Late Morbidity and Mortality in Survivors of Childhood Ependymoma: A Report from the Childhood Cancer Survivor Study (CCSS)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mortality
2.2. Chronic Health Conditions (CHCs)
2.3. Subsequent Neoplasms (SNs)
2.4. Statistical Methods
3. Results
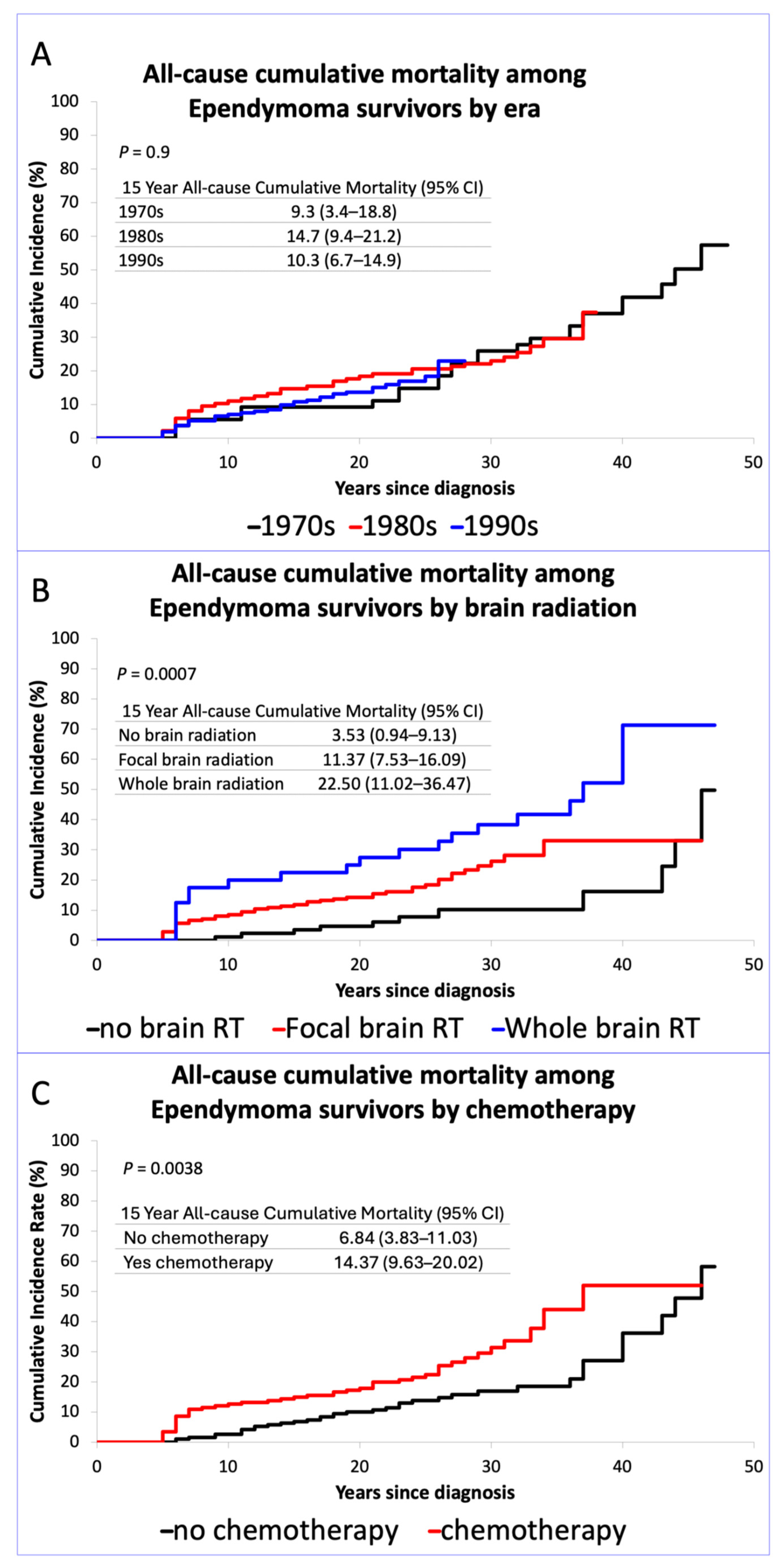
3.1. Late Mortality
3.2. Chronic Health Conditions
3.3. Subsequent Neoplasm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCSS | Childhood Cancer Survivor Study |
| NCI | National Cancer Institute |
| ICD-9 and ICD-10 | International Classification of Diseases, Ninth and Tenth Revisions |
| CHCs | Chronic Health Conditions |
| CTCAE | Common Terminology Criteria for Adverse Events |
| SNs | Subsequent Neoplasms |
| SMNs | Subsequent Malignant Neoplasms |
| ICD-O, 3rd version | International Classification of Diseases for Oncology |
| RR | Relative Risk |
| SIOP | International Society of Pediatric Oncology |
References
- Ostrom, Q.T.; de Blank, P.M.; Kruchko, C.; Petersen, C.M.; Liao, P.; Finlay, J.L.; Stearns, D.S.; Wolff, J.E.; Wolinsky, Y.; Letterio, J.J.; et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncology 2014, 16, x1–x36. [Google Scholar] [CrossRef]
- Hukin, J.; Epstein, F.; Lefton, D.; Allen, J. Treatment of Intracranial Ependymoma by Surgery Alone. Pediatr. Neurosurg. 1998, 29, 40–45. [Google Scholar] [CrossRef]
- Merchant, T.E.; Bendel, A.E.; Sabin, N.D.; Burger, P.C.; Shaw, D.W.; Chang, E.; Wu, S.; Zhou, T.; Eisenstat, D.D.; Foreman, N.K.; et al. Conformal Radiation Therapy for Pediatric Ependymoma, Chemotherapy for Incompletely Resected Ependymoma, and Observation for Completely Resected, Supratentorial Ependymoma. J. Clin. Oncol. 2019, 37, 974–983. [Google Scholar] [CrossRef] [PubMed]
- E Merchant, T.; Zhu, Y.; Thompson, S.J.; Sontag, M.R.; Heideman, R.L.; E Kun, L. Preliminary results from a Phase II trail of conforml radiation therapy for pediatric patients with localised low-grade astrocytoma and ependymoma. Int. J. Radiat. Oncol. 2002, 52, 325–332. [Google Scholar] [CrossRef]
- Merchant, T.E.; Kiehna, E.N.; Li, C.; Xiong, X.; Mulhern, R.K. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Fouladi, M. Ependymoma: New Therapeutic Approaches Including Radiation and Chemotherapy. J. Neuro-Oncology 2005, 75, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Bouffet, E.; Foreman, N. Chemotherapy for intracranial ependymomas. Child’s Nerv. Syst. 1999, 15, 563–570. [Google Scholar] [CrossRef]
- Grill, J.; Kalifa, C.; Doz, F.; Schoepfer, C.; Sainte-Rose, C.; Couanet, D.; Terrier-Lacombe, M.; Valteau-Couanet, D.; Hartmann, O. A High-Dose Busulfan-Thiotepa Combination Followed by Autologous Bone Marrow Transplantation in Childhood Recurrent Ependymoma. Pediatr. Neurosurg. 1996, 25, 7–12. [Google Scholar] [CrossRef]
