The Role of Quantitative Ultrasound in Monitoring Neoadjuvant Chemotherapy in Breast Cancer: A Narrative Review
Simple Summary
Abstract
1. Introduction
2. Quantitative Ultrasound: Physical Principles and Biological Basis for Cancer Therapy Monitoring
3. QUS Parameters and Their Significance in Assessing Tissue Microstructure
3.1. Spectral QUS Parameters
3.2. Amplitude-Based QUS Parameters
3.3. Attenuation Coefficient (AC)
3.4. Parametric Mapping and Texture-Based Assessment of Tumor Heterogeneity
3.5. Integration of QUS with Other Data Sources
4. Translational and Clinical Applications of Quantitative Ultrasound in Breast Cancer
4.1. Preclinical Studies (Mice, Phantoms, In Vitro Models)
4.2. Single-Center Clinical Studies of QUS
4.3. Integration of Quantitative Ultrasound with Multimodal and Multisource Data
4.4. Multi-Institutional Validation of QUS for Monitoring Neoadjuvant Chemotherapy Response
5. Limitations and Future Directions of Quantitative Ultrasound in Neoadjuvant Therapy Monitoring
5.1. Clinical Outlook and Early Predictive Potential
5.2. Technical Challenges and Standardization Issues
5.3. Organizational and Ethical Challenges
6. Summary and Future Vision
Funding
Data Availability Statement
Conflicts of Interest
References
- Harbeck, N.; Penault-Llorca, F.; Cortés, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2021, 94, 20211033. [Google Scholar] [CrossRef]
- Bhushan, A.; Gonsalves, A.; Menon, J.U. Current state of breast cancer diagnosis, treatment, and theranostics. Pharmaceutics 2021, 13, 723. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Hong, R.; Xu, B. Breast cancer: An up-to-date review and future perspectives. Cancer Commun. 2022, 42, 913–936. [Google Scholar] [CrossRef]
- Costa, B.; Amorim, I.; Gärtner, F.; Vale, N. Understanding breast cancer: From conventional therapies to repurposed drugs. Eur. J. Pharm. Sci. 2020, 151, 105401. [Google Scholar] [CrossRef]
- Anastasiadi, Z.; Lianos, G.; Ignatiadou, E.; Harissis, H.; Mitsis, M. Breast cancer in young women: An overview. Updates Surg. 2017, 69, 313–317. [Google Scholar] [CrossRef]
- Zhu, J.; Charkhchi, P.; Adekunte, S.; Akbari, M. What is known about breast cancer in young women? Cancers 2023, 15, 541. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Anders, C.; Litton, J.; Ruddy, K.; Bleyer, A. Breast cancer in adolescents and young adults. Pediatr. Blood Cancer 2018, 65, e2739. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Castelli, G.; Pelosi, E. Breast cancer: A molecularly heterogenous disease needing subtype-specific treatments. Med. Sci. 2020, 8, 18. [Google Scholar] [CrossRef]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef]
- Carvalho, E.; Vieira, A.F.; Ricardo, S. Molecular subtypes and mechanisms of breast cancer: Precision medicine approaches for targeted therapies. Cancers 2025, 17, 123. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef]
- Prat, A.; Pineda, E.; Adamo, B.; Galván, P.; Fernández, A.; Gaba, L.; Diez, M.; Viladdot, M.; Arance, A.; Muñoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24 (Suppl. S2), S26–S35. [Google Scholar] [CrossRef]
- Russnes, H.G.; Lingjærde, O.C.; Børresen-Dale, A.L.; Caldas, C. Breast cancer molecular stratification: From intrinsic subtypes to integrative clusters. Am. J. Pathol. 2017, 187, 2152–2162. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Castro, A.C.; Lin, N.U.; Polyak, K. Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discov. 2019, 9, 176–198. [Google Scholar] [CrossRef]
- Krijgsman, O.; Roepman, P.; Zwart, W.; Helleman, J.; Berns, E.M.; van’t Veer, L.J. A diagnostic gene profile for molecular subtyping of breast cancer associated with treatment response. Breast Cancer Res. Treat. 2012, 133, 37–47. [Google Scholar] [CrossRef]
- Wolf, D.M.; Yau, C.; Sanil, A.; Park, J.W.; van’t Veer, L.J. Redefining breast cancer subtypes to guide treatment prioritization and maximize response: Predictive biomarkers across 10 cancer therapies. Cancer Cell 2022, 40, 579–594. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast cancer—Epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—An updated review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Vicini, E.; Munzone, E.; Intra, M.; Galimberti, V. Shifting from axillary dissection to targeted axillary surgery after neoadjuvant treatment: The evolving management of occult breast cancer in a monoinstitutional series of 114 patients. Breast Cancer Res. Treat. 2025, 210, 661–672. [Google Scholar] [CrossRef]
- Cipolla, C.; Gebbia, V.; D’Agati, E.; Greco, M.; Mesi, C.; Scandurra, G.; Valerio, M.R. Comprehensive axillary management of clinically node-positive (cN+) breast cancer patients: A narrative review on neoadjuvant chemotherapy. Cancers 2024, 16, 3354. [Google Scholar] [CrossRef]
- Derks, M.; van de Velde, C.J.H. Neoadjuvant chemotherapy in breast cancer: More than just downsizing. Lancet Oncol. 2018, 19, 27–28. [Google Scholar] [CrossRef]
- Franceschini, G.; Di Leone, A.; Natale, M.; Sanchez, M.A.; Masetti, R. Conservative surgery after neoadjuvant chemotherapy in patients with operable breast cancer. Ann. Ital. Chir. 2018, 89, 290. [Google Scholar]
- King, T.A.; Morrow, M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat. Rev. Clin. Oncol. 2015, 12, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.M.; Mankoff, D.A.; Joe, B.N. Imaging neoadjuvant therapy response in breast cancer. Radiology 2017, 285, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Romeo, V.; Accardo, G.; Perillo, T.; Basso, L.; Garbino, N.; Nicolai, E.; Maurea, S.; Salvatore, M. Assessment and prediction of response to neoadjuvant chemotherapy in breast cancer: A comparison of imaging modalities and future perspectives. Cancers 2021, 13, 114. [Google Scholar] [CrossRef]
