Monoclonal Gammopathy Prevalence in Newly Diagnosed Prostate Cancer Patients: A Correlative Perspective Observational Study
Simple Summary
Abstract
1. Introduction
1.1. Prostate Cancer (PCa)
1.2. Monoclonal Gammopathy of Undetermined Significance (MGUS)
2. Materials and Methods
2.1. Samples Collection
2.2. Staining Procedures
2.3. Statistical Analysis
3. Results
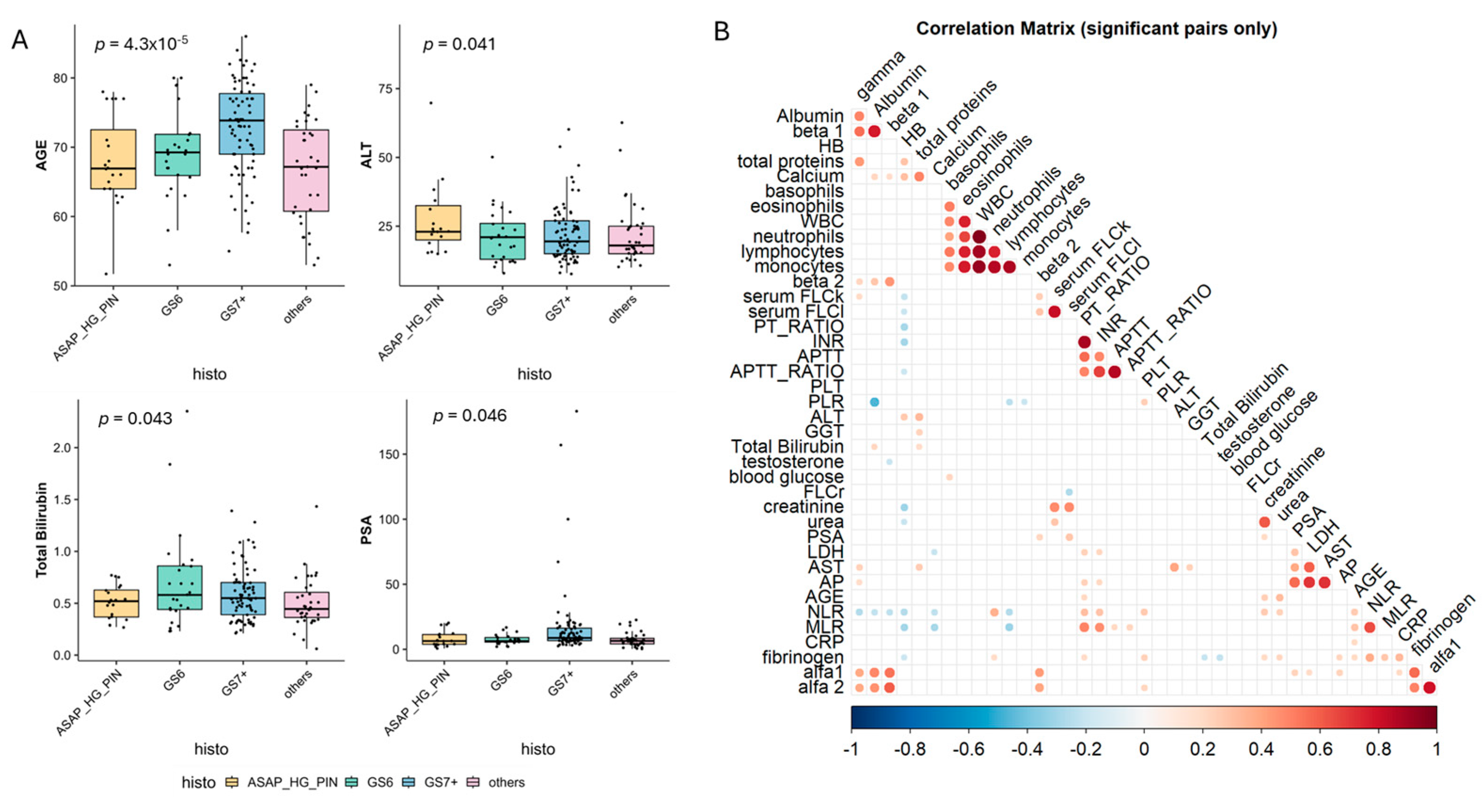
3.1. Patients’ Characteristics
3.2. Biochemical Predictors of MGUS
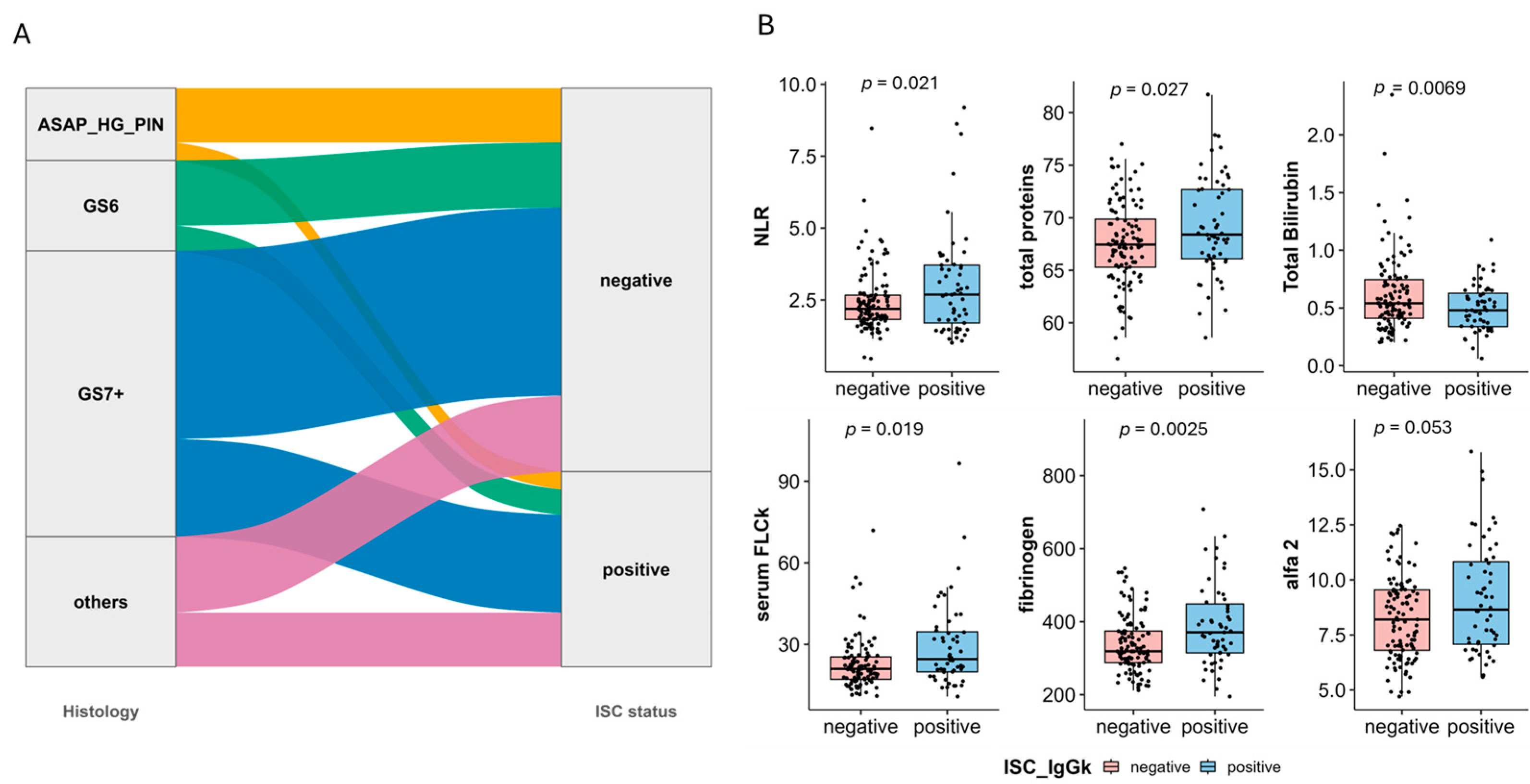
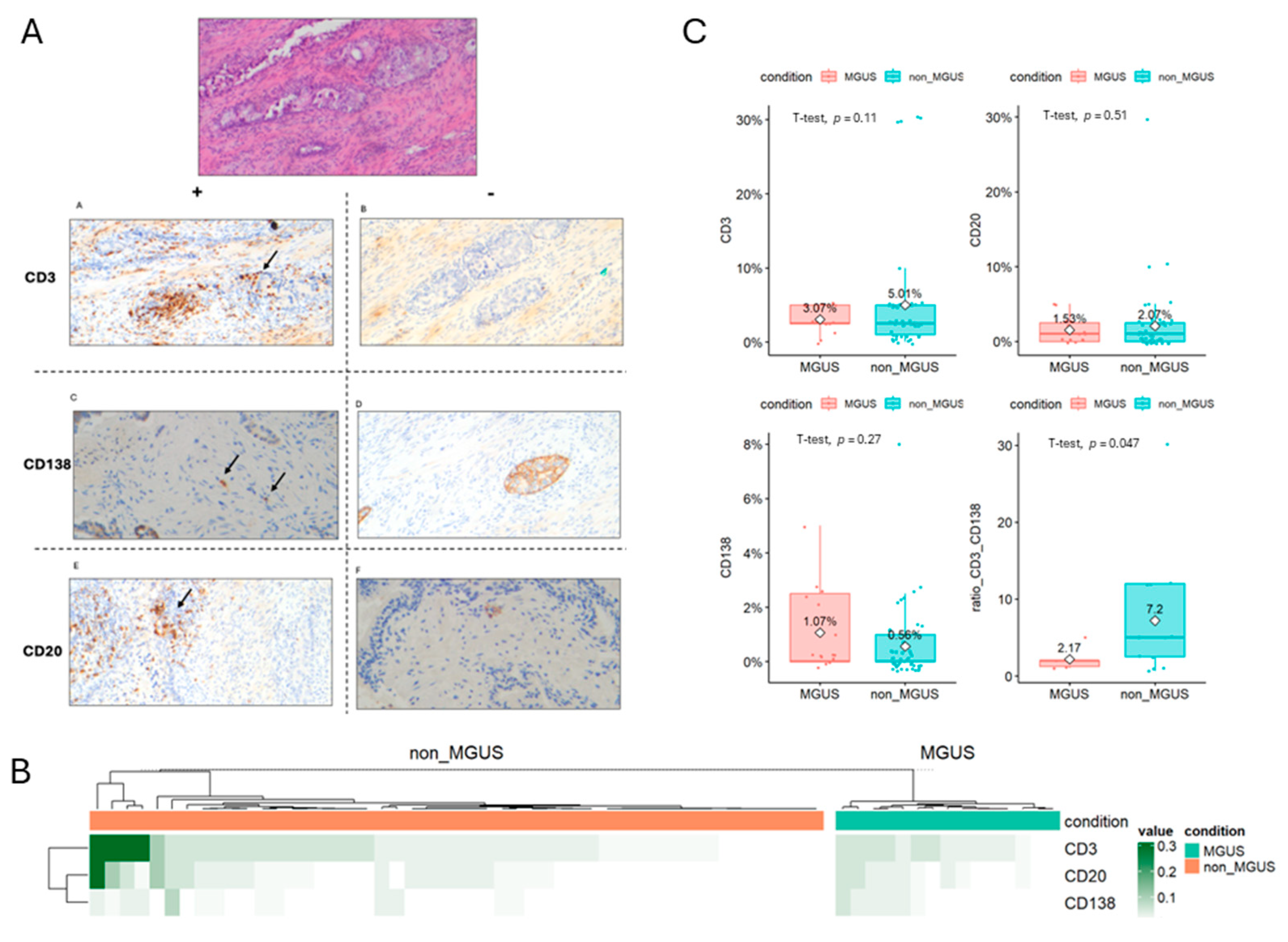
3.3. Prostate Microenvironment Associated with MGUS Incidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | ISC Negative | ISC Positive | p-Val |
|---|---|---|---|
| AGE | 69.84 (68.58–71.09) | 71.32 (69.17–73.48) | 0.24 |
| WBC | 7.50 (6.56–8.44) | 8.19 (7.47–8.90) | 0.25 |
| neutrophils | 4.62 (4.05–5.19) | 5.31 (4.69–5.92) | 0.11 |
| lymphocytes | 2.04 (1.76–2.33) | 2.01 (1.79–2.24) | 0.87 |
| monocytes | 0.61 (0.54–0.69) | 0.63 (0.58–0.67) | 0.71 |
| eosinophils | 0.26 (0.10–0.43) | 0.20 (0.16–0.24) | 0.46 |
| basophils | 0.04 (0.04–0.05) | 0.05 (0.04–0.06) | 0.32 |
| HB | 14.54 (14.28–14.80) | 14.31 (13.86–14.77) | 0.39 |
| PLT | 223.74 (212.19–235.28) | 248.51 (222.34–274.68) | 0.09 |
| creatinine | 1.01 (0.97–1.06) | 1.07 (0.96–1.18) | 0.33 |
| total proteins | 67.58 (66.83–68.32) | 69.21 (67.96–70.46) | 0.03 |
| blood glucose | 102.45 (97.54–107.37) | 107.20 (100.53–113.87) | 0.26 |
| urea | 42.26 (40.19–44.33) | 44.02 (39.43–48.61) | 0.49 |
| AST | 21.83 (19.61–24.05) | 23.33 (21.00–25.67) | 0.35 |
| ALT | 22.29 (20.04–24.54) | 23.54 (20.28–26.80) | 0.53 |
| AP | 96.84 (58.55–135.12) | 78.32 (70.58–86.06) | 0.35 |
| GGT | 30.17 (24.09–36.25) | 29.02 (22.82–35.22) | 0.79 |
| Calcium | 9.42 (9.23–9.60) | 9.48 (9.35–9.61) | 0.56 |