- Smith, A.; Onar-Thomas, A.; Ellison, D.; Owens-Pickle, E.; Wu, S.; Leary, S.E.S.; Fouladi, M.; Merchant, T.; Gajjar, A.; Foreman, N. EPEN-54. ACNS0831, PHASE III RANDOMIZED TRIAL OF POST-RADIATION CHEMOTHERAPY IN PATIENTS WITH NEWLY DIAGNOSED EPENDYMOMA AGES 1 TO 21 YEARS. Neuro-Oncology 2020, 22, iii318–iii319. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Liu, Q.; Yasui, Y.; Huang, S.; Ness, K.K.; Leisenring, W.; Hudson, M.M.; Donaldson, S.S.; King, A.A.; Stovall, M.; et al. Long-Term Outcomes Among Adult Survivors of Childhood Central Nervous System Malignancies in the Childhood Cancer Survivor Study. JNCI J. Natl. Cancer Inst. 2009, 101, 946–958. [Google Scholar] [CrossRef]
- Coltin, H.; Pequeno, P.; Liu, N.; Tsang, D.S.; Gupta, S.; Taylor, M.D.; Bouffet, E.; Ramaswamy, V.; Nathan, P.C. The medical and functional burden of surviving childhood ependymoma: A population-based study in Ontario, Canada. Pediatr. Blood Cancer 2024, 71, e31275. [Google Scholar] [CrossRef] [PubMed]
- Kenborg, L.; Winther, J.F.; Linnet, K.M.; Krøyer, A.; Albieri, V.; Holmqvist, A.S.; Tryggvadottir, L.; Madanat-Harjuoja, L.M.; Stovall, M.; Hasle, H.; et al. Neurologic disorders in 4858 survivors of central nervous system tumors in childhood—An Adult Life after Childhood Cancer in Scandinavia (ALiCCS) study. Neuro-Oncology 2019, 21, 125–136. [Google Scholar] [CrossRef]
- Roddy, E.; Mueller, S. Late Effects of Treatment of Pediatric Central Nervous System Tumors. J. Child Neurol. 2015, 31, 237–254. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Chen, Y.; Yasui, Y.; Leisenring, W.; Gibson, T.M.; Mertens, A.C.; Stovall, M.; Oeffinger, K.C.; Bhatia, S.; Krull, K.R.; et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. New Engl. J. Med. 2016, 374, 833–842. [Google Scholar] [CrossRef] [PubMed]
- de Blank, P.M.K.; Lange, K.R.; Xing, M.; Salehabadi, S.M.; Srivastava, D.; Brinkman, T.M.; Ness, K.K.; Oeffinger, K.C.; Neglia, J.; Krull, K.R.; et al. Temporal changes in treatment and late mortality and morbidity in adult survivors of childhood glioma: A report from the Childhood Cancer Survivor Study. Nat. Cancer 2024, 5, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Salloum, R.; Chen, Y.; Yasui, Y.; Packer, R.; Leisenring, W.; Wells, E.; King, A.; Howell, R.; Gibson, T.M.; Krull, K.R.; et al. Late Morbidity and Mortality Among Medulloblastoma Survivors Diagnosed Across Three Decades: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2019, 37, 731–740. [Google Scholar] [CrossRef]
- Leisenring, W.M.; Mertens, A.C.; Armstrong, G.T.; Stovall, M.A.; Neglia, J.P.; Lanctot, J.Q.; Boice, J.D.; Whitton, J.A.; Yasui, Y. Pediatric Cancer Survivorship Research: Experience of the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009, 27, 2319–2327. [Google Scholar] [CrossRef]
