A Comprehensive Evaluation of Lymph Node Staging and a Proposal to Subdivide N2b Category in Colorectal Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.1.1. NCC Cohort
2.1.2. SEER Cohort (M0 Cases)
2.1.3. SEER Cohort (M1 Cases)
2.2. Statistical Analysis
3. Results
3.1. Clinicopathologic Characteristics of Patients
3.2. Optimal PLN Cutoff Value for Substage N2b Patients
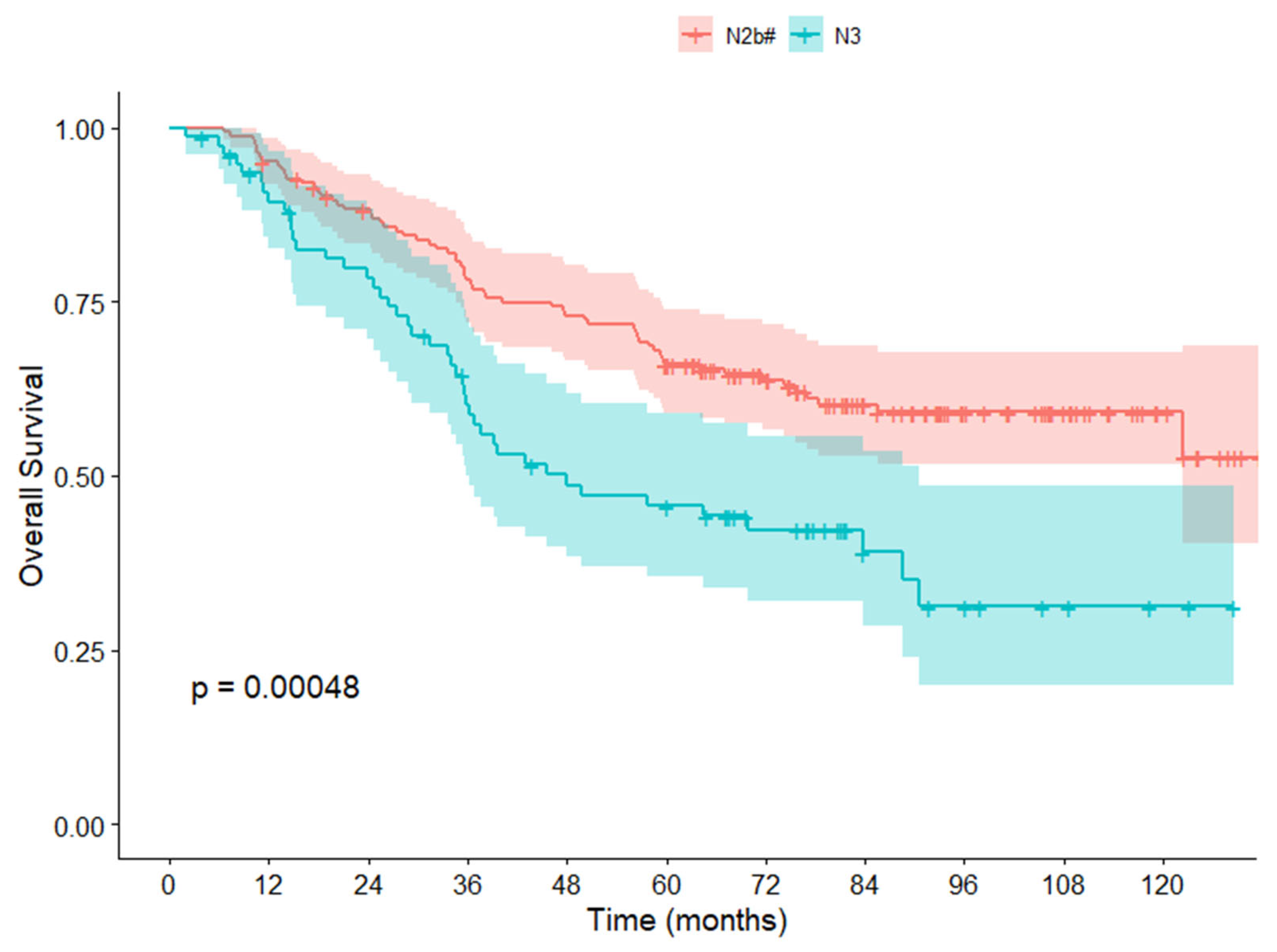
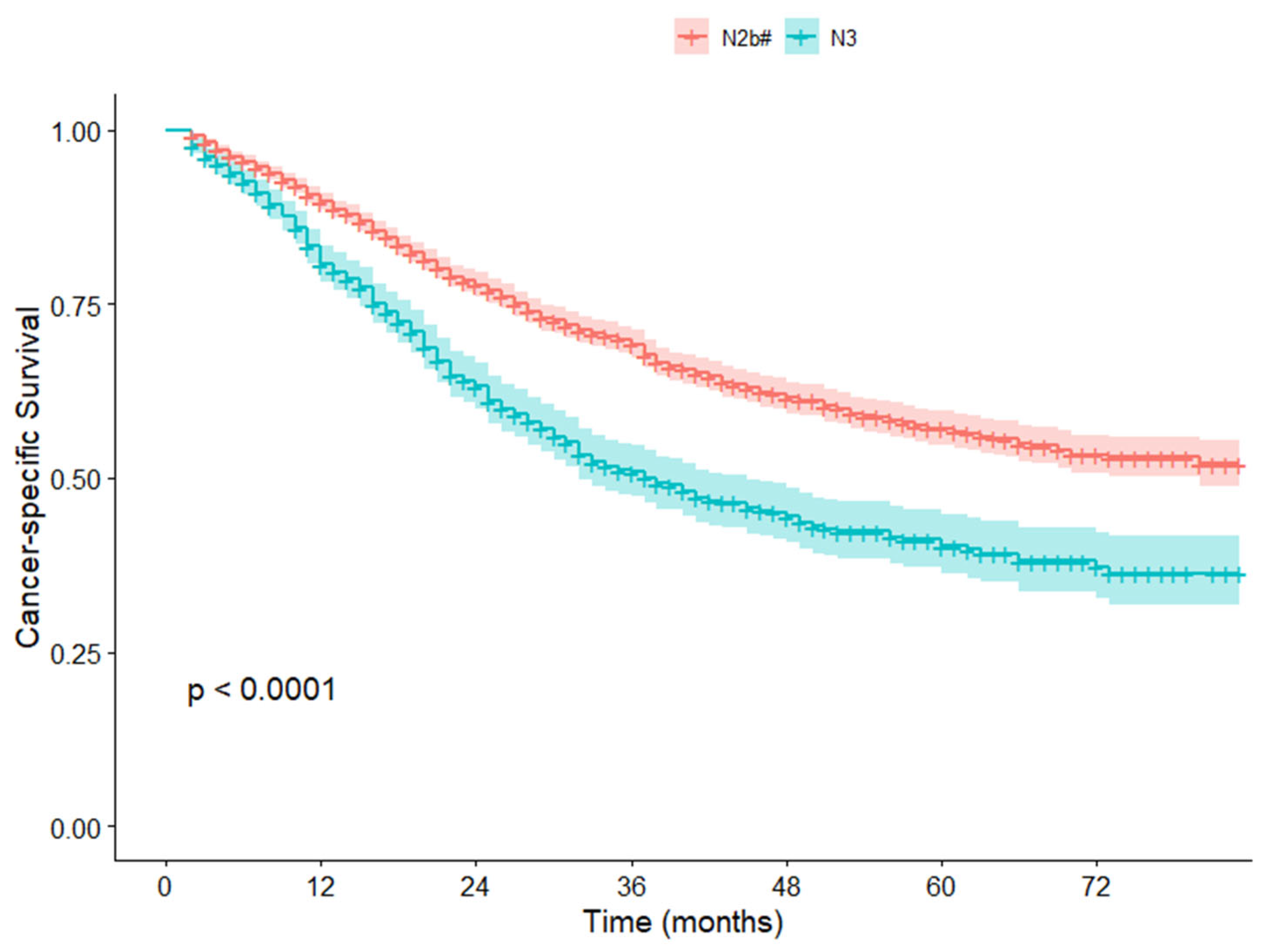
3.3. Survival Analysis in the NCC Cohort
3.4. Validation in the SEER Cohort
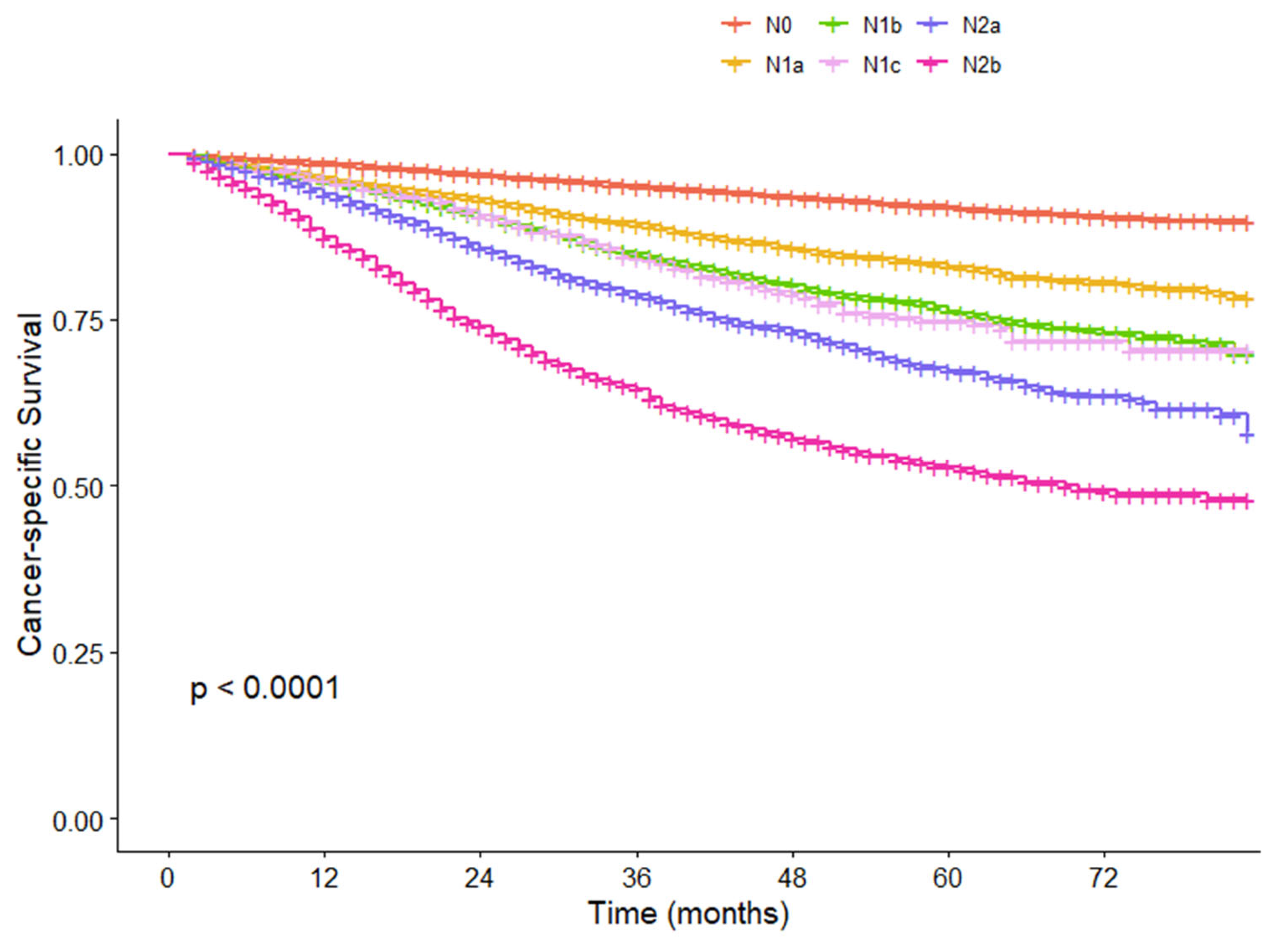
3.5. Cox Analysis for CSS and Comparison of Survival Differences Between N2b Substage and Other N Groups in the SEER Cohort
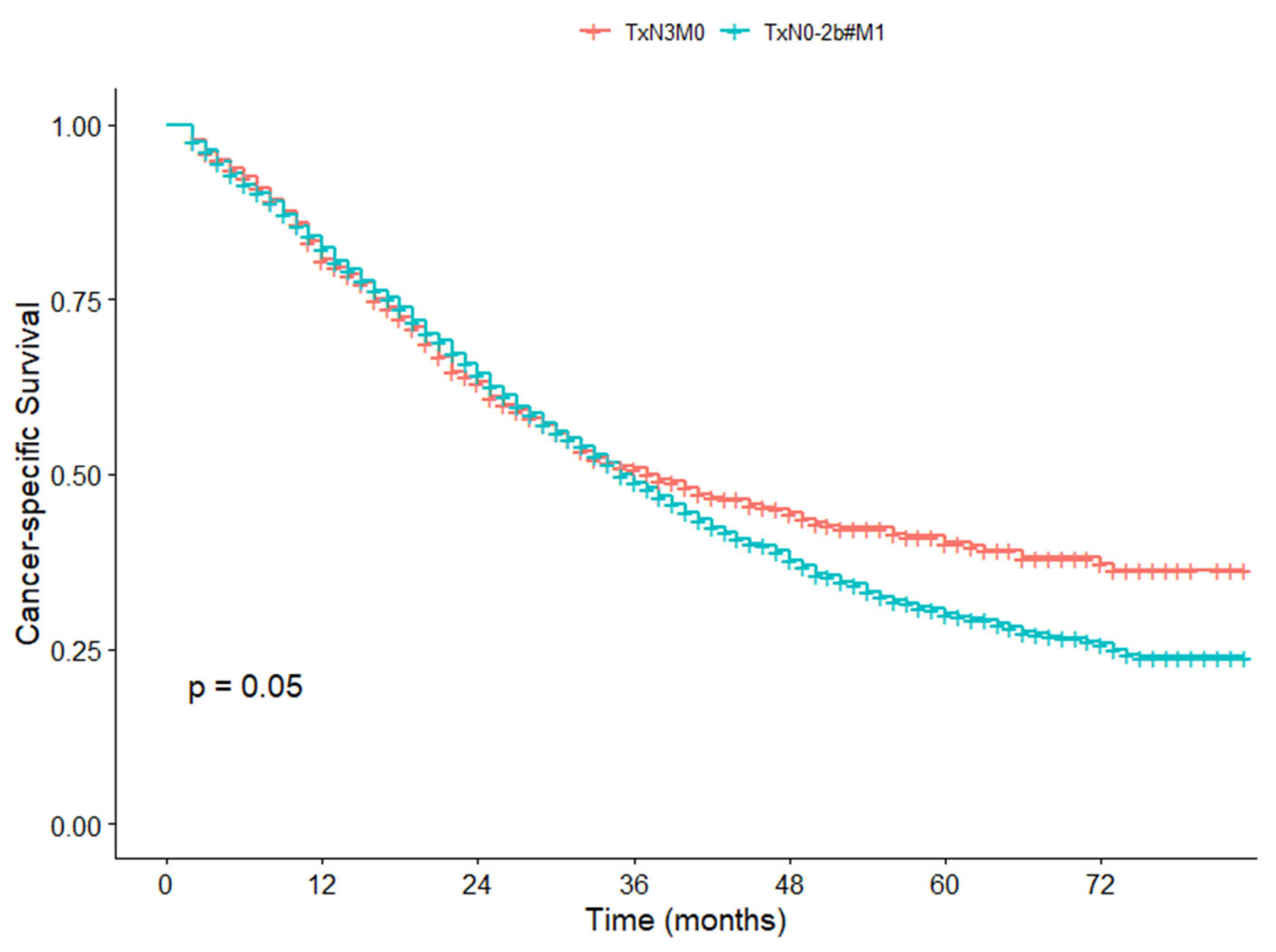
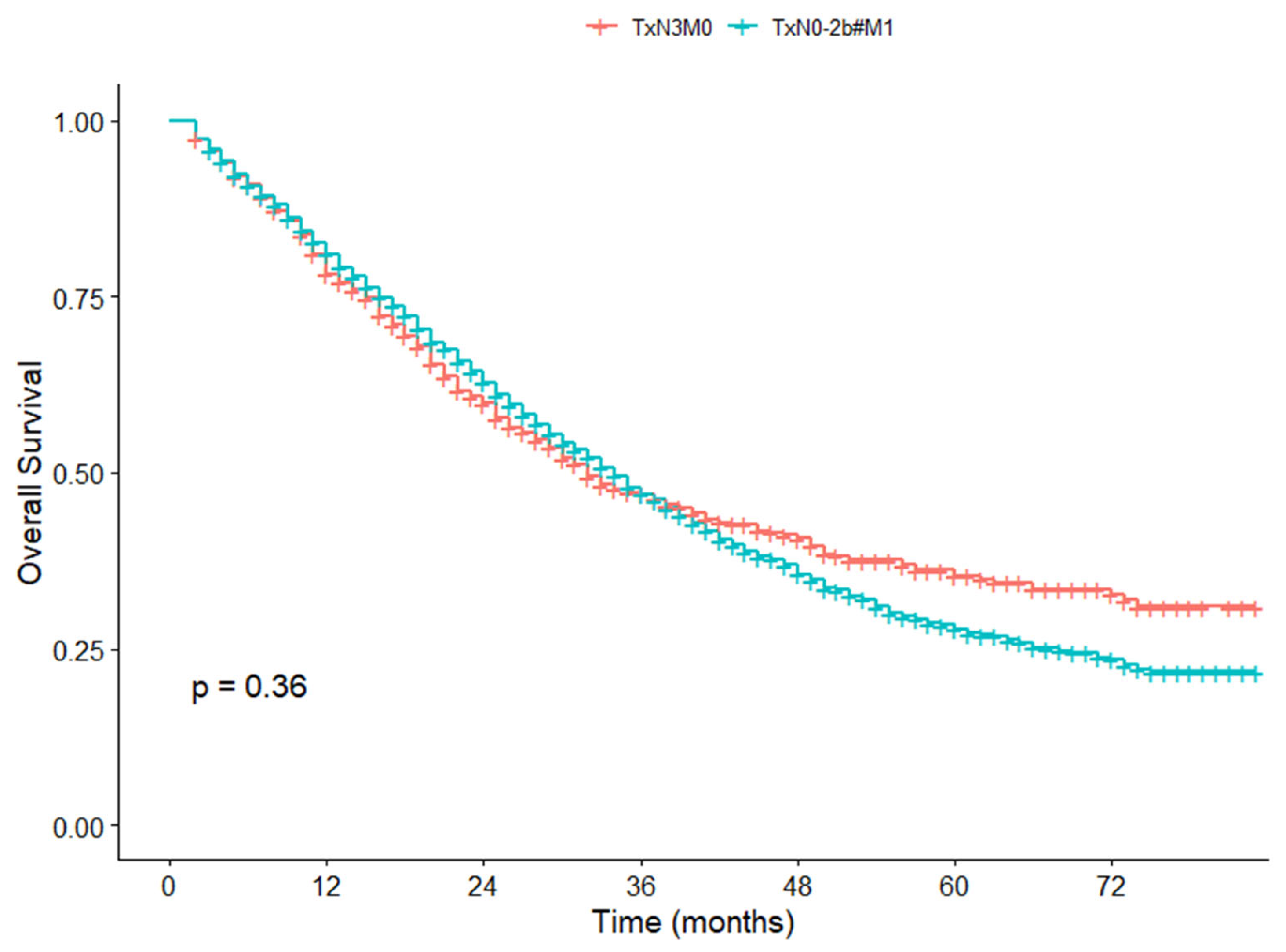
3.6. Survival Difference Between TxN3M0 and TxN0-2b#M1 in the SEER Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B., 3rd; Venook, A.P.; Cederquist, L.; Chan, E.; Chen, Y.J.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; Enzinger, P.C.; Fichera, A.; et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 874–901. [Google Scholar] [CrossRef] [PubMed]