- Portnow, L.H.; Kochkodan-Self, J.M.; Maduram, A.; Barrios, M.; Onken, A.M.; Hong, X.; Mittendorf, E.A.; Giess, C.S.; Chikarmane, S.A. Multimodality imaging review of HER2-positive breast cancer and response to neoadjuvant chemotherapy. Radiographics 2023, 43, e220103. [Google Scholar] [CrossRef]
- Rezai, M.; Kraemer, S. Breast-conserving surgery after neoadjuvant therapy. In Breast Cancer: Innovations in Research and Management; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Scholl, S.M.; Hayes, D.F. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J. Clin. Oncol. 2012, 30, 1747–1749. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Lobbes, M.B.I.; Prevos, R.; Smidt, M.L.; Tjan-Heijnen, V.C.G.; van Goethem, M.; Schipper, R.J.; Beets-Tan, R.G.H.; Wildberger, J.E. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: A systematic review. Eur. J. Radiol. 2013, 82, 512–518. [Google Scholar] [CrossRef]
- Shi, Z.; Chu, C.; Liu, Y.; Zhang, L.; Zhu, L.; Liu, Y.; Liang, C.; Lu, C.; Cui, Y.; Han, C.; et al. MRI-based quantification of intratumoral heterogeneity for predicting treatment response to neoadjuvant chemotherapy in breast cancer. Radiology 2023, 309, e222445. [Google Scholar]
- Yeh, E.; Slanetz, P.; Kopans, D.B.; Rafferty, E.; Georgian-Smith, D.; Moore, R.H.; Kuter, I.; Taghian, A. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am. J. Roentgenol. 2005, 184, 868–877. [Google Scholar] [CrossRef]
- Tadayyon, H.; Sannachi, L.; Gangeh, M.J.; Trudeau, M.; Pritchard, K.; Tran, W.T.; Slodkowska, E.; Sadeghi-Naini, A.; Czarnota, G.J. A priori prediction of neoadjuvant chemotherapy response and survival in breast cancer patients using quantitative ultrasound. Sci. Rep. 2017, 7, 45733. [Google Scholar] [CrossRef] [PubMed]
- Klimonda, Z.; Karwat, P.; Dobruch-Sobczak, K.; Piotrzkowska-Wróblewska, H.; Litniewski, J. On the assessment of local tumor response to neoadjuvant chemotherapy. In Proceedings of the 2023 IEEE International Ultrasonics Symposium (IUS), Montreal, QC, Canada, 3–8 September 2023; pp. 1–4. [Google Scholar]
- Chan, A.W.; Sannachi, L.; Moore-Palhares, D.; Dasgupta, A.; Gandhi, S.; Pezo, R.; Eisen, A.; Warner, E.; Wright, F.C.; Look Hong, N.; et al. Validation of quantitative ultrasound and texture derivative analyses-based model for upfront prediction of neoadjuvant chemotherapy response in breast cancer. J. Imaging 2025, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Piotrzkowska-Wróblewska, H.; Dobruch-Sobczak, K.; Gumowska, M.; Litniewski, J. Changes in quantitative ultrasound imaging as the predictor of response to neoadjuvant chemotherapy in patients with breast cancer. In Proceedings of the IEEE International Ultrasonics Symposium (IUS), Venice, Italy, 10–13 October 2022; pp. 1–4. [Google Scholar]
- Sadeghi-Naini, A.; Sannachi, L.; Tadayyon, H.; Tran, W.; Slodkowska, E.; Trudeau, M.; Gandhi, S.; Pritchard, K.; Kolios, M.C.; Czarnota, G. Chemotherapy-response monitoring of breast cancer patients using quantitative ultrasound-based intra-tumour heterogeneities. Sci. Rep. 2017, 7, 10352. [Google Scholar] [CrossRef]
- Sannachi, L.; Gangeh, M.; Tadayyon, H.; Sadeghi-Naini, A.; Gandhi, S.; Wright, F.; Slodkowska, E.; Curpen, B.; Tran, W.; Czarnota, G. Response monitoring of breast cancer patients receiving neoadjuvant chemotherapy using quantitative ultrasound, texture, and molecular features. PLoS ONE 2018, 13, e0190394. [Google Scholar] [CrossRef]
- Falou, O.; Sannachi, L.; Haque, M.; Czarnota, G.; Kolios, M.C. Transfer learning of pre-treatment quantitative ultrasound multi-parametric images for the prediction of breast cancer response to neoadjuvant chemotherapy. Sci. Rep. 2024, 14, 2340. [Google Scholar] [CrossRef]
- Yu, F.; Miao, S.; Li, C.; Hang, J.; Deng, J.; Ye, X.; Liu, Y. Pretreatment ultrasound-based deep learning radiomics model for the early prediction of pathologic response to neoadjuvant chemotherapy in breast cancer. Eur. Radiol. 2023, 33, 5634–5644. [Google Scholar] [CrossRef]
- Gu, J.; Tong, T.; He, C.; Xu, M.; Yang, X.; Tian, J.; Jiang, T.; Wang, K. Deep learning radiomics of ultrasonography can predict response to neoadjuvant chemotherapy in breast cancer at an early stage of treatment: A prospective study. Eur. Radiol. 2021; in press. [Google Scholar]
- Huang, J.X.; Shi, J.; Ding, S.; Zhang, H.L.; Wang, X.Y.; Lin, S.Y.; Xu, Y.F.; Wei, M.J.; Liu, L.Z.; Pei, X. Deep learning model based on dual-modal ultrasound and molecular data for predicting response to neoadjuvant chemotherapy in breast cancer. Acad. Radiol. 2023, 30, e1–e10. [Google Scholar] [CrossRef]
- Taleghamar, H.; Jalalifar, S.; Czarnota, G.; Sadeghi-Naini, A. Deep learning of quantitative ultrasound multi-parametric images at pre-treatment to predict breast cancer response to chemotherapy. Sci. Rep. 2022, 12, 2302. [Google Scholar] [CrossRef] [PubMed]
- Oelze, M.L.; Mamou, J. Review of quantitative ultrasound: Envelope statistics and backscatter coefficient imaging and contributions to diagnostic ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 336–351. [Google Scholar] [CrossRef]
- Cloutier, G.; Destrempes, F.; Yu, F.; Tang, A. Quantitative ultrasound imaging of soft biological tissues: A primer for radiologists and medical physicists. Insights Imaging 2021, 12, 127. [Google Scholar] [CrossRef]
