The Unfolded Protein Response—Novel Mechanisms, Challenges, and Key Considerations for Therapeutic Intervention
Simple Summary
Abstract
1. Background and Introduction
2. UPR
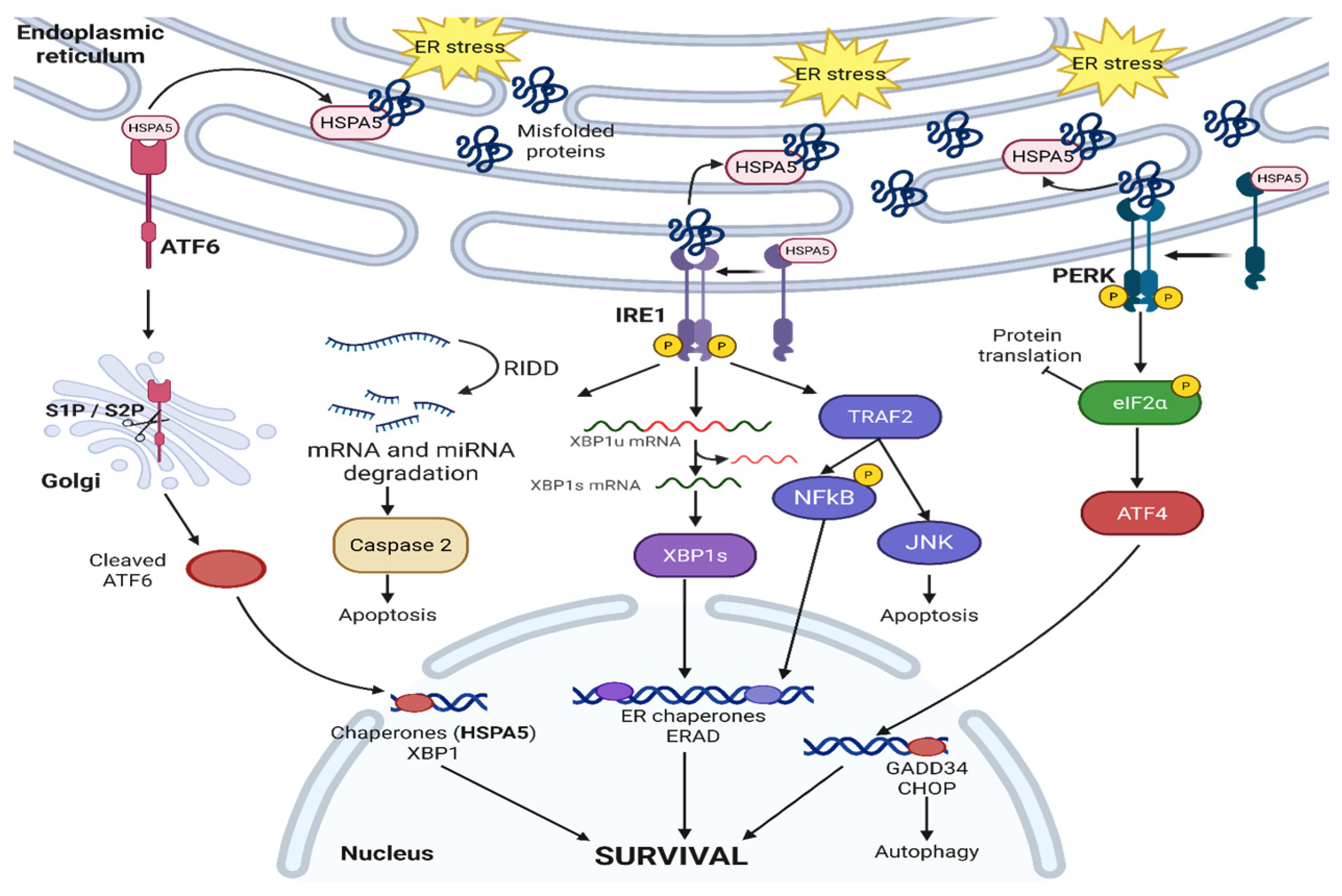
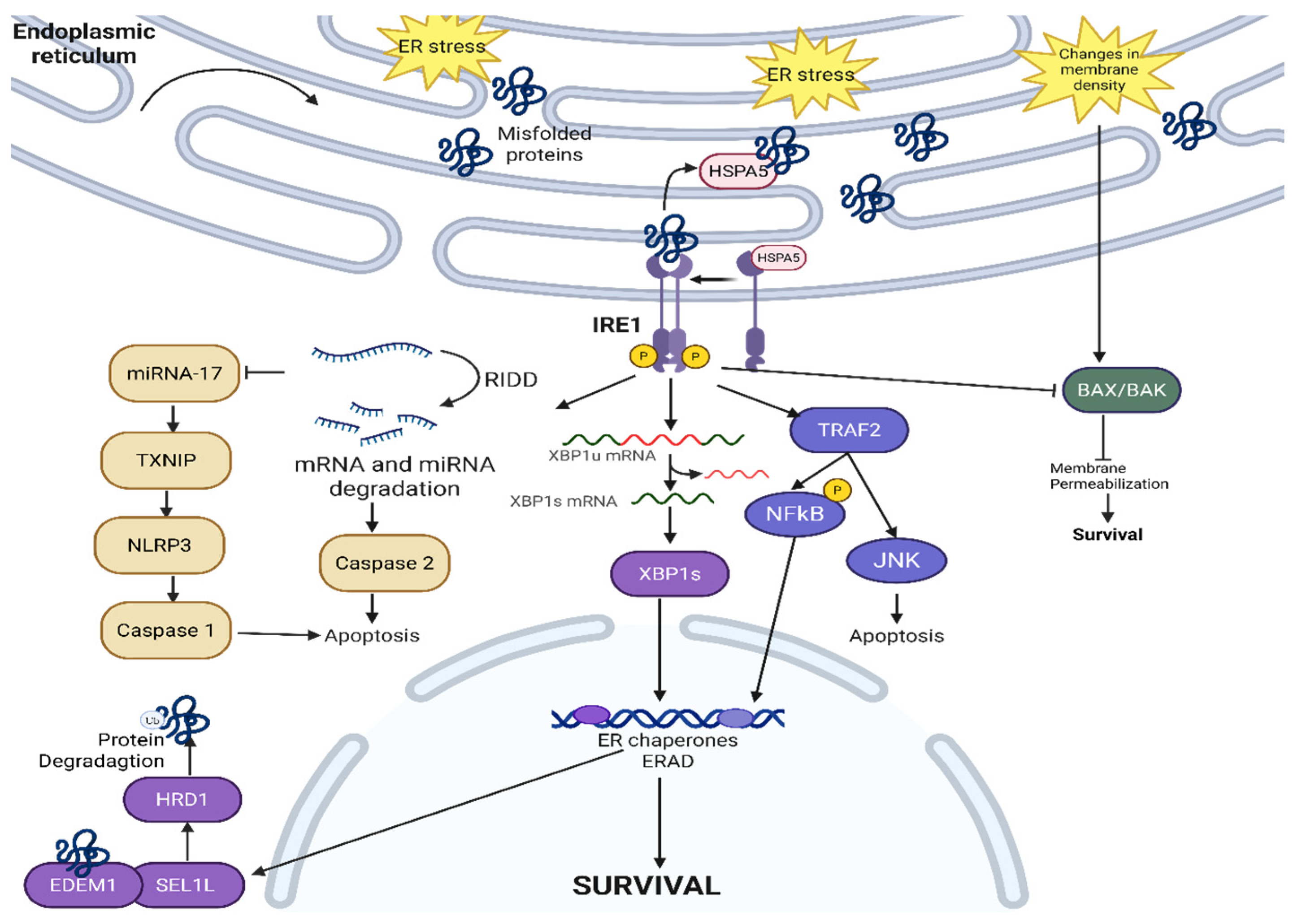
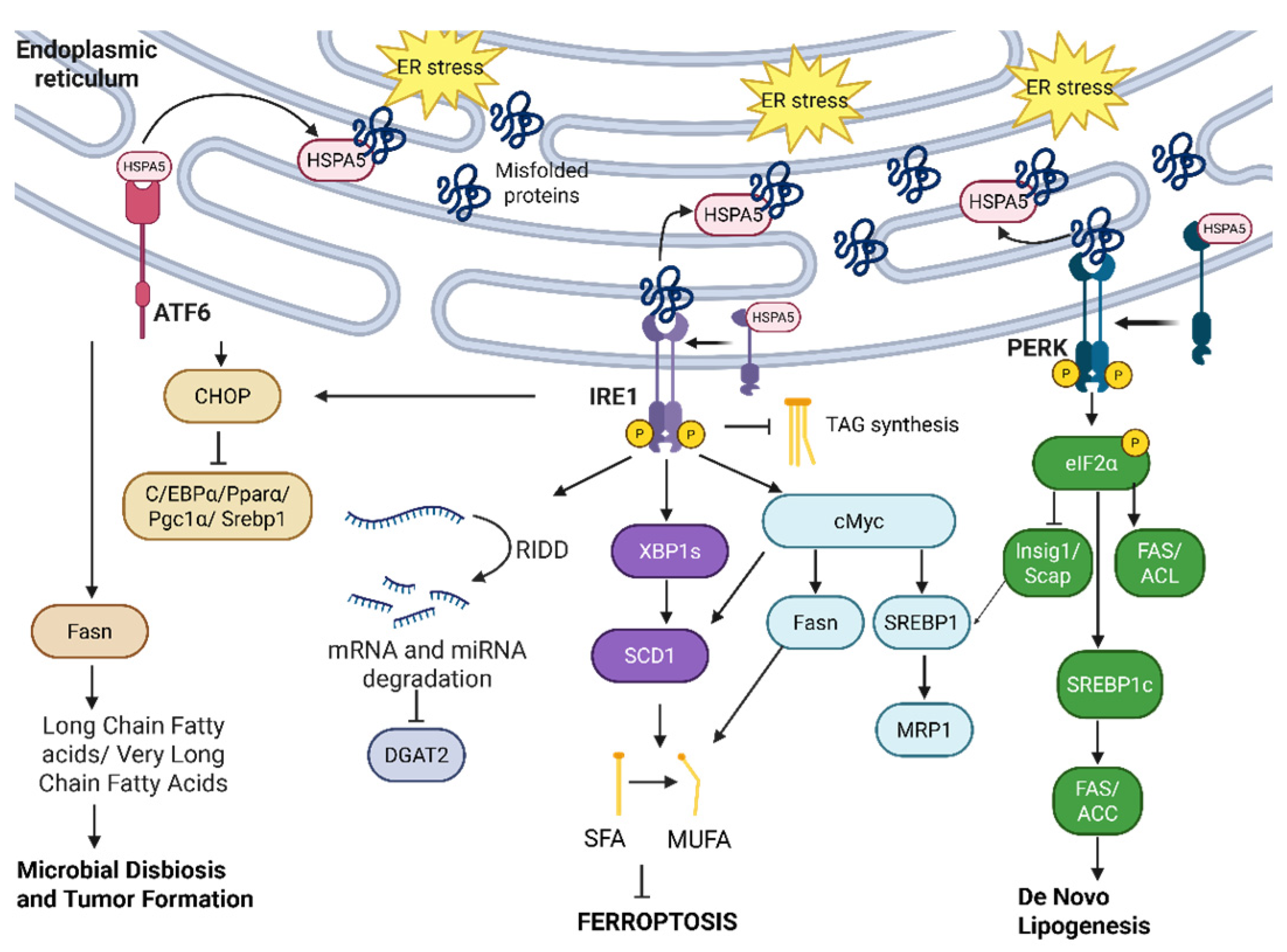
2.1. IRE1—Canonical Signaling
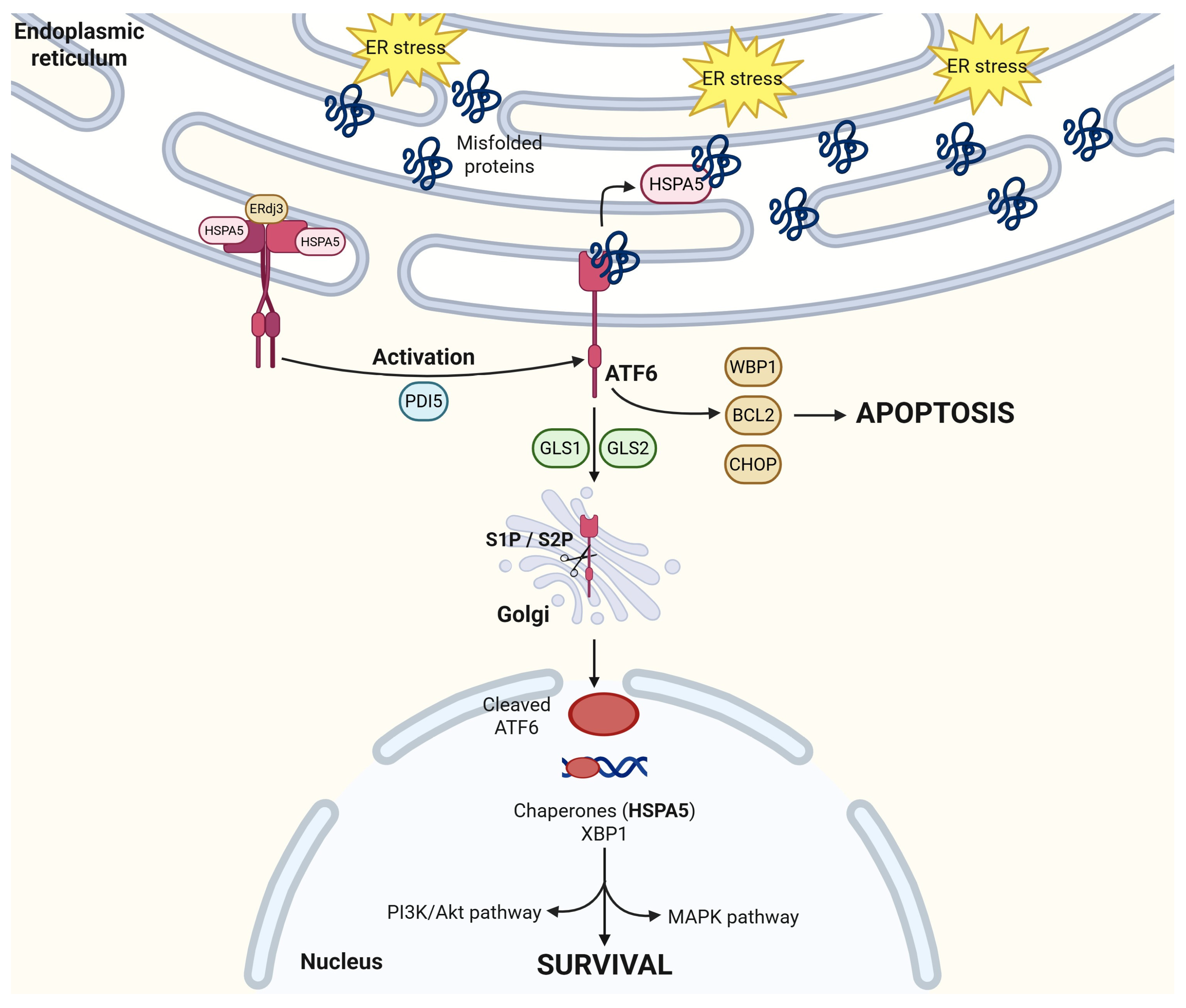
2.2. ATF6
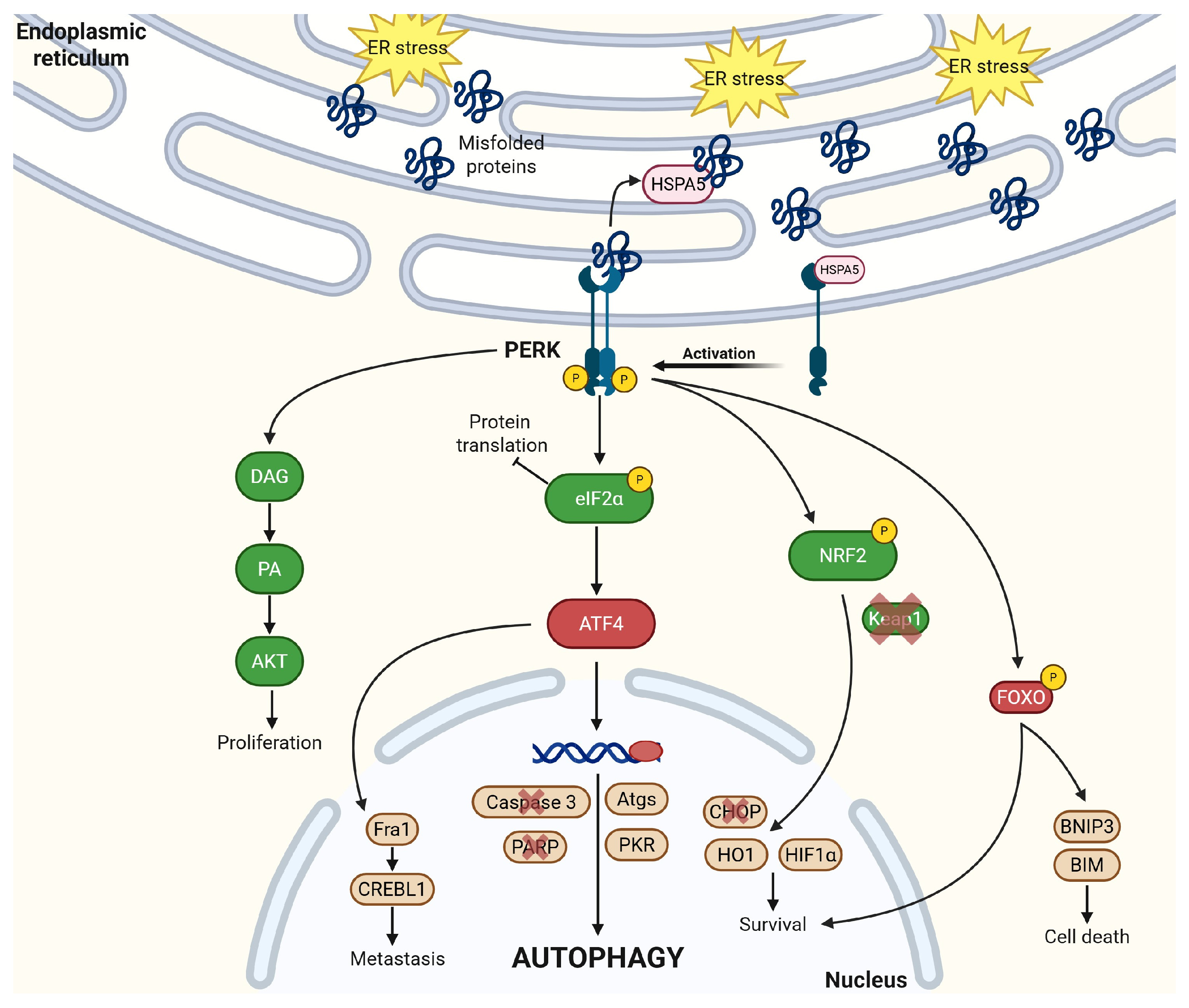
2.3. PERK
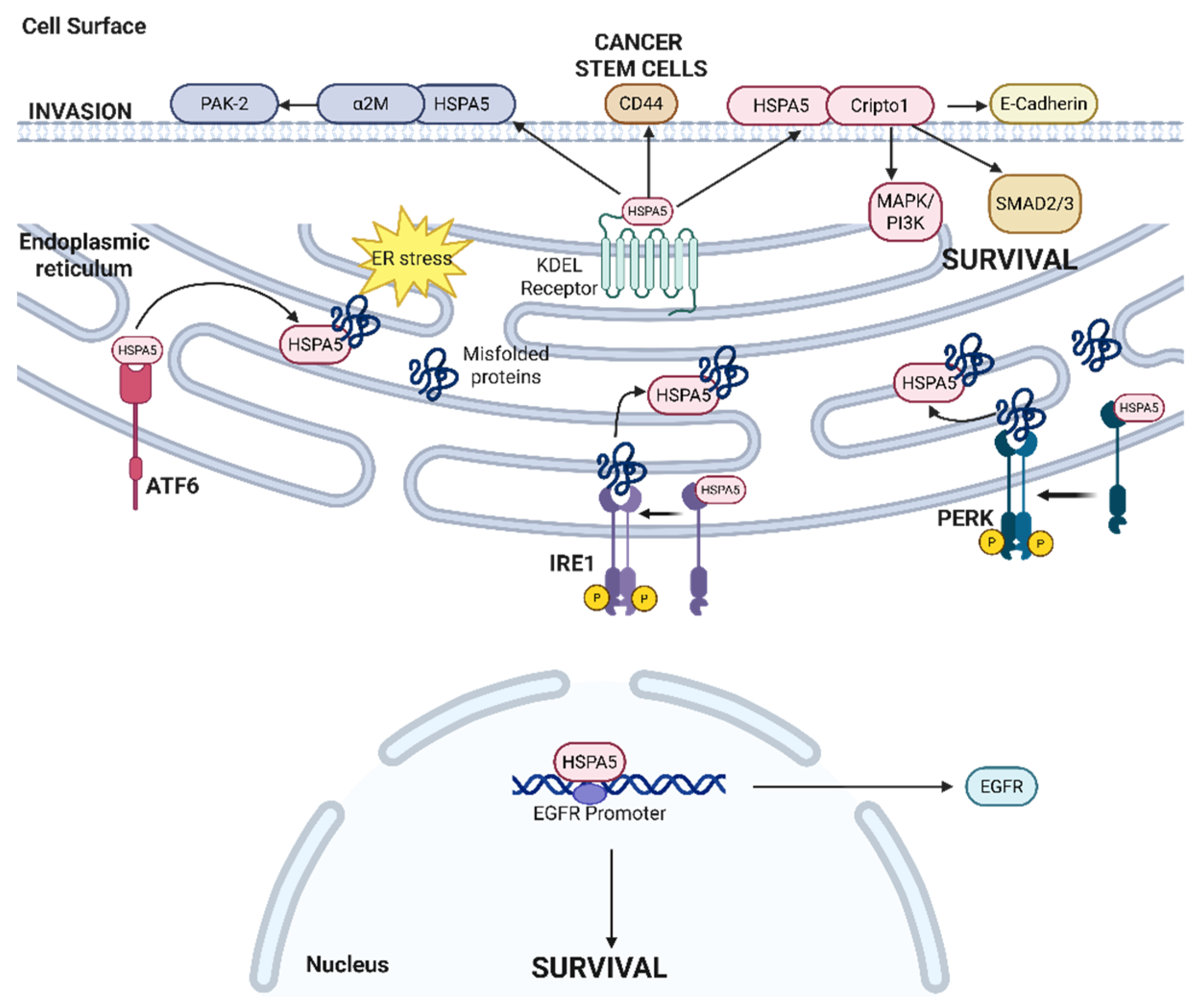
2.4. HSPA5
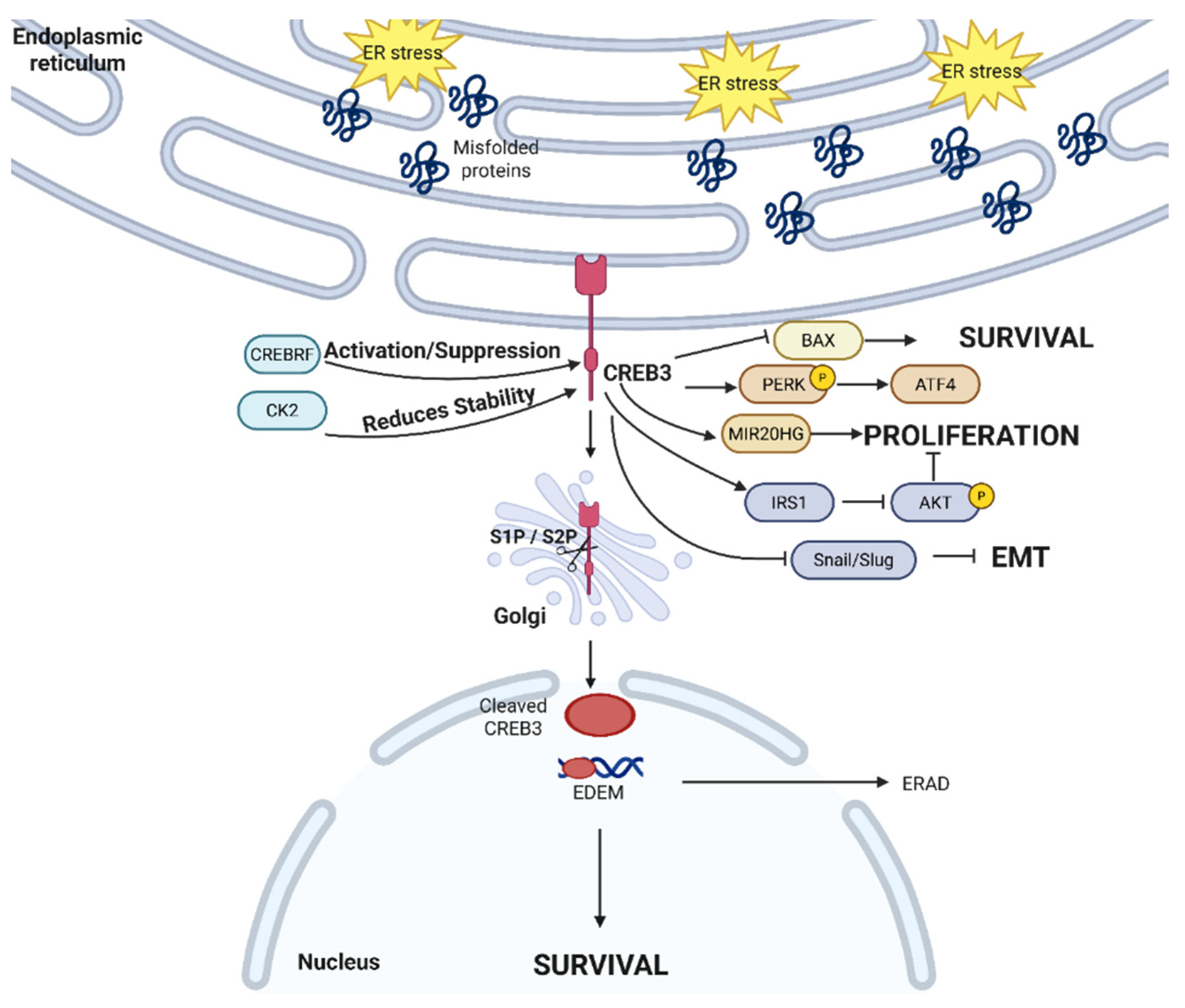
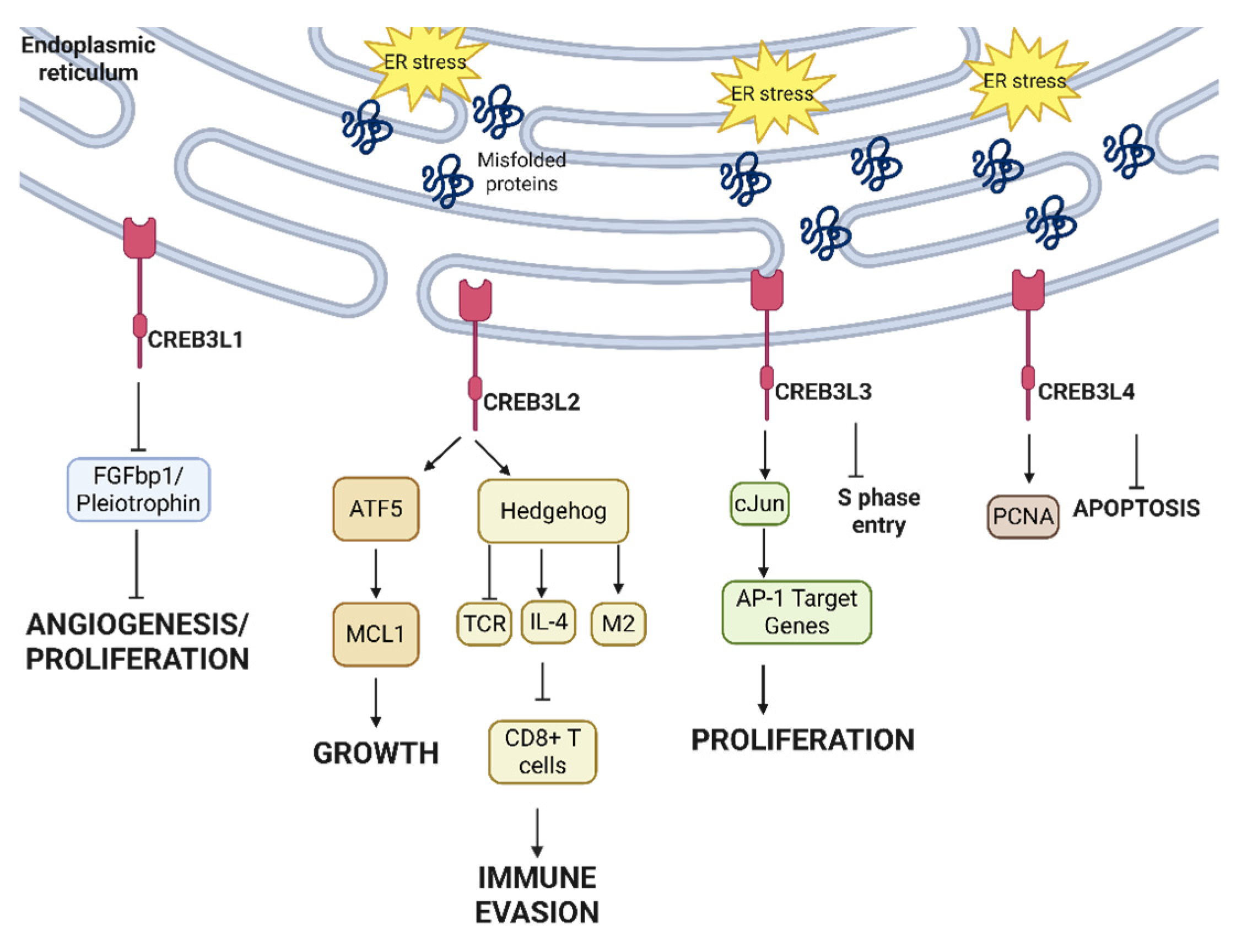
2.5. Non-Canonical Transcription Factors in UPR—CREB3 Family
2.6. UPR in Cancer Biology as a Whole
3. Novel Impacts of UPR Outside of Protein Homeostasis
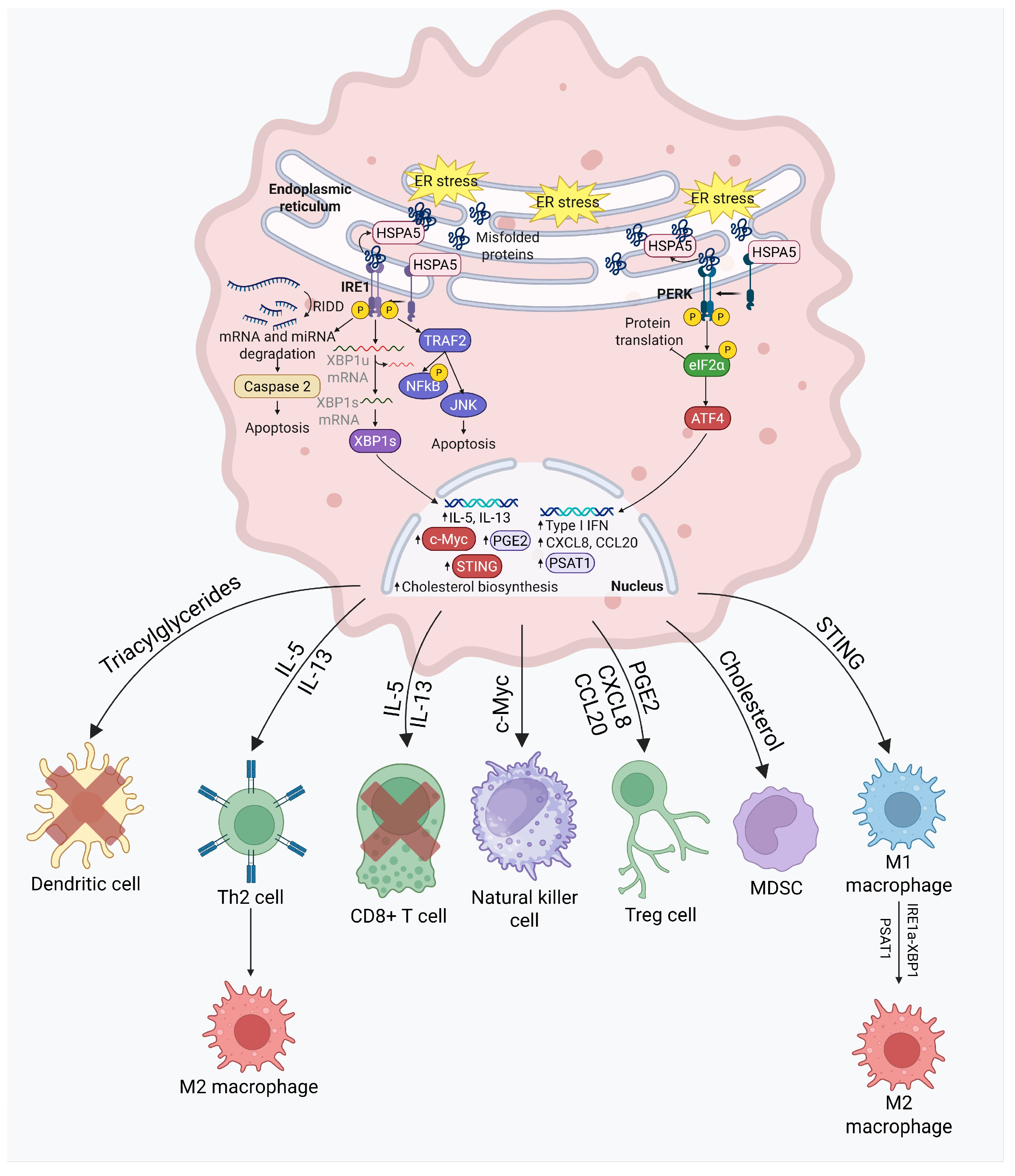
3.1. UPR and Immune Cells
3.2. UPR and Lipid Metabolism
3.3. UPR and ER-Phagy
4. Therapeutically Targeting UPR Pathways in Cancer
4.1. Overcoming Chemoresistance Regulated by UPR Using Combinatorial Approaches
4.2. Overcoming Immunotherapy Resistance by Targeting UPR Pathways
4.3. Specifically Targeting the IRE1α Arm
4.4. Specifically Targeting the PERK Arm
4.5. Specifically Targeting the EIF2α Arm
4.6. Specifically Targeting HSPA5
4.7. Specifically Targeting ATF6
4.8. Nanoparticles
| Agents | Mechanism | Pathway(s) Targeted | References |
|---|---|---|---|
| Camptothecin | Inhibits NRF2 signaling, sensitizing cancer cells (e.g., hepatocellular carcinoma) to chemotherapy and anti-tumor drugs by blocking the NRF2–ARE activation pathway. | NRF2 | [186,187,188] |