| LDH | 158.12 (149.54–166.69) | 160.15 (152.53–167.77) | 0.72 |
| CRP | 6.64 (1.26–12.03) | 8.28 (3.92–12.64) | 0.64 |
| Total Bilirubin | 0.61 (0.55–0.68) | 0.50 (0.45–0.55) | 0.01 |
| PSA | 13.07 (8.48–17.66) | 11.33 (7.66–15.00) | 0.56 |
| PSA RATIO % | 15.95 (14.16–17.74) | 17.32 (13.59–21.05) | 0.50 |
| serum FLCk | 23.61 (21.17–26.04) | 29.58 (25.22–33.93) | 0.02 |
| serum FLCl | 18.99 (16.49–21.50) | 23.40 (16.80–30.00) | 0.21 |
| FLCr | 1.27 (1.21–1.32) | 1.39 (1.24–1.53) | 0.13 |
| Albumin | 51.16 (48.92–53.40) | 48.63 (45.95–51.30) | 0.15 |
| alfa1 | 3.38 (3.24–3.52) | 3.67 (3.41–3.94) | 0.06 |
| alfa 2 | 8.35 (7.97–8.73) | 9.11 (8.44–9.79) | 0.05 |
| beta 1 | 5.33 (5.08–5.58) | 5.42 (5.02–5.82) | 0.71 |
| beta 2 | 4.21 (4.04–4.38) | 4.17 (3.87–4.47) | 0.81 |
| gamma | 11.61 (11.05–12.17) | 12.40 (11.37–13.43) | 0.18 |
| testosterone | 5.22 (4.79–5.66) | 4.73 (4.11–5.35) | 0.19 |
| BMI | 26.96 (25.83–28.08) | 25.96 (23.91–28.01) | 0.38 |
| PT_RATIO | 0.98 (0.95–1.00) | 1.01 (0.96–1.06) | 0.22 |
| INR | 0.98 (0.95–1.00) | 0.99 (0.93–1.05) | 0.57 |
| APTT | 31.42 (30.81–32.03) | 31.65 (30.02–33.29) | 0.79 |
| APTT_RATIO | 1.05 (1.03–1.07) | 1.04 (0.98–1.11) | 0.80 |
| fibrinogen | 338.28 (323.86–352.70) | 397.76 (362.51–433.02) | 0.00 |
| NLR | 2.44 (2.24–2.65) | 3.12 (2.58–3.65) | 0.02 |
| MLR | 0.32 (0.30–0.35) | 0.36 (0.31–0.41) | 0.14 |
| PLR | 165.18 (105.41–224.94) | 151.14 (121.37–180.90) | 0.68 |
| A_G_ratio | 1.05 (1.00–1.10) | 1.10 (1.00–1.19) | 0.37 |
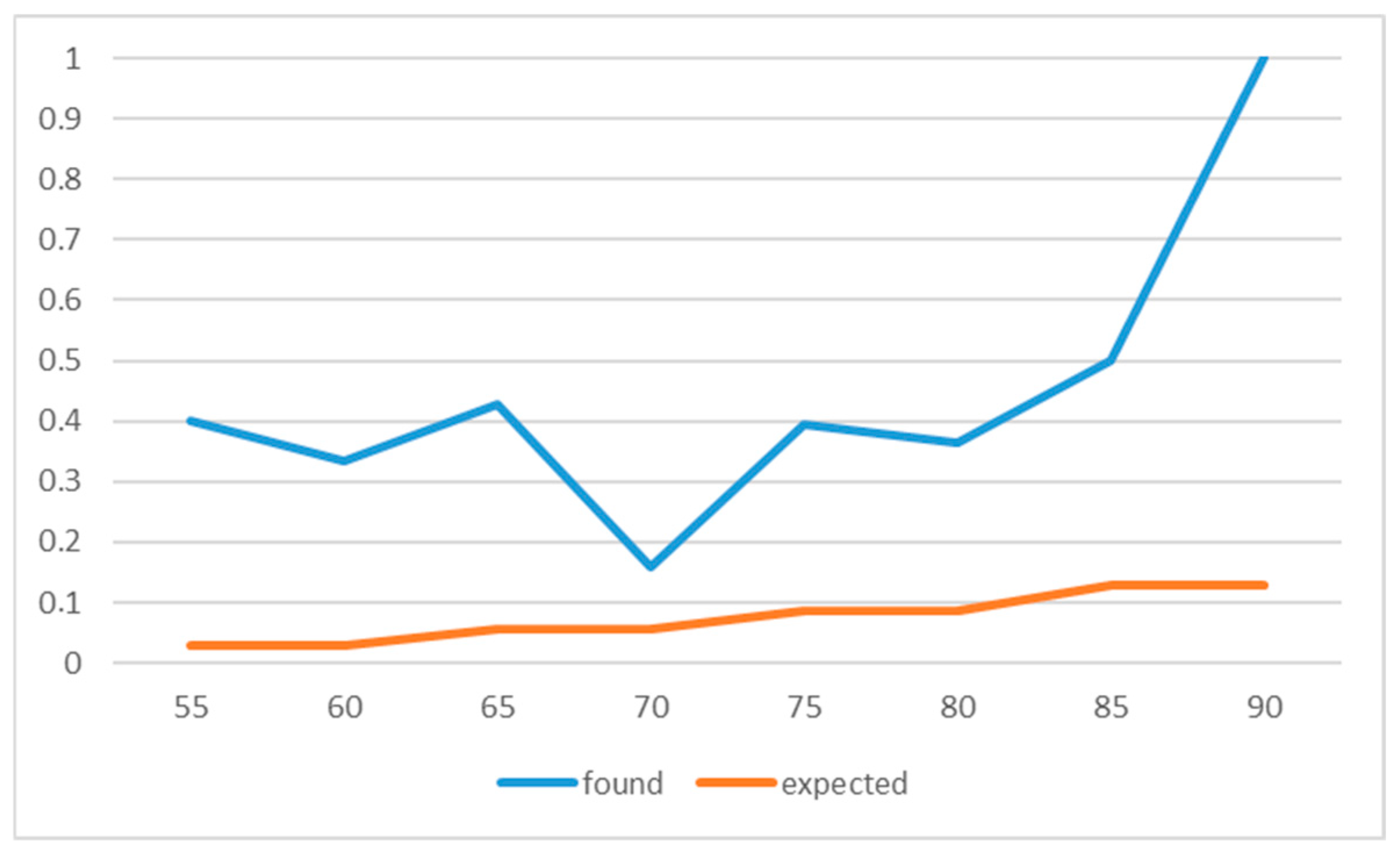
| Age_Band | n | MGUS | Expected Prev | Expected | Obs Prev |
|---|---|---|---|---|---|
| 50–59 | 12 | 4 | 0.017 | 0.2 | 0.3333333333333333 |
| 60–69 | 38 | 8 | 0.03 | 1.14 | 0.21052631578947367 |
| 70–79 | 54 | 14 | 0.05 | 2.7 | 0.25925925925925924 |
| 80+ | 14 | 9 | 0.075 | 1.05 | 0.6428571428571429 |