- Destrempes, F.; Franceschini, E.; Yu, F.; Cloutier, G. Unifying concepts of statistical and spectral quantitative ultrasound techniques. IEEE Trans. Med. Imaging 2016, 35, 488–500. [Google Scholar] [CrossRef]
- Lavarello, R.; Oelze, M. Quantitative ultrasound estimates from populations of scatterers with continuous size distributions. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2011, 58, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Naini, A.; Suraweera, H.; Tran, W.; Hadizad, F.; Bruni, G.; Rastegar, R.; Curpen, B.; Czarnota, G.J. Breast-lesion characterization using textural features of quantitative ultrasound parametric maps. Sci. Rep. 2017, 7, 13638. [Google Scholar] [CrossRef] [PubMed]
- Klimonda, Z.; Karwat, P.; Dobruch-Sobczak, K.; Piotrzkowska-Wróblewska, H.; Litniewski, J. Breast-lesions characterization using quantitative ultrasound features of peritumoral tissue. Sci. Rep. 2019, 9, 7963. [Google Scholar] [CrossRef]
- Sharma, D.; Osapoetra, L.; Czarnota, G. Implementation of non-invasive quantitative ultrasound in clinical cancer imaging. Cancers 2022, 14, 6012. [Google Scholar] [CrossRef]
- Nizam, N.; Ara, S.; Hasan, M.K. Classification of breast lesions using quantitative ultrasound biomarkers. Biomed. Signal Process. Control 2019, 49, 396–406. [Google Scholar] [CrossRef]
- Jia, F.; Sun, S.; Li, J.; Wang, W.; Huang, H.; Hu, X.; Pan, S.; Chen, W.; Shen, L.; Yao, Y.; et al. Neoadjuvant chemotherapy-induced remodeling of human hormonal receptor-positive breast cancer revealed by single-cell RNA sequencing. Cancer Lett. 2024, 585, 216656. [Google Scholar] [CrossRef]
- Hoshina, H.; Sakatani, T.; Kawamoto, Y.; Ohashi, R.; Takei, H. Cytomorphological disparities in invasive breast cancer cells following neoadjuvant endocrine therapy and chemotherapy. Pathobiology 2024, 91, 288–298. [Google Scholar] [CrossRef]
- Derouane, F.; Ambroise, J.; Van Marcke, C.; Van Bockstal, M.; Berlière, M.; Galant, C.; Dano, H.; Lougué, M.; Benidovskaya, E.; Jerusalem, G.; et al. Response to neoadjuvant chemotherapy in early breast cancers is associated with epithelial-mesenchymal transition and tumor-infiltrating lymphocytes. Mol. Oncol. 2025, 19, 2330–2347. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Naini, A.; Papanicolau, N.; Falou, O.; Zubovits, J.; Dent, R.; Verma, S.; Trudeau, M.; Boileau, J.; Spayne, J.; Iradji, S.; et al. Quantitative ultrasound evaluation of tumor cell death response in locally advanced breast cancer patients receiving chemotherapy. Clin. Cancer Res. 2013, 19, 2163–2174. [Google Scholar] [CrossRef]
- Tadayyon, H.; Sadeghi-Naini, A.; Wirtzfeld, L.; Wright, F.C.; Czarnota, G. Quantitative ultrasound characterization of locally advanced breast cancer by estimation of its scatterer properties. Med. Phys. 2014, 41, 012903. [Google Scholar] [CrossRef] [PubMed]
- Sannachi, L.; Gangeh, M.; Tadayyon, H.; Sadeghi-Naini, A.; Gandhi, S.; Wright, F.; Slodkowska, E.; Curpen, B.; Tran, W.; Czarnota, G.J. Breast cancer treatment response monitoring using quantitative ultrasound and texture analysis: Comparative analysis of analytical models. Transl. Oncol. 2019, 12, 1031–1041. [Google Scholar] [CrossRef]
- Sannachi, L.; Tadayyon, H.; Sadeghi-Naini, A.; Tran, W.T.; Gandhi, S.; Wright, F.C.; Oelze, M.; Czarnota, G.J. Non-invasive evaluation of breast cancer response to chemotherapy using quantitative ultrasonic backscatter parameters. Med. Image Anal. 2015, 20, 224–236. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, C.; Santa-Maria, C.; Emens, L.; Popel, A. Dynamics of tumor-associated macrophages in a quantitative systems pharmacology model of immunotherapy in triple-negative breast cancer. iScience 2022, 25, 105. [Google Scholar] [CrossRef] [PubMed]
- Du Terrail, O.; Leopold, A.; Joly, C.; Béguier, C.; Andreux, M.; Maussion, C.; Schmauch, B.; Tramel, E.; Bendjebbar, E.; Zaslavskiy, M.; et al. Federated learning for predicting histological response to neoadjuvant chemotherapy in triple-negative breast cancer. Nat. Med. 2023, 29, 135–146. [Google Scholar] [CrossRef]
- Dasgupta, S.; Feleppa, E.J.; Mamou, J.; Rondeau, M. Validating the theoretical framework relating ultrasonic spectrum-analysis parameters to scatterer properties. J. Acoust. Soc. Am. 2006, 120, EL55–EL61. [Google Scholar] [CrossRef]
- Dasgupta, S.; Feleppa, E.J.; Mamou, J.; Rondeau, M. 2G-3 Validating the theory relating ultrasonic spectral-parameter values to scatterer properties. In Proceedings of the 2006 IEEE Ultrasonics Symposium, Vancouver, BC, Canada, 3–6 October 2006; pp. 1509–1512. [Google Scholar]
- Lizzi, F.L.; Astor, M.; Liu, T.; Deng, C.; Coleman, D.J.; Silverman, R.H. Ultrasonic spectrum analysis for tissue assays and therapy evaluation. Int. J. Imaging Syst. Technol. 1997, 8, 310. [Google Scholar] [CrossRef]
- Trumpaitis, J.; Jurkonis, R.; Imbrasiene, D.; Grizickaitė, A.; Paunksnis, A. Application of ultrasound spectral analysis for intraocular tissues differentiation. J. Vibroeng. 2014, 16, 1197–1205. [Google Scholar]
- Dasgupta, S.; Feleppa, E.J. 4C-1 Empirical validation of the theoretical frameworks underlying ultrasound scattering in tissue. In Proceedings of the 2007 IEEE Ultrasonics Symposium Proceedings, New York, NY, USA, 28–31 October 2007; pp. 1103–1106. [Google Scholar]
- Destrempes, F.; Cloutier, G. Review of envelope statistics models for quantitative ultrasound imaging and tissue characterization. Adv. Exp. Med. Biol. 2023, 1403, 107–152. [Google Scholar]
