Biomarker-Based Responder Selection and Early Prediction of Treatment Response in Hepatocellular Carcinoma: Dynamic Changes in Alpha-Fetoprotein and Des-Gamma-Carboxy Prothrombin During Atezolizumab Plus Bevacizumab Therapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment and Adverse Events
2.3. Radiological Assessment
2.4. Handling of Missing Data and Non-Evaluable (NE) Cases
2.5. Tumor Marker Measurement and Analysis
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Baseline Characteristics
3.2. Overall Treatment Outcomes
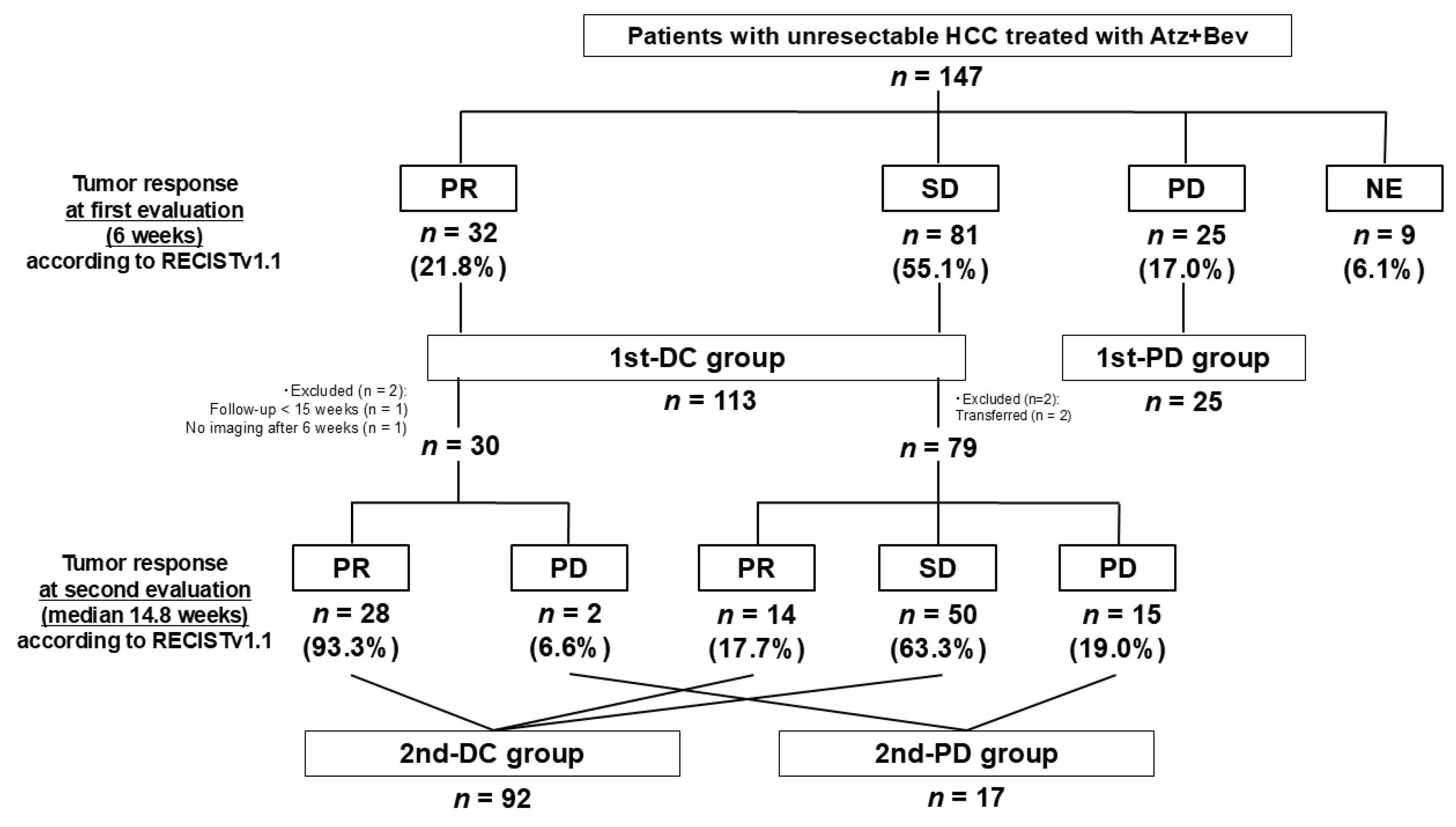
3.2.1. Tumor Response at First Evaluation (6 Weeks)
3.2.2. Tumor Response at Second Evaluation (Median 14.8 Weeks)
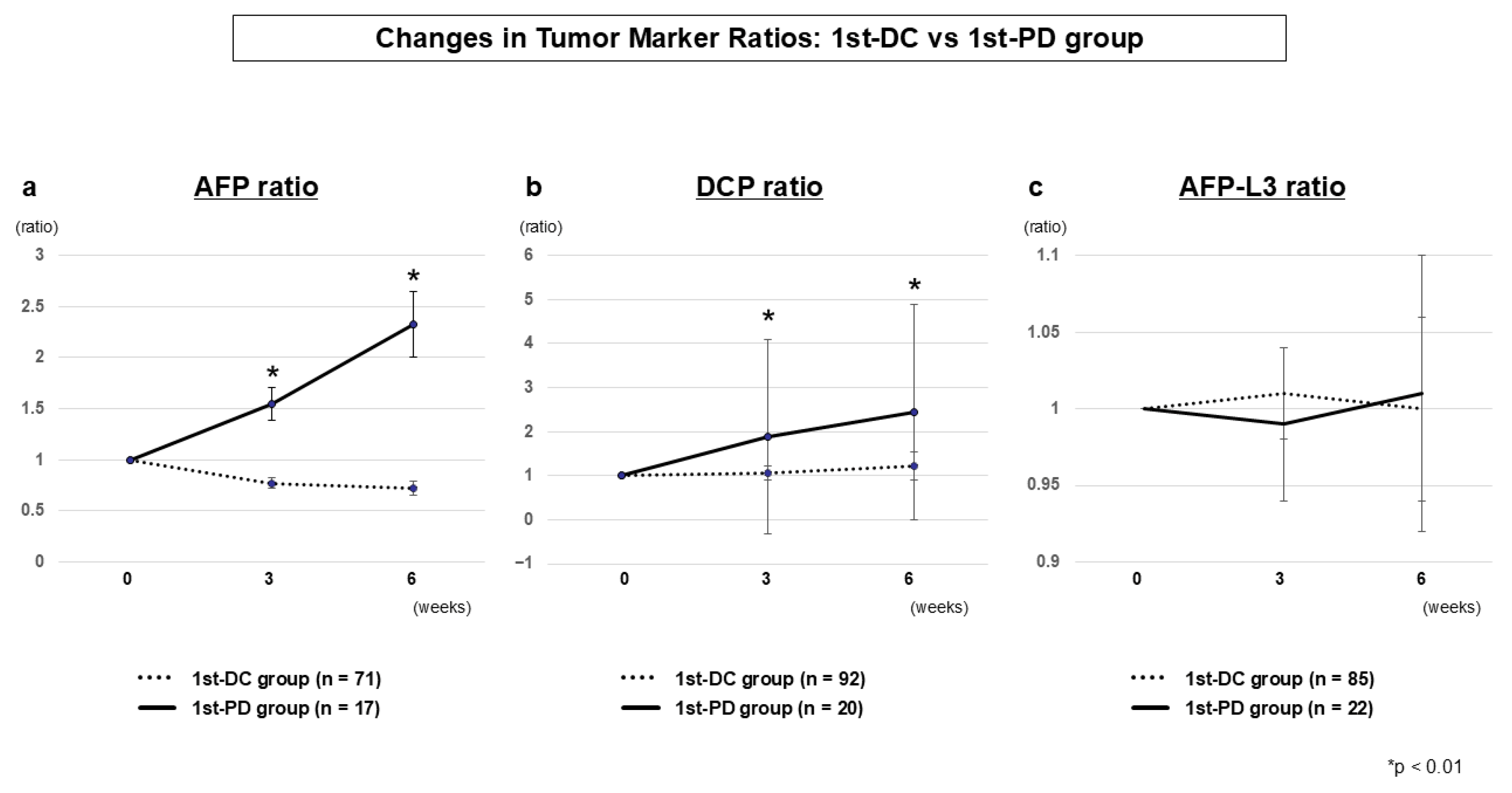
3.2.3. Tumor Marker Dynamics (1st-DC vs. 1st-PD)
3.2.4. Tumor Marker Dynamics (2nd-DC vs. 2nd-PD)
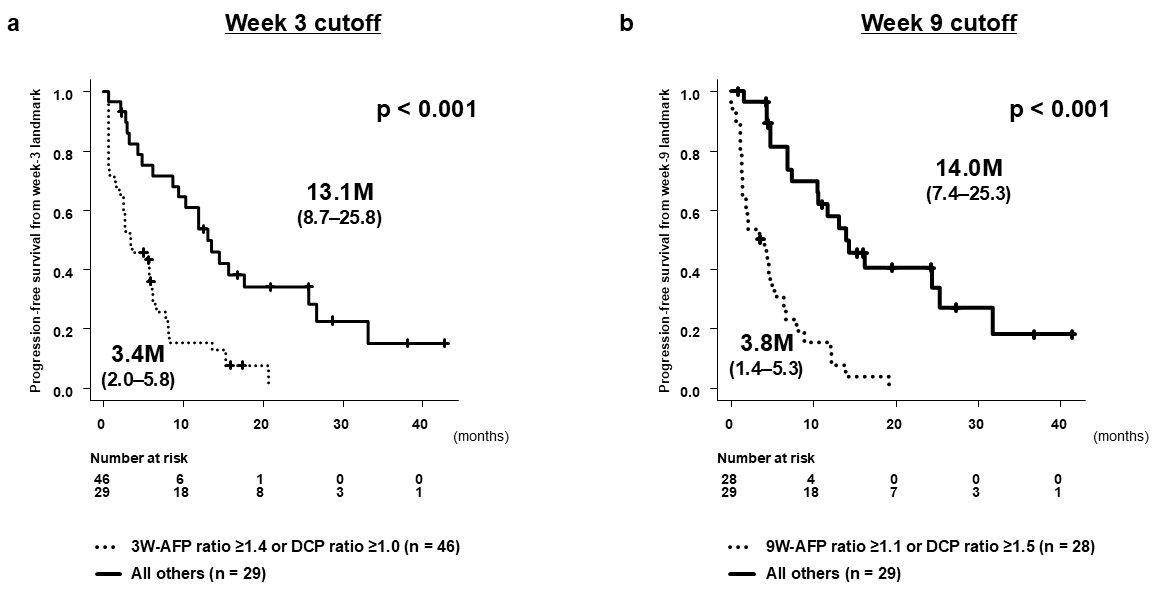
3.3. Predictive Performance of Tumor Marker Ratios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | alpha-fetoprotein |
| AFP-L3 | lens culinaris agglutinin-reactive alpha-fetoprotein |
| AE | adverse event |
| Atz + Bev | atezolizumab plus bevacizumab |
| BCLC | Barcelona Clinic Liver Cancer |
| CI | confidence interval |
| CR | complete response |
| DCP | des-gamma-carboxy prothrombin |
| DC | disease control |
| DCR | disease control rate |
| ECOG | Eastern Cooperative Oncology Group |
| FGFR | fibroblast growth factor receptor |
| HCC | hepatocellular carcinoma |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| ICI | immune checkpoint inhibitor |
| IMPACT | Immunotherapy and TACE Combination Trial (jRCTs051230037) |
| NE | not evaluable |
| ORR | overall response rate |
| OS | overall survival |
| PD | progressive disease |
| PFS | progression-free survival |
| PR | partial response |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| SD | stable disease |
| TACE | transarterial chemoembolization |
| TKI | tyrosine kinase inhibitor |