| Variable | Variable | MGUS Yes | MGUS No | Prevalence in Exposed % | p Value |
|---|---|---|---|---|---|
| Prior_malig | prior_malig | 0 | 0 | nan | 1.0 |
| Diabetes | dm | 0 | 0 | nan | 1.0 |
| High blood pressure | htn | 1 | 5 | 16.7 | 0.6696 |
| Cardio vascular desease | cvd | 3 | 4 | 42.9 | 0.4332 |
| Chronic Obstructive Pulmonary Disease | copd | 2 | 1 | 66.7 | 0.2174 |
| Liver pathology | liver | 0 | 0 | nan | 1.0 |
| Renal desease | ckd | 1 | 0 | 100.0 | 0.3022 |
References
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Rohrmann, S. Risk factors for the onset of prostatic cancer: Age, location, and behavioral correlates. Clin. Epidemiol. 2012, 4, 1–11. [Google Scholar] [CrossRef]
- Hemminki, K. Familial risk and familial survival in prostate cancer. World J. Urol. 2011, 30, 143–148. [Google Scholar] [CrossRef]
- Jansson, K.F.; Akre, O.; Garmo, H.; Bill-Axelson, A.; Adolfsson, J.; Stattin, P.; Bratt, O. Concordance of Tumor Differentiation Among Brothers with Prostate Cancer. Eur. Urol. 2012, 62, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Mahal, B.A.; Gerke, T.; Awasthi, S.; Soule, H.R.; Simons, J.W.; Miyahira, A.; Halabi, S.; George, D.; Platz, E.A.; Mucci, L.; et al. Prostate Cancer Racial Disparities: A Systematic Review by the Prostate Cancer Foundation Panel. Eur. Urol. Oncol. 2022, 5, 18–29. [Google Scholar] [CrossRef]
- Randazzo, M.; Müller, A.; Carlsson, S.; Eberli, D.; Huber, A.; Grobholz, R.; Manka, L.; Mortezavi, A.; Sulser, T.; Recker, F.; et al. A positive family history as a risk factor for prostate cancer in a population-based study with organised prostate-specific antigen screening: Results of the Swiss European Randomised Study of Screening for Prostate Cancer (ERSPC, Aarau). BJU Int. 2015, 117, 576–583. [Google Scholar] [CrossRef]
