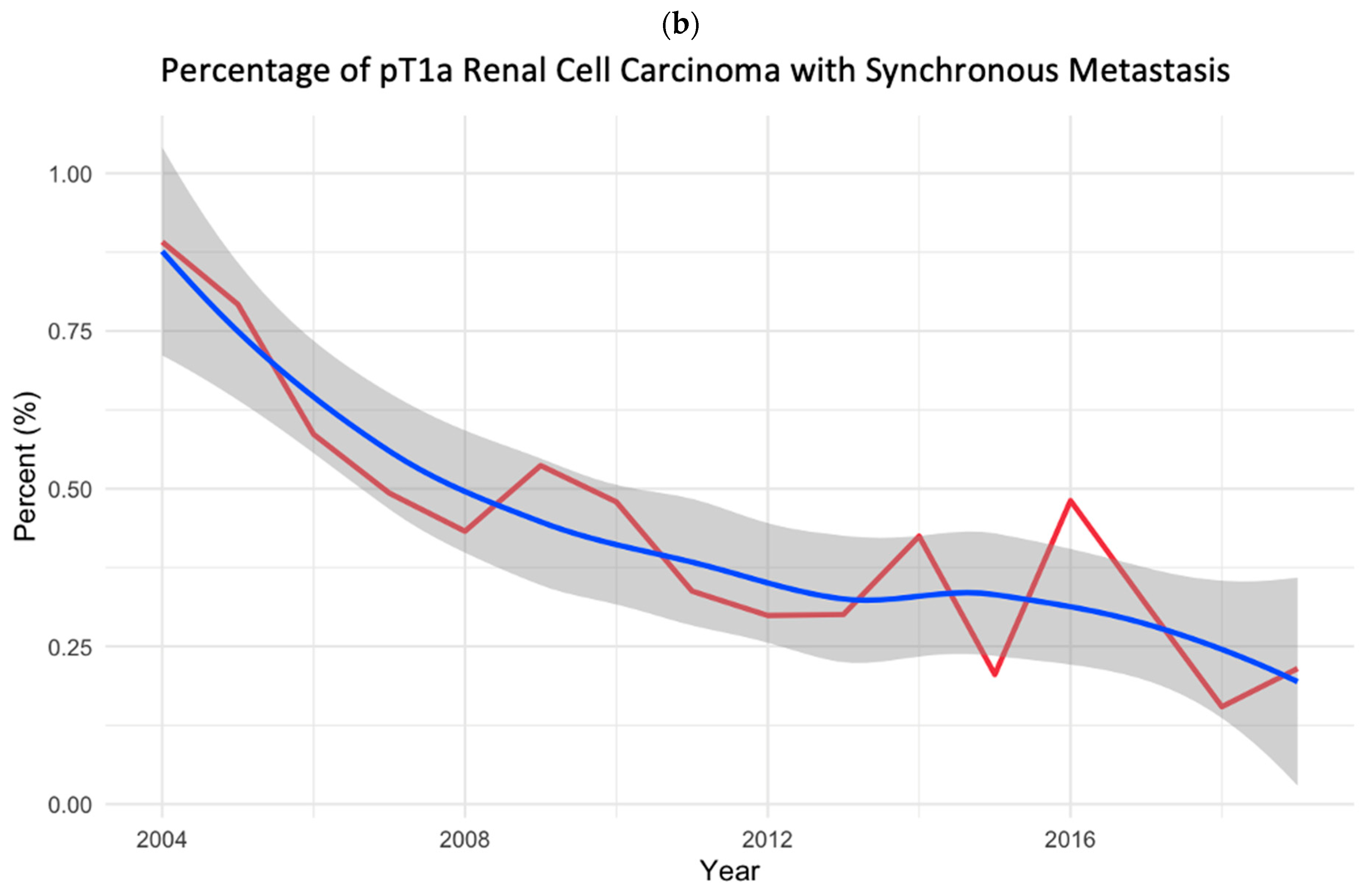
3.1. Study Cohort and Temporal Trend Analyses
3.2. Patient Characteristics
3.3. Variables Associated with Synchronous Metastasis
3.4. Variables Associated with All-Cause Mortality
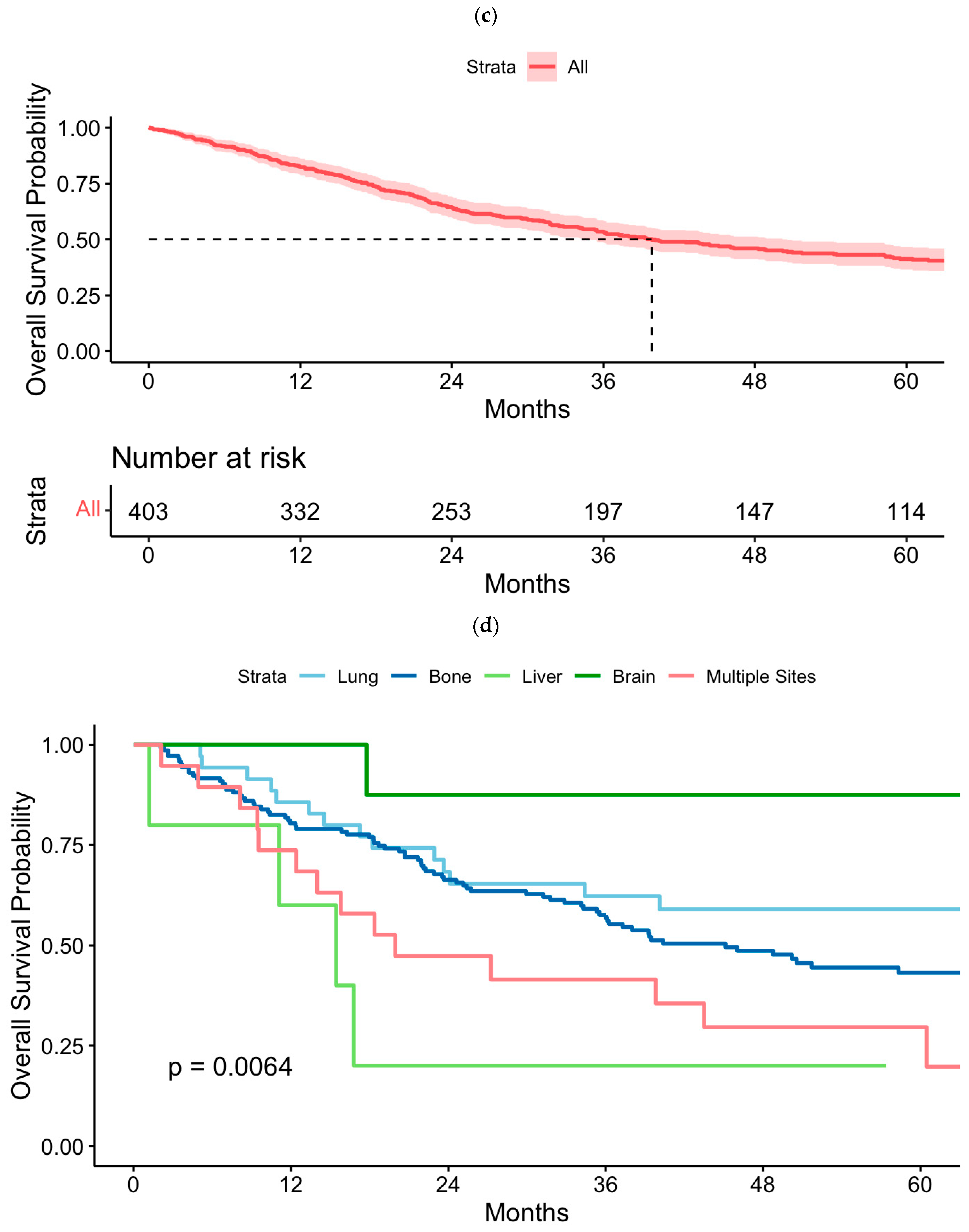
3.5. Kaplan–Meier Analysis of Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kane, C.J.; Mallin, K.; Ritchey, J.; Cooperberg, M.R.; Carroll, P.R. Renal Cell Cancer Stage Migration: Analysis of the National Cancer Data Base. Cancer 2008, 113, 78–83. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2024; American Cancer Society: Atlanta, GA, USA, 2024. [Google Scholar]
- Ko, J.J.; Xie, W.; Kroeger, N.; Lee, J.-L.; I Rini, B.; Knox, J.J.; Bjarnason, G.A.; Srinivas, S.; Pal, S.K.; Yuasa, T.; et al. The International Metastatic Renal Cell Carcinoma Database Consortium Model as a Prognostic Tool in Patients with Metastatic Renal Cell Carcinoma Previously Treated with First-Line Targeted Therapy: A Population-Based Study. Lancet Oncol. 2015, 16, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Tannir, N.; Albigès, L.; McDermott, D.; Burotto, M.; Choueiri, T.; Hammers, H.; Barthélémy, P.; Plimack, E.; Porta, C.; George, S.; et al. Nivolumab Plus Ipilimumab versus Sunitinib for First-Line Treatment of Advanced Renal Cell Carcinoma: Extended 8-Year Follow-Up Results of Efficacy and Safety from the Phase III CheckMate 214 Trial. Ann. Oncol. 2024, 35, 1026–1038. [Google Scholar] [CrossRef]
- Frank, I.; Blute, M.L.; Leibovich, B.C.; Cheville, J.C.; Lohse, C.M.; Zincke, H. Independent Validation of the 2002 American Joint Committee on Cancer Primary Tumor Classification for Renal Cell Carcinoma Using a Large, Single Institution Cohort. J. Urol. 2005, 173, 1889–1892. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Gill, I.S. Effect of Renal Cancer Size on the Prevalence of Metastasis at Diagnosis and Mortality. J. Urol. 2009, 181, 1020–1027. [Google Scholar] [CrossRef]
- Renner, A.; Samtani, S.; Marín, A.; Burotto, M. Is Cytoreductive Nephrectomy Still a Standard of Care in Metastatic Renal Cell Carcinoma? J. Kidney Cancer VHL 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Méjean, A.; Ravaud, A.; Thezenas, S.; Colas, S.; Beauval, J.-B.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 417–427. [Google Scholar] [CrossRef]
- Bex, A.; Mulders, P.; Jewett, M.; Wagstaff, J.; Van Thienen, J.V.; Blank, C.U.; Van Velthoven, R.; Del Pilar Laguna, M.; Wood, L.; Van Melick, H.H.E.; et al. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients with Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol. 2019, 5, 164–170. [Google Scholar] [CrossRef]
- Lerro, C.C.; Robbins, A.S.; Phillips, J.L.; Stewart, A.K. Comparison of Cases Captured in the National Cancer Data Base with Those in Population-Based Central Cancer Registries. Ann. Surg. Oncol. 2013, 20, 1759–1765. [Google Scholar] [CrossRef]
- American College of Surgeons. National Cancer Database Participant User File 2019 Data Dictionary; American College of Surgeons: Chicago, IL, USA, 2020. [Google Scholar]
- American College of Surgeons. Facility Oncology Registry Data Standards (FORDS): Revised for 2016; American College of Surgeons: Chicago, IL, USA, 2016. [Google Scholar]