| VEGFR | vascular endothelial growth factor receptor |
References
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.K.; Van Cutsem, E.; Sangro, B.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2022, 386, 1302–1314. [Google Scholar] [CrossRef]
- Yau, T.; Galle, P.R.; Decaens, T.; Sangro, B.; Qin, S.; da Fonseca, L.G.; Karachiwala, H.; Blanc, J.F.; Park, J.W.; Gane, E.; et al. Nivolumab plus Ipilimumab versus Lenvatinib or Sorafenib as First-Line Treatment for Unresectable Hepatocellular Carcinoma (CheckMate 9DW): An Open-Label, Randomised, Phase 3 Trial. Lancet 2025, 405, 1851–1864. [Google Scholar] [CrossRef]
- Vogel, A.; Chan, S.L.; Dawson, L.A.; Kelley, R.K.; Llovet, J.M.; Meyer, T.; Ricke, J.; Rimassa, L.; Sapisochin, G.; Vilgrain, V.; et al. Hepatocellular Carcinoma: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2025, 36, 491–506. [Google Scholar] [CrossRef]
- Lau, G.; Obi, S.; Zhou, J.; Tateishi, R.; Qin, S.; Zhao, H.; Otsuka, M.; Ogasawara, S.; George, J.; Chow, P.K.H.; et al. APASL Clinical Practice Guidelines on Systemic Therapy for Hepatocellular Carcinoma—2024. Hepatol. Int. 2024, 18, 1661–1683. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on Prevention, Diagnosis, and Treatment of Hepatocellular Carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Treatment Decision-Making in Unresectable Hepatocellular Carcinoma: Importance of Understanding the Different Response Patterns between IO plus Anti-VEGF and IO plus IO Regimens. Liver Cancer 2025, 14, 119–126. [Google Scholar] [CrossRef]
- Akyildiz, A.; Yavuz, B.G.; Ismayilov, R.; Buyukaksoy, M.; Sozen, S.; Eid, J.R.; Avritscher, R.; Sunyoung, L.; Yalcin, S.; Kaseb, A.O. Comprehensive Review of Transarterial Chemoembolization Plus Systemic Therapy in Advanced Hepatocellular Carcinoma. J. Gastrointest. Cancer 2025, 56, 194. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Lee, Y.T.; Tseng, H.R.; Zhu, Y.; You, S.; Agopian, V.G.; Yang, J.D. Alpha-Fetoprotein: Past, Present, and Future. Hepatol. Commun. 2024, 8, e0422. [Google Scholar] [CrossRef]
