Efficacy of First-Line Nivolumab Plus Chemotherapy in Advanced Gastric Cancer Stratified by PD-L1 Expression: A Real-World Comparison
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Biomarker and Genomic Analysis
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Survival Outcomes According to PD-L1 Expression Status
3.3. Subgroup for PFS
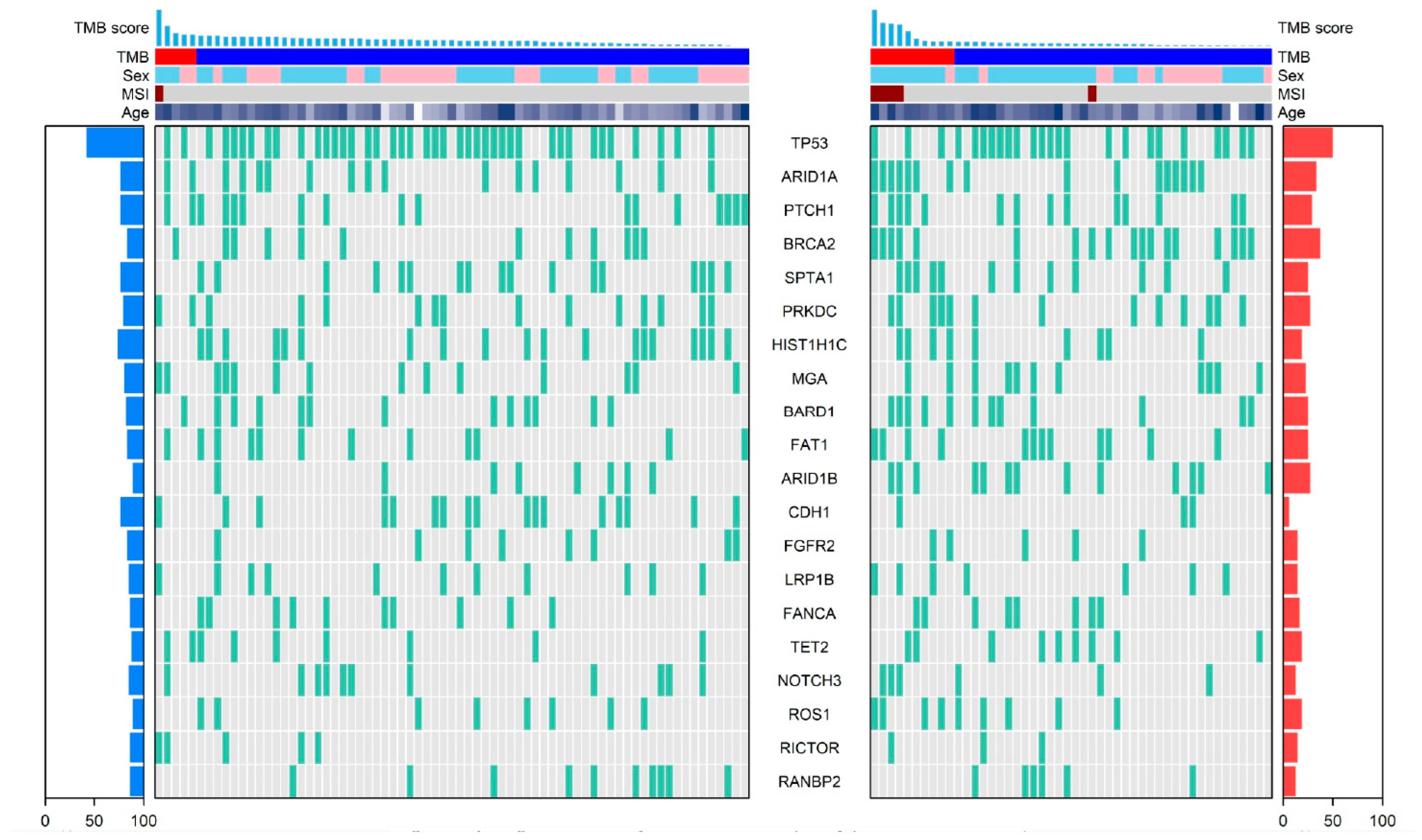
3.4. Genomic Landscape by PD-L1 Expression Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGC | Advanced Gastric Cancer |
| CPS | Combined Positive Score |
| EBV | Epstein–Barr Virus |
| EMR | Electronic Medical Records |
| ECOG | Eastern Cooperative Oncology Group |
| ICI | Immune Checkpoint Inhibitor |
| ISH | In Situ Hybridization |
| MSI | Microsatellite Instability |
| MSI-H | Microsatellite Instability-High |
| NGS | Next-Generation Sequencing |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| TMB | Tumor Mutational Burden |
References
- Shin, W.S.; Xie, F.; Chen, B.; Yu, P.; Yu, J.; To, K.F.; Kang, W. Updated Epidemiology of Gastric Cancer in Asia: Decreased Incidence but Still a Big Challenge. Cancers 2023, 15, 2639. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Zhao, H.P.; Yu, Y.; Wang, J.H.; Guo, L.; Liu, J.Y.; Pu, J.; Lv, J. Updates on global epidemiology, risk and prognostic factors of gastric cancer. World J. Gastroenterol. 2023, 29, 2452–2468. [Google Scholar] [CrossRef] [PubMed]
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Ajani, J.A.; Moehler, M.; Shen, L.; Garrido, M.; Gallardo, C.; Wyrwicz, L.; Yamaguchi, K.; Cleary, J.M.; Elimova, E. First-line nivolumab plus chemotherapy for advanced gastric, gastroesophageal junction, and esophageal adenocarcinoma: 3-year follow-up of the phase III CheckMate 649 trial. J. Clin. Oncol. 2024, 42, 2012–2020. [Google Scholar] [CrossRef]
- Rha, S.Y.; Oh, D.Y.; Yañez, P.; Bai, Y.; Ryu, M.H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.K.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1181–1195. [Google Scholar] [CrossRef]
- Qiu, M.Z.; Oh, D.Y.; Kato, K.; Arkenau, T.; Tabernero, J.; Correa, M.C.; Zimina, A.V.; Bai, Y.; Shi, J.; Lee, K.W.; et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial. BMJ 2024, 385, e078876. [Google Scholar] [CrossRef]
- Shin, M.; Ahn, S.; Jung, J.; Hyung, S.; Kim, K.M.; Kim, S.T.; Kang, W.K.; Lee, J. Impact of programmed death-ligand 1 (PD-L1) positivity on clinical and molecular features of patients with metastatic gastric cancer. Cancer Med. 2023, 12, 18633–18642. [Google Scholar] [CrossRef]
- Zhao, J.J.; Yap, D.W.T.; Chan, Y.H.; Tan, B.K.J.; Teo, C.B.; Syn, N.L.; Smyth, E.C.; Soon, Y.Y.; Sundar, R. Low Programmed Death-Ligand 1-Expressing Subgroup Outcomes of First-Line Immune Checkpoint Inhibitors in Gastric or Esophageal Adenocarcinoma. J. Clin. Oncol. 2022, 40, 392–402. [Google Scholar] [CrossRef]