- Compton, C.C.; Fielding, L.P.; Burgart, L.J.; Conley, B.; Cooper, H.S.; Hamilton, S.R.; Hammond, M.E.; Henson, D.E.; Hutter, R.V.; Nagle, R.B.; et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch. Pathol. Lab. Med. 2000, 124, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, e240029. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.H.; Valsdottir, E.B.; Rather, A.A.; Nweze, I.C.; Newman, D.A.; Chernick, M.R. Fewer than 12 lymph nodes can be expected in a surgical specimen after high-dose chemoradiation therapy for rectal cancer. Dis. Colon Rectum 2010, 53, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Tsikitis, V.L.; Larson, D.L.; Wolff, B.G.; Kennedy, G.; Diehl, N.; Qin, R.; Dozois, E.J.; Cima, R.R. Survival in stage III colon cancer is independent of the total number of lymph nodes retrieved. J. Am. Coll. Surg. 2009, 208, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.N.; Virnig, D.J.; Rothenberger, D.A.; Morris, A.M.; Jessurun, J.; Virnig, B.A. Lymph node evaluation in colorectal cancer patients: A population-based study. J. Natl. Cancer Inst. 2005, 97, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, L.L.; Jessup, J.M.; Sargent, D.J.; Greene, F.L.; Stewart, A. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J. Clin. Oncol. 