- Robison, L.L.; Armstrong, G.T.; Boice, J.D.; Chow, E.J.; Davies, S.M.; Donaldson, S.S.; Green, D.M.; Hammond, S.; Meadows, A.T.; Mertens, A.C.; et al. The Childhood Cancer Survivor Study: A National Cancer Institute–Supported Resource for Outcome and Intervention Research. J. Clin. Oncol. 2009, 27, 2308–2318. [Google Scholar] [CrossRef]
- Packer, R.J.; Gurney, J.G.; Punyko, J.A.; Donaldson, S.S.; Inskip, P.D.; Stovall, M.; Yasui, Y.; Mertens, A.C.; Sklar, C.A.; Nicholson, H.S.; et al. Long-Term Neurologic and Neurosensory Sequelae in Adult Survivors of a Childhood Brain Tumor: Childhood Cancer Survivor Study. J. Clin. Oncol. 2003, 21, 3255–3261. [Google Scholar] [CrossRef]
- Howell, R.M.; Smith, S.A.; Weathers, R.E.; Kry, S.F.; Stovall, M. Adaptations to a Generalized Radiation Dose Reconstruction Methodology for Use in Epidemiologic Studies: An Update from the MD Anderson Late Effect Group. Radiat. Res. 2019, 192, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic health conditions in adult survivors of childhood cancer. New Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Gibson, T.M.; Mostoufi-Moab, S.; Stratton, K.L.; Leisenring, W.M.; Barnea, D.; Chow, E.J.; Donaldson, S.S.; Howell, R.M.; Hudson, M.M.; Mahajan, A.; et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–99: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018, 19, 1590–1601. [Google Scholar] [CrossRef]
- Neglia, J.P.; Friedman, D.L.; Yasui, Y.; Mertens, A.C.; Hammond, S.; Stovall, M.; Donaldson, S.S.; Meadows, A.T.; Robison, L.L. Second Malignant Neoplasms in Five-Year Survivors of Childhood Cancer: Childhood Cancer Survivor Study. JNCI J. Natl. Cancer Inst. 2001, 93, 618–629. [Google Scholar] [CrossRef]
- Leblond, P.; Massimino, M.; English, M.; Ritzmann, T.A.; Gandola, L.; Calaminus, G.; Thomas, S.; Pérol, D.; Gautier, J.; Grundy, R.G.; et al. Toward Improved Diagnosis Accuracy and Treatment of Children, Adolescents, and Young Adults with Ependymoma: The International SIOP Ependymoma II Protocol. Front. Neurol. 2022, 13, 887544. [Google Scholar] [CrossRef]
- Pajtler, K.W.; Witt, H.; Sill, M.; Jones, D.T.; Hovestadt, V.; Kratochwil, F.; Wani, K.; Tatevossian, R.; Punchihewa, C.; Johann, P.; et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 2015, 27, 728–743. [Google Scholar] [CrossRef]
- Marinoff, A.E.; Ma, C.; Guo, D.; Snuderl, M.; Wright, K.D.; Manley, P.E.; Al-Sayegh, H.; Sinai, C.E.; Ullrich, N.J.; Marcus, K.; et al. Rethinking childhood ependymoma: A retrospective, multi-center analysis reveals poor long-term overall survival. J. Neuro-Oncology 2017, 135, 201–211. [Google Scholar] [CrossRef]