- Yu, B.; Ma, W. Biomarker Discovery in Hepatocellular Carcinoma (HCC) for Personalized Treatment and Enhanced Prognosis. Cytokine Growth Factor Rev. 2024, 79, 29–38. [Google Scholar] [CrossRef]
- Kuzuya, T.; Kawabe, N.; Muto, H.; Wada, Y.; Komura, G.; Nakano, T.; Tanaka, H.; Nakaoka, K.; Ohno, E.; Funasaka, K.; et al. Early Changes in Alpha-Fetoprotein and Des-γ-Carboxy Prothrombin Are Useful Predictors of Antitumor Response to Durvalumab plus Tremelimumab Therapy for Advanced Hepatocellular Carcinoma. Curr. Oncol. 2024, 31, 4225–4240. [Google Scholar] [CrossRef]
- Kuzuya, T.; Kawabe, N.; Hashimoto, S.; Miyahara, R.; Sawaki, A.; Nakano, T.; Nakaoka, K.; Tanaka, H.; Miyachi, Y.; Mii, A.; et al. Early Changes in Alpha-Fetoprotein Are a Useful Predictor of Efficacy of Atezolizumab plus Bevacizumab Treatment in Patients with Advanced Hepatocellular Carcinoma. Oncology 2022, 100, 12–21. [Google Scholar] [CrossRef]
- Saeki, I.; Shimose, S.; Tomonari, T.; Ito, T.; Tani, J.; Takeuchi, Y.; Yoshioka, N.; Naito, T.; Takeuchi, M.; Kakizaki, S.; et al. Alpha-Fetoprotein and Des-Gamma-Carboxy Prothrombin Can Predict the Objective Response of Patients with Hepatocellular Carcinoma Receiving Durvalumab plus Tremelimumab Therapy. PLoS ONE 2024, 19, e0311084. [Google Scholar] [CrossRef]
- Tanabe, N.; Saeki, I.; Aibe, Y.; Matsuda, T.; Hanazono, T.; Nishi, M.; Hidaka, I.; Kuwashiro, S.; Shiratsuki, S.; Matsuura, K.; et al. Early Prediction of Response Focused on Tumor Markers in Atezolizumab plus Bevacizumab Therapy for Hepatocellular Carcinoma. Cancers 2023, 15, 2927. [Google Scholar] [CrossRef]
- Tamaki, N.; Tada, T.; Kurosaki, M.; Yasui, Y.; Ochi, H.; Mashiba, T.; Sakamoto, A.; Marusawa, H.; Narita, R.; Uchida, Y.; et al. Optimal Threshold of Alpha-Fetoprotein Response in Patients with Unresectable Hepatocellular Carcinoma Treated with Atezolizumab and Bevacizumab. Investig. New Drugs 2022, 40, 1290–1297. [Google Scholar] [CrossRef]
