Ovarian Cancer Ascites Enriched for CCL23 Reduces Macrophage-Derived CXCL10 Secretion and Is Associated with Poor Patient Outcomes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
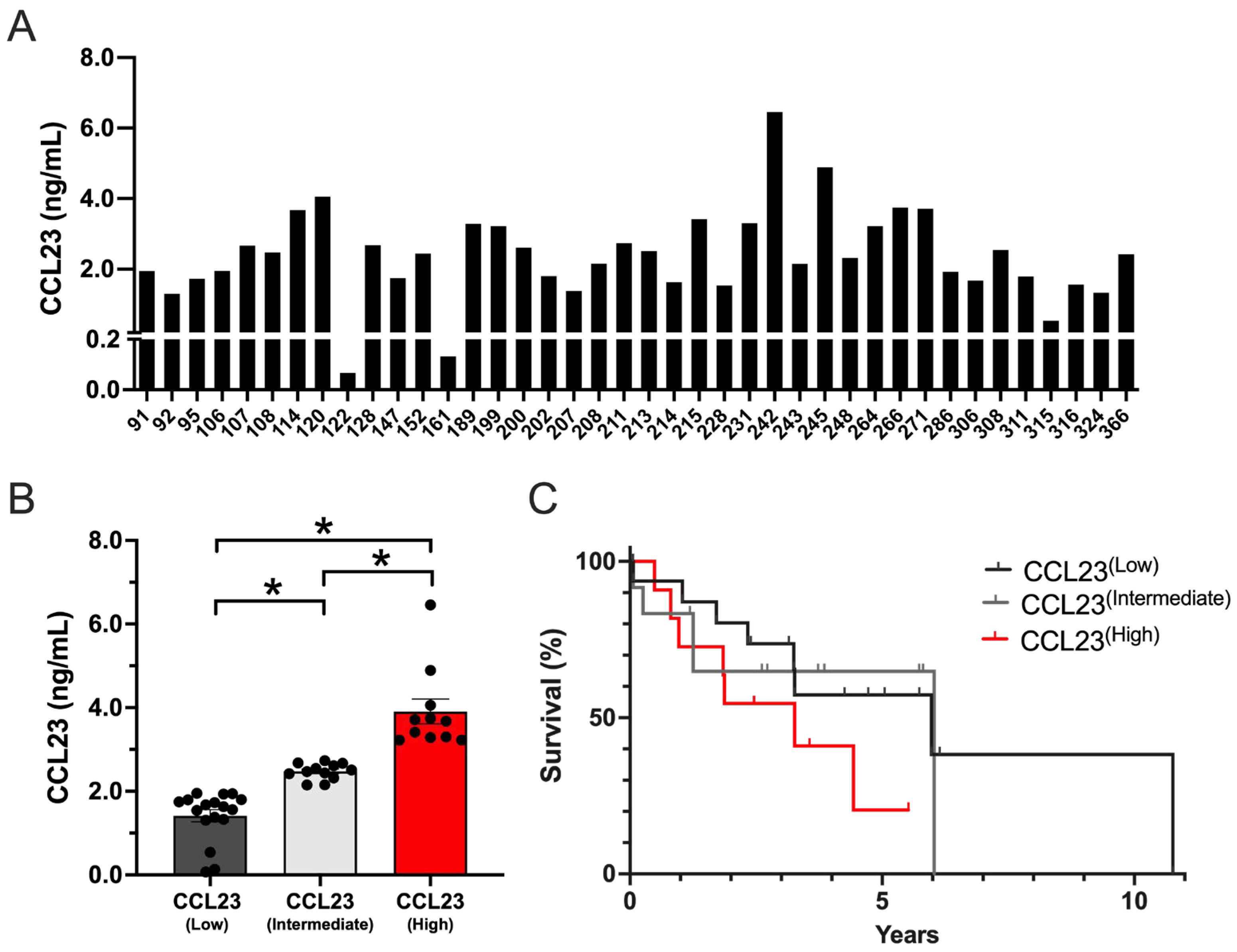
3.1. Ovarian Cancer Ascites with High CCL23 Concentrations Associated with Poor Patient Outcomes
3.2. CXCL10 and Soluble PD-1 Concentrations Are Reduced in Ovarian Ascites Samples Containing High Concentrations of CCL23
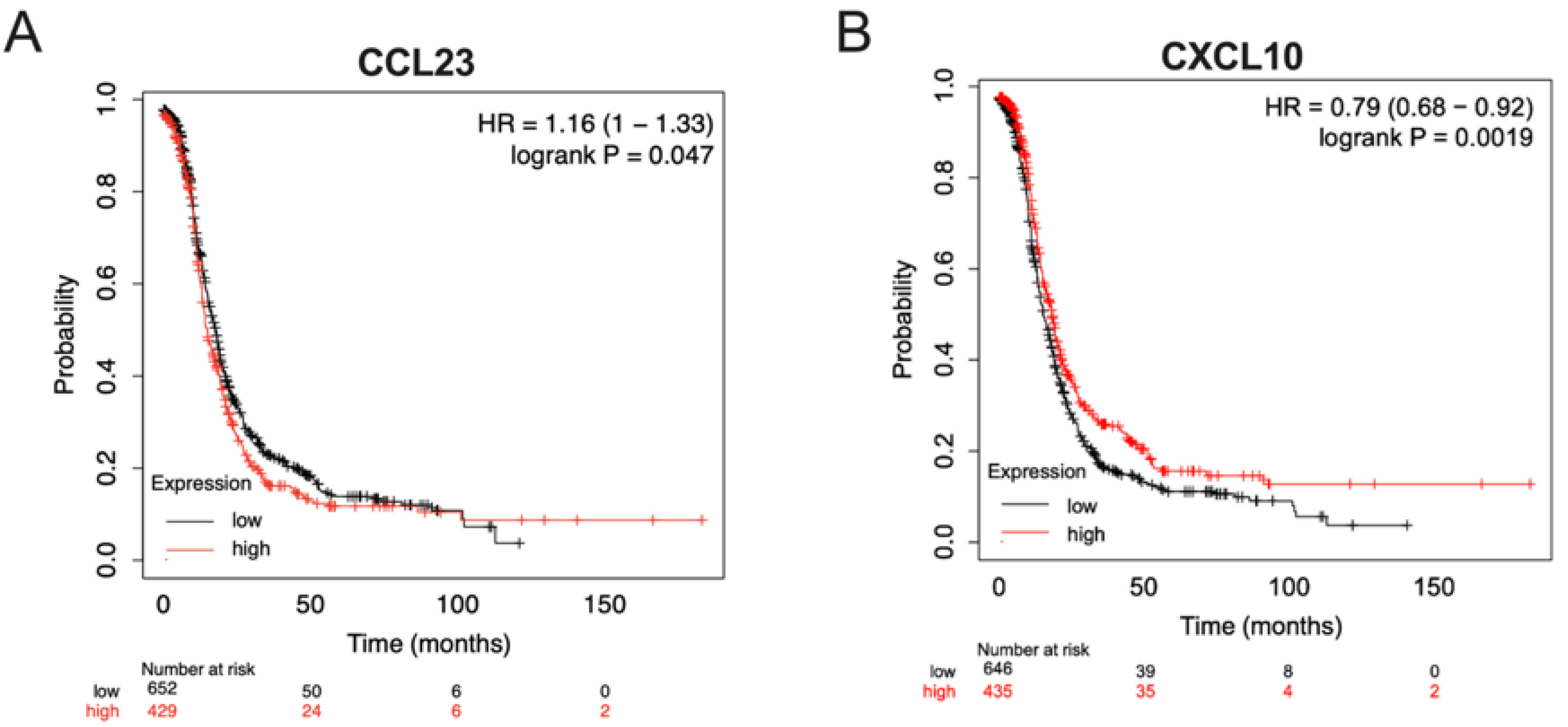
3.3. Ovarian Tumors with Low CCL23 or High CXCL10 Expression Correlate with Improved Patient Survival
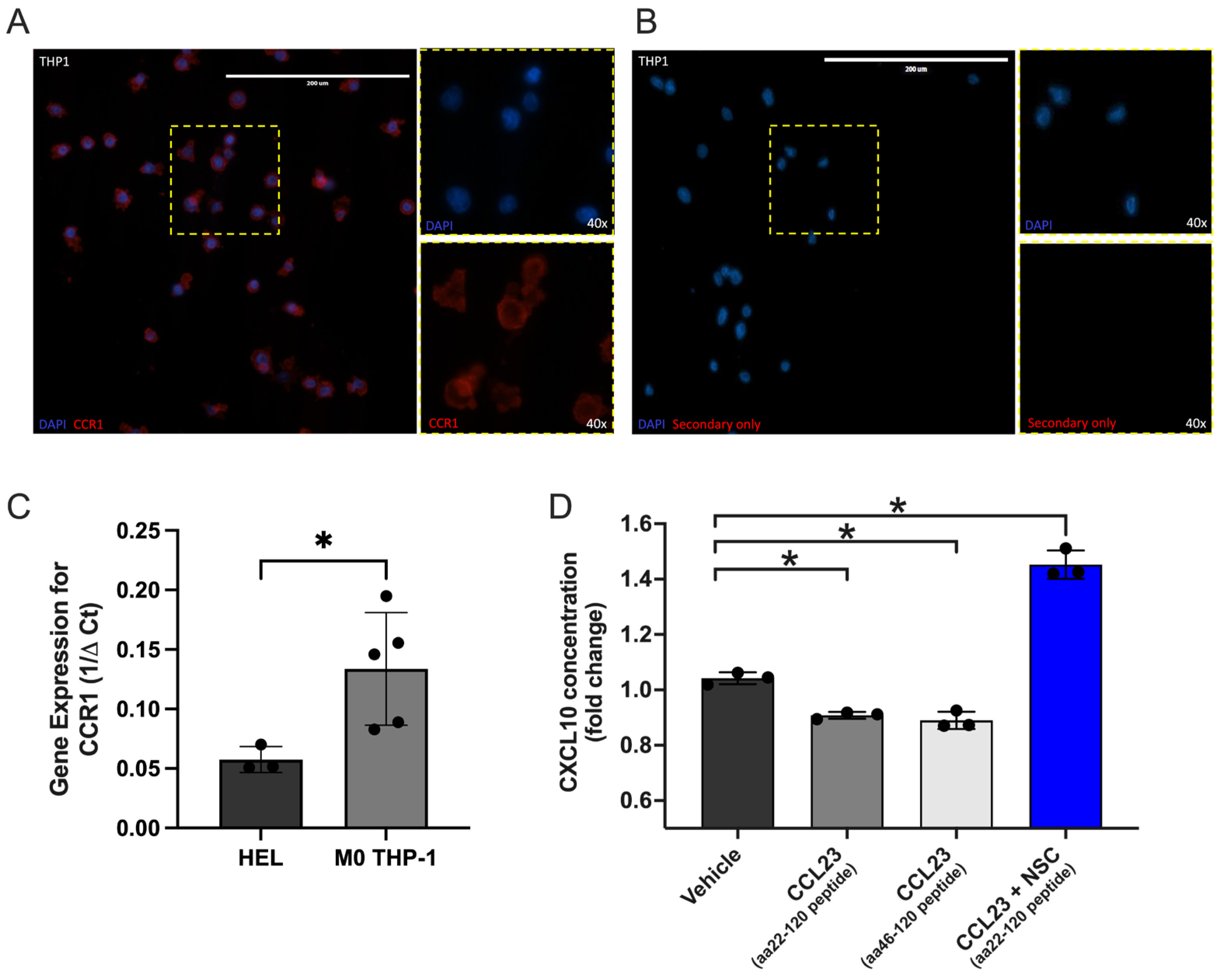
3.4. CCL23 Stimulation Reduces CXCL10 Secretion from Myeloid Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| BSA | Bovine Serum Albumin |
| CCR1 | CC Chemokine Receptor 1 |
| CCL23 | CC Chemokine Ligand 23 |
| CD8+ | Cluster of Differentiation 8 Positive |
| cDNA | Complementary Deoxyribonucleic Acid |
| CXCL10 | C-X-C Motif Chemokine Ligand 10 |
| CXCR3 | C-X-C Motif Chemokine Receptor 3 |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FBS | Fetal Bovine Serum |
| GSK3 | Glycogen Synthase Kinase 3 |
| GSK3β | Glycogen Synthase Kinase 3 Beta |
| HGF | Hepatocyte Growth Factor |
| HGS | High Grade Serous |
| HIMC | Human Immune Monitoring Center |
| HR | Hazard Ratio |
| ICC | Immunocytochemistry |
| IFN | Interferon |
| IRB | Institutional Review Board |
| KM | Kaplan–Meier |
| Luminex | Multiplexed Bead-Based Immunoassay System |
| mOS | Median Overall Survival |
| mRNA | Messenger Ribonucleic Acid |
| NACT | Neoadjuvant Chemotherapy |
| NCCN | National Comprehensive Cancer Network |
| NSC 74859 | STAT3 Pathway Inhibitor (Small Molecule Compound) |
| PBS | Phosphate-Buffered Saline |
| PCR | Polymerase Chain Reaction |
| PD-1 | Programmed Cell Death Protein 1 |
| PFA | Paraformaldehyde |