- Oelze, M.L.; Zachary, J.F. Examination of cancer in mouse models using high-frequency quantitative ultrasound. Ultrasound Med. Biol. 2006, 32, 1639–1648. [Google Scholar] [CrossRef]
- Tehrani, A.K.Z.; Cloutier, G.; Tang, A.; Rosado-Méndez, I.; Rivaz, H. Homodyned K-distribution parameter estimation in quantitative ultrasound: Autoencoder and Bayesian neural network approaches. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2024, 71, 354–365. [Google Scholar] [CrossRef]
- Mori, S.; Arakawa, M.; Kanai, H.; Hachiya, H. Quantification of limitations in statistical analysis of ultrasound echo envelope amplitudes. Jpn. J. Appl. Phys. 2023, 62, SGGE01. [Google Scholar] [CrossRef]
- Omura, M.; Yoshida, K.; Kohta, M.; Kubo, T.; Ishiguro, T.; Kobayashi, K.; Hozumi, N.; Yamaguchi, T. Tissue characterization of skin ulcer for bacterial infection by multiple statistical analysis of echo amplitude envelope. Jpn. J. Appl. Phys. 2016, 55, 07KF13. [Google Scholar] [CrossRef]
- Dobruch-Sobczak, K.; Piotrzkowska-Wróblewska, H.; Karwat, P.; Klimonda, Z.; Markiewicz-Grodzicka, E.; Litniewski, J. Quantitative assessment of the echogenicity of a breast tumor predicts the response to neoadjuvant chemotherapy. Cancers 2021, 13, 3242. [Google Scholar] [CrossRef]
- Nasief, H.; Rosado-Méndez, I.; Zagzebski, J.; Hall, T. A quantitative ultrasound-based multi-parameter classifier for breast masses. Ultrasound Med. Biol. 2019, 45, 1803–1815. [Google Scholar] [CrossRef]
- Tsui, P.H.; Chang, C.C.; Shung, K.K. Ultrasonic Nakagami imaging: A strategy to visualize the scatterer properties of benign and malignant breast tumors. Ultrasound Med. Biol. 2010, 36, 209–217. [Google Scholar] [CrossRef]
- Shankar, P.M.; Narayanan, V.; Reid, J.; Klemow, S.; Piccoli, C.; Forsberg, F. Classification of ultrasonic B-mode images of breast masses using Nakagami distribution. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2001, 48, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Destrempes, F.; Cloutier, G. A critical review and uniformized representation of statistical distributions modeling the ultrasound echo envelope. Ultrasound Med. Biol. 2010, 36, 1037–1051. [Google Scholar] [CrossRef]
- Tsui, P.H. Small-window parametric imaging based on information entropy for ultrasound tissue characterization. Sci Rep. 2017, 7, 41004. [Google Scholar] [CrossRef]
- Tsui, P.H. Ultrasound detection of scatterer concentration by weighted entropy. Entropy 2015, 17, 6598–6611. [Google Scholar] [CrossRef]
- Vajihi, Z.; Rosado-Méndez, I.; Hall, T.; Rivaz, H. Low variance estimation of backscatter quantitative ultrasound parameters using dynamic programming. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, Y.; Zhan, Y.; Li, R.; Bi, Y.; Gao, L.; Wu, X.; Shao, J.; Chen, Y.; Ye, L.; et al. Intratumoral and peritumoral ultrasound radiomics analysis for predicting HER2-low expression in HER2-negative breast cancer patients: A retrospective analysis of dual-central study. Discov. Oncol. 2025, 16, 1007. [Google Scholar] [CrossRef]
- Nam, K.; Zagzebski, J.A.; Hall, T.J. Quantitative assessment of in vivo breast masses using ultrasound attenuation and backscatter. Ultrason. Imaging 2013, 35, 146–161. [Google Scholar] [CrossRef]
- D’Astous, F.; Foster, F.S. Frequency dependence of ultrasound attenuation and backscatter in breast tissue. Ultrasound Med. Biol. 1986, 12, 795–808. [Google Scholar] [CrossRef]
- Huang, S.W.; Li, P.C. Ultrasonic computed tomography reconstruction of the attenuation coefficient using a linear array. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 2011–2022. [Google Scholar] [CrossRef]
- Sarno, D.; Baker, C.; Curtis, S.; Hodnett, M.; Zeqiri, B. In vivo measurements of the bulk ultrasonic attenuation coefficient of breast tissue using a novel phase-insensitive receiver. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 2562–2572. [Google Scholar] [CrossRef]
- Dobruch-Sobczak, K.; Piotrzkowska-Wróblewska, H.; Klimonda, Z.; Karwat, P.; Roszkowska-Purska, K.; Clauser, P.; Baltzer, P.; Litniewski, J. Multiparametric ultrasound examination for response assessment in breast cancer patients undergoing neoadjuvant therapy. Sci. Rep. 2021, 11, 2501. [Google Scholar] [CrossRef] [PubMed]
- Tadayyon, H.; Sannachi, L.; Czarnota, G.J. Quantitative ultrasound monitoring of breast tumor response to chemotherapy by analysis of frequency-dependent attenuation and backscattered power. SPIE Med. Imaging 2014, 9040, 904009. [Google Scholar]
- Oh, S.; Kim, M.G.; Kim, Y.; Jung, G.J.; Kwon, H.; Bae, H.M. Spatio-temporal quantitative ultrasound imaging for breast cancer identification. In Proceedings of the 2023 IEEE 20th International Symposium on Biomedical Imaging (ISBI), Cartagena, Colombia, 17–21 April 2023; pp. 1–4. [Google Scholar]
- Bhardwaj, D.; Dasgupta, A.; Dicenzo, D.; Brade, S.; Fatima, K.; Quiaoit, K.; Trudeau, M.; Gandhi, S.; Eisen, A.; Wright, F.; et al. Early changes in quantitative ultrasound imaging parameters during neoadjuvant chemotherapy to predict recurrence in patients with locally advanced breast cancer. Cancers 2022, 14, 1247. [Google Scholar] [CrossRef] [PubMed]
- Alberico, D.; Sannachi, L.; Anzola Pena, M.L.; Yip, J.; Osapoetra, L.O.; Halstead, S.; DiCenzo, D.; Gandhi, S.; Wright, F.; Oelze, M.; et al. Quantitative ultrasound texture analysis of breast tumors: A comparison of a cart-based and a wireless ultrasound scanner. J. Imaging 2025, 11, 146. [Google Scholar] [CrossRef]
- Osapoetra, L.; Sannachi, L.; Dicenzo, D.; Quiaoit, K.; Fatima, K.; Czarnota, G. Breast lesion characterization using quantitative ultrasound (QUS) and derivative texture methods. Transl. Oncol. 2020, 13, 100776. [Google Scholar] [CrossRef]