- Yoon, H.H.; Jin, Z.; Kour, O.; Kankeu Fonkoua, L.A.; Shitara, K.; Gibson, M.K.; Prokop, L.J.; Moehler, M.; Kang, Y.K.; Shi, Q.; et al. Association of PD-L1 Expression and Other Variables With Benefit From Immune Checkpoint Inhibition in Advanced Gastroesophageal Cancer: Systematic Review and Meta-analysis of 17 Phase 3 Randomized Clinical Trials. JAMA Oncol. 2022, 8, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Siemers, N.O.; Pandya, D.; Chang, H.; Sanchez, T.; Harbison, C.; Szabo, P.M.; Janjigian, Y.; Ott, P.A.; Sharma, P. Analyses of PD-L1 and inflammatory gene expression association with efficacy of nivolumab±ipilimumab in gastric cancer/gastroesophageal junction cancer. Clin. Cancer Res. 2021, 27, 3926–3935. [Google Scholar] [CrossRef]
- Cho, Y.; Ahn, S.; Kim, K.-M. PD-L1 as a biomarker in gastric cancer immunotherapy. J. Gastric Cancer 2024, 25, 177. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Chen, L.-T.; Ryu, M.-H.; Oh, D.-Y.; Oh, S.C.; Chung, H.C.; Lee, K.-W.; Omori, T.; Shitara, K.; Sakuramoto, S. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar]
- Boku, N.; Omori, T.; Shitara, K.; Sakuramoto, S.; Yamaguchi, K.; Kato, K.; Kadowaki, S.; Tsuji, K.; Ryu, M.-H.; Oh, D.-Y. Nivolumab plus chemotherapy in patients with HER2-negative, previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: 3-year follow-up of the ATTRACTION-4 randomized, double-blind, placebo-controlled, phase 3 trial. Gastric Cancer 2024, 27, 1287–1301. [Google Scholar]
- Sun, J.-M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.-P.; Li, Z.; Kim, S.-B. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, H.; Pan, Y.; Gu, K.; Cang, S.; Han, L.; Shu, Y.; Li, J.; Zhao, J.; Pan, H. Sintilimab plus chemotherapy for unresectable gastric or gastroesophageal junction cancer: The ORIENT-16 randomized clinical trial. JAMA 2023, 330, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Zhang, Z.; Zhang, X.; Qi, C.; Shen, L.; Peng, Z. Appropriate PD-L1 cutoff value for gastric cancer immunotherapy: A systematic review and meta-analysis. Front. Oncol. 2021, 11, 646355. [Google Scholar] [CrossRef]
- Park, Y.; Koh, J.; Na, H.Y.; Kwak, Y.; Lee, K.-W.; Ahn, S.-H.; Park, D.J.; Kim, H.-H.; Lee, H.S. PD-L1 testing in gastric cancer by the combined positive score of the 22C3 PharmDx and SP263 assay with clinically relevant cut-offs. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2020, 52, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Kim, K.M. PD-L1 expression in gastric cancer: Interchangeability of 22C3 and 28-8 pharmDx assays for responses to immunotherapy. Mod. Pathol. 2021, 34, 1719–1727. [Google Scholar] [CrossRef]