| PMA | Phorbol 12-Myristate-13-Acetate |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| RPM | Revolutions Per Minute |
| RPMI | Roswell Park Memorial Institute |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| S.E.M. | Standard Error of the Mean |
| TCGA | The Cancer Genome Atlas |
| TCR | T-Cell Receptor |
| THP-1 | Human Monocytic Cell Line |
| TME | Tumor Microenvironment |
| 18S | 18S Ribosomal RNA |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Cristea, M.; DeRosa, M.; Eisenhauer, E.L.; et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 191–226. [Google Scholar] [CrossRef]
- Borella, F.; Ghisoni, E.; Giannone, G.; Cosma, S.; Benedetto, C.; Valabrega, G.; Katsaros, D. Immune Checkpoint Inhibitors in Epithelial Ovarian Cancer: An Overview on Efficacy and Future Perspectives. Diagnostics 2020, 10, 146. [Google Scholar] [CrossRef]
- Leary, A.; Tan, D.; Ledermann, J. Immune checkpoint inhibitors in ovarian cancer: Where do we stand? Ther. Adv. Med. Oncol. 2021, 13, 17588359211039899. [Google Scholar] [CrossRef]
- Rodriguez, G.M.; Galpin, K.J.C.; McCloskey, C.W.; Vanderhyden, B.C. The Tumor Microenvironment of Epithelial Ovarian Cancer and Its Influence on Response to Immunotherapy. Cancers 2018, 10, 242. [Google Scholar] [CrossRef]
- Ahmed, N.; Stenvers, K.L. Getting to know ovarian cancer ascites: Opportunities for targeted therapy-based translational research. Front. Oncol. 2013, 3, 256. [Google Scholar] [CrossRef] [PubMed]
- Nasioudis, D.; Byrne, M.; Ko, E.M.; Haggerty, A.F.; Cory, L.; Giuntoli Ii, R.L.; Kim, S.H.; Latif, N.A. Ascites volume at the time of primary debulking and overall survival of patients with advanced epithelial ovarian cancer. Int. J. Gynecol. Cancer 2021, 31, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Zhou, S.; Liu, Y.; Yin, W.; Liao, Q.; Ren, S.; Zhang, F.; Meng, Y.; Mu, X. Relationship between ascites volume and clinical outcomes in epithelial ovarian cancer. J. Obstet. Gynaecol. Res. 2021, 47, 1527–1535. [Google Scholar] [CrossRef]
