Survival Outcomes in Hepatocellular Carcinoma Patients Undergoing TARE: A Comparative Analysis Before and After Single Admission Order–Map–Treat Protocol Implementation
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
3. Statistical Analysis
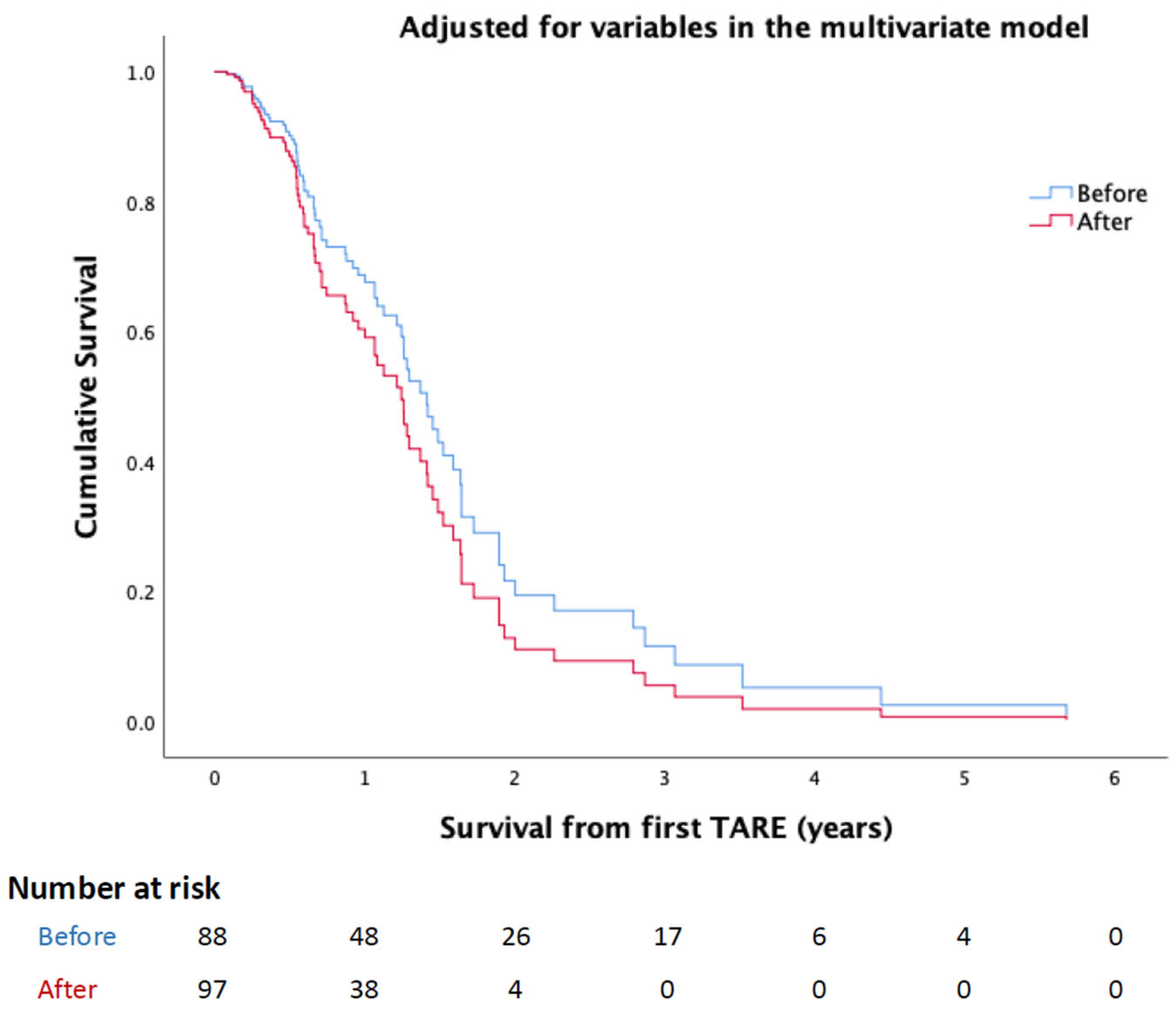
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ungtrakul, T.; Mahidol, C.; Chun-On, P.; Laohapand, C.; Siripongsakun, S.; Worakitsitisatorn, A.; Vidhayakorn, S.; Boonchuay, W.; Dechma, J.; Sornsamdang, G.; et al. Hepatocellular Carcinoma Screening and Surveillance in 2293 Chronic Hepatitis B Patients in an Endemic Area. World J. Gastroenterol. 2016, 22, 7806–7812. [Google Scholar] [CrossRef]
- Wang, W.; Wei, C. Advances in the Early Diagnosis of Hepatocellular Carcinoma. Genes. Dis. 2020, 7, 308–319. [Google Scholar] [CrossRef]
- Reig, M.; Sanduzzi-Zamparelli, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Díaz, A.; Llarch, N.; Iserte, G.; et al. Bclc Strategy for Prognosis Prediction and Treatment Recommendations: The 2025 Update. J. Hepatol. 2025, in press. [Google Scholar] [CrossRef]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular Carcinoma: Esmo Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2025, 36, 491–506. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M.; Diseases American Association for the Study of Liver. Management of Hepatocellular Carcinoma: An Update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- An, C.; Choi, Y.A.; Choi, D.; Paik, Y.H.; Ahn, S.H.; Kim, M.J.; Paik, S.W.; Han, K.H.; Park, M.S. Growth Rate of Early-Stage Hepatocellular Carcinoma in Patients with Chronic Liver Disease. Clin. Mol. Hepatol. 2015, 21, 279–286. [Google Scholar] [CrossRef]
- Nathani, P.; Gopal, P.; Rich, N.; Yopp, A.; Yokoo, T.; John, B.; Marrero, J.; Parikh, N.; Singal, A.G. Hepatocellular Carcinoma Tumour Volume Doubling Time: A Systematic Review and Meta-Analysis. Gut 2021, 70, 401–407. [Google Scholar] [CrossRef]
- Rich, N.E.; John, B.V.; Parikh, N.D.; Rowe, I.; Mehta, N.; Khatri, G.; Thomas, S.M.; Anis, M.; Mendiratta-Lala, M.; Hernandez, C.; et al. Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients with Cirrhosis. Hepatology 2020, 72, 1654–1665. [Google Scholar] [CrossRef]