- Uchikawa, S.; Kawaoka, T.; Murakami, S.; Miura, R.; Shirane, Y.; Johira, Y.; Kosaka, M.; Fujii, Y.; Fujino, H.; Ono, A.; et al. Significance of Changes in Tumor Markers in Patients Treated with Durvalumab plus Tremelimumab Combination Therapy as a Surrogate Marker for Tumor Response to Unresectable Hepatocellular Carcinoma. Hepatol. Res. 2024; in press. [Google Scholar]
- National Institutes of Health; National Cancer Institute, U.S.A.; Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5 × 11.pdf (accessed on 20 June 2020).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. 64th General Assembly, Fortaleza, Brazil, October 2013. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 20 June 2025).
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and Hot Tumors: From Molecular Mechanisms to Targeted Therapy. Signal Transduct. Target Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Liang, J.; Liu, Y.; Hou, X.; Zhang, M.; Li, Y.; Jiang, X. Immunotherapy for Hepatocellular Carcinoma: Current Status and Future Prospects. Front. Immunol. 2021, 12, 765101. [Google Scholar] [CrossRef]
- Salani, F.; Genovesi, V.; Vivaldi, C.; Massa, V.; Cesario, S.; Bernardini, L.; Caccese, M.; Graziani, J.; Berra, D.; Fornaro, L.; et al. Primary Resistance to Immunotherapy-Based Regimens in First Line Hepatocellular Carcinoma: Perspectives on Jumping the Hurdle. Cancers 2022, 14, 4896. [Google Scholar] [CrossRef]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC Guidelines) 2019 Update. Hepatol. Res. 2019, 49, 1109–1113. [Google Scholar] [CrossRef]
- The Japan Society of Hepatology (JSH). Manual for the Diagnosis and Treatment of Hepatocellular Carcinoma, 5th ed.; The Japan Society of Hepatology: Tokyo, Japan, 2025; Chapter 5; p. 249. (In Japanese) [Google Scholar]
- Xie, Q.; Zhang, P.; Wang, Y.; Mei, W.; Zeng, C. Overcoming Resistance to Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Challenges and Opportunities. Front. Oncol. 2022, 12, 958720. [Google Scholar] [CrossRef]