- Shu, H.G.; Sall, W.F.; Maity, A.; Tochner, Z.A.; Janss, A.J.; Belasco, J.B.; Rorke-Adams, L.B.; Phillips, P.C.; Sutton, L.N.; Fisher, M.J. Childhood intracranial ependymoma. Cancer 2007, 110, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Robertson, P.L.; Zeltzer, P.M.; Boyett, J.M.; Rorke, L.B.; Allen, J.C.; Geyer, J.R.; Stanley, P.; Li, H.; Albright, A.L.; McGuire-Cullen, P.; et al. Survival and prognostic factors following radiation therapy and chemotherapy for ependymomas in children: A report of the Children’s Cancer Group. J. Neurosurg. 1998, 88, 695–703. [Google Scholar] [CrossRef]
- Garvin, J.H.; Selch, M.T.; Holmes, E.; Berger, M.S.; Finlay, J.L.; Flannery, A.; Goldwein, J.W.; Packer, R.J.; Rorke-Adams, L.B.; Shiminski-Maher, T.; et al. Phase II study of pre-irradiation chemotherapy for childhood intracranial ependymoma. Children’s Cancer Group protocol 9942: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2012, 59, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- A Healey, E.; Barnes, P.D.; Kupsky, W.J.; Scott, R.M.; E Sallan, S.; Black, P.M.; Tarbell, N.J. The prognostic significance of postoperative residual tumor in ependymoma. Neurosurgery 1991, 28, 666. [Google Scholar] [CrossRef] [PubMed]
- Nazar, G.B.; Hoffman, H.J.; Becker, L.E.; Jenkin, D.; Humphreys, R.P.; Hendrick, E.B. Infratentorial ependymomas in childhood: Prognostic factors and treatment. J. Neurosurg. 1990, 72, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Mulhern, R.K.; Krasin, M.J.; Kun, L.E.; Williams, T.; Li, C.; Xiong, X.; Khan, R.B.; Lustig, R.H.; Boop, F.A.; et al. Preliminary Results From a Phase II Trial of Conformal Radiation Therapy and Evaluation of Radiation-Related CNS Effects for Pediatric Patients with Localized Ependymoma. J. Clin. Oncol. 2004, 22, 3156–3162. [Google Scholar] [CrossRef] [PubMed]
- Zapotocky, M.; Beera, K.; Adamski, J.; Laperierre, N.; Guger, S.; Janzen, L.; Lassaletta, A.; Nobre, L.F.; Bartels, U.; Tabori, U.; et al. Survival and functional outcomes of molecularly defined childhood posterior fossa ependymoma: Cure at a cost. Cancer 2019, 125, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
| Total | 1970–1979 | 1980–1989 | 1990–1999 | ||
|---|---|---|---|---|---|
| Characteristic | n = 404 | n = 55 | n = 136 | n = 213 | p-Value a |
| Diagnosis | |||||
| 9383.1—subependymoma | 4 (0.99) | 1 (1.8) | 1 (0.7) | 2 (0.9) | 0.6 |
| 9391.3—ependymoma NOS | 342 (84.65) | 48 (87.3) | 118 (86.8) | 176 (82.6) | |
| 9392.3—anaplastic ependymoma, | 36 (8.91) | 2 (3.6) | 10 (7.4) | 24 (11.3) | |
| 9393.1—papillary ependymoma | 6 (1.49) | 2 (3.6) | 1 (0.7) | 3 (1.4) | |
| 9394.1—myxopapillary ependymoma | 16 (3.96) | 2 (3.6) | 6 (4.4) | 8 (3.8) | |
| Sex (n,%): Male | 212 (52.5) | 32 (58.2) | 65 (47.8) | 115 (54.0) | 0.35 |