- Dudani, S.; de Velasco, G.; Wells, J.C.; Gan, C.L.; Donskov, F.; Porta, C.; Fraccon, A.; Pasini, F.; Lee, J.L.; Hansen, A.; et al. Evaluation of Clear Cell, Papillary, and Chromophobe Renal Cell Carcinoma Metastasis Sites and Association with Survival. JAMA Netw. Open 2021, 4, e2021869. [Google Scholar] [CrossRef]
- Ullah, A.; Yasinzai, A.Q.K.; Sakhalkar, O.V.; Lee, K.T.; Khan, I.; Tareen, B.; Wali, A.; Waheed, A.; Khan, J.; Andam, G.; et al. Demographic Patterns and Clinicopathological Analysis of Sarcomatoid Renal Cell Carcinoma in US Population. Clin. Genitourin. Cancer 2024, 22, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, T.; Klaassen, Z.; Goldberg, H.; Kulkarni, G.S.; Hamilton, R.J.; Fleshner, N.E. Metastatic Renal Cell Carcinoma: Patterns and Predictors of Metastases—A Contemporary Population-Based Series. Urol. Oncol. 2017, 35, 661.e7–661.e14. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Sun, M.; Jeldres, C.; Shariat, S.F.; Trinh, Q.-D.; Briganti, A.; Tian, Z.; Schmitges, J.; Graefen, M.; Perrotte, P.; et al. Distribution of Metastatic Sites in Renal Cell Carcinoma: A Population-Based Analysis. Ann. Oncol. 2012, 23, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, A.; Palumbo, C.; Knipper, S.; Mistretta, F.A.; Rosiello, G.; Tian, Z.; St-Hilaire, P.-A.; Shariat, S.F.; Saad, F.; Lavallée, L.; et al. Synchronous Metastasis Rates in T1 Renal Cell Carcinoma: A Surveillance, Epidemiology, and End Results Database-Based Study. Eur. Urol. Focus 2021, 7, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Sugiyama, T.; Kai, F.; Suzuki, T.; Nagata, M.; Imanishi, T.; Mizuno, T.; Sato, S.; Furuse, H.; Mugiya, S.; et al. Characteristics of Aggressive Variants in T1a Renal Cell Carcinoma. J. Cancer Res. Clin. Oncol. 2011, 137, 1653–1659. [Google Scholar] [CrossRef]
- Grünwald, V.; Eberhardt, B.; Bex, A.; Flörcken, A.; Gauler, T.; Derlin, T.; Panzica, M.; Dürr, H.R.; Grötz, K.A.; Giles, R.H.; et al. An Interdisciplinary Consensus on the Management of Bone Metastases from Renal Cell Carcinoma. Nat. Rev. Urol. 2018, 15, 511–521. [Google Scholar] [CrossRef]
- McKay, R.R.; Kroeger, N.; Xie, W.; Lee, J.-L.; Knox, J.J.; Bjarnason, G.A.; MacKenzie, M.J.; Wood, L.; Srinivas, S.; Vaishampayan, U.N.; et al. Impact of Bone and Liver Metastases on Patients with Renal Cell Carcinoma Treated with Targeted Therapy. Eur. Urol. 2014, 65, 577. [Google Scholar] [CrossRef]
- Santoni, M.; Conti, A.; Procopio, G.; Porta, C.; Ibrahim, T.; Barni, S.; Guida, F.M.; Fontana, A.; Berruti, A.; Berardi, R.; et al. Bone Metastases in Patients with Metastatic Renal Cell Carcinoma: Are They Always Associated with Poor Prognosis? J. Exp. Clin. Cancer Res. 2015, 34, 10. [Google Scholar] [CrossRef]
- Beuselinck, B.; Oudard, S.; Rixe, O.; Wolter, P.; Blesius, A.; Ayllon, J.; Elaidi, R.; Schöffski, P.; Barrascout, E.; Morel, A.; et al. Negative Impact of Bone Metastasis on Outcome in Clear-Cell Renal Cell Carcinoma Treated with Sunitinib. Ann. Oncol. 2011, 22, 794–800. [Google Scholar] [CrossRef]
- Powles, T.; Albiges, L.; Bex, A.; Comperat, E.; Grünwald, V.; Kanesvaran, R.; Kitamura, H.; McKay, R.; Porta, C.; Procopio, G.; et al. Renal Cell Carcinoma: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2024, 35, 692–706. [Google Scholar] [CrossRef]