| ISRIB | Inhibits eIF2α phosphorylation, enhancing gemcitabine-induced pancreatic cancer cell death. | eIF2α | [189] |
| MKC-3946 | Inhibits IRE1α ribonuclease activity, enhancing tumor-killing effects of bortezomib or arsenic trioxide in acute myeloid leukemia cells. | IRE1α | [190] |
| EGCG | Targets HSPA5 to resensitize glioma cells to temozolomide treatment. | HSPA5 | [191] |
| KDM4B inhibitors (methylstat) | Inhibit KDM4B, upregulating UPR target ATF4 and triggering apoptosis in PTEN-deficient breast cancer cells. | KDM4B | [196] |
| PI3K/AKT inhibitors | Used in breast cancer, but combined with KDM4B inhibitors to sensitize PTEN-deficient tumors to apoptosis by inducing UPR and activating ATF4. | KDM4B, ATF4 | [196] |
| IRE1α inhibitors | Target IRE1α signaling to reduce tumor proliferation and alter the tumor microenvironment; effective for both solid and liquid cancers. ORIN1001, a notable IRE1α-XBP1 blocker, is progressing in clinical trials. | IRE1α | [200,201] |
| Fulvestrant | Indirectly inhibits IRE1α-XBP1 axis and induces selective apoptosis in breast cancer cells | IRE1α | [202] |
| Fenofibrate | Activates IRE1α and PERK, acting as a PPAR-γ antagonist, in prostate cancer cells. | IRE1α, PERK | [203] |
| Sunitinib | FDA-approved for blood cancers; inhibits IRE1α autophosphorylation, reducing XBP1 splicing in blood cancer cells. | IRE1α | [15,204] |
| PERK inhibitors (HC4, GSK2656157, etc.) | Block PERK signaling, preventing metastasis in cancers like HER2+ breast cancer and colorectal cancer; their application must consider side effects such as viral infection susceptibility. | PERK | [207,210,211,212,213] |