| Female | 192 (47.5) | 23 (41.8) | 71 (52.2) | 98 (46.0) | |
| Race/Ethnicity (n,%): Non-Hispanic White | 326 (80.7) | 50 (90.9) | 117 (86.0) | 159 (74.6) | 0.006 |
| Non-Hispanic Black | 30 (7.4) | 0 (0.0) | 6 (4.4) | 24 (11.3) | |
| Hispanic | 33 (8.2) | 2 (3.6) | 8 (5.9) | 23 (10.8) | |
| Other | 15 (3.7) | 3 (5.5) | 5 (3.7) | 7 (3.3) | |
| Age at diagnosis (median, min–max) years | 6 (0–20) | 10 (0–19) | 6 (0–20) | 5 (0–20) | 0.0001 |
| Time since diagnosis (median, min–max) years | 22 (5–49) | 34 (6–49) | 27.5 (5–37) | 20 (5–28) | <0.001 |
| Chemotherapy Exposure (n,%) | 174 (47.7) | 13 (29.5) | 59 (50) | 102 (50.2) | 0.037 |
| Any surgery (n,%) | 367 (100) | 45 (100) | 118 (100) | 204 (100) | NA b |
| Any brain RT (n,%) | 265 (75.7) | 28 (65.1) | 97 (84.3) | 140 (72.9) | 0.0174 |
| Radiation treatment group, dose ≥40 Gy | |||||
| Dose in Gy, among those ≥40 Gy (median, min–max) | 54 (40–300) | 50 (40–69) | 54 (43.2–90) | 56 (40–300) | <0.0001 |
| No brain radiation | 85 (25.2) | 15 (35.7) | 18 (16.1) | 52 (28.4) | <0.0001 |
| Focal brain radiation | 212 (62.9) | 9 (21.4) | 77 (68.8) | 126 (68.9) | |
| Whole-brain radiation | 40 (11.9) | 18 (42.9) | 17 (15.2) | 5 (2.7) | |
| Recurrence within 5 years (n,%) | 48 (11.9) | 3 (5.5) | 16 (11.8) | 29 (13.6) | 0.25 |
| Age at radiation in years (median, min–max) | 5.45 (0.8–21.35) | 9.33 (1.19–16.96) | 5.39 (0.8–20.96) | 5.41 (0.92–21.35) | 0.1815 |
| All-Cause Mortality | Death due to Recurrence or Progression of Primary Malignancy | Health-Related Cause Mortality | |
|---|---|---|---|
| RR [95%CI] | RR [95%CI] | RR [95%CI] | |
| Treatment Era | |||
| 1970–1979 | 1.0 | 1.0 | 1.0 |
| 1980–1989 | 0.95 (0.54–1.69) | 1.74 (0.54–5.64) | 0.68 (0.31–1.5) |
| 1990–1999 | 0.88 (0.47–1.65) | 1.51 (0.46–4.99) | 0.85 (0.35–2.09) |
| Sex | |||
| Male | 1.0 | 1.0 | 1.0 |
| Female | 0.95 (0.63–1.41) | 1.21 (0.64–2.28) | 0.84 (0.46–1.51) |
| Race | |||
| Non-Hispanic White | 1.0 | 1.0 | 1.0 |
| Other | 0.92 (0.52–1.61) | 0.93 (0.4–2.13) | 0.85 (0.35–2.04) |
| All-Cause | Recurrence or Progression of Primary Malignancy | Health-Related Causes | |
|---|---|---|---|
| RR [95%CI] | RR [95%CI] | RR [95%CI] | |
| Sex | |||
| Male | 1.0 | 1.0 | 1.0 |
| female | 0.92 (0.58–1.46) | 1.24 (0.58–2.67) | 0.78 (0.39–1.55) |
| Race | |||
| Non-Hispanic White | 1.0 | 1.0 | 1.0 |
| Other | 0.71 (0.36–1.4) | 1.13 (0.45–2.83) | 0.29 (0.07–1.21) |
| Chemotherapy | |||
| No | 1.0 | 1.0 | 1.0 |
| Yes | 1.87 (1.11–3.14) | 1.56 (0.68–3.58) | 2.93 (1.31–6.54) |
| Brain Radiation | |||
| No brain radiation | 1.0 | 1.0 | 1.0 |
| Focal brain radiation | 2.25 (1.11–4.58) | 3.98 (0.92–17.33) | 1.44 (0.54–3.85) |
| Whole-brain radiation | 3.93 (1.81–8.54) | 5.2 (0.94–28.6) | 3.86 (1.43–10.43) |