- Jang, H.J.; Choi, S.H.; Wee, S.; Choi, S.J.; Byun, J.H.; Won, H.J.; Shin, Y.M.; Sirlin, C.B. Ct-and Mri-Based Factors Associated with Rapid Growth in Early-Stage Hepatocellular Carcinoma. Radiology 2024, 313, e240961. [Google Scholar] [CrossRef]
- Zhao, K.; Blume, H.; Petre, E.N.; Xenos, D.; Kobus, Z.; Alexander, E.S.; Sotirchos, V.S.; Geevarghese, R.; Covey, A.; Erinjeri, J.P.; et al. Hepatocellular Carcinoma Growth Kinetics and Outcomes after Transarterial Embolization: A Single-Center Analysis. Cancers 2025, 17, 3346. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The Redcap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inf. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Hao, K.; Paik, A.J.; Han, L.H.; Makary, M.S. Yttrium-90 Radioembolization Treatment Strategies for Management of Hepatocellular Carcinoma. World J. Radiol. 2024, 16, 512–527. [Google Scholar] [CrossRef]
- Fite, E.L.; Makary, M.S. Advances and Emerging Techniques in Y-90 Radioembolization for Hepatocellular Carcinoma. Cancers 2025, 17, 1494. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (Redcap)--a Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inf. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Shuqun, C.; Mengchao, W.; Han, C.; Feng, S.; Jiahe, Y.; Guanghui, D.; Wenming, C.; Peijun, W.; Yuxiang, Z. Tumor Thrombus Types Influence the Prognosis of Hepatocellular Carcinoma with the Tumor Thrombi in the Portal Vein. Hepatogastroenterology 2007, 54, 499–502. [Google Scholar]
- Frantz, S.; Matsuoka, L.; Vaheesan, K.; Petroziello, M.; Golzarian, J.; Wang, E.; Gandhi, R.; Collins, Z.; Brower, J.; Rachakonda, V.M.; et al. Multicenter Evaluation of Survival and Toxicities of Hepatocellular Carcinoma Following Radioembolization: Analysis of the Resin Registry. J. Vasc. Interv. Radiol. 2021, 32, 845–852. [Google Scholar] [CrossRef]
- Kolligs, F.; Arnold, D.; Golfieri, R.; Pech, M.; Peynircioglu, B.; Pfammatter, T.; Ronot, M.; Sangro, B.; Schaefer, N.; Maleux, G.; et al. Factors Impacting Survival after Transarterial Radioembolization in Patients with Hepatocellular Carcinoma: Results from the Prospective Cirt Study. JHEP Rep. 2023, 5, 100633. [Google Scholar] [CrossRef]
- Levillain, H.; Bagni, O.; Deroose, C.M.; Dieudonne, A.; Gnesin, S.; Grosser, O.S.; Kappadath, S.C.; Kennedy, A.; Kokabi, N.; Liu, D.M.; et al. International Recommendations for Personalised Selective Internal Radiation Therapy of Primary and Metastatic Liver Diseases with Yttrium-90 Resin Microspheres. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1570–1584. [Google Scholar] [CrossRef]