| Doxorubicin | Blocks cancer proliferation via CREB3L1, a downstream mediator of PERK in metastasis. Target of ER stress induction by nanoparticles. | CREB3L1 (PERK) | [208,209] |
| Taxanes (e.g., paclitaxel and docetaxel) | Activate PERK/eIF2α axis, inducing cancer cell apoptosis by targeting microtubules; resistance can be mitigated by targeting EIF2α/ATF4 pathway. | IRE1α, PERK | [216,217,218,219,220,221,222,223] |
| GCN2 inhibitors (SP600215, BCR-ABL) | Inhibit ISR (Integrated Stress Response) by blocking EIF2α phosphorylation, which can overcome drug resistance in cancers. | EIF2α | [225,226] |
| Ceapin-A7 | Selectively inhibits ATF6a in UPR, with potential for colorectal and pancreatic cancer treatment. | ATF6a | [235,236] |
| Melatonin, PF−429242, combined therapy, 4-PBA | Inhibits ATF6, demonstrating potential to sensitize colon cancer cells to cytotoxic effects in combination with Adriamycin; targets HSPA5-ATF6-CHOP axis. | ATF6 and related axes | [237,238,239,240] |
| HSPA5 inhibitors (PAT-SM6) | Target HSPA5, a chaperone protein involved in tumorigenesis; currently in phase I trials for tumors. | HSPA5 | [249] |
| Nanoparticles (e.g., graphene oxide-based) | Deliver drugs to cancer cells to induce ER stress and autophagy; can encapsulate chemotherapeutic agents (e.g., doxorubicin and cisplatin) for targeted delivery. | N/A | [241,242,243,244,245,246,247,248] |
5. Limitations and Challenges in Targeting UPR
