Optimal Timing of Treatment Initiation in Non-Metastatic Castration-Resistant Prostate Cancer Based on PSA Level and Doubling Time for Prognostic Benefit
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
3.1. Patient Characteristics
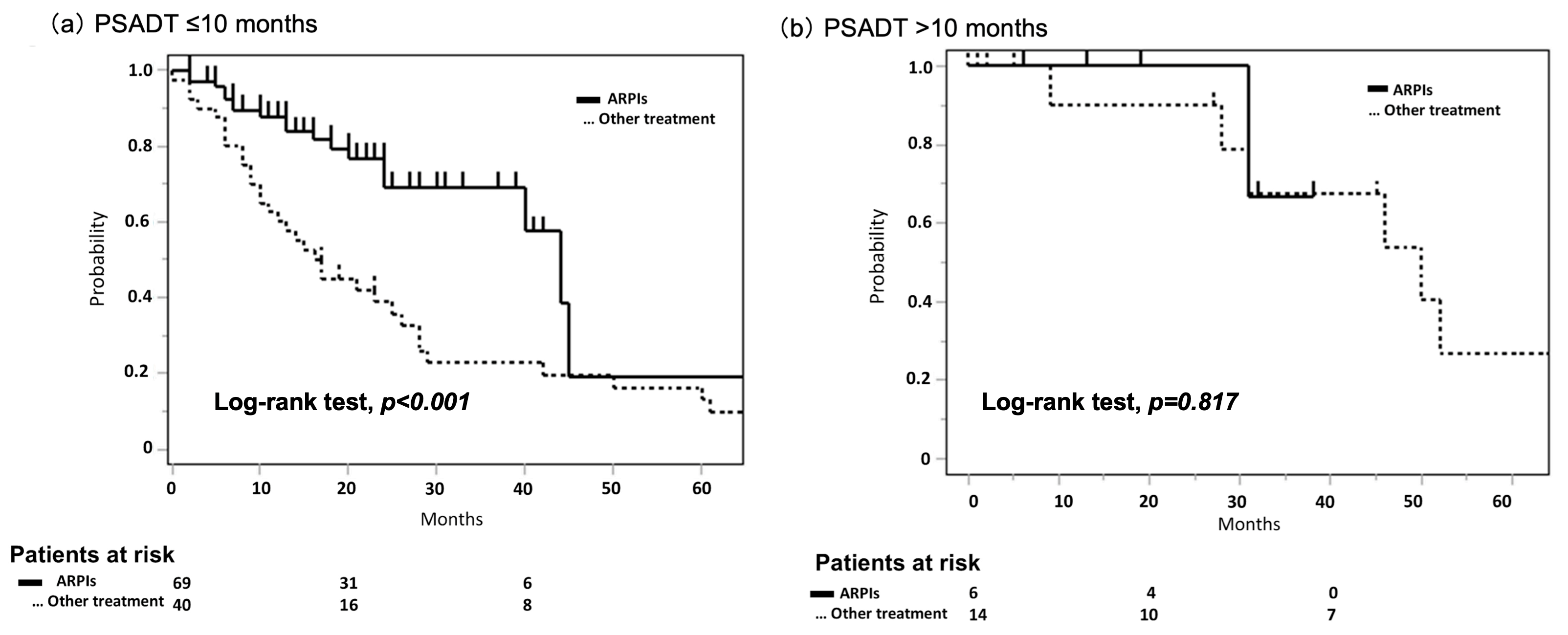
3.2. The Significance of PSADT in nmCRPC for Prognosis
3.3. Stability of PSADT
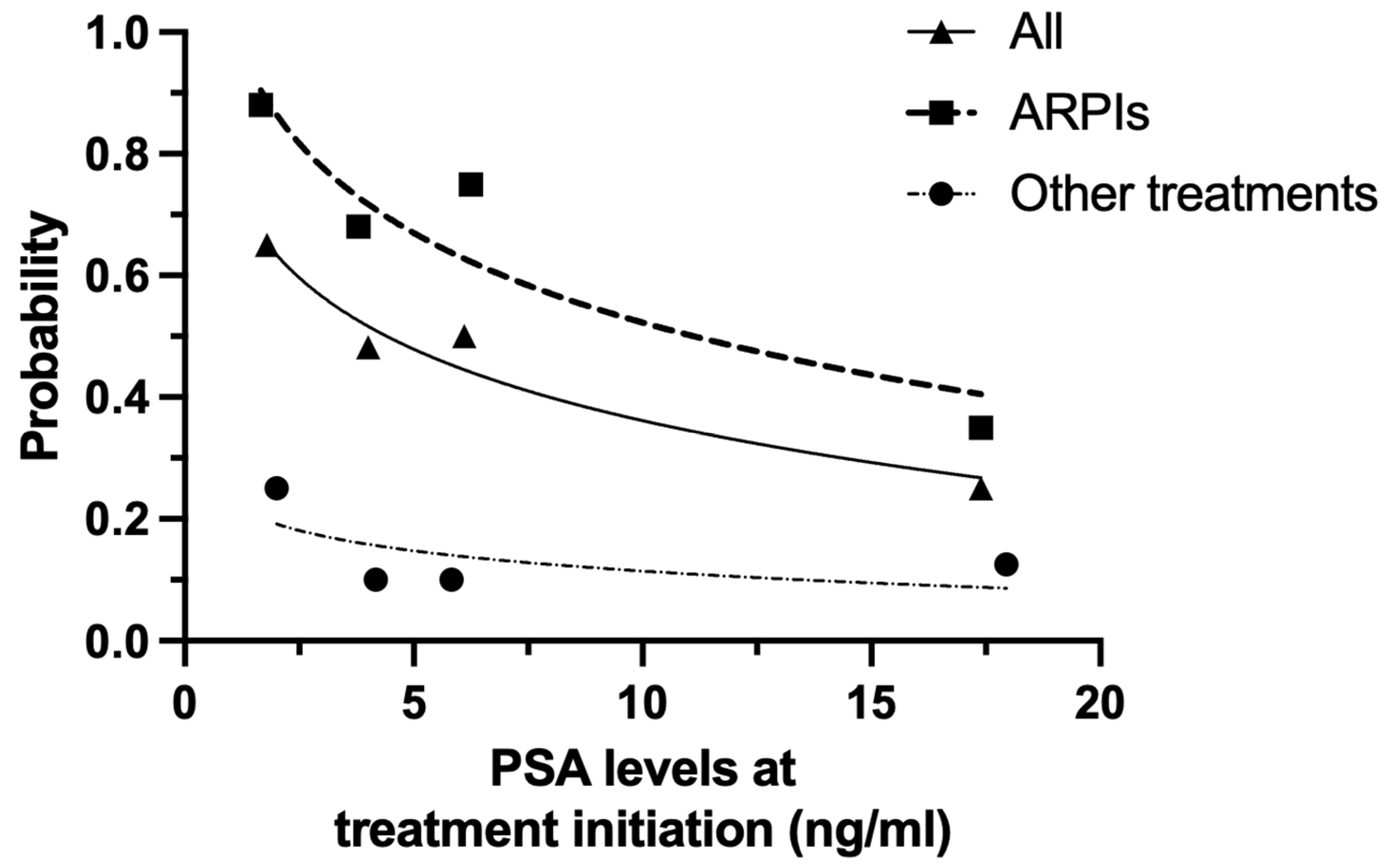
3.4. PSA-PFS According to Baseline PSA Level at Treatment Initiation in Patients with PSADT ≤ 10 Months
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef]
- Freedland, S.J.; Humphreys, E.B.; Mangold, L.A.; Eisenberger, M.; Dorey, F.J.; Walsh, P.C.; Partin, A.W. Risk of Prostate Cancer-Specific Mortality Following Biochemical Recurrence after Radical Prostatectomy. JAMA 2005, 294, 433–439. [Google Scholar] [CrossRef]
- Kupelian, P.A.; Buchsbaum, J.C.; Elshaikh, M.; Reddy, C.A.; Zippe, C.; Klein, E.A. Factors Affecting Recurrence Rates after Prostatectomy or Radiotherapy in Localized Prostate Carcinoma Patients with Biopsy Gleason Score 8 or Above. Cancer 2002, 95, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining Biochemical Failure Following Radiotherapy with or without Hormonal Therapy in Men with Clinically Localized Prostate Cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Crook, J.M.; O’Callaghan, C.J.; Duncan, G.; Dearnaley, D.P.; Higano, C.S.; Horwitz, E.M.; Frymire, E.; Malone, S.; Chin, J.; Nabid, A.; et al. Intermittent Androgen Suppression for Rising PSA Level after Radiotherapy. N. Engl. J. Med. 2012, 367, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Paller, C.J.; Antonarakis, E.S.; Eisenberger, M.A.; Carducci, M.A. Management of Patients with Biochemical Recurrence after Local Therapy for Prostate Cancer. Hematol. Oncol. Clin. N. Am. 2013, 27, 1205–1219. [Google Scholar] [CrossRef]
- Duchesne, G.M.; Woo, H.H.; Bassett, J.K.; Bowe, S.J.; D’Este, C.; Frydenberg, M.; King, M.; Ledwich, L.; Loblaw, A.; Malone, S.; et al. Timing of Androgen-Deprivation Therapy in Patients with Prostate Cancer with a Rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): A Randomised, Multicentre, Non-Blinded, Phase 3 Trial. Lancet Oncol. 2016, 17, 727–737. [Google Scholar] [CrossRef]