- Weber, M.; Lam, M.; Chiesa, C.; Konijnenberg, M.; Cremonesi, M.; Flamen, P.; Gnesin, S.; Bodei, L.; Kracmerova, T.; Luster, M.; et al. Eanm Procedure Guideline for the Treatment of Liver Cancer and Liver Metastases with Intra-Arterial Radioactive Compounds. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1682–1699. [Google Scholar] [CrossRef] [PubMed]
- Kokabi, N.; Arndt-Webster, L.; Chen, B.; Brandon, D.; Sethi, I.; Davarpanahfakhr, A.; Galt, J.; Elsayed, M.; Bercu, Z.; Cristescu, M.; et al. Voxel-Based Dosimetry Predicting Treatment Response and Related Toxicity in Hcc Patients Treated with Resin-Based Y90 Radioembolization: A Prospective, Single-Arm Study. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1743–1752. [Google Scholar] [CrossRef]
- Villalobos, A.; Arndt, L.; Cheng, B.; Dabbous, H.; Loya, M.; Majdalany, B.; Bercu, Z.; Kokabi, N. Yttrium-90 Radiation Segmentectomy of Hepatocellular Carcinoma: A Comparative Study of the Effectiveness, Safety, and Dosimetry of Glass-Based Versus Resin-Based Microspheres. J. Vasc. Interv. Radiol. 2023, 34, 1226–1234. [Google Scholar] [CrossRef]
- Kim, H.C.; Suh, M.; Paeng, J.C.; Lee, J.H.; Lee, M.; Chung, J.W.; Choi, J.W. Streamlining Radioembolization without Lung Shunt Estimation Versus Regular Radioembolization in Patients with Hepatocellular Carcinoma within the Milan Criteria. J. Vasc. Interv. Radiol. 2025, 36, 78–87.e1. [Google Scholar] [CrossRef] [PubMed]
- Mohnasky, M.; Gad, S.; Fanous, M.; Pisanie, J.L.D.; Ivanovic, M.; Mauro, D.M.; Yu, H.; Villalobos, A.; Moon, A.M.; Sanoff, H.K.; et al. Initial Experience with Single-Session Resin-Based Transarterial Radioembolization Mapping and Treatment of Small Hepatocellular Carcinomas. Cancers 2025, 17, 1265. [Google Scholar] [CrossRef]
- Paladini, A.; Spinetta, M.; Matheoud, R.; D’Alessio, A.; Sassone, M.; Di Fiore, R.; Coda, C.; Carriero, S.; Biondetti, P.; Lagana, D.; et al. Role of Flex-Dose Delivery Program in Patients Affected by Hcc: Advantages in Management of Tare in Our Experience. J. Clin. Med. 2024, 13, 2188. [Google Scholar] [CrossRef]