2010, 28, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, I.; Warschkow, R.; Hackert, T.; Schmied, B.M.; Buchler, M.W.; Strobel, O.; Ulrich, A. Staging of pancreatic cancer based on the number of positive lymph nodes. Br. J. Surg. 2017, 104, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Tsuboi, M.; Yoshida, K.; Kato, Y.; Nomura, M.; Matsubayashi, J.; Nagao, T.; Kakihana, M.; Usuda, J.; Kajiwara, N.; et al. Prognostic impact of number of resected and involved lymph nodes at complete resection on survival in non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Zlobec, I.; Lugli, A.; Baker, K.; Roth, S.; Minoo, P.; Hayashi, S.; Terracciano, L.; Jass, J.R. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J. Pathol. 2007, 212, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Hlubek, F.; Spaderna, S.; Schmalhofer, O.; Hiendlmeyer, E.; Jung, A.; Kirchner, T. Invasion and metastasis in colorectal cancer: Epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs 2005, 179, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Quasar Collaborative, G.; Gray, R.; Barnwell, J.; McConkey, C.; Hills, R.K.; Williams, N.S.; Kerr, D.J. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007, 370, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Sobrero, A.F.; Shields, A.F.; Yoshino, T.; Paul, J.; Taieb, J.; Souglakos, J.; Shi, Q.; Kerr, R.; Labianca, R.; et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N. Engl. J. Med. 2018, 378, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| No. | HR (95% CI) | p | HR (95% CI) | p | |