- Chun, F.K.-H.; Graefen, M.; Zacharias, M.; Haese, A.; Steuber, T.; Schlomm, T.; Walz, J.; Karakiewicz, P.I.; Huland, H. Anatomic Radical Retropubic Prostatectomy-Long-Term Recurrence-Free Survival Rates for Localized Prostate Cancer. World J. Urol. 2006, 24, 273–280. [Google Scholar] [CrossRef]
- Saad, F.; Bögemann, M.; Suzuki, K.; Shore, N. Treatment of Nonmetastatic Castration-Resistant Prostate Cancer: Focus on Second-Generation Androgen Receptor Inhibitors. Prostate Cancer Prostatic Dis. 2021, 24, 323–334. [Google Scholar] [CrossRef]
- Smith, M.R.; Kabbinavar, F.; Saad, F.; Hussain, A.; Gittelman, M.C.; Bilhartz, D.L.; Wynne, C.; Murray, R.; Zinner, N.R.; Schulman, C.; et al. Natural History of Rising Serum Prostate-Specific Antigen in Men with Castrate Nonmetastatic Prostate Cancer. J. Clin. Oncol. 2005, 23, 2918–2925. [Google Scholar] [CrossRef]
- Howard, L.E.; Moreira, D.M.; De Hoedt, A.; Aronson, W.J.; Kane, C.J.; Amling, C.L.; Cooperberg, M.R.; Terris, M.K.; Freedland, S.J. Thresholds for PSA Doubling Time in Men with Non-Metastatic Castration-Resistant Prostate Cancer. BJU Int. 2017, 120, E80–E86. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide Treatment and Metastasis-Free Survival in Prostate Cancer. N. Engl. J. Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivashchenko, P.; Demirhan, E.; Modelska, K.; Phung, D.; et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide and Overall Survival in Prostate Cancer. Eur. Urol. 2021, 79, 150–158. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Fizazi, K.; Saad, F.; Shore, N.D.; De Giorgi, U.; Penson, D.F.; Ferreira, U.; Efstathiou, E.; Madziarska, K.; Kolinsky, M.P.; et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2197–2206. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N. Engl. J. Med. 2020, 383, 1040–1049. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kosaka, T.; Terada, N.; Kimura, T.; Nonomura, N.; Suzuki, H.; Uemura, H. Current Issues and Management Consensus of Advanced Prostate Cancer: Report of the Advanced Prostate Cancer Consensus Conference-JAPAN 2023. Int. J. Urol. 2024, 31, 975–985. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef]