- European Association of Urology. EAU Guidelines. In Proceedings of the EAU Annual Congress, Paris, France, 5–8 April 2024; ISBN 978-94-92671-23-3. [Google Scholar]
- Singla, N.; Hutchinson, R.C.; Ghandour, R.A.; Freifeld, Y.; Fang, D.; Sagalowsky, A.I.; Lotan, Y.; Bagrodia, A.; Margulis, V.; Hammers, H.J.; et al. Improved Survival after Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma in the Contemporary Immunotherapy Era: An Analysis of the National Cancer Database. Urol. Oncol. 2020, 38, 604.e9–604.e17. [Google Scholar] [CrossRef]
- Zarba, M.; Fujiwara, R.; Yuasa, T.; Koga, F.; Heng, D.Y.C.; Takemura, K. Multidisciplinary Systemic and Local Therapies for Metastatic Renal Cell Carcinoma: A Narrative Review. Expert Rev. Anticancer Ther. 2024, 24, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.; Wells, J.C.; Rini, B.I.; Beuselinck, B.; Lee, J.-L.; Knox, J.J.; Bjarnason, G.A.; Pal, S.K.; Kollmannsberger, C.K.; Yuasa, T.; et al. Cytoreductive Nephrectomy in Patients with Synchronous Metastases from Renal Cell Carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur. Urol. 2014, 66, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Tangen, C.M.; Vaishampayan, U.; Tripathi, A.; Patel, S.K.; Shuch, B.; Barata, P.; Tan, A.; Esfeller, L.; Lara, P.N.; et al. SWOG S1931 (PROBE): Phase III Randomized Trial of Immune Checkpoint Inhibitor (ICI) Combination Regimen with or without Cytoreductive Nephrectomy (CN) in Advanced Renal Cancer [NCT04510597]. Urol. Oncol. 2024, 42 (Suppl. 1), S4. [Google Scholar] [CrossRef]
- Hall, W.A.; Karrison, T.; McGregor, B.A.; Barata, P.C.; Nagar, H.; Tang, C.; Siva, S.; Morgan, T.M.; Lang, J.M.; Kamran, S.C.; et al. NRG-GU012: Randomized Phase II Stereotactic Ablative Radiation Therapy (SABR) for Patients with Metastatic Unresected Renal Cell Carcinoma (RCC) Receiving Immunotherapy (SAMURAI). J. Clin. Oncol. 2023, 41, TPS4604. [Google Scholar] [CrossRef]
- Zaid, H.B.; Parker, W.P.; Safdar, N.S.; Gershman, B.; Erwin, P.J.; Murad, M.H.; Boorjian, S.A.; Costello, B.A.; Thompson, R.H.; Leibovich, B.C. Outcomes Following Complete Surgical Metastasectomy for Patients with Metastatic Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. J. Urol. 2017, 197, 44–49. [Google Scholar] [CrossRef]
- Ouzaid, I.; Capitanio, U.; Staehler, M.; Wood, C.G.; Leibovich, B.C.; Ljungberg, B.; Van Poppel, H.; Bensalah, K. Surgical Metastasectomy in Renal Cell Carcinoma: A Systematic Review. Eur. Urol. Oncol. 2019, 2, 141–149. [Google Scholar] [CrossRef]

| cT1a RCC with Synchronous Metastasis | pT1a RCC with Synchronous Metastasis | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| Age | 1.02 | 1.02, 1.03 | <0.001 | 1.01 | 1.01, 1.02 | 0.002 |
| Year of diagnosis | ||||||
| 2004–2009 | — | — | — | — | ||
| 2010–2015 | 1.00 | 0.86, 1.16 | 1.000 | 0.75 | 0.57, 0.99 | 0.042 |
| 2016–2019 | 1.02 | 0.89, 1.18 | 1.000 | 0.58 | 0.44, 0.76 | <0.001 |
| Sex | ||||||
| Female | — | — | — | — | ||
| Male | 1.49 | 1.34, 1.65 | <0.001 | 1.61 | 1.30, 2.00 | <0.001 |
| Race | ||||||
| White | — | — | — | — | ||
| Black | 0.99 | 0.85, 1.15 | 1.000 | 0.78 | 0.53, 1.11 | 0.715 |
| Native American | 0.83 | 0.37, 1.64 | 1.000 | 0.35 | 0.02, 1.67 | 0.925 |
| Asian/Pacific Islander | 1.00 | 0.67, 1.44 | 1.000 | 0.98 | 0.46, 1.81 | 0.947 |
