Novel CTC Detection Method in Patients with Pancreatic Cancer Using High-Resolution Image Scanning
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment and Sample Collections
2.2. Sample Collection and CTC Enrichment
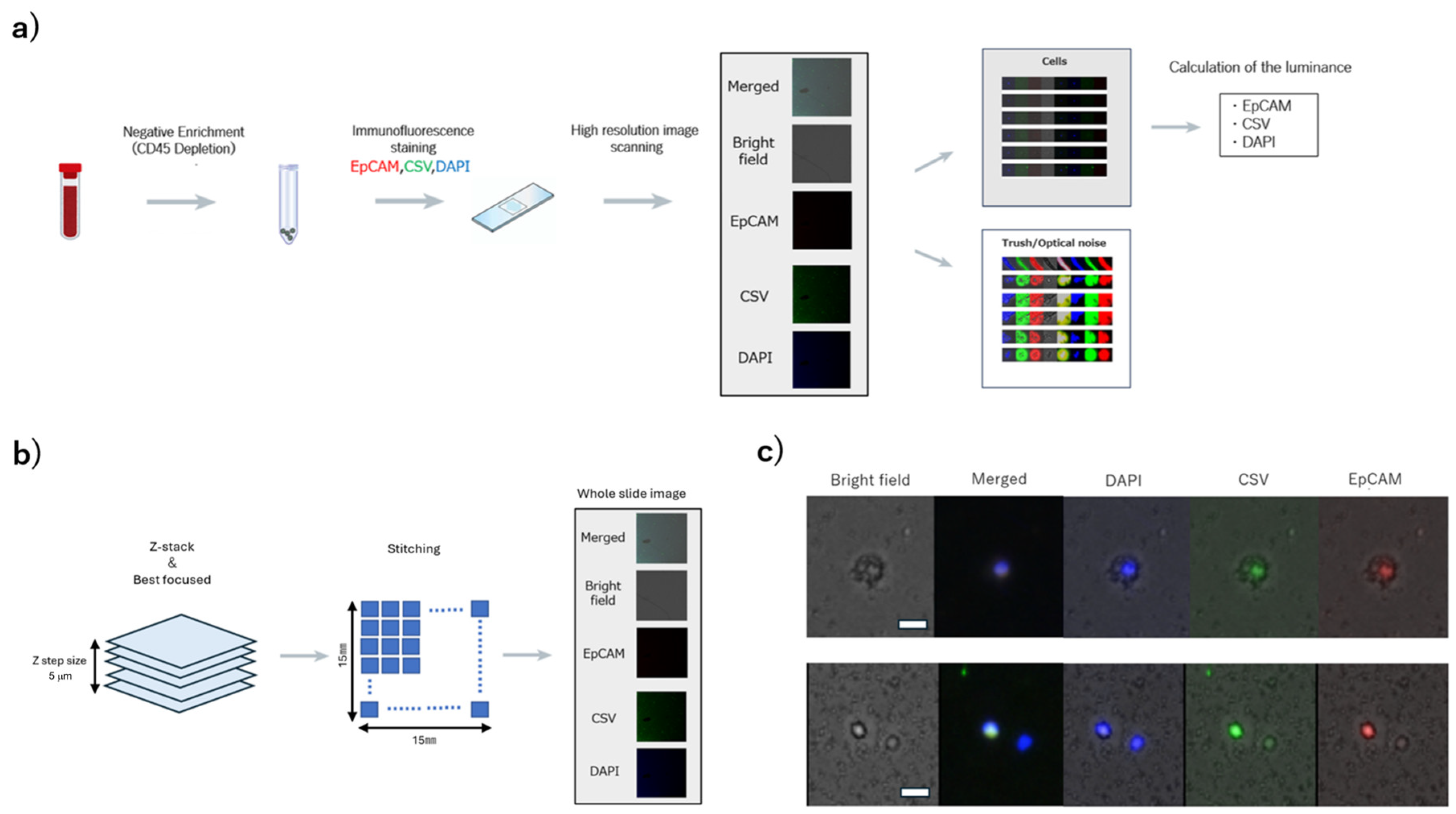
2.3. High-Resolution Image Scanning and Image Processing
2.3.1. Detection of Cellular Regions
2.3.2. Calculation of the Average Luminance of Cellular Regions
2.4. Threshold Settings
2.5. Statistical Analysis
3. Results
3.1. Acquisition of Cell Images and Measurement of Luminance
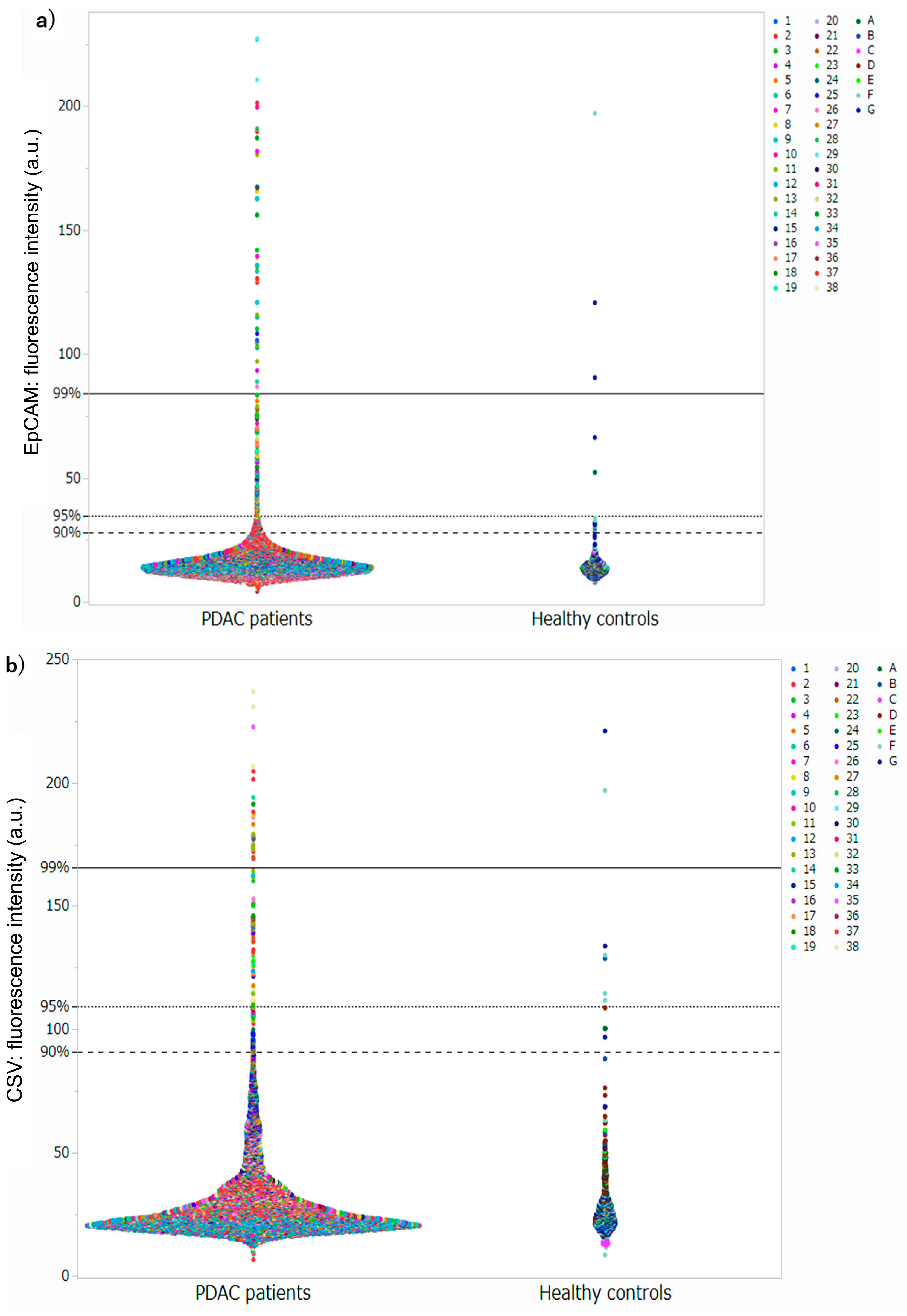
3.2. Setting Thresholds and Counting CTC Candidate Cells at Each Threshold
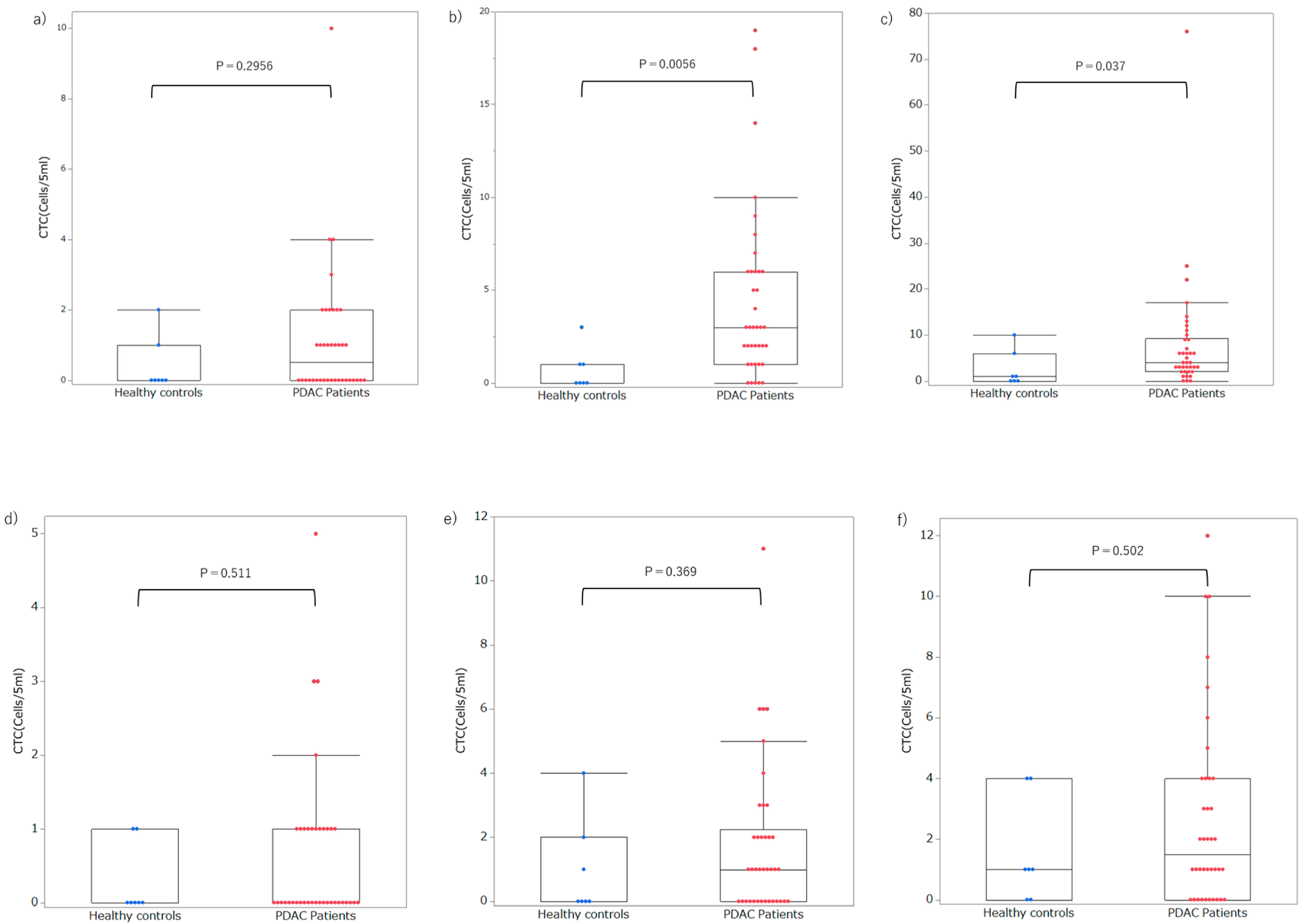
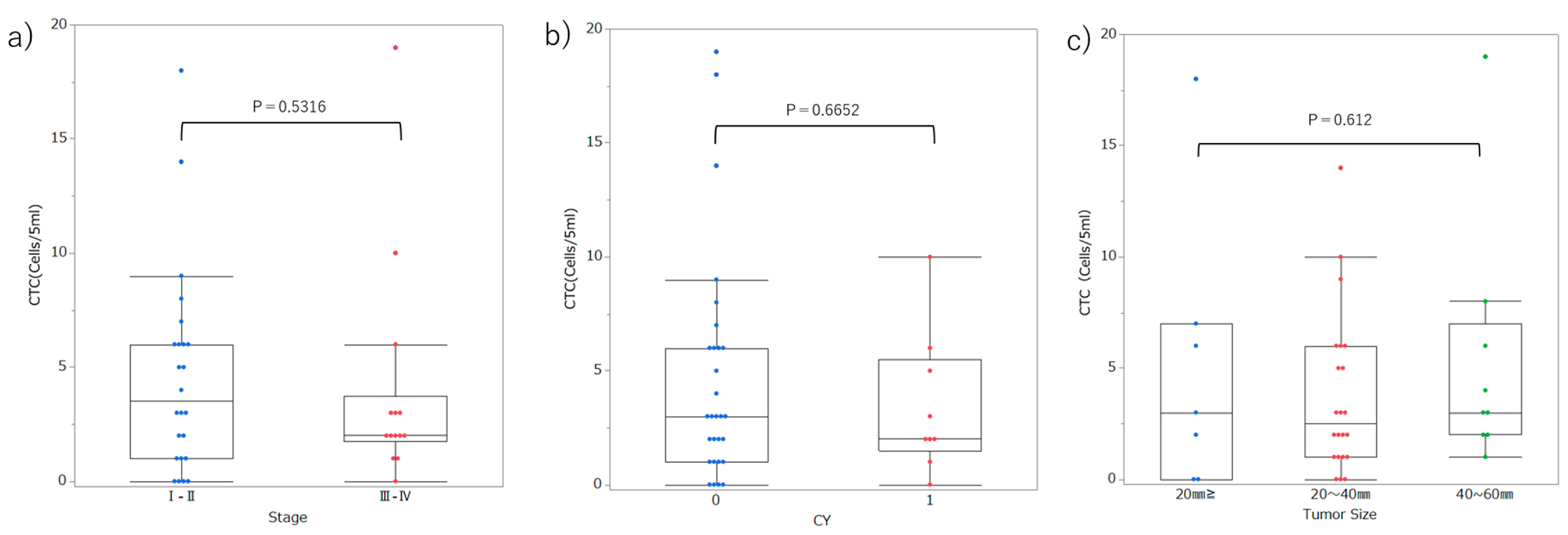
3.3. Setting Optimal Thresholds and Distributions of CTC in Patients with PDAC
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDAC | Pancreatic ductal adenocarcinoma |
| CTC | Circulating tumor cells |
| CSV | Cell surface vimentin |
| UICC | Union for International Cancer Control |
| DAPI | 4′, 1, 6′-diamidino-2-phenylindole |
| ROC | Receiver Operating Characteristic |
| AUC | Areas under the curve |
| SN | Sensitivity |
| SP | Specificity |
| DL | Deep learning |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Yamada, S.; Hoshino, Y.; Murotani, K.; Baba, H.; Takami, H.; Yoshioka, I.; Shibuya, K.; Kodera, Y.; Fujii, T. Prognostic factors in conversion surgery following nab-paclitaxel with gemcitabine and subsequent chemoradiotherapy for unresectable locally advanced pancreatic cancer: Results of a dual-center study. Ann. Gastroenterol. Surg. 2023, 7, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Fujii, T.; Takami, H.; Suenaga, M.; Inokawa, Y.; Yamada, S.; Nakayama, G.; Sugimoto, H.; Koike, M.; Nomoto, S.; et al. Combination of the serum carbohydrate antigen 19–9 and carcinoembryonic antigen is a simple and accurate predictor of mortality in pancreatic cancer patients. Surg. Today 2014, 44, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Cabel, L.; Proudhon, C.; Gortais, H.; Loirat, D.; Coussy, F.; Pierga, J.Y.; Bidard, F.C. Circulating tumor cells: Clinical validity and utility. Int. J. Clin. Oncol. 2017, 22, 421–430. [Google Scholar] [CrossRef]
- Yeo, D.; Bastian, A.; Strauss, H.; Saxena, P.; Grimison, P.; Rasko, J.E.J. Exploring the clinical utility of pancreatic cancer circulating tumor cells. Int. J. Mol. Sci. 2022, 23, 1671. [Google Scholar] [CrossRef]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.J.; Uhr, J.W.; Terstappen, L.W.M.M. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef]
- Svensson, C.M.; Hübler, R.; Figge, M.T. Automated classification of circulating tumor cells and the impact of Interobsever variability on classifier training and performance. J. Immunol. Res. 2015, 2015, 573165. [Google Scholar] [CrossRef]
- Akashi, T.; Okumura, T.; Terabayashi, K.; Yoshino, Y.; Tanaka, H.; Yamazaki, T.; Numata, Y.; Fukuda, T.; Manabe, T.; Baba, H.; et al. The use of an artificial intelligence algorithm for circulating tumor cell detection in patients with esophageal cancer. Oncol. Lett. 2023, 26, 320. [Google Scholar] [CrossRef]
- Zeune, L.L.; Boink, Y.E.; van Dalum, G.; Nanou, A.; de Wit, S.; Andree, K.C.; Swennenhuis, J.F.; van Gils, S.A.; Terstappen, L.W.M.M.; Brune, C. Deep learning of circulating tumour cells. Nat. Mach. Intell. 2020, 2, 124–133. [Google Scholar] [CrossRef]