- Alvarenga, A.; Pereira, W.; Infantosi, A.; Azevedo, C. Complexity curve and grey level co-occurrence matrix in the texture evaluation of breast tumor on ultrasound images. Med. Phys. 2007, 34, 379–387. [Google Scholar] [CrossRef]
- Gómez, W.; Pereira, W.; Infantosi, A. Analysis of co-occurrence texture statistics as a function of gray-level quantization for classifying breast ultrasound. IEEE Trans. Med. Imaging 2012, 31, 1889–1899. [Google Scholar] [CrossRef]
- Djunaidi, K.; Agtriadi, H.B.; Kuswardani, D.; Purwanto, Y. Gray level co-occurrence matrix feature extraction and histogram in breast cancer classification with ultrasonographic imagery. Indones. J. Electr. Eng. Comput. Sci. 2021, 22, 1012–1018. [Google Scholar] [CrossRef]
- Czarnota, G.J.; Tadayyon, H.; Gangeh, M.J.; Sannachi, L.; Sadeghi-Naini, A.; Tran, W.T. Quantitative ultrasound and texture predictors of breast tumour response to chemotherapy. In Proceedings of the 2018 IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018; pp. 1–4. [Google Scholar]
- Dasgupta, A.; Brade, S.; Sannachi, L.; Quiaoit, K.; Fatima, K.; Dicenzo, D.; Osapoetra, L.; Saifuddin, M.; Trudeau, M.; Gandhi, S.; et al. Quantitative ultrasound radiomics using texture derivatives in prediction of treatment response to neo-adjuvant chemotherapy for locally advanced breast cancer. Oncotarget 2020, 11, 3968–3979. [Google Scholar] [CrossRef]
- Taleghamar, H.; Moghadas-Dastjerdi, H.; Czarnota, G.; Sadeghi-Naini, A. Characterizing intra-tumor regions on quantitative ultrasound parametric images to predict breast cancer response to chemotherapy at pre-treatment. Sci. Rep. 2021, 11, 14865. [Google Scholar] [CrossRef]
- Karwat, P.; Piotrzkowska-Wróblewska, H.; Klimonda, Z.; Dobruch-Sobczak, K.; Litniewski, J. Monitoring breast cancer response to neoadjuvant chemotherapy using probability maps derived from quantitative ultrasound parametric images. IEEE Trans. Biomed. Eng. 2024, 71, 1202–1211. [Google Scholar] [CrossRef]
- Dicenzo, D.; Quiaoit, K.; Fatima, K.; Bhardwaj, D.; Sannachi, L.; Gangeh, M.; Sadeghi-Naini, A.; Dasgupta, A.; Kolios, M.C.; Trudeau, M.; et al. Quantitative ultrasound radiomics in predicting response to neoadjuvant chemotherapy in patients with locally advanced breast cancer: Results from multi-institutional study. Cancer Med. 2020, 9, 4296–4306. [Google Scholar] [CrossRef]
- Dasgupta, A.; Dicenzo, D.; Sannachi, L.; Gandhi, S.; Pezo, R.C.; Eisen, A.; Tran, W.T.; Look Hong, N.; Wright, F.C.; Curpen, B.; et al. Quantitative ultrasound radiomics guided adaptive neoadjuvant chemotherapy in breast cancer: Early results from a randomized feasibility study. Front. Oncol. 2024, 14, 1350132. [Google Scholar] [CrossRef]
- Sannachi, L.; Osapoetra, L.; Dicenzo, D.; Halstead, S.; Wright, F.; Look-Hong, N.; Slodkowska, E.; Gandhi, S.; Curpen, B.; Kolios, M.C.; et al. A priori prediction of breast cancer response to neoadjuvant chemotherapy using quantitative ultrasound, texture derivative and molecular subtype. Sci. Rep. 2023, 13, 22113. [Google Scholar] [CrossRef] [PubMed]
- Czarnota, G.J.; Kolios, M.C.; Abraham, J.; Portnoy, M.; Ottensmeyer, F.P.; Hunt, J.W.; Sherar, M.D. Ultrasound imaging of apoptosis: High-resolution non-invasive monitoring of programmed cell death in vitro, in situ and in vivo. Br. J. Cancer 1999, 81, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Carter, H.; Sannachi, L.; Czarnota, G.J. Quantitative ultrasound for evaluation of tumour response to ultrasound-microbubbles and hyperthermia. Technol. Cancer Res. Treat. 2023, 22, 15330338231157344. [Google Scholar] [CrossRef]
- Czarnota, G.J. Role of ultrasound in the detection of apoptosis. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Czarnota, G.J.; Kolios, M.C.; Vaziri, H.; Benchimol, S.; Lee, K.C.; Hunt, J.W.; Sherar, M.D. Ultrasonic spectral parameter characterization of apoptosis. Cancer Res. 2005, 65, 3561–3567. [Google Scholar]
- Dighe, S.; Shinde, R.; Shinde, S.; Verma, P. Assessment of response of neoadjuvant chemotherapy in carcinoma breast patients by high-frequency ultrasound. J. Fam. Med. Prim. Care 2022, 11, 4717–4722. [Google Scholar] [CrossRef]
- Sannachi, L.; Gangeh, M.; Tadayyon, H.; Sadeghi-Naini, A.; Gandhi, S.; Wright, F.C.; Bhargava, P.; Jain, A.; Tran, W.T.; Czarnota, G.J. Repeatability of quantitative ultrasound parameters in the assessment of breast tumor response to neoadjuvant chemotherapy using two clinical ultrasound systems. Ultrasound Med. Biol. 2020, 46, 1142–1157. [Google Scholar] [CrossRef] [PubMed]
- Piotrzkowska-Wróblewska, H.; Dobruch-Sobczak, K.; Klimonda, Z.; Karwat, P.; Sznajder, M.; Litniewski, J. Predicting response to neoadjuvant chemotherapy in breast cancer—Repeatability of quantitative ultrasound parameters. PLoS ONE 2019, 14, e0213749. [Google Scholar] [CrossRef]
- Klimonda, Z.; Piotrzkowska-Wróblewska, H.; Dobruch-Sobczak, K.; Litniewski, J. Detecting early response to neoadjuvant chemotherapy in breast cancer using quantitative ultrasound parametric maps of tumour microstructure. Med. Phys. 2022, 49, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Moore-Palhares, D.; Sannachi, L.; Bhardwaj, D.; Gangeh, M.; Tadayyon, H.; Sadeghi-Naini, A.; Dasgupta, A.; Chan, A.W.; Eisen, A.; Gandhi, S.; et al. Prospective validation of quantitative ultrasound and texture analysis for early prediction of breast cancer treatment response. Cancers 2025, 17, 2594. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Adaptive Neoadjuvant Chemotherapy Based on Quantitative Ultrasound Biomarkers in Locally Advanced Breast Cancer. Identifier: NCT04050228. Available online: https://clinicaltrials.gov/study/NCT04050228 (accessed on 14 October 2025).