- Al Olama, A.A.; Dadaev, T.; Hazelett, D.J.; Li, Q.; Leongamornlert, D.; Saunders, E.J.; Stephens, S.; Cieza-Borrella, C.; Whitmore, I.; Garcia, S.B.; et al. Multiple novel prostate cancer susceptibility signals identified by fine-mapping of known risk loci among Europeans. Hum. Mol. Genet. 2015, 24, 5589–5602. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Cheng, H.H.; Lange, P.H.; Nelson, P.S.; Etzioni, R. Screening Men at Increased Risk for Prostate Cancer Diagnosis: Model Estimates of Benefits and Harms. Cancer Epidemiol. Biomark. Prev. 2017, 26, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Giri, V.N.; Hegarty, S.E.; Hyatt, C.; O’Leary, E.; Garcia, J.; Knudsen, K.E.; Kelly, W.K.; Gomella, L.G. Germline genetic testing for inherited prostate cancer in practice: Implications for genetic testing, precision therapy, and cascade testing. Prostate 2018, 79, 333–339. [Google Scholar] [CrossRef]
- Davies, N.M.; Gaunt, T.R.; Lewis, S.J.; Holly, J.; Donovan, J.L.; Hamdy, F.C.; Kemp, J.P.; Eeles, R.; Easton, D.; Kote-Jarai, Z.; et al. The effects of height and BMI on prostate cancer incidence and mortality: A Mendelian randomization study in 20,848 cases and 20,214 controls from the PRACTICAL consortium. Cancer Causes Control. 2015, 26, 1603–1616. [Google Scholar] [CrossRef]
- Rivera-Izquierdo, M.; de Rojas, J.P.; Martínez-Ruiz, V.; Pérez-Gómez, B.; Sánchez, M.-J.; Khan, K.S.; Jiménez-Moleón, J.J. Obesity as a Risk Factor for Prostate Cancer Mortality: A Systematic Review and Dose-Response Meta-Analysis of 280,199 Patients. Cancers 2021, 13, 4169. [Google Scholar] [CrossRef]