- Feigenberg, T.; Clarke, B.; Virtanen, C.; Plotkin, A.; Letarte, M.; Rosen, B.; Bernardini, M.Q.; Kollara, A.; Brown, T.J.; Murphy, K.J. Molecular profiling and clinical outcome of high-grade serous ovarian cancer presenting with low-versus high-volume ascites. BioMed. Res. Int. 2014, 2014, 367103. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.; Wicha, M.S.; Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 2017, 17, 559–572. [Google Scholar] [CrossRef]
- Korbecki, J.; Kojder, K.; Siminska, D.; Bohatyrewicz, R.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4. Int. J. Mol. Sci. 2020, 21, 8412. [Google Scholar] [CrossRef] [PubMed]
- Kamat, K.; Krishnan, V.; Dorigo, O. Macrophage-derived CCL23 Upregulates Expression of T-cell Exhaustion Markers in Ovarian Cancer. Br. J. Cancer 2022. in Press. [Google Scholar] [CrossRef]
- Hwang, J.; Son, K.N.; Kim, C.W.; Ko, J.; Na, D.S.; Kwon, B.S.; Gho, Y.S.; Kim, J. Human CC chemokine CCL23, a ligand for CCR1, induces endothelial cell migration and promotes angiogenesis. Cytokine 2005, 30, 254–263. [Google Scholar] [CrossRef]
- Nardelli, B.; Tiffany, H.L.; Bong, G.W.; Yourey, P.A.; Morahan, D.K.; Li, Y.; Murphy, P.M.; Alderson, R.F. Characterization of the signal transduction pathway activated in human monocytes and dendritic cells by MPIF-1, a specific ligand for CC chemokine receptor 1. J. Immunol. 1999, 162, 435–444. [Google Scholar] [CrossRef]
- Krishnan, V.; Tallapragada, S.; Schaar, B.; Kamat, K.; Chanana, A.M.; Zhang, Y.; Patel, S.; Parkash, V.; Rinker-Schaeffer, C.; Folkins, A.K.; et al. Omental macrophages secrete chemokine ligands that promote ovarian cancer colonization of the omentum via CCR1. Commun. Biol. 2020, 3, 524. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, K.; Zhang, S.; Guida, W.C.; Blaskovich, M.A.; Greedy, B.; Lawrence, H.R.; Yip, M.L.; Jove, R.; McLaughlin, M.M.; Lawrence, N.J.; et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc. Natl. Acad. Sci. USA 2007, 104, 7391–7396. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jove, R. The STATs of cancer--new molecular targets come of age. Nat. Rev. Cancer 2004, 4, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kortylewski, M.; Pardoll, D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007, 7, 41–51. [Google Scholar] [CrossRef]
- Gotthardt, D.; Putz, E.M.; Straka, E.; Kudweis, P.; Biaggio, M.; Poli, V.; Strobl, B.; Muller, M.; Sexl, V. Loss of STAT3 in murine NK cells enhances NK cell-dependent tumor surveillance. Blood 2014, 124, 2370–2379. [Google Scholar] [CrossRef]
- Yue, C.; Shen, S.; Deng, J.; Priceman, S.J.; Li, W.; Huang, A.; Yu, H. STAT3 in CD8+ T Cells Inhibits Their Tumor Accumulation by Downregulating CXCR3/CXCL10 Axis. Cancer Immunol. Res. 2015, 3, 864–870. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Rainczuk, A.; Rao, J.; Gathercole, J.; Stephens, A.N. The emerging role of CXC chemokines in epithelial ovarian cancer. Reproduction 2012, 144, 303–317. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Bronger, H.; Singer, J.; Windmuller, C.; Reuning, U.; Zech, D.; Delbridge, C.; Dorn, J.; Kiechle, M.; Schmalfeldt, B.; Schmitt, M.; et al. CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br. J. Cancer 2016, 115, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Kryczek, I.; Nagarsheth, N.; Zhao, L.; Wei, S.; Wang, W.; Sun, Y.; Zhao, E.; Vatan, L.; Szeliga, W.; et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015, 527, 249–253. [Google Scholar] [CrossRef]
- Beurel, E.; Jope, R.S. Differential regulation of STAT family members by glycogen synthase kinase-3. J. Biol. Chem. 2008, 283, 21934–21944. [Google Scholar] [CrossRef]