- Freedland, S.J.; Ramaswamy, K.; Huang, A.; Sandin, R.; Mardekian, J.; Schultz, N.M.; Janjan, N.; George, D.J. Survival and Economic Impact of Rapid Prostate-Specific Antigen Doubling Time in Patients with Nonmetastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2023, 21, 419–429. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Oudard, S.; Shore, N.; Fizazi, K.; Sieber, P.; Tombal, B.; Damiao, R.; Marx, G.; Miller, K.; et al. Denosumab and Bone Metastasis-Free Survival in Men with Nonmetastatic Castration-Resistant Prostate Cancer: Exploratory Analyses by Baseline Prostate-Specific Antigen Doubling Time. J. Clin. Oncol. 2013, 31, 3800–3806. [Google Scholar] [CrossRef] [PubMed]
- Penson, D.F.; Armstrong, A.J.; Concepcion, R.; Agarwal, N.; Olsson, C.; Karsh, L.; Dunshee, C.; Wang, F.; Wu, K.; Krivoshik, A.; et al. Enzalutamide versus Bicalutamide in Castration-Resistant Prostate Cancer: The STRIVE Trial. J. Clin. Oncol. 2016, 34, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Fendler, W.P.; Ravi Kumar, A.S.; Calais, J.; Czernin, J.; Ilhan, H.; Saad, F.; Kretschmer, A.; Hekimsoy, T.; Brookman-May, S.D.; et al. Prostate-Specific Membrane Antigen Positron Emission Tomography-Detected Disease Extent and Overall Survival of Patients with High-Risk Nonmetastatic Castration-Resistant Prostate Cancer: An International Multicenter Retrospective Study. Eur. Urol. 2024, 85, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, C.; Wei, Y.; Meng, J.; Zhang, Y.; Gan, H.; Xu, X.; Wan, F.; Pan, J.; Ma, X.; et al. A Prospective Trial of 68Ga-PSMA and 18F-FDG PET/CT in Nonmetastatic Prostate Cancer Patients with an Early PSA Progression during Castration. Clin. Cancer Res. 2020, 26, 4551–4558. [Google Scholar] [CrossRef]
- Arnold, P.; Penaloza-Ramos, M.C.; Adedokun, L.; Rees, S.; Lockhat, M.; Spary, L.; Watkins, A.; Gnanapragasam, V.; Crabb, S.J. Clinical Characteristics and Outcomes for Patients with Non-metastatic Castration-Resistant Prostate Cancer. Sci. Rep. 2021, 11, 22151. [Google Scholar] [CrossRef]
- Sakamoto, S.; Sato, K.; Kimura, T.; Matsui, Y.; Shiraishi, Y.; Hashimoto, K.; Miyake, H.; Narita, S.; Miki, J.; Matsumoto, R.; et al. PSA Doubling Time 4.65 Months as an Optimal Cut-off of Japanese Nonmetastatic Castration-Resistant Prostate Cancer. Sci. Rep. 2024, 14, 15307. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-Specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-Specific Membrane Antigen-Avid Lesions: A Systematic Review and Meta-Analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [CrossRef]