6. Conclusions, Perspectives, and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The Endoplasmic Reticulum. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Phillips, M.J.; Voeltz, G.K. Structure and Function of ER Membrane Contact Sites with Other Organelles. Nat. Rev. Mol. Cell Biol. 2016, 17, 69–82. [Google Scholar] [CrossRef]
- Hetz, C. The Unfolded Protein Response: Controlling Cell Fate Decisions under ER Stress and Beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bhattacharya, A.; Qi, L. Endoplasmic Reticulum Quality Control in Cancer: Friend or Foe. Semin. Cancer Biol. 2015, 33, 25–33. [Google Scholar] [CrossRef]
- Madden, E.; Logue, S.E.; Healy, S.J.; Manie, S.; Samali, A. The Role of the Unfolded Protein Response in Cancer Progression: From Oncogenesis to Chemoresistance. Biol. Cell 2019, 111, 1–17. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lee, D.-H.; Choudry, H.A.; Bartlett, D.L.; Lee, Y.J. Ferroptosis-Induced Endoplasmic Reticulum Stress: Cross-Talk between Ferroptosis and Apoptosis. Mol. Cancer Res. 2018, 16, 1073–1076. [Google Scholar] [CrossRef]
- Wang, J.; Lee, J.; Liem, D.; Ping, P. HSPA5 Gene Encoding Hsp70 Chaperone BiP in the Endoplasmic Reticulum. Gene 2017, 618, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic Interaction of BiP and ER Stress Transducers in the Unfolded-Protein Response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER Stress Regulation of ATF6 Localization by Dissociation of BiP/GRP78 Binding and Unmasking of Golgi Localization Signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Siwecka, N.; Rozpędek-Kamińska, W.; Wawrzynkiewicz, A.; Pytel, D.; Diehl, J.A.; Majsterek, I. The Structure, Activation and Signaling of IRE1 and Its Role in Determining Cell Fate. Biomedicines 2021, 9, 156. [Google Scholar] [CrossRef]
