Development and Validation of a Site-Specific Tumor Burden Score for Predicting Surgical Outcomes in Advanced Ovarian Cancer
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. MRI and CT Acquisition and Evaluation
2.3. Preoperative Imaging Evaluation Protocol
2.4. Intraoperative Evaluation
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
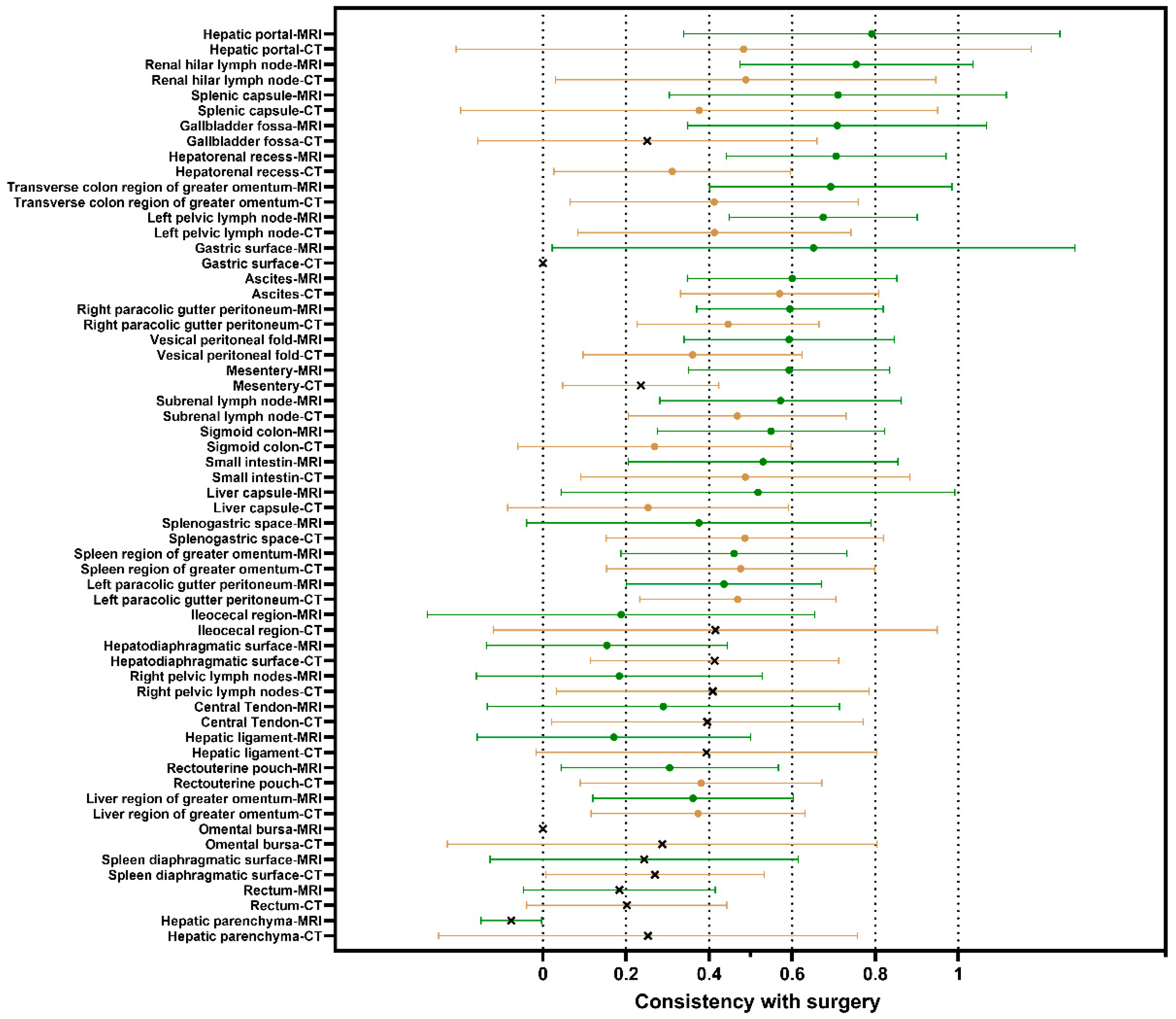
3.2. Comparisons Between Preoperative MRI/CT Site-Specific Tumor Burdens and Intraoperative Observations
3.3. Determination of the Suboptimal Cytoreduction Related Site-Specific Tumor Burden
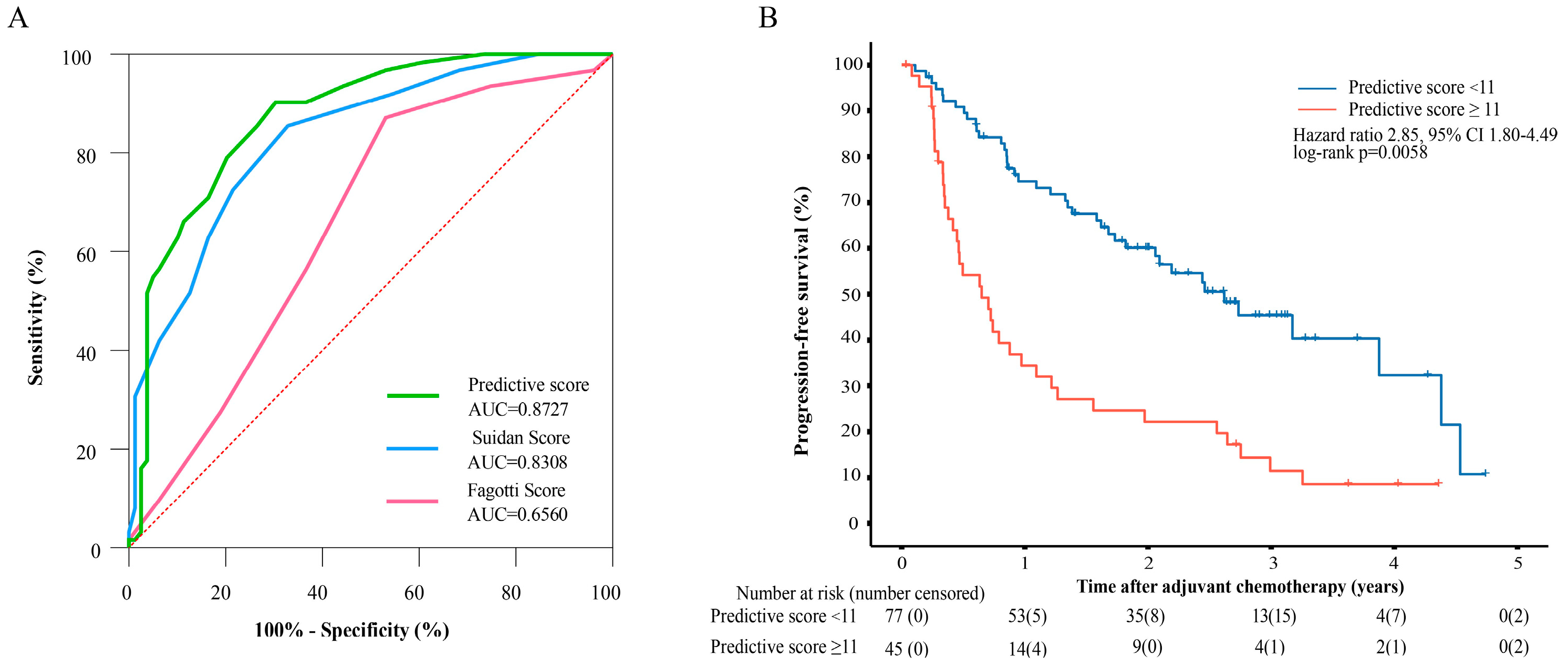
3.4. Evaluating the Performance of the Tumor Burden-Integrated Suboptimal Cytoreduction Predictive Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival Effect of Maximal Cytoreductive Surgery for Advanced Ovarian Carcinoma During the Platinum Era: A Meta-Analysis. J. Clin. Oncol. 2023, 41, 4065–4076. [Google Scholar] [CrossRef] [PubMed]
- Lyons, Y.A.; Reyes, H.D.; McDonald, M.E.; Newtson, A.; Devor, E.; Bender, D.P.; Goodheart, M.J.; Gonzalez Bosquet, J. Interval debulking surgery is not worth the wait: A National Cancer Database study comparing primary cytoreductive surgery versus neoadjuvant chemotherapy. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Coens, C.; Nankivell, M.; Kristensen, G.B.; Parmar, M.K.B.; Ehlen, T.; Jayson, G.C.; Johnson, N.; Swart, A.M.; Verheijen, R.; et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: Pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018, 19, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, N.; Devor, E.J.; Pedra Nobre, S.; Newtson, A.; Leslie, K.; Bender, D.P.; Smith, B.J.; Goodheart, M.J.; Gonzalez-Bosquet, J. Integrated Clinical and Genomic Models to Predict Optimal Cytoreduction in High-Grade Serous Ovarian Cancer. Cancers 2022, 14, 3554. [Google Scholar] [CrossRef]
- Lan, W.; Hong, J.; Huayun, T. Advances in ovarian cancer radiomics: A bibliometric analysis from 2010 to 2024. Front. Oncol. 2024, 14, 1456932. [Google Scholar] [CrossRef]
- Csikos, C.; Czina, P.; Molnár, S.; Kovács, A.R.; Garai, I.; Krasznai, Z.T. Predicting Complete Cytoreduction with Preoperative [18F]FDG PET/CT in Patients with Ovarian Cancer: A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 1740. [Google Scholar] [CrossRef]