- Kim, H.C.; Suh, M.; Paeng, J.C.; Choi, J.W. Same-Day Versus Multiday Planning/Treatment Radioembolization with Yttrium-90 Resin Microspheres in Patients with Liver Cancer >/=5 Cm. J. Vasc. Interv. Radiol. 2025, 36, 2010–2020.e2. [Google Scholar] [CrossRef]
- Frost, J.P.; Bell, J.; Lawrance, J.; Najran, P.; Mullan, D. Ambulatory Same-Day Map-and-Treat Angiography for Selective Internal Radiation Therapy Using a Transradial Approach. Cureus 2022, 14, e27741. [Google Scholar] [CrossRef] [PubMed]
- Li, M.D.; Chu, K.F.; DePietro, A.; Wu, V.; Wehrenberg-Klee, E.; Zurkiya, O.; Liu, R.W.; Ganguli, S. Same-Day Yttrium-90 Radioembolization: Feasibility with Resin Microspheres. J. Vasc. Interv. Radiol. 2019, 30, 314–319. [Google Scholar] [CrossRef]
- Gabr, A.; Ranganathan, S.; Mouli, S.K.; Riaz, A.; Gates, V.L.; Kulik, L.; Ganger, D.; Maddur, H.; Moore, C.; Hohlastos, E.; et al. Streamlining Radioembolization in Unos T1/T2 Hepatocellular Carcinoma by Eliminating Lung Shunt Estimation. J. Hepatol. 2020, 72, 1151–1158. [Google Scholar] [CrossRef]
- Elsayed, M.; Loya, M.; Galt, J.; Schuster, D.M.; Bercu, Z.L.; Newsome, J.; Brandon, D.; Benenati, S.; Behbahani, K.; Duszak, R.; et al. Same Day Yttrium-90 Radioembolization with Single Photon Emission Computed Tomography/Computed Tomography: An Opportunity to Improve Care during the Covid-19 Pandemic and Beyond. World J. Gastrointest. Oncol. 2021, 13, 440–452. [Google Scholar] [CrossRef]
- Berman, Z.T.; Pianka, K.; Qaseem, Y.; Redmond, J.; Minocha, J. Single-Session Ablative Transarterial Radioembolization for Patients with Hepatocellular Carcinoma to Streamline Care: An Initial Experience. Cardiovasc. Interv. Radiol. 2024, 47, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Pollock, R.F.; Shergill, S.; Carion, P.L.; von Oppen, N.; Agirrezabal, I.; Brennan, V.K. Advances in Delivery of Selective Internal Radiation Therapy (SIRT): Economic and Logistical Effects of Same-Stay Work-Up and Procedure in the Treatment of Unresectable Liver Tumors in England. Adv. Ther. 2023, 40, 294–309. [Google Scholar] [CrossRef] [PubMed]
| Total | <2021 | ≥2021 | p-Value | |
|---|---|---|---|---|
| N | 185 | 88 (47.6%) | 97 (52.4%) | |
| Age (years) | 71 ± 12 | 71 ± 12 | 71 ± 12 | 0.807 |
| Gender | ||||
| Female | 57 (30.8%) | 25 (28.4%) | 32 (33%) | 0.527 |
| Male | 128 (69.2%) | 63 (71.6%) | 65 (67%) | |
| Body Mass Index | 29 ± 8 | 30 ± 9 | 28 ± 7 | 0.682 |
| ECOG Performance Status | ||||