| Gender | 0.284 | ||||
| Men | 141 | 1.000 | |||
| Women | 99 | 0.807 (0.545–1.194) | |||
| Age (year) | <0.001 | 0.001 | |||
| ≤60 | 147 | 1.000 | 1.000 | ||
| >60 | 93 | 2.008 (1.373–2.936) | 1.949 (1.300–2.923) | ||
| Preoperative CEA | 0.031 | ||||
| Positive | 67 | 1.000 | |||
| Negative | 123 | 0.605 (0.389–0.941) | 0.026 | ||
| Unknown | 50 | 1.035 (0.628–1.705) | 0.892 | ||
| Preoperative CA199 | 0.043 | 0.007 | |||
| Positive | 29 | 1.000 | 1.000 | ||
| Negative | 159 | 0.550 (0.318–0.953) | 0.033 | 0.382 (0.215–0.676) | 0.001 |
| Unknown | 52 | 0.830 (0.449–1.536) | 0.554 | 0.528 (0.276–1.010) | 0.054 |
| Surgical procedure | 0.891 | ||||
| Open | 87 | 1.000 | |||
| Laparoscope | 153 | 1.028 (0.694–1.522) | |||
| Tumor location | 0.009 | 0.004 | |||
| Rectum | 151 | 1.000 | 1.000 | ||
| Colon | 89 | 0.561 (0.364–0.864) | 0.512 (0.324–0.811) | ||
| Vascular invasion | 0.011 | ||||
| Positive | 97 | 1.000 | |||
| Negative | 143 | 0.611 (0.417–0.893) | |||
| Perineural invasion | 0.025 | 0.007 | |||
| Positive | 77 | 1.000 | 1.000 | ||