- De Lorenzo, S.; Tovoli, F.; Trevisani, F. Mechanisms of Primary and Acquired Resistance to Immune Checkpoint Inhibitors in Patients with Hepatocellular Carcinoma. Cancers 2022, 14, 4616. [Google Scholar] [CrossRef]
- Vogel, A.; Saborowski, A.; Rimassa, L.; El-Khoueiry, A. Navigating Second-Line Therapy after Immunotherapy in Advanced HCC. JHEP Rep. 2025, 101630. [Google Scholar] [CrossRef]
- Kudo, M. Immune Checkpoint Inhibitor plus Anti-VEGF/TKI Combined with Transarterial Chemoembolization in Locally Advanced Nonmetastatic Hepatocellular Carcinoma: Real-World Treatment Strategy Based on Phase 3 Clinical Trial Results. Liver Cancer 2025, 14, 241–247. [Google Scholar] [CrossRef]
- Kudo, M.; Aoki, T.; Ueshima, K.; Tsuchiya, K.; Morita, M.; Chishina, H.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Achievement of Complete Response and Drug-Free Status by Atezolizumab plus Bevacizumab Combined with or without Curative Conversion in Patients with Transarterial Chemoembolization-Unsuitable, Intermediate-Stage Hepatocellular Carcinoma: A Multicenter Proof-of-Concept Study. Liver Cancer 2023, 12, 321–338. [Google Scholar] [CrossRef]
- Kuzuya, T.; Kawabe, N.; Muto, H.; Tachi, Y.; Ukai, T.; Wada, Y.; Komura, G.; Nakano, T.; Tanaka, H.; Nakaoka, K.; et al. Characteristics and Prognosis of Patients with Advanced Hepatocellular Carcinoma Treated with Atezolizumab/Bevacizumab Combination Therapy Who Achieved Complete Response. Curr. Oncol. 2024, 31, 6218–6231. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Tsuchiya, K.; Yamashita, T.; Shimose, S.; Numata, K.; Kodama, Y.; Itoh, S.; Tanaka, Y.; Kuroda, H.; et al. Primary Analysis of a Phase II Study of Atezolizumab plus Bevacizumab for TACE-Unsuitable Patients with Tumor Burden beyond Up-To-Seven Criteria in Intermediate-Stage Hepatocellular Carcinoma: REPLACEMENT Study. Liver Cancer, 2025; in press. [Google Scholar]