| Any Grade 3–4 | >1 Grade 3–4 | Any Hearing CHC Grade 3–4 | |
|---|---|---|---|
| RR [95%CI] | RR [95%CI] | RR [95%CI] | |
| Sex | |||
| Male | 1.0 | 1.0 | 1.0 |
| Female | 1.13 (0.69–1.84) | 1.09 (0.52–2.27) | 0.43 (0.18–1.06) |
| Race | |||
| Non-Hispanic White | 1.0 | 1.0 | 1.0 |
| Other | 1.13 (0.61–2.1) | 1.42 (0.60–3.37) | 0.56 (0.17–1.90) |
| Chemotherapy | |||
| No | 1.0 | 1.0 | 1.0 |
| Yes | 1.15 (0.69–1.91) | 2.17 (0.99–4.73) | 0.55 (0.22–1.33) |
| Brain Radiation | |||
| No Brain Radiation | 1.0 | 1.0 | 1.0 |
| Focal Brain Radiation | 2.6 (1.25–5.39) | 4.39 (1.0–19.31) | 12.54 (1.60–98.14) |
| Whole-Brain Radiation | 3.5 (1.48–8.11) | 6.47 (1.32–31.64) | 15.65 (1.79–137.07) |
| Any Subsequent Neoplasm | Any Subsequent Malignant Neoplasm | |
|---|---|---|
| RR [95%CI] | RR [95%CI] | |
| Sex | ||
| Male | 1.0 | 1.0 |
| Female | 1.73 (0.86–3.49) | 1.16 (0.49–2.75) |
| Race | ||
| Non-Hispanic White | 1.0 | 1.0 |
| Other | 0.45 (0.14–1.47) | 0.48 (0.11–2.08) |
| Chemotherapy | ||
| No | 1.0 | 1.0 |
| Yes | 1.26 (0.62–2.58) | 1.67 (0.68–4.11) |
| RT | ||
| No Brain Radiation | 1.0 | 1.0 |
| Focal Brain Radiation | 2.78 (1.0–7.73) | 2.70 (0.74–9.93) |
| Whole-Brain Radiation | 3.82 (1.17–12.41) | 3.05 (0.66–13.98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lange, K.R.; de Blank, P.; Xing, M.; Mirzaei, S.; Srivastava, D.K.; Oeffinger, K.; Neglia, J.; Krull, K.; Nathan, P.C.; Howell, R.; et al. Late Morbidity and Mortality in Survivors of Childhood Ependymoma: A Report from the Childhood Cancer Survivor Study (CCSS). Cancers 2025, 17, 3669. https://doi.org/10.3390/cancers17223669
Lange KR, de Blank P, Xing M, Mirzaei S, Srivastava DK, Oeffinger K, Neglia J, Krull K, Nathan PC, Howell R, et al. Late Morbidity and Mortality in Survivors of Childhood Ependymoma: A Report from the Childhood Cancer Survivor Study (CCSS). Cancers. 2025; 17(22):3669. https://doi.org/10.3390/cancers17223669
Chicago/Turabian StyleLange, Katharine R., Peter de Blank, Mengqi Xing, Sedigheh Mirzaei, Deo Kumar Srivastava, Kevin Oeffinger, Joseph Neglia, Kevin Krull, Paul C. Nathan, Rebecca Howell, and et al. 2025. "Late Morbidity and Mortality in Survivors of Childhood Ependymoma: A Report from the Childhood Cancer Survivor Study (CCSS)" Cancers 17, no. 22: 3669. https://doi.org/10.3390/cancers17223669
APA StyleLange, K. R., de Blank, P., Xing, M., Mirzaei, S., Srivastava, D. K., Oeffinger, K., Neglia, J., Krull, K., Nathan, P. C., Howell, R., Ness, K. K., Turcotte, L. M., Leisenring, W., Armstrong, G. T., Brinkman, T., Bowers, D. C., & Okcu, M. F. (2025). Late Morbidity and Mortality in Survivors of Childhood Ependymoma: A Report from the Childhood Cancer Survivor Study (CCSS). Cancers, 17(22), 3669. https://doi.org/10.3390/cancers17223669