- Angeles, M.A.; Rychlik, A.; Cabarrou, B.; Spagnolo, E.; Guyon, F.; Pérez-Benavente, A.; Gil-Moreno, A.; Siegrist, J.; Querleu, D.; Mery, E.; et al. A multivariate analysis of the prognostic impact of tumor burden, surgical timing and complexity after complete cytoreduction for advanced ovarian cancer. Gynecol. Oncol. 2020, 158, 614–621. [Google Scholar] [CrossRef]
- Horowitz, N.S.; Miller, A.; Rungruang, B.; Richard, S.D.; Rodriguez, N.; Bookman, M.A.; Hamilton, C.A.; Krivak, T.C.; Maxwell, G.L. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: An analysis of GOG 182. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.C.; Vasey, P.A.; Paul, J.; Hay, A.; Davis, J.A.; Kaye, S.B. Does aggressive surgery only benefit patients with less advanced ovarian cancer? Results from an international comparison within the SCOTROC-1 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 8802–8811. [Google Scholar] [CrossRef]
- Martinez, A.; Ngo, C.; Leblanc, E.; Gouy, S.; Luyckx, M.; Darai, E.; Classe, J.M.; Guyon, F.; Pomel, C.; Ferron, G.; et al. Surgical Complexity Impact on Survival After Complete Cytoreductive Surgery for Advanced Ovarian Cancer. Ann. Surg. Oncol. 2016, 23, 2515–2521. [Google Scholar] [CrossRef]
- Seifert, H.; Georgiou, A.; Alexander, H.; McLachlan, J.; Bodla, S.; Kaye, S.; Barton, D.; Nobbenhuis, M.; Gore, M.; Banerjee, S. Poor performance status (PS) is an indication for an aggressive approach to neoadjuvant chemotherapy in patients with advanced epithelial ovarian cancer (EOC). Gynecol. Oncol. 2015, 139, 216–220. [Google Scholar] [CrossRef]
- Engbersen, M.P.; Van’ TSant, I.; Lok, C.; Lambregts, D.M.J.; Sonke, G.S.; Beets-Tan, R.G.H.; van Driel, W.J.; Lahaye, M.J. MRI with diffusion-weighted imaging to predict feasibility of complete cytoreduction with the peritoneal cancer index (PCI) in advanced stage ovarian cancer patients. Eur. J. Radiol. 2019, 114, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Suidan, R.S.; Ramirez, P.T.; Sarasohn, D.M.; Teitcher, J.B.; Iyer, R.B.; Zhou, Q.; Iasonos, A.; Denesopolis, J.; Zivanovic, O.; Long Roche, K.C.; et al. A multicenter assessment of the ability of preoperative computed tomography scan and CA-125 to predict gross residual disease at primary debulking for advanced epithelial ovarian cancer. Gynecol. Oncol. 2017, 145, 27–31. [Google Scholar] [CrossRef]
- Fagotti, A.; Ferrandina, G.; Fanfani, F.; Ercoli, A.; Lorusso, D.; Rossi, M.; Scambia, G. A laparoscopy-based score to predict surgical outcome in patients with advanced ovarian carcinoma: A pilot study. Ann. Surg. Oncol. 2006, 13, 1156–1161. [Google Scholar] [CrossRef]