| Other/Unknown | 0.92 | 0.61, 1.35 | 1.000 | 0.75 | 0.32, 1.49 | 0.925 |
| Hispanic | ||||||
| No | — | — | — | — | ||
| Yes | 0.83 | 0.67, 1.03 | 0.209 | 1.03 | 0.68, 1.51 | 1.000 |
| Unclassified | 0.87 | 0.66, 1.13 | 0.306 | 0.97 | 0.59, 1.53 | 1.000 |
| Facility Location | ||||||
| New England | — | — | — | — | ||
| Middle Atlantic | 1.09 | 0.83, 1.44 | 1.000 | 1.37 | 0.82, 2.45 | 1.000 |
| South Atlantic | 1.13 | 0.87, 1.48 | 1.000 | 1.16 | 0.69, 2.08 | 1.000 |
| East North Central | 1.34 | 1.04, 1.76 | 0.257 | 1.48 | 0.88, 2.64 | 1.000 |
| East South Central | 1.32 | 0.99, 1.78 | 0.507 | 1.53 | 0.85, 2.85 | 1.000 |
| West North Central | 1.24 | 0.92, 1.67 | 0.954 | 1.38 | 0.77, 2.58 | 1.000 |
| West South Central | 1.15 | 0.86, 1.54 | 1.000 | 1.30 | 0.73, 2.41 | 1.000 |
| Mountain | 1.20 | 0.85, 1.69 | 1.000 | 2.14 | 1.13, 4.14 | 0.165 |
| Pacific | 1.12 | 0.84, 1.49 | 1.000 | 1.20 | 0.68, 2.22 | 1.000 |
| Unclassified | 0.68 | 0.41, 1.10 | 0.869 | 0.10 | 0.02, 0.40 | 0.034 |
| Facility Type | ||||||
| Community | — | — | — | — | ||
| Community Comprehensive | 0.83 | 0.68, 1.01 | 0.054 | 0.75 | 0.48, 1.22 | 0.450 |
| Academic | 0.62 | 0.51, 0.76 | <0.001 | 0.92 | 0.60, 1.48 | 0.703 |
| Integrated Cancer Network | 0.64 | 0.52, 0.80 | <0.001 | 0.70 | 0.44, 1.16 | 0.450 |
| Median Income | ||||||
| First Quartile (lowest) | — | — | — | — | ||
| Second Quartile | 0.98 | 0.83, 1.14 | 0.843 | 1.16 | 0.84, 1.62 | 1.000 |
| Third Quartile | 0.94 | 0.80, 1.10 | 0.843 | 0.9 | 0.64, 1.26 | 1.000 |
| Fourth Quartile | 0.7 | 0.59, 0.83 | <0.001 | 1.00 | 0.72, 1.39 | 1.000 |
| Unclassified | 0.83 | 0.68, 1.00 | 0.140 | 0.9 | 0.59, 1.34 | 1.000 |
| Charlson Score | ||||||
| Charlson 0 to 1 | — | — | — | — | ||
| Charlson 2 or higher | 0.97 | 0.84, 1.11 | 0.628 | 0.69 | 0.47, 0.98 | 0.047 |
| Tumor Size | 1.70 | 1.60, 1.82 | <0.001 | 1.79 | 1.56, 2.06 | <0.001 |
| Clinical N Stage | ||||||
| cN0 | — | — | — | — | ||
| cN1 | 319 | 237, 436 | <0.001 | 95.3 | 55.0, 160 | <0.001 |
| Unclassified | 8.22 | 6.92, 9.73 | <0.001 | 2.16 | 1.70, 2.75 | <0.001 |
| Histology | ||||||
| Clear Cell | — | — | — | — | ||
| Papillary | 0.39 | 0.31, 0.49 | <0.001 | 0.40 | 0.26, 0.58 | <0.001 |
| Chromophobe | 0.09 | 0.05, 0.14 | <0.001 | 0.32 | 0.16, 0.59 | 0.002 |
| Collecting Duct | 11.9 | 5.24, 25.1 | <0.001 | — | — | — |
| Medullary | 73.7 | 20.2, 303 | <0.001 | — | — | — |
| RCC NOS | 1.24 | 1.10, 1.40 | <0.001 | 1.09 | 0.86, 1.36 | 0.477 |
| Other | 2.1 | 1.73, 2.54 | <0.001 | 0.58 | 0.23, 1.23 | 0.409 |
| Sarcomatoid dedifferentiation | 2.41 | 1.69, 3.37 | <0.001 | 5.41 | 3.24, 8.77 | <0.001 |
| Tumor Grade | ||||||
| Grade 1 | — | — | — | — | ||
| Grade 2 | 1.09 | 0.80, 1.53 | 0.593 | 1.73 | 1.13, 2.79 | 0.017 |
| Grade 3 | 4.08 | 2.99, 5.68 | <0.001 | 5.09 | 3.31, 8.23 | <0.001 |
| Grade 4 | 12.2 | 8.15, 18.3 | <0.001 | 16.7 | 10.0, 28.7 | <0.001 |
| Unclassified | 17 | 12.8, 23.2 | <0.001 | 2.99 | 1.73, 5.25 | <0.001 |
| cT1a RCC with Synchronous Metastases | pT1a RCC with Synchronous Metastases | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age | 1.01 | 1.01, 1.02 | <0.001 | 1.03 | 1.01, 1.06 | 0.005 |
| Sex | ||||||
| Female | — | — | — | — | ||
| Male | 1.06 | 0.92, 1.22 | 0.402 | 1.07 | 0.67, 1.71 | 0.774 |
| Race | ||||||
| White | — | — | — | — | ||