- Zhang, T.; Xiaohan, C. Unveiling the Role of JAK2/STAT3 signaling in chemoresistance of gynecological cancers: From mechanisms to therapeutic implications. Crit. Rev. Oncol. Hematol. 2025, 211, 104712. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.H.; Jia, Y.; Guo, F.J.; Chen, J.; Zhang, X.W.; Cui, M.H. Phosphorylation of STAT3 at Tyr705 regulates MMP-9 production in epithelial ovarian cancer. PLoS ONE 2017, 12, e0183622. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Hsieh, F.C.; Lieblein, J.C.; Brown, J.; Chan, C.; Wallace, J.A.; Cheng, G.; Hall, B.M.; Lin, J. Stat3 activation in human endometrial and cervical cancers. Br. J. Cancer 2007, 96, 591–599. [Google Scholar] [CrossRef]
- Vajavaara, H.; Mortensen, J.B.; Leivonen, S.K.; Hansen, I.M.; Ludvigsen, M.; Holte, H.; Jorgensen, J.; Bjerre, M.; d’Amore, F.; Leppa, S. Soluble PD-1 but Not PD-L1 Levels Predict Poor Outcome in Patients with High-Risk Diffuse Large B-Cell Lymphoma. Cancers 2021, 13, 398. [Google Scholar] [CrossRef]
- Dillman, R.O.; Nistor, G.I.; McLelland, B.T.; Hsieh, C.; Poole, A.J.; Cornforth, A.N.; Keirstead, H.S. Preliminary observations on soluble programmed cell death protein-1 as a prognostic and predictive biomarker in patients with metastatic melanoma treated with patient-specific autologous vaccines. Oncotarget 2019, 10, 5359–5371. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Chamoto, K.; Suzuki, S.; Kanemura, H.; Mitani, S.; Tanaka, K.; Kawakami, H.; Kishimoto, Y.; Haku, Y.; Ito, K.; et al. The combination of soluble forms of PD-1 and PD-L1 as a predictive marker of PD-1 blockade in patients with advanced cancers: A multicenter retrospective study. Front. Immunol. 2023, 14, 1325462. [Google Scholar] [CrossRef] [PubMed]
- Buderath, P.; Schwich, E.; Jensen, C.; Horn, P.A.; Kimmig, R.; Kasimir-Bauer, S.; Rebmann, V. Soluble Programmed Death Receptor Ligands sPD-L1 and sPD-L2 as Liquid Biopsy Markers for Prognosis and Platinum Response in Epithelial Ovarian Cancer. Front. Oncol. 2019, 9, 1015. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | No. | Percent (%) |