- Wenwen, J.; Jiang, Z.; Liu, J.; Liu, D.; Li, Y.; He, Y.; Zhao, H.; Li, L.; Zhu, Y.; Long, Q.; et al. Integrating ultrasound radiomics and clinicopathological features for machine learning-based survival prediction in patients with nonmetastatic triple-negative breast cancer. BMC Cancer 2025, 25, 291. [Google Scholar] [CrossRef]
- Mao, N.; Zhang, L.; Wang, Y.; Zhao, J.; Li, X.; Yang, Q.; Dai, Y.; Zhou, H.; Lin, F.; Zheng, T.; et al. A multimodal and fully automated system for prediction of pathological complete response to neoadjuvant chemotherapy in breast cancer. Sci. Adv. 2025, 11, eadj9381. [Google Scholar] [CrossRef]
- Yang, M.; Liu, H.; Dai, Q.; Yao, L.; Zhang, S.; Wang, Z.; Li, J.; Duan, Q. Treatment response prediction using ultrasound-based pre-, post-early, and delta radiomics in neoadjuvant chemotherapy in breast cancer. Front. Oncol. 2022, 12, 825210. [Google Scholar] [CrossRef]
- Byra, M.; Dobruch-Sobczak, K.; Klimonda, Z.; Piotrzkowska-Wróblewska, H.; Litniewski, J. Early prediction of response to neoadjuvant chemotherapy in breast cancer sonography using Siamese convolutional neural networks. IEEE J. Biomed. Health Inform. 2021, 25, 797–805. [Google Scholar] [CrossRef]
- Byra, M.; Dobruch-Sobczak, K.; Piotrzkowska-Wróblewska, H.; Klimonda, Z.; Litniewski, J. Prediction of response to neoadjuvant chemotherapy in breast cancer with recurrent neural networks and raw ultrasound signals. Phys. Med. Biol. 2022, 67, ac8c82. [Google Scholar] [CrossRef]
- Feng, X.; Shi, Y.; Wu, M.; Cui, G.; Du, Y.; Yang, J.; Xu, Y.; Wang, W.; Liu, F. Predicting the efficacy of neoadjuvant chemotherapy in breast cancer patients based on ultrasound longitudinal temporal-depth network fusion model. Breast Cancer Res. 2025, 27, 34. [Google Scholar] [CrossRef]
- Huang, J.X.; Wu, L.; Wang, X.Y.; Lin, S.Y.; Xu, Y.F.; Wei, M.J.; Pei, X.Q. Delta radiomics based on longitudinal dual-modal ultrasound can early predict response to neoadjuvant chemotherapy in breast cancer patients. Acad. Radiol. 2023, 30, e377–e386. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhong, X.; Fang, C.; Lou, W.; Fu, P.; Woodruff, H.; Wang, B.; Jiang, T.; Lambin, P. Deep learning of multimodal ultrasound: Stratifying the response to neoadjuvant chemotherapy in breast cancer before treatment. Oncologist 2023, 28, e1001–e1012. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Wang, Y.; Xie, Y.; Cui, Y.; Feng, S.; Yao, M.; Qiu, B.; Shen, W.; Chen, D.; et al. Early prediction of treatment response to neoadjuvant chemotherapy based on longitudinal ultrasound images of HER2-positive breast cancer patients by Siamese multi-task network: A multicentre, retrospective cohort study. eClinicalMedicine 2022, 52, 101601. [Google Scholar] [CrossRef]
- Sadeghi-Naini, A.; Sannachi, L.; Pritchard, K.; Trudeau, M.; Gandhi, S.; Wright, F.; Zubovits, J.; Yaffe, M.; Kolios, M.; Czarnota, G. Early prediction of therapy responses and outcomes in breast cancer patients using quantitative ultrasound spectral texture. Oncotarget 2014, 5, 3497–3511. [Google Scholar] [CrossRef]
- Tadayyon, H.; Sannachi, L.; Gangeh, M.; Sadeghi-Naini, A.; Tran, W.; Trudeau, M.; Pritchard, K.; Ghandi, S.; Verma, S.; Czarnota, G. Quantitative ultrasound assessment of breast tumor response to chemotherapy using a multi-parameter approach. Oncotarget 2016, 7, 45094–45111. [Google Scholar] [CrossRef]
- Adrada, B.E.; Candelaria, R.; Moulder, S.; Thompson, A.; Wei, P.; Whitman, G.; Valero, V.; Litton, J.K.; Santiago, L.; Scoggins, M.E.; et al. Early ultrasound evaluation identifies excellent responders to neoadjuvant systemic therapy among patients with triple-negative breast cancer. Cancer 2021, 127, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Peréz-García, J.; Gebhart, G.; Ruíz Borrego, M.; Stradella, A.; Bermejo, B.; Schmid, P.; Marmé, F.; Escrivá-de-Romani, S.; Calvo, L.; Ribelles, N.; et al. Chemotherapy de-escalation using an 18F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): A multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021, 22, 858–871. [Google Scholar] [CrossRef]
- Fu, Y.; Lei, Y.T.; Huang, Y.; Mei, F.; Wang, S.; Yan, K.; Wang, Y.H.; Ma, Y.H.; Cui, L.G. Longitudinal ultrasound-based AI model predicts axillary lymph node response to neoadjuvant chemotherapy in breast cancer: A multicenter study. Eur. Radiol. 2024, 34, 7080–7089. [Google Scholar] [CrossRef]
- Li, Z.; Tong, Y.; Chen, X.; Shen, K. Accuracy of ultrasonographic changes during neoadjuvant chemotherapy to predict axillary lymph node response in clinical node-positive breast cancer patients. Front. Oncol. 2022, 12, 954282. [Google Scholar] [CrossRef] [PubMed]
- Tinterri, C.; Fernandes, B.; Zambelli, A.; Sagona, A.; Barbieri, E.; Di Maria Grimaldi, S.; Darwish, S.S.; Jacobs, F.; De Carlo, C.; Iuzzolino, M.; et al. The Impact of Different Patterns of Residual Disease on Long-Term Oncological Outcomes in Breast Cancer Patients Treated with Neo-Adjuvant Chemotherapy. Cancers 2024, 16, 376. [Google Scholar] [CrossRef] [PubMed]
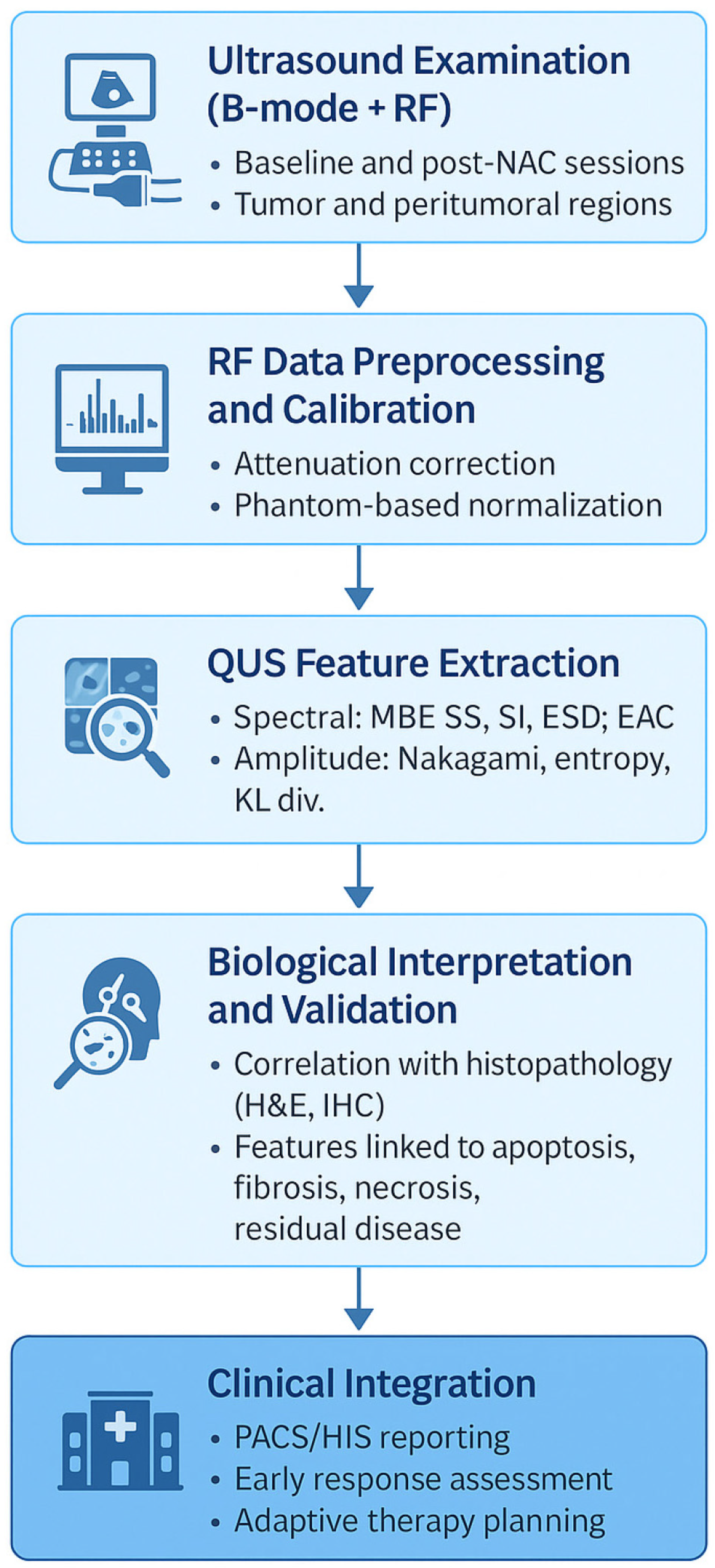
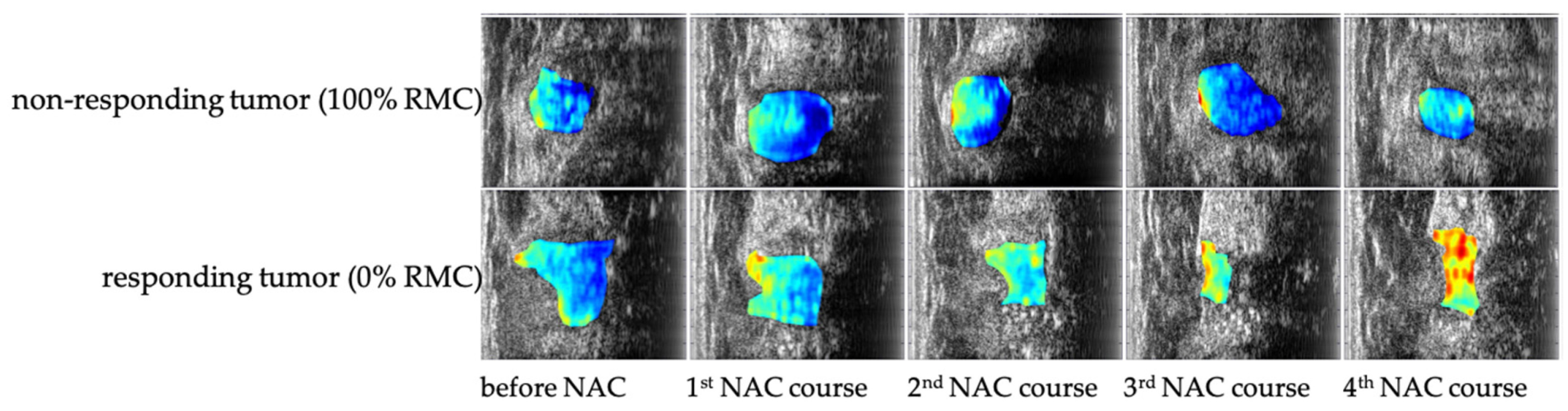
| Imaging Modality | Primary Assessment | Sensitivity to Early Biological Changes | Accessibility | Key Limitations |
|---|---|---|---|---|