- Brookman-May, S.D.; Campi, R.; Henríquez, J.D.; Klatte, T.; Langenhuijsen, J.F.; Brausi, M.; Linares-Espinós, E.; Volpe, A.; Marszalek, M.; Akdogan, B.; et al. Latest Evidence on the Impact of Smoking, Sports, and Sexual Activity as Modifiable Lifestyle Risk Factors for Prostate Cancer Incidence, Recurrence, and Progression: A Systematic Review of the Literature by the European Association of Urology Section of Oncological Urology (ESOU). Eur. Urol. Focus 2019, 5, 756–787. [Google Scholar] [CrossRef]
- Pabalan, N.; Singian, E.; Jarjanazi, H.; Paganini-Hill, A. Association of male circumcision with risk of prostate cancer: A meta-analysis. Prostate Cancer Prostatic Dis. 2015, 18, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Kaseb, H.; Annamaraju, P. Monoclonal Gammopathy of Undetermined Significance. In StatPearls; Babiker, H.M., Ed.; StatPearls: Petersburg, FL, USA, 2022. [Google Scholar]
- Liu, Y.; Parks, A.L. Diagnosis and Management of Monoclonal Gammopathy of Undetermined Significance. JAMA Intern. Med. 2025, 185, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Zanwar, S.; Rajkumar, S.V. Current risk stratification and staging of multiple myeloma and related clonal plasma cell disorders. Leukemia 2025, 39, 2610–2617. [Google Scholar] [CrossRef]
- Kaur, J.; Valisekka, S.S.; Hameed, M.; Bandi, P.S.; Varma, S.; Onwughalu, C.J.; Ibrahim, H.; Mongia, H. Monoclonal Gammopathy of Undetermined Significance: A Comprehensive Review. Clin. Lymphoma Myeloma Leuk. 2023, 23, e195–e212. [Google Scholar] [CrossRef] [PubMed]
- Rögnvaldsson, S.; Thorsteinsdóttir, S.; Syriopoulou, E.; Sverrisdottir, I.; Turesson, I.; Eythorsson, E.; Oskarsson, J.T.; Long, T.E.; Vidarsson, B.; Onundarson, P.T.; et al. Prior cancer and risk of monoclonal gammopathy of undetermined significance: A population-based study in Iceland and Sweden. Haematologica 2024, 109, 2250–2255. [Google Scholar] [CrossRef]