- Yeong, J.; Lum, H.Y.J.; Teo, C.B.; Tan, B.K.J.; Chan, Y.H.; Tay, R.Y.K.; Choo, J.R.; Jeyasekharan, A.D.; Miow, Q.H.; Loo, L.H.; et al. Choice of PD-L1 immunohistochemistry assay influences clinical eligibility for gastric cancer immunotherapy. Gastric Cancer 2022, 25, 741–750. [Google Scholar] [CrossRef]
- Kim, H.D.; Shin, J.; Song, I.H.; Hyung, J.; Lee, H.; Ryu, M.H.; Park, Y.S. Discordant PD-L1 results between 28-8 and 22C3 assays are associated with outcomes of gastric cancer patients treated with nivolumab plus chemotherapy. Gastric Cancer 2024, 27, 819–826. [Google Scholar] [CrossRef]
- Li, J.B.; Lai, M.Y.; Lin, Z.C.; Guan, W.L.; Sun, Y.T.; Yang, J.; Wang, W.X.; Yang, Z.R.; Qiu, M.Z. The optimal threshold of PD-L1 combined positive score to predict the benefit of PD-1 antibody plus chemotherapy for patients with HER2-negative gastric adenocarcinoma: A meta-analysis. Cancer Immunol. Immunother. 2024, 73, 132. [Google Scholar] [CrossRef]
- Chao, J.; Fuchs, C.S.; Shitara, K.; Tabernero, J.; Muro, K.; Van Cutsem, E.; Bang, Y.J.; De Vita, F.; Landers, G.; Yen, C.J.; et al. Assessment of Pembrolizumab Therapy for the Treatment of Microsatellite Instability-High Gastric or Gastroesophageal Junction Cancer Among Patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 Clinical Trials. JAMA Oncol. 2021, 7, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Ogura, G.; Tanabe, M.; Hayashi, T.; Ohbayashi, C.; Azuma, M.; Kunisaki, C.; Akazawa, Y.; Ozawa, S.; Matsumoto, S.; et al. Clinicopathological features of PD-L1 protein expression, EBV positivity, and MSI status in patients with advanced gastric and esophagogastric junction adenocarcinoma in Japan. Cancer Biol. Ther. 2022, 23, 191–200. [Google Scholar] [CrossRef]
- Khan, H.; Judd, J.; Xiu, J.; Ullah, A.; Raval, G.G.; Ma, P.C.; Nieva, J.J.; Radovich, M.; Oberley, M.J.; Kim, S.Y.; et al. Co-mutational status and PD-L1 expression in KRAS mutant non-small cell lung cancer (NSCLC): Role in treatment selection and association with clinical outcomes. J. Clin. Oncol. 2023, 41, 9038. [Google Scholar] [CrossRef]
- Lindsay, C.R.; Veluswamy, R.; Castro, G.; Tan, D.S.-W.; Caparica, R.; Glaser, S.; Malhotra, S.; Boran, A.; Felip, E. A phase II trial of JDQ443 in KRAS G12C-mutated NSCLC with PD-L1 expression < 1% or PD-L1 expression ≥ 1% and an STK11 co-mutation. J. Clin. Oncol. 2023, 41, TPS9158. [Google Scholar] [CrossRef]
- Jiang, W.; Ouyang, X.; Li, C.; Long, Y.; Chen, W.; Ji, Z.; Shen, X.; Xiang, L.; Yang, H. Targeting PI3Kalpha increases the efficacy of anti-PD-1 antibody in cervical cancer. Immunology 2023, 170, 419–438. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ju, Q.; Jia, K.; Yu, J.; Shi, H.; Wu, H.; Jiang, M. Correlation between sex and efficacy of immune checkpoint inhibitors (PD-1 and CTLA-4 inhibitors). Int. J. Cancer 2018, 143, 45–51. [Google Scholar] [CrossRef]