- Kodama, Y.; Ueshima, K.; Moriguchi, M.; Inaba, Y.; Yamashita, T.; Iwamoto, H.; Ueno, M.; Ogasawara, S.; Kuzuya, T.; Kodama, T.; et al. Protocol of the IMPACT Study: Randomized, Multicenter, Phase 3 Study Evaluating the Efficacy of Immunotherapy (Atezolizumab) plus Anti-VEGF Therapy (Bevacizumab) in Combination with Transcatheter Arterial Chemoembolization for Unresectable Hepatocellular Carcinoma. BMC Cancer 2025, 25, 434. [Google Scholar] [CrossRef]

| Characteristic | |
|---|---|
| Age (years), median (range) | 74 (38–90) |
| Sex (men/women), n | 119/28 |
| Etiology (HBV/HCV/non-viral), n | 18/29/100 |
| ECOG PS (0/1/2), n | 112/29/6 |
| Child–Pugh score (5/6/7), n | 97/37/13 |
| BCLC stage (A/B/C), n | 3/65/79 |
| AFP level (ng/mL), median (range) | 50.1 (1.8–2,037,310) |
| AFP level (<10/≥10 ng/mL), n | 51/96 |
| DCP level (mAU/mL), median (range) | 613 (10–403,328) |
| DCP level (<40/≥40 mAU/mL), n | 26/121 |
| AFP-L3 level (%), median (range) | 16.4 (<0.5–99.6) |
| AFP-L3 level (<0.5/≥0.5%), n | 29/117 |
| Treatment line of Atz + Bev (1st/2nd/3rd/4th), n | 106/38/2/1 |
| Observation period (months), median (range) | 14.5 (0.63–53.6) |
| Marker | 1st-DC Median ± SE (n) | 1st-PD Median ± SE (n) | p-Value |
|---|---|---|---|
| AFP ratio 3W | 0.77 ± 0.05 (n = 71) | 1.55 ± 0.16 (n = 17) | 0.0001 |
| AFP ratio 6W | 0.72 ± 0.07 (n = 71) | 2.33 ± 0.32 (n = 16) | <0.0001 |
| DCP ratio 3W | 1.05 ± 0.16 (n = 92) | 1.88 ± 2.21 (n = 20) | 0.0049 |
| DCP ratio 6W | 1.22 ± 0.32 (n = 91) | 2.44 ± 2.45 (n = 19) | 0.0022 |
| AFP-L3 ratio 3W | 1.01 ± 0.03 (n = 85) | 1.00 ± 0.06 (n = 22) | 0.4247 |
| AFP-L3 ratio 6W | 0.99 ± 0.05 (n = 84) | 1.01 ± 0.09 (n = 21) | 0.1743 |
| Marker | 2nd-DC Median ± SE (n) | 2nd-PD Median ± SE (n) | p-Value |
|---|---|---|---|
| AFP ratio 3W | 0.75 ± 0.05 (n = 52) | 0.99 ± 0.12 (n = 16) | 0.0192 |
| AFP ratio 6W | 0.72 ± 0.06 (n = 52) | 0.83 ± 0.21 (n = 16) | 0.0429 |