| 0 | 114 (62.3%) | 69 (80.2%) | 45 (46.4%) | <0.001 |
| 1 | 51 (27.9%) | 13 (15.1%) | 38 (39.2%) | |
| 2 | 12 (6.6%) | 3 (3.5%) | 9 (9.3%) | |
| 3 | 6 (3.3%) | 1 (1.2%) | 5 (5.2%) | |
| Diabetes Mellitus | 130 (70.3%) | 66 (75%) | 64 (66%) | 0.200 |
| Prior interventions | ||||
| None | 118 (63.8%) | 55 (62.5%) | 63 (64.9%) | 0.011 |
| Ablation | 6 (3.2%) | 0 (0%) | 6 (6.2%) | |
| Surgery | 2 (1.1%) | 0 (0%) | 2 (2.1%) | |
| Systemic | 5 (2.7%) | 1 (1.1%) | 4 (4.1%) | |
| Transarterial Chemoembolization/ Transarterial Embolization | 52 (28.1%) | 30 (34.1%) | 22 (22.7%) | |
| TARE | 2 (1.1%) | 2 (2.3%) | 0 (0%) | |
| Cirrhosis | 151 (81.6%) | 73 (83%) | 78 (80.4%) | 0.707 |
| Child score | ||||
| A5 | 52 (28.1%) | 18 (20.5%) | 34 (35.1%) | 0.218 |
| A6 | 79 (42.7%) | 44 (50%) | 35 (36.1%) | |
| B7 | 32 (17.3%) | 16 (18.2%) | 16 (16.5%) | |
| B8 | 10 (5.4%) | 4 (4.5%) | 6 (6.2%) | |
| B9 | 3 (1.6%) | 1 (1.1%) | 2 (2.1%) | |
| C10 | 1 (0.5%) | 0 (0%) | 1 (1%) | |
| Etiology of cirrhosis | ||||
| Cryptogenic | 84 (45.4%) | 40 (45.5%) | 44 (45.4%) | 0.029 |
| Hemochromatosis | 1 (0.5%) | 1 (1.1%) | 0 (0%) | |
| Hep B | 34 (18.4%) | 16 (18.2%) | 18 (18.6%) | |
| Hep C | 50 (27%) | 28 (31.8%) | 22 (22.7%) | |
| Nonalcoholic fatty liver disease | 15 (8.1%) | 2 (2.3%) | 13 (13.4%) | |
| Schistosomiasis | 1 (0.5%) | 1 (1.1%) | 0 (0%) | |
| BCLC stage | ||||
| 0 | 20 (10.8%) | 15 (17%) | 5 (5.2%) | 0.091 |
| A | 56 (30.3%) | 23 (26.1%) | 33 (34%) | |
| B | 64 (34.6%) | 31 (35.2%) | 33 (34%) | |
| C | 42 (22.7%) | 18 (20.5%) | 24 (24.7%) | |
| D | 3 (1.6%) | 1 (1.1%) | 2 (2.1%) | |
| Extent of intrahepatic disease | ||||
| Bilobar | 28 (15.5%) | 13 (15.5%) | 15 (15.5%) | 0.041 |
| Multifocal | 55 (30.4%) | 33 (39.3%) | 22 (22.7%) | |
| Segmental | 43 (23.8%) | 20 (23.8%) | 23 (23.7%) | |
| Unilobar | 55 (30.4%) | 18 (21.4%) | 37 (38.1%) | |
| Replacement of liver by tumor | ||||
| Less than 25% | 128 (70.3%) | 55 (63.2%) | 73 (76.8%) | 0.184 |
| 25–50% | 36 (19.8%) | 21 (24.1%) | 15 (15.8%) | |
| 50–75% | 16 (8.8%) | 10 (11.5%) | 6 (6.3%) | |
| >75% | 2 (1.1%) | 1 (1.1%) | 1 (1.1%) | |
| Largest lesion size (cm) | 5.8 ± 4 | 6.3 ± 4 | 5.5 ± 3.9 | 0.181 |
| Portal Vein Tumoral Thrombosis | 55 (30.6%) | 26 (30.6%) | 29 (30.5%) | |
| PVTT 1 | 6 (3.2%) | 4 (4.5%) | 2 (2.1%) | 0.009 |
| PVTT 2 | 24 (13%) | 16 (18.2%) | 8 (8.2%) | |