| Black | 0.86 | 0.71, 1.05 | 0.447 | 1.28 | 0.53, 3.10 | 1.000 |
| Native American | 2.21 | 0.88, 5.56 | 0.376 | |||
| Asian/Pacific Islander | 0.94 | 0.59, 1.51 | 0.802 | 1.51 | 0.41, 5.52 | 1.000 |
| Other/Unknown | 0.79 | 0.46, 1.37 | 0.802 | 0.84 | 0.21, 3.32 | 1.000 |
| Hispanic | ||||||
| No | — | — | — | — | ||
| Yes | 0.85 | 0.63, 1.15 | 0.584 | 0.71 | 0.27, 1.88 | 0.990 |
| Unclassified | 1.20 | 0.70, 2.06 | 0.584 | 1.51 | 0.28, 7.99 | 0.990 |
| Facility Location | ||||||
| New England | — | — | — | — | ||
| Middle Atlantic | 0.76 | 0.54, 1.06 | 0.875 | 0.37 | 0.11, 1.17 | 0.721 |
| South Atlantic | 0.87 | 0.63, 1.20 | 1.000 | 0.41 | 0.13, 1.36 | 1.000 |
| East North Central | 1.28 | 0.93, 1.77 | 0.918 | 0.61 | 0.20, 1.80 | 1.000 |
| East South Central | 1.11 | 0.78, 1.60 | 1.000 | 0.83 | 0.25, 2.76 | 1.000 |
| West North Central | 1.25 | 0.87, 1.81 | 1.000 | 0.47 | 0.15, 1.46 | 1.000 |
| West South Central | 0.95 | 0.66, 1.37 | 1.000 | 0.46 | 0.12, 1.75 | 1.000 |
| Mountain | 1.49 | 0.96, 2.32 | 0.663 | 0.47 | 0.11, 1.95 | 1.000 |
| Pacific | 0.96 | 0.68, 1.36 | 1.000 | 0.32 | 0.09, 1.11 | 0.648 |
| Unclassified | 0.75 | 0.38, 1.47 | 1.000 | 2.38 | 0.14, 39.5 | 1.000 |
| Facility Type | ||||||
| Community | — | — | — | — | ||
| Community Comprehensive | 0.88 | 0.69, 1.11 | 0.279 | 2.59 | 0.71, 9.41 | 0.255 |
| Academic | 0.66 | 0.51, 0.84 | 0.003 | 2.75 | 0.75, 10.1 | 0.255 |
| Integrated Cancer Network | 0.76 | 0.58, 1.00 | 0.095 | 3.49 | 0.88, 13.7 | 0.223 |
| Median Income | ||||||
| First Quartile (lowest) | — | — | — | — | ||
| Second Quartile | 1.02 | 0.84, 1.25 | 1.000 | 0.71 | 0.32, 1.59 | 1.000 |
| Third Quartile | 1.03 | 0.85, 1.25 | 1.000 | 0.91 | 0.43, 1.93 | 1.000 |
| Fourth Quartile | 0.96 | 0.77, 1.18 | 1.000 | 1.07 | 0.51, 2.22 | 1.000 |
| Unclassified | 0.97 | 0.76, 1.23 | 1.000 | 0.39 | 0.15, 1.00 | 0.198 |
| Charlson Score | ||||||
| Charlson 0 to 1 | — | — | — | — | ||
| Charlson 2 or higher | 1.28 | 1.08, 1.51 | 0.004 | 1.73 | 0.92, 3.25 | 0.09 |
| Tumor Size | 1.04 | 0.96, 1.13 | 0.325 | 0.8 | 0.55, 1.14 | 0.218 |
| cN Stage | ||||||
| cN0 | — | — | — | — | ||
| cN1 | 1.61 | 1.37, 1.90 | <0.001 | 3.37 | 1.32, 8.56 | 0.021 |
| Unclassified | 0.84 | 0.65, 1.09 | 0.196 | 1.31 | 0.72, 2.40 | 0.379 |
| Synchronous Metastatic Site * | ||||||
| Lung | — | — | — | — | ||
| Bone | 1.08 | 0.90, 1.31 | 0.812 | 1.36 | 0.72, 2.56 | 0.537 |
| Liver | 1.24 | 0.91, 1.68 | 0.504 | 3.64 | 0.84, 15.8 | 0.338 |
| Brain | 1.02 | 0.74, 1.42 | 0.890 | 0.23 | 0.03, 1.96 | 0.537 |
| Multiple Organs | 1.77 | 1.46, 2.16 | <0.001 | 1.67 | 0.71, 3.93 | 0.537 |
| Histology | ||||||
| Clear Cell | — | — | — | — | ||
| Papillary | 1.00 | 0.74, 1.35 | 1.000 | 0.66 | 0.28, 1.56 | 1.000 |
| Chromophobe | 1.45 | 0.45, 4.71 | 1.000 | 0.51 | 0.06, 4.09 | 1.000 |
| Collecting Duct | 1.02 | 0.52, 2.01 | 1.000 | |||
| Medullary | 2.04 | 0.71, 5.88 | 0.739 | |||
| RCC NOS | 1.29 | 1.11, 1.51 | 0.006 | 1.24 | 0.74, 2.09 | 1.000 |
| Other | 1.86 | 1.48, 2.34 | <0.001 | |||
| Sarcomatoid Dedifferentiation | 2.04 | 1.37, 3.06 | <0.001 | 2.03 | 0.75, 5.49 | 0.165 |
| Tumor Grade | ||||||
| 1 | — | — | — | — | ||
| 2 | 1.38 | 0.76, 2.51 | 0.580 | 2.56 | 0.59, 11.1 | 0.662 |
| 3 | 1.63 | 0.92, 2.91 | 0.379 | 2.36 | 0.58, 9.59 | 0.662 |