| AFP ratio 9W | 0.63 ± 0.07 (n = 52) | 1.12 ± 0.29 (n = 16) | 0.0008 |
| AFP ratio 12W | 0.61 ± 0.14 (n = 52) | 1.16 ± 0.51 (n = 16) | 0.0003 |
| AFP ratio 15W | 0.63 ± 0.10 (n = 48) | 1.76 ± 0.56 (n = 15) | 0.0002 |
| DCP ratio 3W | 0.92 ± 0.13 (n = 73) | 1.58 ± 0.48 (n = 16) | 0.0157 |
| DCP ratio 6W | 0.98 ± 0.14 (n = 73) | 2.02 ± 0.69 (n = 16) | 0.0178 |
| DCP ratio 9W | 0.68 ± 0.09 (n = 73) | 1.84 ± 0.54 (n = 16) | 0.0020 |
| DCP ratio 12W | 0.72 ± 0.13 (n = 71) | 1.75 ± 0.47 (n = 16) | 0.0019 |
| DCP ratio 15W | 0.72 ± 0.14 (n = 71) | 1.68 ± 0.46 (n = 15) | 0.0020 |
| AFP-L3 ratio 3W | 0.98 ± 0.02 (n = 66) | 1.03 ± 0.04 (n = 16) | 0.2123 |
| AFP-L3 ratio 6W | 0.99 ± 0.02 (n = 66) | 1.01 ± 0.04 (n = 16) | 0.2285 |
| AFP-L3 ratio 9W | 1.02 ± 0.02 (n = 66) | 1.08 ± 0.05 (n = 16) | 0.1448 |
| AFP-L3 ratio 12W | 1.00 ± 0.02 (n = 66) | 1.11 ± 0.06 (n = 16) | 0.0698 |
| AFP-L3 ratio 15W | 1.03 ± 0.02 (n = 63) | 1.18 ± 0.06 (n = 15) | 0.0790 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Factor | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| ECOG PS (0) | 0.755 (0.487–1.171) | 0.2100 | — | — |
| Child–Pugh score (5) | 0.729 (0.495–1.074) | 0.1097 | — | — |
| BCLC stage (A or B) | 0.896 (0.618–1.298) | 0.5613 | — | — |
| AFP ratio 3W (continuous) | 3.893 (2.428–6.242) | <0.0001 | 3.822 (2.198–6.644) | <0.0001 |
| DCP ratio 3W (continuous) | 1.065 (1.027–1.104) | 0.0006 | 1.019 (0.972–1.068) | 0.4294 |
| Treatment line (first line) | 0.694 (0.467–1.031) | 0.0708 | 0.890 (0.494–1.604) | 0.6976 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Factor | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| ECOG PS (0) | 0.875 (0.514–1.491) | 0.6232 | ||
| Child–Pugh score (5) | 0.712 (0.455–1.112) | 0.1354 | ||
| BCLC stage (A or B) | 1.208 (0.782–1.866) | 0.3934 | ||
| AFP ratio 9W (continuous) | 1.929 (1.481–2.512) | <0.0001 | 1.725 (1.251–2.378) | 0.0009 |
| DCP ratio 9W (continuous) | 1.155 (1.068–1.249) | 0.0003 | 1.140 (1.022–1.273) | 0.0188 |