- Shen, C.; Rawal, S.; Brown, R.; Zhou, H.; Agarwal, A.; Watson, M.A.; Cote, R.J.; Yang, C. Automatic detection of circulating tumor cells and cancer associated fibroblasts using deep learning. Sci. Rep. 2023, 13, 5708. [Google Scholar] [CrossRef]
- Brierley, J.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumor; John Wiley & Sons, Inc.: Chichester, West Sussex, UK, 2025. [Google Scholar]
- Akita, H.; Nagano, H.; Takeda, Y.; Eguchi, H.; Wada, H.; Kobayashi, S.; Marubashi, S.; Tanemura, M.; Takahashi, H.; Ohigashi, H.; et al. Ep-CAM is a significant prognostic factor in pancreatic cancer patients by suppressing cell activity. Oncogene 2011, 30, 3468–3476. [Google Scholar] [CrossRef] [PubMed]
- Mentink, A.; Isebia, K.T.; Kraan, J.; Terstappen, L.W.M.M.; Stevens, M. Measuring antigen expression of cancer cell lines and circulating tumour cells. Sci. Rep. 2023, 13, 6051. [Google Scholar] [CrossRef]
- Nicolazzo, C.; Gradilone, A.; Loreni, F.; Raimondi, C.; Gazzaniga, P. EpCAMlow circulating tumor cells: Gold in the waste. Dis. Markers 2019, 2019, 1718920. [Google Scholar] [CrossRef]
- Rao, C.G.; Chianese, D.; Doyle, G.V.; Miller, M.C.; Russell, T.; Sanders, R.A.; Terstappen, L.W.M.M. Expression of epithelial cell adhesion molecule in carcinoma cells present in blood and primary and metastatic tumors. Int. J. Oncol. 2005, 27, 49–57. [Google Scholar] [CrossRef]
- Satelli, A.; Mitra, A.; Brownlee, Z.; Xia, X.; Bellister, S.; Overman, M.J.; Kopetz, S.; Ellis, L.M.; Meng, Q.H.; Li, S. Epithelial-mesenchymal transitioned circulating tumor cells capture for detecting tumor progression. Clin. Cancer Res. 2015, 21, 899–906. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, X.; Zhang, Q.; Yang, J.; Chen, Q.; Wang, J.; Li, X.; Chen, J.; Ma, T.; Li, G.; et al. Vimentin-positive circulating tumor cells as a biomarker for diagnosis and treatment monitoring in patients with pancreatic cancer. Cancer Lett. 2019, 452, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Gemenetzis, G.; Groot, V.P.; Yu, J.; Ding, D.; Teinor, J.A.; Javed, A.A.; Wood, L.D.; Burkhart, R.A.; Cameron, J.L.; Makary, M.A.; et al. Circulating tumor cells dynamics in pancreatic adenocarcinoma correlate with disease status: Results of the prospective CLUSTER study. Ann. Surg. 2018, 268, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Poruk, K.E.; Valero, V., 3rd; Saunders, T.; Blackford, A.L.; Griffin, J.F.; Poling, J.; Hruban, R.H.; Anders, R.A.; Herman, J.; Zheng, L.; et al. Circulating tumor cell phenotype predicts recurrence and survival in pancreatic adenocarcinoma. Ann. Surg. 2016, 264, 1073–1081. [Google Scholar] [CrossRef]
- Munnings, R.; Gibbs, P.; Lee, B. Evolution of Liquid Biopsies for Detecting Pancreatic Cancer. Cancers 2024, 16, 3335. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, H.-Y.; Liu, F.-C.; Gu, F.-M.; Yuan, S.-X.; Huang, J.; Pan, Z.-Y.; Wang, W.-J. Circulating Tumor Cells Expressing Kruppel-Like Factor 8 and Vimentin as Predictors of Poor Prognosis in Pancreatic Cancer Patients. Cancer Control J. Moffitt Cancer Cent. 2021, 28, 10732748211027163. [Google Scholar] [CrossRef]
- Gasparini-Junior, J.L.; Fanelli, M.F.; Abdallah, E.A.; Chinen, L.T.D. Evaluating Mmp-2 and Tgfs-Ri Expression in Circulating Tumor Cells of Pancreatic Cancer Patients and Their Correlation with Clinical Evolution. Arq. Bras. Cir. Dig. ABCD Braz. Arch. Dig. Surg. 2019, 32, e1433. [Google Scholar] [CrossRef]