- Tian, B.-W.; Han, C.-L.; Wang, H.-C.; Yan, L.-J.; Ding, Z.-N.; Liu, H.; Mao, X.-C.; Tian, J.-C.; Xue, J.-S.; Yang, L.-S. Effect of liver metastasis on the efficacy of immune checkpoint inhibitors in cancer patients: A systemic review and meta-analysis. Clin. Exp. Metastasis 2023, 40, 255–287. [Google Scholar] [CrossRef]
- Kadowaki, S.; Otsuka, T.; Minashi, K.; Nishina, S.; Yabusaki, H.; Inagaki, C.; Nishina, T.; Yasui, H.; Matsuoka, H.; Machida, N. An observational study of the effectiveness and safety of nivolumab plus chemotherapy for untreated advanced or recurrent gastric cancer in Japanese real-world settings: The G-KNIGHT study. Gastric Cancer 2025, 28, 955–967. [Google Scholar] [CrossRef]
- Mildanoglu, M.M.; Kutlu, Y.; Bas, O.; Koylu, B.; Dae, S.A.; Sakin, A.; Erdem, D.; Sendur, M.A.N.; Tasci, E.S.; Dane, F. Prognostic and predictive value of systemic inflammatory markers in patients with metastatic gastric and GEJ adenocarcinoma with PD-L1 CPS score≥ 5: Turkish Oncology Group (TOG) study. Sci. Rep. 2025, 15, 25336. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Liu, Y.; Zhang, Z.; Zhang, X.; Gong, J.; Qi, C.; Li, J.; Shen, L.; Peng, Z. Positive status of Epstein-Barr virus as a biomarker for gastric cancer immunotherapy: A prospective observational study. J. Immunother. 2020, 43, 139–144. [Google Scholar] [CrossRef]
- Giampieri, R.; Maccaroni, E.; Mandolesi, A.; Del Prete, M.; Andrikou, K.; Faloppi, L.; Bittoni, A.; Bianconi, M.; Scarpelli, M.; Bracci, R. Mismatch repair deficiency may affect clinical outcome through immune response activation in metastatic gastric cancer patients receiving first-line chemotherapy. Gastric Cancer 2017, 20, 156–163. [Google Scholar] [CrossRef]
- Ke, L.; Li, S.; Huang, D. The predictive value of tumor mutation burden on survival of gastric cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Int. Immunopharmacol. 2023, 124, 110986. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Zhao, Y.; Zhu, H. Predictive biomarkers for immunotherapy in gastric cancer: Current status and emerging prospects. Int. J. Mol. Sci. 2023, 24, 15321. [Google Scholar] [CrossRef]
- Khan, B.; Qahwaji, R.M.; Alfaifi, M.S.; Mobashir, M. Nivolumab and Ipilimumab Acting as Tormentors of Advanced Tumors by Unleashing Immune Cells and Associated Collateral Damage. Pharmaceutics 2024, 16, 732. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | PD-L1 < 5 (n = 87) | PD-L1 ≥ 5 (n = 56) | p-Value |
|---|---|---|---|
| Median age (range) | 58 (24–76) | 58 (21–79) | 0.22 |
| Age < 65 years | 71 (81.6%) | 40 (71.4%) | |
| Age ≥ 65 years | 16 (18.4%) | 16 (28.6%) | |