- Tan, X.; Chen, H. The Prognostic Value of Prognostic Nutritional Index in Patients with Ovarian Cancer: A Systematic Review and Meta-Analysis. Nutr. Cancer 2023, 75, 73–81. [Google Scholar] [CrossRef]
- Timmerman, D.; Planchamp, F.; Bourne, T.; Landolfo, C.; du Bois, A.; Chiva, L.; Cibula, D.; Concin, N.; Fischerova, D.; Froyman, W.; et al. ESGO/ISUOG/IOTA/ESGE Consensus Statement on pre-operative diagnosis of ovarian tumors. Int. J. Gynecol. Cancer 2021, 31, 961–982. [Google Scholar] [CrossRef]
- Feng, Z.; Fu, Y.; Li, R.; Li, H.; Lu, J.; Chen, X.; Ju, X.; Wu, X.; Wen, H. Diffusion-weighted magnetic resonance imaging for the pre-operative evaluation of epithelial ovarian cancer patients. Gynecol. Oncol. 2023, 174, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.Y.; Cheah, W.K.; Ramli Hamid, M.T.; Md Shah, M.N.; Fadzli, F.; Kaur, S.; See, M.H.; Mohd Taib, N.A.; Rahmat, K. Impact of preoperative magnetic resonance imaging on surgery and eligibility for intraoperative radiotherapy in early breast cancer. PLoS ONE 2022, 17, e0274385. [Google Scholar] [CrossRef]
- Coccolini, F.; Catena, F.; Glehen, O.; Yonemura, Y.; Sugarbaker, P.H.; Piso, P.; Montori, G.; Ansaloni, L. Complete versus incomplete cytoreduction in peritoneal carcinosis from gastric cancer, with consideration to PCI cut-off. Systematic review and meta-analysis. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2015, 41, 911–919. [Google Scholar] [CrossRef]
- Dottino, J.A.; He, W.; Sun, C.C.; Zhao, H.; Fu, S.; Rauh-Hain, J.A.; Suidan, R.S.; Lu, K.H.; Giordano, S.H.; Meyer, L.A. National trends in bowel and upper abdominal procedures in ovarian cancer surgery. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 1195–1202. [Google Scholar] [CrossRef]
- Rush, S.K.; Lees, B.F.; Huang, D.S.; Peterson, M.F.; Al-Niaimi, A. Splenectomy at the time of primary or interval cytoreductive surgery for epithelial ovarian carcinoma: A review of outcomes. Gynecol. Oncol. 2022, 167, 283–288. [Google Scholar] [CrossRef]
- Kostov, S.; Selçuk, I.; Watrowski, R.; Dineva, S.; Kornovski, Y.; Slavchev, S.; Ivanova, Y.; Yordanov, A. Neglected Anatomical Areas in Ovarian Cancer: Significance for Optimal Debulking Surgery. Cancers 2024, 16, 285. [Google Scholar] [CrossRef]
- Llueca, A.; Serra, A.; Rivadulla, I.; Gomez, L.; Escrig, J.; MUAPOS working group (Multidisciplinary Unit of Abdominal Pelvic Oncology Surgery). Prediction of suboptimal cytoreductive surgery in patients with advanced ovarian cancer based on preoperative and intraoperative determination of the peritoneal carcinomatosis index. World J. Surg. Oncol. 2018, 16, 37. [Google Scholar] [CrossRef]