| MRI | Morphologic, vascular (contrast-based) | Limited (size, enhancement) | Moderate | Expensive, time-consuming, limited access |
| CT | Morphological (density) | Limited | High | Ionizing radiation, poor soft-tissue contrast |
| B-mode | Morphological (echogenicity, size) | Qualitative only | Excellent | Operator-dependent, subjective |
| Mammography | Morphological (density, calcifications) | None | High | Low accuracy in dense breasts, radiation |
| Quantitative ultrasound (QUS) | Microstructural (spectral, scattering, attenuation parameters) | Quantitative (RF-derived biomarkers) | Excellent | Lower spatial resolution than MRI; limited standardization |
| Parameter | Unit | Sensitivity to Microstructural Changes | Interpretation/Clinical Significance | References |
|---|---|---|---|---|
| spectral slope (SS) | dB/MHz | High (dominant scatterer size) | Steeper (more negative) slopes imply finer dominant structures; useful for lesion characterization and early therapy monitoring. | [64,65,66,67] |
| 0 MHz intercept (SI) | dB | Moderate-high (backscatter amplitude) | Higher SI indicates stronger impedance fluctuations/overall scatter; sensitive to early treatment-induced cell death (after proper calibration). | [64,65,66,69] |
| midband fit (MBF) | dB | Moderate-high (backscatter level) | Mean of the fitted log-spectrum within the mid band; increases may reflect apoptosis/early microstructural disorganization; useful for early response. | [50,64,65,66,67] |
| backscatter coefficient (BSC) | 1/(cm·sr) | High (total backscatter intensity) | System-independent physical measure after reference-phantom normalization and attenuation compensation; supports longitudinal, quantitative comparisons. | [49,64,66,69] |
| effective scatterer diameter (ESD) | mm | High (structure size) | Larger ESD → coarser dominant microstructure (e.g., edema/necrosis); smaller ESD → finer subcellular features (e.g., nuclear condensation). Trends are context-dependent. | [50,64,66] |
| effective acoustic concentration (EAC) | 1/mm3 | High (number of scatterers) | Higher EAC reflects more or higher-contrast scatterers; may decrease with necrosis or increase with inflammatory/fibrotic changes; interpret with SI/MBF/ESD jointly. | [50,64,66] |
| Parameter | Unit | Sensitivity to Microstructural Changes | Interpretation/Clinical Significance | References |
|---|---|---|---|---|
| Nakagami parameter (m) | - | Moderate–high (homogeneity/clustering) | m ≈ 1: Rayleigh (fully developed speckle, random scattering); m < 1: pre-Rayleigh (sparse/heterogeneous); m > 1: post-Rayleigh (more coherent/ordered microstructure). Useful for tracking organization/fibrosis and malignancy-related heterogeneity. | [48,76,77] |
| homodyned K distribution | – | High (mixed scatterer populations) | Flexibly models mixtures from Rayleigh-like speckle to signals dominated by strong, discrete scatterers (e.g., fibrous or calcified inclusions coexisting with cellular tissue). | [48,71,72,78] |
| envelope entropy | bit | High (tissue heterogeneity) | Higher entropy → greater disorder/heterogeneity; decline during NAC may indicate homogenization/acoustic normalization. | [72,79,80] |
| Kullback–Leibler (KL) divergence | – | Moderate–high (distributional shift vs. reference) | Statistical distance between tumor amplitude histogram and reference tissue (e.g., contralateral/peritumoral); sensitive to subtle microstructural alterations. | [48,74,75] |
| skewness | – | Moderate (asymmetry/strong scatterers) | Positive skewness suggests presence of strong scatterers (e.g., calcifications); negative skewness indicates predominance of finer structures. | [75] |
| kurtosis | – | Moderate (tail/peakedness) | Higher kurtosis → more peaked/“uniform” scattering; lower → broader tails/marked variability and heterogeneity. | [75] |
| effective number of scatterers (ENS) | – | High (effective scatterer population) | Estimates effective scatterer count; decreases with necrosis or structural homogenization during effective NAC. | [81] |
| generalized gamma distribution parameters (α, β) | – | High (flexible fit to real histograms) | Shape parameters capture changes in microstructural organization; more flexible than Rayleigh/Nakagami for real amplitude histograms. | [78] |
| Model | QUS Application | Analyzed QUS Parameters | Key Findings | References |
|---|---|---|---|---|
| Murine tumor models | Monitoring response to chemotherapy, radiotherapy, and targeted therapies; detection of apoptosis and microstructural remodeling | MBF, SS, SI, ESD, EAC | Early detection of microstructural alterations associated with apoptosis and cell death; strong correlation with histopathology | [36,40,58,98,103,104,105] |
| In vitro cellular models | Assessment of apoptosis and necrosis effects on ultrasonic backscatter | MBF, SS, SI, ESD, EAC | QUS detects morphological changes such as chromatin condensation and nuclear fragmentation during programmed cell death | [47,48,58,103,104] |
| Acoustic phantoms | Calibration and validation of QUS systems; repeatability and cross-platform testing | BSC, AC, ESD, EAC | Provide standardized reference media for system calibration; enable evaluation of scatterer-related effects on spectral and statistical parameters | [47,48] |
| Ex vivo tissue models | Validation of QUS parameters in excised tumor specimens | MBF, SS, SI, ESD, EAC | Confirmed correlation between QUS-derived parameters and tissue microstructural organization in resected samples | [47,48,103,104] |
| Year (Journal) | No. of Patients | Assessment Time | Analyzed QUS Parameters | Key Findings | References |