- Flippo, K.; Mahmud, S.; Tribolet, G.M.; Lisi, M.; Hudson, K.; Brisbin, L.; Paulson, R.S.; Mamo, S.; Shelley, B. Increased Incidence of Non-Hematologic Malignancy in Patients with Monoclonal Gammopathy of Undetermined Significance. Blood 2024, 144 (Suppl. S1), 6884. [Google Scholar] [CrossRef]
- de Falco, R.; Togo, G.; Minopoli, A.; Cuomo, M.; Rea, D.; Meola, S.; Cavalcanti, E. Prevalence and distribution of M-proteins in the oncologic population affected by solid tumor. Blood Cancer J. 2024, 14, 121. [Google Scholar] [CrossRef]
- Kyle, R.A. Monoclonal gammopathy of undetermined significance. Am. J. Med. 1978, 64, 814–826. [Google Scholar] [CrossRef]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Larson, D.R.; Plevak, M.F.; Melton, L.J. Long-term Follow-up of 241 Patients With Monoclonal Gammopathy of Undetermined Significance: The Original Mayo Clinic Series 25 Years Later. Mayo Clin. Proc. 2004, 79, 859–866. [Google Scholar] [CrossRef]
- Rögnvaldsson, S.; Love, T.J.; Thorsteinsdottir, S.; Reed, E.R.; Óskarsson, J.Þ.; Pétursdóttir, Í.; Sigurðardóttir, G.Á.; Viðarsson, B.; Önundarson, P.T.; Agnarsson, B.A.; et al. Iceland screens, treats, or prevents multiple myeloma (iStopMM): A population-based screening study for monoclonal gammopathy of undetermined significance and randomized controlled trial of follow-up strategies. Blood Cancer J. 2021, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Jehangir, W.; Tulpule, S.; Sanabria, F.; Bhatt, H.; Zafar, S.; Osman, M.; Enakuaa, S.; Yousif, A. Prostate cancer leading to monoclonal gammopathy of undetermined significance: A case report. Mol. Clin. Oncol. 2018, 9, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Tulone, G.; Giannone, S.; Mannone, P.; Tognarelli, A.; Di Vico, T.; Giaimo, R.; Zucchi, A.; Rossanese, M.; Abrate, A.; Pavan, N.; et al. Comparison of Fluoroquinolones and Other Antibiotic Prophylaxis Regimens for Preventing Complications in Patients Undergoing Transrectal Prostate Biopsy. Antibiotics 2022, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, M.; Hara, T.; Fukasawa, R.; Koiso, K. Prostatic cancer presenting monoclonal gammopathy: Report of two cases. Acta Urol. Jpn. 1993, 39, 569–572. [Google Scholar]