| 4 | 1.15 | 0.59, 2.26 | 0.681 | 1.84 | 0.34, 10.1 | 0.662 |
| Unclassified | 1.44 | 0.84, 2.47 | 0.562 | 3.46 | 0.60, 20.0 | 0.662 |
| Surgery of Primary Site | ||||||
| Nephrectomy | — | — | — | — | ||
| No Surgery of Primary Site | 2.04 | 1.06, 3.89 | 0.127 | |||
| Cryosurgery/Thermal Ablation | 0.76 | 0.38, 1.53 | 0.866 | |||
| Partial Nephrectomy | 0.74 | 0.48, 1.13 | 0.482 | 1.35 | 0.85, 2.16 | 0.208 |
| Unclassified/Other | 1.66 | 0.47, 5.89 | 0.866 | |||
| Margin Status | ||||||
| Negative | — | — | — | — | ||
| Positive | 2.07 | 1.16, 3.70 | 0.028 | 2.21 | 0.86, 5.67 | 0.198 |
| Unclassified/Not Applicable | 1.22 | 0.64, 2.32 | 0.546 | 0.88 | 0.09, 8.37 | 0.910 |
| Metastasectomy | ||||||
| No | — | — | — | — | ||
| Yes | 0.83 | 0.69, 1.00 | 0.110 | 0.94 | 0.57, 1.54 | 0.805 |
| Unclassified | 0.87 | 0.27, 2.86 | 0.823 | |||
| Systemic Therapy | ||||||
| No | — | — | — | — | ||
| Yes | 0.49 | 0.42, 0.56 | <0.001 | 1.57 | 1.00, 2.47 | 0.097 |
| Unclassified | 0.42 | 0.25, 0.71 | 0.001 | 1.45 | 0.39, 5.33 | 0.576 |
| cT1a Synchronous Metastases to Lung | cT1a Synchronous Metastases to Bone | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age | 0.98 | 0.96, 1.01 | 0.178 | 1.03 | 1.02, 1.04 | <0.001 |
| Sex | ||||||
| Female | — | — | — | — | ||
| Male | 0.59 | 0.37, 0.95 | 0.029 | 0.97 | 0.74, 1.27 | 0.814 |
| Race | ||||||
| White | — | — | — | — | ||
| Black | 0.50 | 0.25, 0.99 | 0.192 | 0.70 | 0.45, 1.09 | 0.471 |
| Native American | 2.65 | 0.71, 9.94 | 0.443 | 3.83 | 0.39, 37.5 | 0.745 |
| Asian/Pacific Islander | 1.69 | 0.29, 9.65 | 1.000 | 1.73 | 0.65, 4.61 | 0.745 |
| Other/Unknown | 0.76 | 0.06, 9.61 | 1.000 | 0.90 | 0.37, 2.14 | 0.806 |
| Hispanic | ||||||
| No | — | — | — | — | ||
| Yes | 2.59 | 0.97, 6.95 | 0.117 | 1.05 | 0.59, 1.86 | 0.863 |
| Unclassified | 2.27 | 0.34, 15.2 | 0.398 | 1.84 | 0.81, 4.20 | 0.292 |
| Facility Location | ||||||
| New England | — | — | — | — | ||
| Middle Atlantic | 1.01 | 0.36, 2.89 | 1.000 | 1.00 | 0.53, 1.90 | 1.000 |
| South Atlantic | 0.76 | 0.30, 1.90 | 1.000 | 1.09 | 0.59, 2.01 | 1.000 |
| East North Central | 0.86 | 0.35, 2.09 | 1.000 | 0.97 | 0.52, 1.82 | 1.000 |
| East South Central | 1.92 | 0.65, 5.66 | 1.000 | 1.83 | 0.89, 3.78 | 0.911 |
| West North Central | 2.05 | 0.83, 5.10 | 1.000 | 1.06 | 0.54, 2.08 | 1.000 |
| West South Central | 0.56 | 0.15, 2.07 | 1.000 | 1.26 | 0.61, 2.60 | 1.000 |
| Mountain | 1.78 | 0.39, 8.19 | 1.000 | 1.33 | 0.57, 3.13 | 1.000 |
| Pacific | 0.79 | 0.30, 2.09 | 1.000 | 1.31 | 0.68, 2.51 | 1.000 |
| Unclassified | 0.83 | 0.17, 4.17 | 1.000 | 2.64 | 0.79, 8.77 | 0.911 |
| Facility Type | ||||||
| Community | — | — | — | — | ||
| Community Comprehensive | 1.90 | 0.85, 4.22 | 0.349 | 1.19 | 0.69, 2.07 | 1.000 |
| Academic | 0.62 | 0.29, 1.33 | 0.438 | 0.85 | 0.48, 1.51 | 1.000 |
| Integrated Cancer Network | 1.50 | 0.65, 3.49 | 0.438 | 1.14 | 0.63, 2.08 | 1.000 |
| Median Income | ||||||
| First Quartile (lowest) | — | — | — | — | ||
| Second Quartile | 0.77 | 0.34, 1.74 | 1.000 | 0.83 | 0.55, 1.26 | 1.000 |
| Third Quartile | 1.21 | 0.57, 2.55 | 1.000 | 0.94 | 0.63, 1.40 | 1.000 |
| Fourth Quartile | 1.05 | 0.44, 2.53 | 1.000 | 0.75 | 0.49, 1.14 | 0.715 |
| Unclassified | 0.70 | 0.27, 1.81 | 1.000 | 0.90 | 0.56, 1.44 | 1.000 |
| Charlson Score | ||||||