- Cheng, H.; He, W.; Yang, J.; Ye, Q.; Cheng, L.; Pan, Y.; Mao, L.; Chu, X.; Lu, C.; Li, G.; et al. Ligand-targeted polymerase chain reaction for the detection of folate receptor-positive circulating tumour cells as a potential diagnostic biomarker for pancreatic cancer. Cell Prolif. 2020, 53, e12880. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Zhou, L.; Liu, Z.; Tan, X. A combination of circulating tumor cells and CA19-9 improves the diagnosis of pancreatic cancer. J. Clin. Lab. Anal. 2022, 36, e24341. [Google Scholar] [CrossRef] [PubMed]
- Ankeny, J.S.; Court, C.M.; Hou, S.; Li, Q.; Song, M.; Wu, D.; Chen, J.F.; Lee, T.; Lin, M.; Sho, S.; et al. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br. J. Cancer 2016, 114, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, M.; Watanabe, T.; Tanaka, H.; Itoh, A.; Kimura, N.; Shibuya, K.; Yoshioka, I.; Murotani, K.; Hirabayashi, K.; Fujii, T. Efficacy of staging laparoscopy for resectable pancreatic cancer on imaging and the therapeutic effect of systemic chemotherapy for positive peritoneal cytology. J. Hepato-Bil. Pancreat. Sci. 2023, 30, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Court, C.M.; Ankeny, J.S.; Sho, S.; Winograd, P.; Hou, S.; Song, M.; Wainberg, Z.A.; Girgis, M.D.; Graeber, T.G.; Agopian, V.G.; et al. Circulating tumor cells predict occult metastatic disease and prognosis in pancreatic cancer. Ann. Surg. Oncol. 2018, 25, 1000–1008. [Google Scholar] [CrossRef]
- Bissolati, M.; Sandri, M.T.; Burtulo, G.; Zorzino, L.; Balzano, G.; Braga, M. Portal vein-circulating tumor cells predict liver metastases in patients with resectable pancreatic cancer. Tumour Biol. 2015, 36, 991–996. [Google Scholar] [CrossRef]