- Pramanik, S.; Gazi, J.; Das, A.K.; Debnath, N.B.; Pal, S.K. Monoclonal gammopathy in prostate carcinoma: A case report and review of literature. J. Med. Case Rep. 2018, 12, 325. [Google Scholar] [CrossRef]
- Botta, C.; Di Martino, M.T.; Ciliberto, D.; Cucè, M.; Correale, P.; Rossi, M.; Tagliaferri, P.; Tassone, P. A gene expression inflammatory signature specifically predicts multiple myeloma evolution and patients survival. Blood Cancer J. 2016, 6, e511. [Google Scholar] [CrossRef]
- Plano, F.; Gigliotta, E.; Corsale, A.M.; Azgomi, M.S.; Santonocito, C.; Ingrascì, M.; Di Carlo, L.; Augello, A.E.; Speciale, M.; Vullo, C.; et al. Ferritin Metabolism Reflects Multiple Myeloma Microenvironment and Predicts Patient Outcome. Int. J. Mol. Sci. 2023, 24, 8852. [Google Scholar] [CrossRef]
- Desantis, V.; Andriano, A.; Düking, T.; Hartwig, O.; Ingravallo, G.; Biondo, M.; Botta, C.; Ria, R.; Vacca, A.; Solimando, A.G. Spatial imaging unlocks the potential of charting multiple myeloma and extramedullary disease. J. Hematol. Oncol. 2025, 18, 47. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.S.; Han, S.R.; Kang, Y.H.; Mun, J.Y.; Shin, D.W.; Oh, H.-W.; Cho, Y.-K.; Lee, M.-S.; Park, J. Alpha-2-macroglobulin as a novel diagnostic biomarker for human bladder cancer in urinary extracellular vesicles. Front. Oncol. 2022, 12, 976407. [Google Scholar] [CrossRef] [PubMed]
- Facchiano, F.; D’aRcangelo, D.; Facchiano, A. Alpha-2-Macroglobulin Is a Novel Anticancer Agent. Oncology 2023, 102, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance. Br. J. Haematol. 2006, 134, 573–589. [CrossRef] [PubMed]