| Sex | 0.08 | ||
| Men | 45 (51.7%) | 38 (67.9%) | |
| Women | 42 (48.3%) | 18 (32.1%) | |
| ECOG performance status | 0.70 | ||
| 0–1 | 86 (98.9%) | 54 (96.4%) | |
| ≥2 | 1 (1.1%) | 2 (3.6%) | |
| Primary tumor location at initial diagnosis | 0.03 | ||
| Cardia | 12 (13.8%) | 6 (10.7%) | |
| Fundus | 2 (2.3%) | 2 (3.6%) | |
| Body | 58 (66.7%) | 26 (46.4%) | |
| Antrum | 15 (17.2%) | 22 (39.3%) | |
| Previous surgery (for definitive aim) | 0.03 | ||
| Yes | 29 (33.3%) | 9 (16.1%) | |
| No | 58 (67.7%) | 47 (83.9%) | |
| Site of metastases | |||
| Liver | 11 (12.5%) | 16 (28.6%) | 0.03 |
| Peritoneum | 55 (63.2%) | 26 (46.4%) | 0.07 |
| Lung | 8 (9.1%) | 2 (3.6%) | 0.34 |
| Bone | 10 (11.4%) | 1 (1.8%) | 0.07 |
| Signet ring cell carcinoma | 0.07 | ||
| Yes | 17 (19.5%) | 4 (7.1%) | |
| No | 70 (80.5%) | 52 (92.9%) | |
| EBV ISH | 0.69 | ||
| Positive | 1 (1.4%) | 2 (4.7%) | |
| Negative | 68 (98.6%) | 41 (95.3%) | |
| Unknown | 18 | 13 | |
| MLH1 IHC | 0.02 | ||
| Intact | 62 (96.9%) | 40 (81.6%) | |
| Loss | 2 (3.1%) | 9 (18.4%) | |
| Unknown | 23 | 7 | |
| Tumor mutation burden | 0.008 | ||
| Low (<10 mut/Mb) | 75 (90.4%) | 39 (70.9%) | |
| High (≥10 mut/Mb) | 8 (9.6%) | 16 (29.1%) | |
| Unknown | 4 | 1 | |
| Co-mutation confirmed by NGS | |||
| PIK3CA | 9 (10.6%) | 10 (18.2%) | 0.430 |
| KRAS | 5 (5.9%) | 11 (20.0%) | 0.036 |
| TP53 | 7 (8.2%) | 4 (7.3%) | 0.958 |
| ARID1A | 7 (8.2%) | 3 (5.5%) | 0.805 |
| No tier I/II mutation | 42 (49.4%) | 19 (34.5%) | |
| Chemotherapy regimens | 0.30 | ||
| † FOLFOX | 13 (14.9%) | 13 (23.2%) | |
| ‡ XELOX | 74 (85.1%) | 43 (76.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.-H.; Shin, J.E.; Lee, E.; Kim, S.T.; Lim, S.H. Efficacy of First-Line Nivolumab Plus Chemotherapy in Advanced Gastric Cancer Stratified by PD-L1 Expression: A Real-World Comparison. Cancers 2025, 17, 3716. https://doi.org/10.3390/cancers17223716
Choi D-H, Shin JE, Lee E, Kim ST, Lim SH. Efficacy of First-Line Nivolumab Plus Chemotherapy in Advanced Gastric Cancer Stratified by PD-L1 Expression: A Real-World Comparison. Cancers. 2025; 17(22):3716. https://doi.org/10.3390/cancers17223716
Chicago/Turabian StyleChoi, Dae-Ho, Ji Eun Shin, Eunbyeol Lee, Seung Tae Kim, and Sung Hee Lim. 2025. "Efficacy of First-Line Nivolumab Plus Chemotherapy in Advanced Gastric Cancer Stratified by PD-L1 Expression: A Real-World Comparison" Cancers 17, no. 22: 3716. https://doi.org/10.3390/cancers17223716
APA StyleChoi, D.-H., Shin, J. E., Lee, E., Kim, S. T., & Lim, S. H. (2025). Efficacy of First-Line Nivolumab Plus Chemotherapy in Advanced Gastric Cancer Stratified by PD-L1 Expression: A Real-World Comparison. Cancers, 17(22), 3716. https://doi.org/10.3390/cancers17223716