| Negative | 163 | 0.639 (0.432–0.946) | 0.569 (0.378–0.858) | ||
| Differentiation grade | 0.013 | ||||
| Well/Unknown | 3 | 1.000 | |||
| Moderate | 152 | 0.441 (0.107–1.808) | 0.255 | ||
| Poor | 85 | 0.769 (0.186–3.175) | 0.717 | ||
| pT | 0.004 | 0.002 | |||
| T1–2 | 9 | 1.000 | 1.000 | ||
| T3 | 147 | 0.813 (0.294–2.243) | 0.689 | 0.833 (0.289–2.404) | 0.736 |
| T4 | 84 | 1.576 (0.568–4.379) | 0.382 | 1.774 (0.608–5.182) | 0.294 |
| pN | 0.001 | 0.002 | |||
| N2b# | 163 | 1.000 | 1.000 | ||
| N3 | 77 | 1.966 (1.335–2.895) | 1.869 (1.253–2.787) | ||
| Number of LNs examined < 12 | 0.550 | ||||
| Yes | 6 | 1.000 | |||
| No | 234 | 0.736 (0.270–2.010) | |||
| Adjuvant radiation | 0.970 | ||||
| Yes | 84 | 1.000 | |||
| No | 156 | 1.008 (0.676–1.501) | |||
| Adjuvant chemotherapy | <0.001 | <0.001 | |||
| Yes | 206 | 1.000 | 1.000 | ||
| No | 34 | 3.459 (2.199–5.439) | 3.004 (1.842–4.901) | ||
| Characteristics | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| No. | HR (95% CI) | p | HR (95% CI) | p | |
| Gender | 0.947 | ||||
| Men | 33,588 | 1.000 | |||
| Women | 31,601 | 0.998 (0.953–1.046) | |||
| Age (year) | <0.001 | <0.001 | |||
| ≤60 | 25,207 | 1.000 | 1.000 | ||
| >60 | 39,982 | 1.598 (1.518–1.681) | 1.697 (1.609–1.791) | ||
| Race | <0.001 | <0.001 | |||