| Patients Characteristics | |
|---|---|
| Histological report | ASAP/HG PIN: 20 GS6: 25 GS7+: 79 Others/no cancer: 36 Missing: 8 |
| MGUS | yes: 57 no: 111 |
| MGUS patients characteristics | |
| Isotype | IgG κ: 44 IgG λ: 7 IgM κ: 1 IgM λ: 1 LC κ: 2 LC λ: 2 |
| Risk class | Low Risk: 51 Int-low Risk: 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tulone, G.; Pavan, N.; Giaimo, R.; Martorana, A.; Salvaggio, G.; Cutaia, G.; Claps, F.; Gigliotta, E.; Marmo, D.; Minasola, C.; et al. Monoclonal Gammopathy Prevalence in Newly Diagnosed Prostate Cancer Patients: A Correlative Perspective Observational Study. Cancers 2025, 17, 3790. https://doi.org/10.3390/cancers17233790
Tulone G, Pavan N, Giaimo R, Martorana A, Salvaggio G, Cutaia G, Claps F, Gigliotta E, Marmo D, Minasola C, et al. Monoclonal Gammopathy Prevalence in Newly Diagnosed Prostate Cancer Patients: A Correlative Perspective Observational Study. Cancers. 2025; 17(23):3790. https://doi.org/10.3390/cancers17233790
Chicago/Turabian StyleTulone, Gabriele, Nicola Pavan, Rosa Giaimo, Anna Martorana, Giuseppe Salvaggio, Giuseppe Cutaia, Francesco Claps, Emilia Gigliotta, Dalila Marmo, Cristina Minasola, and et al. 2025. "Monoclonal Gammopathy Prevalence in Newly Diagnosed Prostate Cancer Patients: A Correlative Perspective Observational Study" Cancers 17, no. 23: 3790. https://doi.org/10.3390/cancers17233790
APA StyleTulone, G., Pavan, N., Giaimo, R., Martorana, A., Salvaggio, G., Cutaia, G., Claps, F., Gigliotta, E., Marmo, D., Minasola, C., Siragusa, S., Simonato, A., & Botta, C. (2025). Monoclonal Gammopathy Prevalence in Newly Diagnosed Prostate Cancer Patients: A Correlative Perspective Observational Study. Cancers, 17(23), 3790. https://doi.org/10.3390/cancers17233790