- Rizzo, S.; De Piano, F.; Buscarino, V.; Pagan, E.; Bagnardi, V.; Zanagnolo, V.; Colombo, N.; Maggioni, A.; Del Grande, M.; Del Grande, F.; et al. Pre-operative evaluation of epithelial ovarian cancer patients: Role of whole body diffusion weighted imaging MR and CT scans in the selection of patients suitable for primary debulking surgery. A single-centre study. Eur. J. Radiol. 2020, 123, 108786. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Wang, X.; Zheng, T.; Peng, F.; Xiong, L.; Wang, Y.; Gong, L. Development and validation of radiomics nomogram for metastatic status of epithelial ovarian cancer. Sci. Rep. 2024, 14, 12456. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xiao, Y.; Guo, W.; Yao, J.; Lan, T.; Li, S.; Wen, H.; Zhu, W.; He, G.; Zheng, H.; et al. Development and validation of an ultrasound-based deep learning radiomics nomogram for predicting the malignant risk of ovarian tumours. Biomed. Eng. Online 2024, 23, 41. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]

| R0 Group | Non-R0 Group | p Value | |
|---|---|---|---|
| Number of patients | 91 (53.22%) | 80 (46.78%) | - |
| Age (years) | 57 (20–79) | 56 (33–76) | 0.108 |
| BMI (kg/m2) | 23.27 (17.85–34.24) | 22.66 (14.06–31.98) | 0.112 |
| CA125 (kU/L) | 379.15 (24.15–7055) | 852 (19.25–11619) | 0.887 |
| HE4 (pmol/L) | 286 (42.8–1500) | 248.7 (42.7–1393) | 0.059 |
| VEGF (pg/mL) | 395.47 (24.48–1291.29) | 516.41 (48.32–1311.41) | 0.048 |
| PNI | 46.2 (28.85–58.6) | 45.9 (28.5–56) | 0.284 |
| Pathologic subtypes | 0.539 * | ||
| High-grade serous carcinoma | 79 (86.82%) | 70 (87.50%) | - |
| Low-grade serous carcinoma | 2 (2.20%) | 4 (5.00%) | - |
| Mucinous carcinoma | 4 (4.39%) | 2 (2.50%) | - |
| Endometrioid carcinoma | 4 (4.39%) | 1 (1.25%) | - |
| Clear cell carcinoma | 2 (2.20%) | 3 (3.75%) | - |
| FIGO stage | 0.170 | ||
| III | 73 (80.22%) | 57 (71.25%) | - |
| IV | 18 (19.78%) | 23 (28.75%) | - |
| Imaging examination type before treatment | 0.091 | ||
| MRI only | 59 (64.84%) | 39 (48.75%) | - |
| CT only | 12 (13.19%) | 18 (22.50%) | - |
| MRI and CT | 20 (21.98%) | 23 (28.75%) | - |
| Univariate Analysis | Multivariate Analyze | Predictive Score | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | ||
| Age ≥ 55.5 | 0.68 | 0.348–1.327 | 0.258 | ||||