- Weber, M.; Kurek, C.; Barbato, F.; Eiber, M.; Maurer, T.; Nader, M.; Hadaschik, B.; Grünwald, V.; Herrmann, K.; Wetter, A.; et al. PSMA-Ligand PET for Early Castration-Resistant Prostate Cancer: A Retrospective Single-Center Study. J. Nucl. Med. 2021, 62, 88–91. [Google Scholar] [CrossRef]
- Reese, A.C.; Fradet, V.; Whitson, J.M.; Davis, C.B.; Carroll, P.R. Poor Agreement of Prostate Specific Antigen Doubling Times Calculated Using Ultrasensitive versus Standard Prostate Specific Antigen Values: Important Impact on Risk Assessment. J. Urol. 2011, 186, 2228–2232. [Google Scholar] [CrossRef]
| Overall | PSADT ≤ 10 Months | PSADT > 10 Months | p Value | |
|---|---|---|---|---|
| At diagnosis | ||||
| Age, median (IQR), years | 71 (66–76) | 71 (66–76) | 72 (66–76) | 0.919 |
| PSA, median (IQR), ng/mL | 22.0 (10.6–62.1) | 21.0 (9.4–60.0) | 30.0 (12.1–57.8) | 0.336 |
| T stage, n (%) | 0.834 | |||
| ≤T2 | 83 (64) | 69 (63) | 14(77) | |
| T3 | 29 (23) | 25 (23) | 4 (15) | |
| T4 | 17 (13) | 15 (14) | 2 (8) | |
| N stage, n (%) | 0.525 | |||
| N0 | 106 (82) | 88 (81) | 18 (90) | |
| N1 | 23 (18) | 21 (19) | 2 (10) | |
| M stage, n (%) | 0.690 | |||
| M0 | 116 (89) | 97 (8 9) | 19 (95) | |
| M1 | 13 (11) | 12 (1 1 ) | 1 (5) | |
| ISUP grade, n (%) | 0.626 | |||
| ≤3 | 43 (33) | 35 (32) | 8 (40) | |
| 4 | 34 (26) | 29 (27) | 5 (25) | |
| 5 | 41 (32) | 35 (32) | 6 (30) | |
| NA | 11 (9) | 10 (9) | 1 (5) | |
| Local therapy, n (%) | 0.374 | |||
| prostatectomy | 31 (24) | 28 (26) | 3 (15) | |
| radiation therapy ± ADT | 26 (20) | 23 (21) | 3 (15) | |
| At nmCRPC diagnosis | ||||
| Time from initial ADT to nmCRPC, median (IQR), months | 39 (22–83) | 37 (22–74) | 54 (36–92) | 0.142 |
| Age, median (IQR), years | 78 (72–82) | 77 (75–82) | 78 (72–82) | 0.181 |
| PSA, median (IQR), ng/mL | 2.8 (1.8–5.1) | 3.2 (1.8–5.8) | 2.4 (1.5–2.8) | 0.310 |
| PSADT, median (IQR), months | 5.8 (3.1–9.1) | 4.8 (3.0–7.8) | 19.2 (13.0–24.0) | <0.001 |
| First-line treatment, n (%) | ||||
| ARPIs | 76 (64) | 71 (64) | 5 (25) | 0.003 |
| Apalutamide | 12 (9) | 11 (10) | 1 (5) | |
| Abiraterone | 12 (9) | 11 (10) | 1 (5) | |
| Darolutamide | 41 (37) | 38 (34) | 3 (15) | |
| Enzalutamide | 11 (9) | 11 (10) | 0 | |
| Other treatments | 53 (36) | 38 (36) | 15 (75) | 0.003 |