| Treatment line (first line) | 0.888 (0.547–1.443) | 0.6315 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzuya, T.; Muto, H.; Tachi, Y.; Kobayashi, M.; Sugiyama, H.; Ariga, M.; Morisaki, S.; Komura, G.; Nakano, T.; Tanaka, H.; et al. Biomarker-Based Responder Selection and Early Prediction of Treatment Response in Hepatocellular Carcinoma: Dynamic Changes in Alpha-Fetoprotein and Des-Gamma-Carboxy Prothrombin During Atezolizumab Plus Bevacizumab Therapy. Cancers 2025, 17, 3891. https://doi.org/10.3390/cancers17243891
Kuzuya T, Muto H, Tachi Y, Kobayashi M, Sugiyama H, Ariga M, Morisaki S, Komura G, Nakano T, Tanaka H, et al. Biomarker-Based Responder Selection and Early Prediction of Treatment Response in Hepatocellular Carcinoma: Dynamic Changes in Alpha-Fetoprotein and Des-Gamma-Carboxy Prothrombin During Atezolizumab Plus Bevacizumab Therapy. Cancers. 2025; 17(24):3891. https://doi.org/10.3390/cancers17243891
Chicago/Turabian StyleKuzuya, Teiji, Hisanori Muto, Yoshihiko Tachi, Mariko Kobayashi, Hijiri Sugiyama, Mizuki Ariga, Sayaka Morisaki, Gakushi Komura, Takuji Nakano, Hiroyuki Tanaka, and et al. 2025. "Biomarker-Based Responder Selection and Early Prediction of Treatment Response in Hepatocellular Carcinoma: Dynamic Changes in Alpha-Fetoprotein and Des-Gamma-Carboxy Prothrombin During Atezolizumab Plus Bevacizumab Therapy" Cancers 17, no. 24: 3891. https://doi.org/10.3390/cancers17243891
APA StyleKuzuya, T., Muto, H., Tachi, Y., Kobayashi, M., Sugiyama, H., Ariga, M., Morisaki, S., Komura, G., Nakano, T., Tanaka, H., Nakaoka, K., Ohno, E., Funasaka, K., Nagasaka, M., Miyahara, R., & Hirooka, Y. (2025). Biomarker-Based Responder Selection and Early Prediction of Treatment Response in Hepatocellular Carcinoma: Dynamic Changes in Alpha-Fetoprotein and Des-Gamma-Carboxy Prothrombin During Atezolizumab Plus Bevacizumab Therapy. Cancers, 17(24), 3891. https://doi.org/10.3390/cancers17243891