- Iwawaki, T.; Akai, R.; Yamanaka, S.; Kohno, K. Function of IRE1 Alpha in the Placenta Is Essential for Placental Development and Embryonic Viability. Proc. Natl. Acad. Sci. USA 2009, 106, 16657–16662. [Google Scholar] [CrossRef]
- Lai, C.W.; Otero, J.H.; Hendershot, L.M.; Snapp, E. ERdj4 Protein Is a Soluble Endoplasmic Reticulum (ER) DnaJ Family Protein That Interacts with ER-Associated Degradation Machinery *. J. Biol. Chem. 2012, 287, 7969–7978. [Google Scholar] [CrossRef] [PubMed]
- Amin-Wetzel, N.; Saunders, R.A.; Kamphuis, M.J.; Rato, C.; Preissler, S.; Harding, H.P.; Ron, D. A J-Protein Co-Chaperone Recruits BiP to Monomerize IRE1 and Repress the Unfolded Protein Response. Cell 2017, 171, 1625–1637.e13. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.K.; Dey, M.; Neculai, D.; Cao, C.; Dever, T.E.; Sicheri, F. Structure of the Dual Enzyme Ire1 Reveals the Basis for Catalysis and Regulation in Nonconventional RNA Splicing. Cell 2008, 132, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.U.; Bagratuni, T.; Davenport, E.L.; Nowak, P.R.; Silva-Santisteban, M.C.; Hardcastle, A.; McAndrews, C.; Rowlands, M.G.; Morgan, G.J.; Aherne, W.; et al. Structure of the Ire1 Autophosphorylation Complex and Implications for the Unfolded Protein Response. EMBO J. 2011, 30, 894–905. [Google Scholar] [CrossRef]