- Okubo, K.; Uenosono, Y.; Arigami, T.; Mataki, Y.; Matsushita, D.; Yanagita, S.; Kurahara, H.; Sakoda, M.; Kijima, Y.; Maemura, K.; et al. Clinical impact of circulating tumor cells and therapy response in pancreatic cancer. Eur. J. Surg. Oncol. 2017, 43, 1050–1055. [Google Scholar] [CrossRef]
- Martini, V.; Timme-Bronsert, S.; Fichtner-Feigl, S.; Hoeppner, J.; Kulemann, B. Circulating tumor cells in pancreatic cancer: Current perspectives. Cancers 2019, 11, 1659. [Google Scholar] [CrossRef]

| Cutoff Value (Cells/5 mL) | AUC (95%CI) | SN | SP | PPV | NPV | Delong Test p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EpCAM (99) | EpCAM (95) | EpCAM (90) | CSV (99) | CSV (95) | CSV (90) | |||||||
| EpCAM (99) | 1 | 0.617 (0.414–0.785) | 0.50 | 0.72 | 0.90 | 0.21 | 1.0000 | 0.0002 | 0.0063 | 0.3932 | 0.8864 | 0.6209 |
| EpCAM (95) | 2 | 0.830 (0.634–0.932) | 0.74 | 0.76 | 0.96 | 0.33 | 0.0002 | 1.0000 | 0.2704 | 0.0002 | 0.0201 | 0.0021 |
| EpCAM (90) | 2 | 0.750 (0.456–0.914) | 0.84 | 0.72 | 0.94 | 0.45 | 0.0063 | 0.2704 | 1.0000 | 0.0025 | 0.0551 | 0.0087 |
| CSV (99) | 1 | 0.570 (0.386–0.736) | 0.39 | 0.72 | 0.88 | 0.18 | 0.3932 | 0.0002 | 0.0025 | 1.0000 | 0.5453 | 0.848 |
| CSV (95) | 1 | 0.605 (0.372–0.799) | 0.63 | 0.57 | 0.85 | 0.17 | 0.8864 | 0.0201 | 0.0551 | 0.5453 | 1.0000 | 0.651 |
| CSV (90) | 2 | 0.581 (0.362–0.772) | 0.50 | 0.72 | 0.89 | 0.19 | 0.6209 | 0.0021 | 0.0087 | 0.848 | 0.651 | 1.0000 |
| CTC ≥ 2 | 2 > CTC | p-Value | ||
|---|---|---|---|---|
| Age | 67.1 (58–75) | 72.9 (62–84) | 0.079 | |
| Sex | Male | 19 | 7 | 0.733 |