| White | 50,283 | 1.000 | 1.000 | ||
| Black | 7971 | 1.331 (1.247–1.420) | <0.001 | 1.249 (1.169–1.336) | <0.001 |
| Other/Unknown | 6935 | 0.890 (0.821–0.966) | 0.005 | 0.812 (0.748–0.881) | <0.001 |
| Marital status | <0.001 | <0.001 | |||
| Single | 10,526 | 1.000 | 1.000 | ||
| Married | 36,577 | 0.652 (0.613–0.695) | <0.001 | 0.741 (0.694–0.790) | <0.001 |
| Widowed/divorced | 13,756 | 1.044 (0.974–1.119) | 0.222 | 0.983 (0.915–1.056) | 0.633 |
| Others | 4330 | 0.750 (0.673–0.834) | <0.001 | 0.822 (0.738–0.915) | <0.001 |
| Preoperative CEA | <0.001 | <0.001 | |||
| Positive | 12,305 | 1.000 | 1.000 | ||
| Negative | 25,021 | 0.408 (0.384–0.434) | <0.001 | 0.634 (0.596–0.675) | <0.001 |
| Unknown | 27,863 | 0.570 (0.539–0.602) | <0.001 | 0.825 (0.779–0.873) | <0.001 |
| Tumor location | 0.073 | ||||
| Rectum | 11,462 | 1.000 | |||
| Colon | 53,763 | 1.058 (0.995–1.126) | |||
| Perineural invasion | <0.001 | <0.001 | |||
| Positive | 5612 | 1.000 | 1.000 | ||
| Negative | 52,794 | 0.336 (0.317–0.357) | <0.001 | 0.729 (0.684–0.777) | <0.001 |
| Unknown | 6783 | 0.438 (0.402–0.477) | <0.001 | 0.808 (0.739–0.883) | <0.001 |
| Differentiation grade | <0.001 | <0.001 | |||
| Well | 6033 | 1.000 | 1.000 | ||
| Moderate | 47,135 | 1.683 (1.511–1.874) | <0.001 | 1.238 (1.110–1.380) | <0.001 |