- Jurkin, J.; Henkel, T.; Nielsen, A.F.; Minnich, M.; Popow, J.; Kaufmann, T.; Heindl, K.; Hoffmann, T.; Busslinger, M.; Martinez, J. The Mammalian tRNA Ligase Complex Mediates Splicing of XBP1 mRNA and Controls Antibody Secretion in Plasma Cells. EMBO J. 2014, 33, 2922–2936. [Google Scholar] [CrossRef]
- Fu, F.; Doroudgar, S. IRE1/XBP1 and Endoplasmic Reticulum Signaling—From Basic to Translational Research for Cardiovascular Disease. Curr. Opin. Physiol. 2022, 28, 100552. [Google Scholar] [CrossRef]
- Goupil, S.L.; Laprade, H.; Aubry, M.; Chevet, E. Exploring the IRE1 Interactome: From Canonical Signaling Functions to Unexpected Roles. J. Biol. Chem. 2024, 300, 107169. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Kopito, R.R.; Christianson, J.C. The Mammalian Endoplasmic Reticulum-Associated Degradation System. Cold Spring Harb. Perspect. Biol. 2013, 5, a013185. [Google Scholar] [CrossRef]
- Sheng, X.; Nenseth, H.Z.; Qu, S.; Kuzu, O.F.; Frahnow, T.; Simon, L.; Greene, S.; Zeng, Q.; Fazli, L.; Rennie, P.S.; et al. IRE1α-XBP1s Pathway Promotes Prostate Cancer by Activating c-MYC Signaling. Nat. Commun. 2019, 10, 323. [Google Scholar] [CrossRef]
- Yoshida, H.; Oku, M.; Suzuki, M.; Mori, K. pXBP1(U) Encoded in XBP1 Pre-mRNA Negatively Regulates Unfolded Protein Response Activator pXBP1(S) in Mammalian ER Stress Response. J. Cell Biol. 2006, 172, 565–575. [Google Scholar] [CrossRef]
- Mueller, B.; Klemm, E.J.; Spooner, E.; Claessen, J.H.; Ploegh, H.L. SEL1L Nucleates a Protein Complex Required for Dislocation of Misfolded Glycoproteins. Proc. Natl. Acad. Sci. USA 2008, 105, 12325–12330. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.W.; Han, H.G.; Jeon, Y.J. Protein Quality Control in the Endoplasmic Reticulum and Cancer. Int. J. Mol. Sci. 2018, 19, 3020. [Google Scholar] [CrossRef]