| Bicalutamide | 31 (24) | 19 (18) | 12 (50) | |
| Flutamide | 22 (12) | 19 (18) | 3 (25) | |
| Duration of first-line ARPIs, median (IQR), months | 18 (11–25.5) | 16 (11–24) | 31 (16–35) | 0.125 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95%CI) | p Value | Hazard Ratio (95%CI) | p Value | |
| Non-metastatic disease at initial diagnosis | 0.923 (0.457–2.562) | 0.856 | ||
| Prior definitive local therapy | 0.456 (0.255–0.815) | 0.008 | 0.409 (0.223–0.748) | <0.001 |
| ARPIs as a first-line treatment | 0.362 (0.201–0.650) | <0.001 | 0.421 (0.230–0.766) | <0.001 |
| Longer PSADT at nmCRPC diagnosis | 0.973 (0.874–1.079) | 0.610 | ||
| Lower PSA at treatment initiation | 0.961 (0.946–0.982) | <0.001 | 0.961 (0.944–0.983) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogasawara, T.; Hashimoto, K.; Shindo, T.; Kobayashi, K.; Tanaka, T.; Fukuta, F.; Kobayashi, G.; Kato, R.; Miyamoto, S.; Kunishima, Y.; et al. Optimal Timing of Treatment Initiation in Non-Metastatic Castration-Resistant Prostate Cancer Based on PSA Level and Doubling Time for Prognostic Benefit. Cancers 2025, 17, 3641. https://doi.org/10.3390/cancers17223641
Ogasawara T, Hashimoto K, Shindo T, Kobayashi K, Tanaka T, Fukuta F, Kobayashi G, Kato R, Miyamoto S, Kunishima Y, et al. Optimal Timing of Treatment Initiation in Non-Metastatic Castration-Resistant Prostate Cancer Based on PSA Level and Doubling Time for Prognostic Benefit. Cancers. 2025; 17(22):3641. https://doi.org/10.3390/cancers17223641
Chicago/Turabian StyleOgasawara, Takuto, Kohei Hashimoto, Tetsuya Shindo, Ko Kobayashi, Toshiaki Tanaka, Fumimasa Fukuta, Genki Kobayashi, Ryuichi Kato, Shintaro Miyamoto, Yasuharu Kunishima, and et al. 2025. "Optimal Timing of Treatment Initiation in Non-Metastatic Castration-Resistant Prostate Cancer Based on PSA Level and Doubling Time for Prognostic Benefit" Cancers 17, no. 22: 3641. https://doi.org/10.3390/cancers17223641
APA StyleOgasawara, T., Hashimoto, K., Shindo, T., Kobayashi, K., Tanaka, T., Fukuta, F., Kobayashi, G., Kato, R., Miyamoto, S., Kunishima, Y., & Masumori, N. (2025). Optimal Timing of Treatment Initiation in Non-Metastatic Castration-Resistant Prostate Cancer Based on PSA Level and Doubling Time for Prognostic Benefit. Cancers, 17(22), 3641. https://doi.org/10.3390/cancers17223641