| Charlson 0 to 1 | — | — | — | — | ||
| Charlson 2 or higher | 2.37 | 1.32, 4.26 | 0.004 | 1.56 | 1.08, 2.27 | 0.019 |
| Tumor Size | 1.03 | 0.78, 1.38 | 0.816 | 1.19 | 1.0, 1.41 | 0.057 |
| cN Stage | ||||||
| cN0 | — | — | — | — | ||
| cN1 | 1.13 | 0.69, 1.84 | 1.000 | 1.91 | 1.36, 2.68 | <0.001 |
| Unclassified | 1.29 | 0.53, 3.11 | 1.000 | 0.90 | 0.54, 1.50 | 0.693 |
| Histology | ||||||
| Clear Cell | — | — | — | — | ||
| Papillary | 1.82 | 1.04, 3.20 | 0.183 | 1.17 | 0.79, 1.74 | 1.000 |
| Chromophobe | — | — | 0.95 | 0.21, 4.30 | 1.000 | |
| Collecting Duct | 0.85 | 0.27, 2.73 | 1.000 | 1.11 | 0.50, 2.43 | 1.000 |
| Medullary | 2.84 | 0.62, 13.0 | 0.537 | 10.2 | 2.60, 40.4 | 0.004 |
| RCC NOS | 3.22 | 0.70, 14.9 | 0.537 | 2.65 | 1.07, 6.54 | 0.140 |
| Other | 2.43 | 1.12, 5.28 | 0.147 | 3.63 | 2.15, 6.13 | <0.001 |
| Sarcomatoid Dedifferentiation | 4.09 | 1.35, 12.4 | 0.013 | 2.05 | 1.04, 4.05 | 0.039 |
| Tumor Grade | ||||||
| 1 | — | — | — | — | ||
| 2 | 0.58 | 0.16, 2.08 | 1.000 | 4.24 | 1.14, 15.8 | 0.125 |
| 3 | 1.44 | 0.39, 5.34 | 1.000 | 3.97 | 1.07, 14.8 | 0.125 |
| 4 | 0.95 | 0.24, 3.79 | 1.000 | 1.83 | 0.45, 7.53 | 0.401 |
| Unclassified | 0.78 | 0.27, 2.24 | 1.000 | 3.34 | 0.95, 11.8 | 0.125 |
| Surgery of Primary Site | ||||||
| No Surgery Primary Site | — | — | — | — | ||
| Cryosurgery/Thermal Ablation | 0.47 | 0.13, 1.78 | 0.269 | 0.43 | 0.21, 0.87 | 0.056 |
| Nephrectomy | 0.08 | 0.01, 0.72 | 0.047 | 0.35 | 0.14, 0.86 | 0.056 |
| Partial Nephrectomy | 0.02 | 0.00, 0.31 | 0.013 | 0.23 | 0.09, 0.60 | 0.011 |
| Unclassified/Other | — | — | 0.64 | 0.08, 4.98 | 0.665 | |
| Margin Status | ||||||
| Negative | — | — | — | — | ||
| Positive | 6.75 | 1.24, 36.8 | 0.055 | 2.57 | 1.04, 6.38 | 0.083 |
| Unclassified/Not Applicable | 0.63 | 0.07, 5.71 | 0.682 | 1.03 | 0.44, 2.42 | 0.937 |
| Metastasectomy | ||||||
| No | — | — | — | — | ||
| Yes | 0.91 | 0.50, 1.67 | 0.759 | 0.81 | 0.59, 1.10 | 0.169 |
| Unclassified/Not Applicable | — | — | — | — | ||
| Systemic Therapy | ||||||
| No | — | — | — | — | ||
| Yes | 0.36 | 0.23, 0.57 | <0.001 | 0.57 | 0.44, 0.74 | <0.001 |
| Unclassified | 0.09 | 0.02, 0.43 | 0.003 | 0.78 | 0.29, 2.09 | 0.627 |
| cT1a Synchronous Metastases to Liver | cT1a Synchronous Metastases to Brain | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age | 1 | 0.97, 1.03 | 0.989 | 0.99 | 0.96, 1.03 | 0.748 |
| Sex | ||||||
| Female | — | — | — | — | ||
| Male | 0.80 | 0.38, 1.67 | 0.546 | 1.35 | 0.62, 2.94 | 0.455 |
| Race | ||||||
| White | # | # | ||||
| Black | ||||||
| Native American | ||||||
| Asian/Pacific Islander | ||||||
| Other/Unknown | ||||||
| Hispanic | ||||||
| No | # | # | ||||
| Yes | ||||||
| Unclassified | ||||||
| Facility Location | ||||||
| New England | # | # | ||||
| Middle Atlantic | ||||||
| South Atlantic | ||||||
| East North Central | ||||||
| East South Central | ||||||
| West North Central | ||||||
| West South Central | ||||||
| Mountain | ||||||
| Pacific | ||||||
| Unclassified | ||||||
| Facility Type | ||||||
| Community | # | # | ||||
| Community Comprehensive | ||||||
| Academic | ||||||
| Integrated Cancer Network | ||||||
| Median Income | ||||||
| First Quartile (lowest) | — | — | — | — | ||
| Second Quartile | 3.44 | 1.20, 9.87 | 0.066 | 0.53 | 0.14, 1.99 | 1.000 |