|---|---|---|---|---|---|
| 2005–2013 (Cancer Res, Clin Cancer Res) | ~20–30 | Weeks 1–2 | MBF, SS, SI | Early rise in QUS parameters among responders; no change in resistant tumors; markers of apoptosis and microstructural remodeling | [58,106] |
| 2018 (PLoS One) | 96 | Weeks 1, 4, 8 | QUS + texture + molecular | Combined QUS, texture, and molecular biomarkers yielded 78–86% accuracy for early response prediction | [41] |
| 2020 (Ultrsound Med Biol) | 100100 | Weeks 1, 4, 8 | QUS + texture (2 scanners) | High repeatability and inter-system consistency in clinical measurements | [108] |
| 2017 (Sci Rep) | 56 | Pre-NAC baseline | QUS (tumor core + margin) | Baseline QUS predicts NAC response and 5-year recurrence-free survival | [36] |
| 2022 (Cancers) | 83 | Week 4 | QUS + higher-order texture | Early prediction of recurrence; accuracy ≈ 81% | [90] |
| 2019 (PLoS One) | 16/24 tumors | After each NAC cycle (1–5) | IBSC + envelope statistics (H-K) | AUC 0.82–0.91 (2nd–3rd cycle); response predicted earlier than size reduction | [109] |
| 2022 (Med Phys) | 37 tumors | After 1st and 3rd dose | KLD/KSS from RF envelopes | Non-response detected after 1st dose (AUC ~0.83–0.84); improved accuracy after 3rd (AUC ~0.90–0.91) | [110] |
| 2021 (Cancers) | 24 | Baseline + 7 days after 1–4 cycles | Quantitative echogenicity | Echogenicity changes correlate with treatment outcome | [74] |
| 2020 (PLoS One) | 59 | During NAC | QUS radiomics | In-treatment QUS features outperform baseline in predicting pCR | [100] |
| 2022 (Sci Rep) | 181 | Pre-NAC | Multiparametric QUS with deep learning | Pre-treatment QUS-based deep learning enables accurate response prediction | [46] |
| 2024 (Sci Rep) | 174 | Pre-NAC | Transfer learning on QUS maps | Transfer learning improves pre-treatment response prediction | [42] |
| 2024 (IEEE Trans Biomed Eng) | 56 | Adaptive (serial NAC) | QUS-based probability maps | Serial probabilistic mapping enables adaptive response classification | [99] |
| 2025 (Cancers) | 100 | Weeks 1, 4, 8 | QUS + texture model validation | Prospective validation of early prediction model; supports treatment intensification | [111] |
| Ongoing (ClinicalTrials.gov NCT04050228) | ≥100 (planned) | Adaptive NAC | Parametric QUS + radiomics | Ongoing prospective trial of QUS-guided adaptive NAC | [112] |
| Year (Journal) | No. of Patients/Centers | Assessment Time | Model Type | Key Findings | References |
|---|---|---|---|---|---|
| 2018 (PLos ONE) | 96 | Pretreament, Weeks 1, 4, 8, presurgery | QUS + texture + molecular | Combined QUS, texture, and molecular features predicted response with up to 86% accuracy | [41] |
| 2023 (Sci Rep) | 208 | Pretreatment | QUS, texture derivatives, core-margin, molecular | 87% accuracy in predicting NAC response before treatment; supports risk stratification and personalized therapy | [102] |
| 2023 (Academic Radiology) | 255 | Pretreatment | QUS (B-mode US, SWE), molecular, CNN | LDUR model (B-mode US + SWE): AUC 0.97, Sensitivity 95.5%, Specificity 91.1% | [45] |
| 2023 (Acad Radiol) | 112 | Pre-, 2nd and 4th NAC cycles | QUS (BUS), SWE, delta radiomics | Model LDUR (BUS + SWE): AUC 0.97, Sens 95.5%, Spec 91.1% | [120] |
| 2024 (Sci Rep) | 174100 | PretreatmentWeeks 1, 4, 8 | QUS parametric maps (core + margin), deep learning | Transfer learning: balanced accuracy 86%, F1-score 0.83; effective prediction of non-responders (NR) vs. responders (RR) | [42] |
| 2025 (J Imaging) | 56 | Pretreatment | QUS, texture derivatives, molecular subtype | Sensitivity 94%, Specificity 100% | [38] |
| Year (Journal) | No. of Patients/Centers | Assessment Time | Analyzed QUS Parameters | Key Findings | References |
|---|---|---|---|---|---|
| 2018 (PLoS One) | 96 patients, multi-institutional | Pretreatment, Weeks 1, 4, 8, pre-surgery | QUS + texture + molecular | Combined QUS, texture, and molecular features predicted response with up to 86% accuracy at week 4 | [41] |
| 2020 (Cancer Med) | 82 patients, 4 centers | Pretreatment | Pretreatment QUS radiomics | Radiomic features predicted NAC response with 87% accuracy; supported early risk stratification and personalized therapy | [100] |
| 2022 (eClinicalMedicine) | 393 patients, 3 hospitals | Pre-treatment, after 1st/2nd NAC cycles | Deep learning on US images | Siamese multi-task network predicted pCR with AUC 0.90–0.99 in external validation | [121] |
| 2024 (Front Oncol) | Randomized multi-center trial100 | Pretreatment, Weeks 1, 4 | QUS-guided adaptive NAC | QUS-based model prospectively validated; early prediction enabled therapy adaptation | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrzkowska-Wróblewska, H. The Role of Quantitative Ultrasound in Monitoring Neoadjuvant Chemotherapy in Breast Cancer: A Narrative Review. Cancers 2025, 17, 3676. https://doi.org/10.3390/cancers17223676
Piotrzkowska-Wróblewska H. The Role of Quantitative Ultrasound in Monitoring Neoadjuvant Chemotherapy in Breast Cancer: A Narrative Review. Cancers. 2025; 17(22):3676. https://doi.org/10.3390/cancers17223676
Chicago/Turabian StylePiotrzkowska-Wróblewska, Hanna. 2025. "The Role of Quantitative Ultrasound in Monitoring Neoadjuvant Chemotherapy in Breast Cancer: A Narrative Review" Cancers 17, no. 22: 3676. https://doi.org/10.3390/cancers17223676
APA StylePiotrzkowska-Wróblewska, H. (2025). The Role of Quantitative Ultrasound in Monitoring Neoadjuvant Chemotherapy in Breast Cancer: A Narrative Review. Cancers, 17(22), 3676. https://doi.org/10.3390/cancers17223676