| Poor/undifferentiation | 10,045 | 3.693 (3.299–4.133) | <0.001 | 1.713 (1.527–1.923) | <0.001 |
| Unknown | 1976 | 1.076 (0.882–1.314) | 0.470 | 1.259 (1.030–1.539) | 0.024 |
| pT | <0.001 | <0.001 | |||
| T1 | 11,886 | 1.000 | 1.000 | ||
| T2 | 11,467 | 1.991 (1.706–2.323) | <0.001 | 1.801 (1.541–2.105) | <0.001 |
| T3 | 33,754 | 6.244 (5.490–7.102) | <0.001 | 4.208 (3.681–4.810) | <0.001 |
| T4 | 8082 | 17.679 (15.501–20.164) | <0.001 | 9.455 (8.228–10.865) | <0.001 |
| pN | <0.001 | <0.001 | |||
| N0 | 41,004 | 1.000 | 1.000 | ||
| N1a | 7666 | 2.240 (2.077–2.416) | <0.001 | 1.992 (1.840–2.158) | <0.001 |
| N1b | 7465 | 3.165 (2.956–3.388) | <0.001 | 2.642 (2.454–2.844) | <0.001 |
| N1c | 1017 | 3.301 (2.814–3.873) | <0.001 | 2.298 (1.955–2.702) | <0.001 |
| N2a | 4517 | 4.630 (4.306–4.977) | <0.001 | 3.517 (3.249–3.809) | <0.001 |
| N2b# | 2626 | 6.976 (6.451–7.543) | <0.001 | 4.944 (4.534–5.392) | <0.001 |
| N3 | 894 | 12.237 (11.032–13.575) | <0.001 | 8.170 (7.298–9.146) | <0.001 |
| Number of examined LNs < 12 | <0.001 | <0.001 | |||
| Yes | 9212 | 1.000 | 1.000 | ||
| No | 55,977 | 0.851 (0.799–0.905) | 0.570 (0.535–0.608) | ||
| Adjuvant radiation | <0.001 | 0.004 | |||
| Yes | 2824 | 1.000 | 1.000 | ||