| Third Quartile | 3.47 | 1.06, 11.4 | 0.080 | 0.51 | 0.16, 1.68 | 1.000 |
| Fourth Quartile | 2.40 | 0.79, 7.29 | 0.121 | 1.15 | 0.25, 5.29 | 1.000 |
| Unclassified | 9.50 | 1.98, 45.5 | 0.020 | 0.59 | 0.14, 2.55 | 1.000 |
| Charlson Score | ||||||
| Charlson 0 to 1 | — | — | — | — | ||
| Charlson 2 or higher | 2.14 | 0.91, 5.03 | 0.082 | 2.36 | 0.90, 6.20 | 0.082 |
| Tumor Size | 1.70 | 1.14, 2.55 | 0.010 | 2.44 | 1.30, 4.59 | 0.006 |
| cN Stage | ||||||
| cN0 | — | — | — | — | ||
| cN1 | 0.58 | 0.27, 1.25 | 0.326 | 1 | 0.34, 2.92 | 0.997 |
| Unclassified | 0.63 | 0.13, 3.08 | 0.566 | 0.34 | 0.05, 2.37 | 0.550 |
| Histology | ||||||
| Clear Cell | — | — | — | — | ||
| Papillary | 0.84 | 0.39, 1.78 | 1.000 | 0.49 | 0.17, 1.43 | 0.383 |
| Chromophobe | — | — | — | — | ||
| Collecting Duct | — | — | — | — | ||
| Medullary | 8.42 | 1.67, 42.4 | 0.029 | — | — | |
| RCC NOS | — | — | — | — | ||
| Other | 1.16 | 0.42, 3.22 | 1.000 | 2.14 | 0.63, 7.21 | 0.383 |
| Sarcomatoid Dedifferentiation | 1.00 | 0.05, 21.5 | 0.998 | 1.52 | 0.17, 13.9 | 0.709 |
| Tumor Grade | ||||||
| 1 | # | # | # | |||
| 2 | ||||||
| 3 | ||||||
| 4 | ||||||
| Unclassified | ||||||
| Surgery of Primary Site | ||||||
| No Surgery Primary Site | — | — | — | — | ||
| Cryosurgery/Thermal Ablation | — | — | — | — | ||
| Nephrectomy | 0.22 | 0.06, 0.78 | 0.039 ^ | 0.08 | 0.02, 0.41 | 0.005 ^^ |
| Partial Nephrectomy | 0.13 | 0.01, 2.35 | 0.165 | 0.1 | 0.01, 0.97 | 0.047 ^^ |
| Unclassified/Other | — | — | — | — | ||
| Margin Status | ||||||
| Negative | # | # | ||||
| Positive | ||||||
| Unclassified/Not Applicable | ||||||
| Metastasectomy | ||||||
| No | — | — | — | — | ||
| Yes | 3.14 | 0.90, 11.0 | 0.074 | 0.26 | 0.10, 0.68 | 0.006 |
| Unclassified/Not Applicable | — | — | — | — | ||
| Systemic Therapy | ||||||
| No | — | — | — | — | ||
| Yes | 0.41 | 0.20, 0.85 | 0.033 | 0.22 | 0.10, 0.48 | <0.001 |
| Unclassified | 1.32 | 0.07, 25.8 | 0.853 | — | — | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Guer, M.; Puri, D.; Liu, F.; Dhanji, S.; Meagher, M.F.; Shah, A.; Ghassemzadeh, S.; Javier-DesLoges, J.; Brugarolas, J.; et al. Characteristics and Outcomes of T1a Renal Cell Carcinoma Presenting with Metastasis. Cancers 2025, 17, 364. https://doi.org/10.3390/cancers17030364
Wang L, Guer M, Puri D, Liu F, Dhanji S, Meagher MF, Shah A, Ghassemzadeh S, Javier-DesLoges J, Brugarolas J, et al. Characteristics and Outcomes of T1a Renal Cell Carcinoma Presenting with Metastasis. Cancers. 2025; 17(3):364. https://doi.org/10.3390/cancers17030364
Chicago/Turabian StyleWang, Luke, Melis Guer, Dhruv Puri, Franklin Liu, Sohail Dhanji, Margaret F. Meagher, Aastha Shah, Saeed Ghassemzadeh, Juan Javier-DesLoges, James Brugarolas, and et al. 2025. "Characteristics and Outcomes of T1a Renal Cell Carcinoma Presenting with Metastasis" Cancers 17, no. 3: 364. https://doi.org/10.3390/cancers17030364
APA StyleWang, L., Guer, M., Puri, D., Liu, F., Dhanji, S., Meagher, M. F., Shah, A., Ghassemzadeh, S., Javier-DesLoges, J., Brugarolas, J., Kapur, P., Bagrodia, A., Rose, B., Murphy, J. D., Derweesh, I. H., & McKay, R. R. (2025). Characteristics and Outcomes of T1a Renal Cell Carcinoma Presenting with Metastasis. Cancers, 17(3), 364. https://doi.org/10.3390/cancers17030364