| Female | 9 | 3 | ||
| Location | Head/Neck | 11 | 7 | 0.092 |
| Body/Tail | 18 | 3 | ||
| UICC Stage | I–II | 17 | 7 | 0.598 |
| III–IV | 11 | 3 | ||
| Tumor size | TS1-2 | 20 | 9 | 0.290 |
| TS3-4 | 8 | 1 | ||
| CY | 0 | 21 | 8 | 0.747 |
| 1 | 7 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manabe, T.; Okumura, T.; Terabayashi, K.; Akashi, T.; Rui, T.Y.; Numata, Y.; Takeda, N.; Yamada, A.; Kimura, N.; Fukasawa, M.; et al. Novel CTC Detection Method in Patients with Pancreatic Cancer Using High-Resolution Image Scanning. Cancers 2025, 17, 3640. https://doi.org/10.3390/cancers17223640
Manabe T, Okumura T, Terabayashi K, Akashi T, Rui TY, Numata Y, Takeda N, Yamada A, Kimura N, Fukasawa M, et al. Novel CTC Detection Method in Patients with Pancreatic Cancer Using High-Resolution Image Scanning. Cancers. 2025; 17(22):3640. https://doi.org/10.3390/cancers17223640
Chicago/Turabian StyleManabe, Takahiro, Tomoyuki Okumura, Kenji Terabayashi, Takahisa Akashi, Teo Yi Rui, Yoshihisa Numata, Naoya Takeda, Akane Yamada, Nana Kimura, Mina Fukasawa, and et al. 2025. "Novel CTC Detection Method in Patients with Pancreatic Cancer Using High-Resolution Image Scanning" Cancers 17, no. 22: 3640. https://doi.org/10.3390/cancers17223640
APA StyleManabe, T., Okumura, T., Terabayashi, K., Akashi, T., Rui, T. Y., Numata, Y., Takeda, N., Yamada, A., Kimura, N., Fukasawa, M., Araki, T., Mori, K., Kishi, Y., Tanaka, K., Minagawa, T., Miwa, T., Watanabe, T., Hirano, K., Sekine, S., ... Fujii, T. (2025). Novel CTC Detection Method in Patients with Pancreatic Cancer Using High-Resolution Image Scanning. Cancers, 17(22), 3640. https://doi.org/10.3390/cancers17223640