| No | 62,365 | 0.700 (0.637–0.770) | 0.866 (0.786–0.955) | ||
| Chemotherapy | <0.001 | <0.001 | |||
| Yes | 21,671 | 1.000 | 1.000 | ||
| No | 43,518 | 0.574 (0.547–0.601) | 1.531 (1.448–1.619) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, K.; Wu, Y.; Feng, L.; Zhu, Y.; Fang, H.; Zhang, H. A Comprehensive Evaluation of Lymph Node Staging and a Proposal to Subdivide N2b Category in Colorectal Cancer Patients. Cancers 2025, 17, 4002. https://doi.org/10.3390/cancers17244002
Xi K, Wu Y, Feng L, Zhu Y, Fang H, Zhang H. A Comprehensive Evaluation of Lymph Node Staging and a Proposal to Subdivide N2b Category in Colorectal Cancer Patients. Cancers. 2025; 17(24):4002. https://doi.org/10.3390/cancers17244002
Chicago/Turabian StyleXi, Kexing, Yunlong Wu, Lin Feng, Yuelu Zhu, Hui Fang, and Haizeng Zhang. 2025. "A Comprehensive Evaluation of Lymph Node Staging and a Proposal to Subdivide N2b Category in Colorectal Cancer Patients" Cancers 17, no. 24: 4002. https://doi.org/10.3390/cancers17244002
APA StyleXi, K., Wu, Y., Feng, L., Zhu, Y., Fang, H., & Zhang, H. (2025). A Comprehensive Evaluation of Lymph Node Staging and a Proposal to Subdivide N2b Category in Colorectal Cancer Patients. Cancers, 17(24), 4002. https://doi.org/10.3390/cancers17244002