- Travers, K.J.; Patil, C.K.; Wodicka, L.; Lockhart, D.J.; Weissman, J.S.; Walter, P. Functional and Genomic Analyses Reveal an Essential Coordination between the Unfolded Protein Response and ER-Associated Degradation. Cell 2000, 101, 249–258. [Google Scholar] [CrossRef]
- Darmadi, D.; Saleh, R.O.; Oghenemaro, E.F.; Shakir, M.N.; Hjazi, A.; Hassan, Z.F.; Zwamel, A.H.; Matlyuba, S.; Deorari, M.; Oudah, S.K. Role of SEL1L in the Progression of Solid Tumors, with a Special Focus on Its Recent Therapeutic Potential. Cell Biol. Int. 2025, 49, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Qi, L. Quality Control in the Endoplasmic Reticulum: Crosstalk between ERAD and UPR Pathways. Trends Biochem. Sci. 2018, 43, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Shi, G.; Sha, H.; Ji, Y.; Han, X.; Shu, X.; Ma, H.; Inoue, T.; Gao, B.; Kim, H.; et al. IRE1α Is an Endogenous Substrate of Endoplasmic-Reticulum-Associated Degradation. Nat. Cell Biol. 2015, 17, 1546–1555. [Google Scholar] [CrossRef]
- Martínez-Puente, D.H.; Garza-Morales, R.; Pérez-Trujillo, J.J.; García-García, A.; Villanueva-Olivo, A.; Rodríguez-Rocha, H.; Zavala-Flores, L.M.; Saucedo-Cárdenas, O.; Montes de Oca-Luna, R.; Loera-Arias, M.d.J. Targeting E7 Antigen to the Endoplasmic Reticulum Degradation Pathway Promotes a Potent Therapeutic Antitumor Effect. J. Drug Target. 2021, 29, 1102–1110. [Google Scholar] [CrossRef]