|---|---|---|
| Ovarian Cancer Patients | 40 | |
| Age (years) | ||
| Median | 59.6 | |
| Range | 33–90 | |
| Stage | ||
| IC | 2 | 5.0 |
| IIB | 1 | 2.5 |
| IIIB | 2 | 5.0 |
| IIIC | 19 | 47.5 |
| IV | 13 | 32.5 |
| Unknown | 3 | 7.5 |
| Sample Type | ||
| Primary | 33 | 82.5 |
| Recurrent | 7 | 17.5 |
| Sensitive | 3 | 42.9 |
| Resistant | 4 | 57.1 |
| Refractory | 0 | 0 |
| Histology | ||
| High Grade Serous | 32 | 80.0 |
| Low Grade Serous | 1 | 2.5 |
| Endometrioid | 1 | 2.5 |
| Clear cell | 1 | 2.5 |
| Carcinosarcoma | 1 | 2.5 |
| Rhabdomyosarcoma | 1 | 2.5 |
| Mesonephric-like adenocarcinoma | 1 | 2.5 |
| Adenocarcinoma NOS | 1 | 2.5 |
| Mixed | 1 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, S.M.; Tallapragada, S.; Chan, J.; Dorigo, O. Ovarian Cancer Ascites Enriched for CCL23 Reduces Macrophage-Derived CXCL10 Secretion and Is Associated with Poor Patient Outcomes. Cancers 2025, 17, 3925. https://doi.org/10.3390/cancers17243925
Lang SM, Tallapragada S, Chan J, Dorigo O. Ovarian Cancer Ascites Enriched for CCL23 Reduces Macrophage-Derived CXCL10 Secretion and Is Associated with Poor Patient Outcomes. Cancers. 2025; 17(24):3925. https://doi.org/10.3390/cancers17243925
Chicago/Turabian StyleLang, Susan M., Supreeti Tallapragada, Justine Chan, and Oliver Dorigo. 2025. "Ovarian Cancer Ascites Enriched for CCL23 Reduces Macrophage-Derived CXCL10 Secretion and Is Associated with Poor Patient Outcomes" Cancers 17, no. 24: 3925. https://doi.org/10.3390/cancers17243925
APA StyleLang, S. M., Tallapragada, S., Chan, J., & Dorigo, O. (2025). Ovarian Cancer Ascites Enriched for CCL23 Reduces Macrophage-Derived CXCL10 Secretion and Is Associated with Poor Patient Outcomes. Cancers, 17(24), 3925. https://doi.org/10.3390/cancers17243925