| PVTT 3 | 16 (8.6%) | 3 (3.4%) | 13 (13.4%) | |
| PVTT 4 | 8 (4.3%) | 2 (2.3%) | 6 (6.2%) | |
| Splenomegaly | 64 (34.6%) | 28 (31.8%) | 36 (37.1%) | 0.536 |
| Ascites | 36 (19.5%) | 16 (18.2%) | 20 (20.6%) | 0.713 |
| Varices | 40 (21.6%) | 16 (18.2%) | 24 (24.7%) | 0.290 |
| Whole liver volume | 1653.2 ± 497 | 1654.2 ± 487.3 | 1652.4 ± 507.5 | 0.981 |
| Target perfused liver volume | 638.2 ± 399.6 | 860 ± 457.8 | 523.7 ± 316.6 | 0.005 |
| Tumor volume | 283.2 ± 399.1 | 284.7 ± 321.5 | 282 ± 454.9 | 0.965 |
| Y90 Dose | 1.6 ± 0.8 | 1.7 ± 0.7 | 1.4 ± 0.8 | 0.006 |
| Target tumor dose | 141.2 ± 58 | 146.5 ± 47.9 | 137.4 ± 64.2 | 0.333 |
| Treated lobe | ||||
| Left | 31 (16.8%) | 11 (12.5%) | 20 (20.6%) | 0.165 |
| Rad segmentectomy | 12 (6.5%) | 3 (3.4%) | 9 (9.3%) | |
| Right | 130 (70.3%) | 63 (71.6%) | 67 (69.1%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhussaini, A.A.; AlShreadah, S.; Elzahrani, M.R.; AlTaweel, A.; AlAhmed, M.; Bashir, O.; Al Shehri, S.; Arabi, M. Survival Outcomes in Hepatocellular Carcinoma Patients Undergoing TARE: A Comparative Analysis Before and After Single Admission Order–Map–Treat Protocol Implementation. Cancers 2025, 17, 3930. https://doi.org/10.3390/cancers17243930
Alhussaini AA, AlShreadah S, Elzahrani MR, AlTaweel A, AlAhmed M, Bashir O, Al Shehri S, Arabi M. Survival Outcomes in Hepatocellular Carcinoma Patients Undergoing TARE: A Comparative Analysis Before and After Single Admission Order–Map–Treat Protocol Implementation. Cancers. 2025; 17(24):3930. https://doi.org/10.3390/cancers17243930
Chicago/Turabian StyleAlhussaini, Abdulmohsen Ahmed, Saleh AlShreadah, Mohamed Rajab Elzahrani, Abdulaziz AlTaweel, Mohammed AlAhmed, Omar Bashir, Shaker Al Shehri, and Mohammad Arabi. 2025. "Survival Outcomes in Hepatocellular Carcinoma Patients Undergoing TARE: A Comparative Analysis Before and After Single Admission Order–Map–Treat Protocol Implementation" Cancers 17, no. 24: 3930. https://doi.org/10.3390/cancers17243930
APA StyleAlhussaini, A. A., AlShreadah, S., Elzahrani, M. R., AlTaweel, A., AlAhmed, M., Bashir, O., Al Shehri, S., & Arabi, M. (2025). Survival Outcomes in Hepatocellular Carcinoma Patients Undergoing TARE: A Comparative Analysis Before and After Single Admission Order–Map–Treat Protocol Implementation. Cancers, 17(24), 3930. https://doi.org/10.3390/cancers17243930