| CA125 ≥ 560 | 4.044 | 1.995–8.201 | 0.001 | 2.925 | 1.147–7.464 | 0.025 | 2 |
| HE4 ≥ 150 | 2.726 | 1.164–6.385 | 0.021 | ||||
| PNI ≤ 45.7 | 9.000 | 4.091–19.801 | 0.001 | 2.898 | 1.090–7.707 | 0.033 | 2 |
| Diaphragmatic surface of liver | 1.651 | 1.142–2.388 | 0.008 | ||||
| Diaphragmatic surface of spleen | 7.243 | 3.111–16.866 | 0.001 | 3.970 | 1.582–9.962 | 0.003 | 3 |
| Central tendon of diaphragm | 1.987 | 1.149–3.436 | 0.014 | ||||
| Falciform ligament of liver | 1.901 | 1.111–3.255 | 0.019 | ||||
| Gallbladder fossa | 3.136 | 1.644–5.979 | 0.001 | ||||
| Hepatorenal recess | 2.596 | 1.614–4.176 | 0.009 | 2.053 | 1.140–3.696 | 0.01 | 2 |
| Splenic capsule | 2.661 | 1.520–4.657 | 0.001 | ||||
| Splenic hilum | 2.264 | 1.473–3.481 | 0.001 | ||||
| Omentum | 1.550 | 1.063–2.261 | 0.023 | ||||
| Mesentery | 4.797 | 2.501–9.198 | 0.001 | 2.468 | 1.132–5.380 | 0.023 | 2 |
| Small intestine | 1.639 | 1.007–2.668 | 0.047 | ||||
| MRI total score | 1.103 | 1.052–3.157 | 0.001 | ||||
| Upper abdominal | 1.278 | 1.149–1.421 | 0.001 | 1.278 | 1.149–1.421 | 0.001 * | 1 |
| Middle abdominal | 1.273 | 1.111–1.458 | 0.001 | ||||
| Lower abdominal | 1.100 | 0.970–1.247 | 0.138 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Li, X.; Liu, Y.; Tang, Y.; Hou, W.; Jin, Y.; Cao, G.; Li, L.; Zhao, H.; Lv, X.; et al. Development and Validation of a Site-Specific Tumor Burden Score for Predicting Surgical Outcomes in Advanced Ovarian Cancer. Cancers 2025, 17, 3649. https://doi.org/10.3390/cancers17223649
Xu Z, Li X, Liu Y, Tang Y, Hou W, Jin Y, Cao G, Li L, Zhao H, Lv X, et al. Development and Validation of a Site-Specific Tumor Burden Score for Predicting Surgical Outcomes in Advanced Ovarian Cancer. Cancers. 2025; 17(22):3649. https://doi.org/10.3390/cancers17223649
Chicago/Turabian StyleXu, Zhiyang, Xiaotian Li, Ying Liu, Yongqiang Tang, Weihuan Hou, Yihua Jin, Gaijing Cao, Lingxia Li, Hongxi Zhao, Xiaohui Lv, and et al. 2025. "Development and Validation of a Site-Specific Tumor Burden Score for Predicting Surgical Outcomes in Advanced Ovarian Cancer" Cancers 17, no. 22: 3649. https://doi.org/10.3390/cancers17223649
APA StyleXu, Z., Li, X., Liu, Y., Tang, Y., Hou, W., Jin, Y., Cao, G., Li, L., Zhao, H., Lv, X., & Liu, S. (2025). Development and Validation of a Site-Specific Tumor Burden Score for Predicting Surgical Outcomes in Advanced Ovarian Cancer. Cancers, 17(22), 3649. https://doi.org/10.3390/cancers17223649