- Du, R.; Sullivan, D.K.; Azizian, N.G.; Liu, Y.; Li, Y. Inhibition of ERAD Synergizes with FTS to Eradicate Pancreatic Cancer Cells. BMC Cancer 2021, 21, 237. [Google Scholar] [CrossRef]
- Dreher, L.-S.; Hoppe, T. ERADicate Tumor Progression with Metformin. Mol. Cell 2018, 71, 481–482. [Google Scholar] [CrossRef]
- Hollien, J.; Lin, J.H.; Li, H.; Stevens, N.; Walter, P.; Weissman, J.S. Regulated Ire1-Dependent Decay of Messenger RNAs in Mammalian Cells. J. Cell Biol. 2009, 186, 323–331. [Google Scholar] [CrossRef]
- Moore, K.; Hollien, J. Ire1-Mediated Decay in Mammalian Cells Relies on mRNA Sequence, Structure, and Translational Status. Mol. Biol. Cell 2015, 26, 2873–2884. [Google Scholar] [CrossRef]
- Han, D.; Lerner, A.G.; Vande Walle, L.; Upton, J.-P.; Xu, W.; Hagen, A.; Backes, B.J.; Oakes, S.A.; Papa, F.R. IRE1α Kinase Activation Modes Control Alternate Endoribonuclease Outputs to Determine Divergent Cell Fates. Cell 2009, 138, 562–575. [Google Scholar] [CrossRef]
- Tabas, I.; Ron, D. Integrating the Mechanisms of Apoptosis Induced by Endoplasmic Reticulum Stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Lindner, P.; Christensen, S.B.; Nissen, P.; Møller, J.V.; Engedal, N. Cell Death Induced by the ER Stressor Thapsigargin Involves Death Receptor 5, a Non-Autophagic Function of MAP1LC3B, and Distinct Contributions from Unfolded Protein Response Components. Cell Commun. Signal. 2020, 18, 12. [Google Scholar] [CrossRef]
- Upton, J.-P.; Wang, L.; Han, D.; Wang, E.S.; Huskey, N.E.; Lim, L.; Truitt, M.; McManus, M.T.; Ruggero, D.; Goga, A.; et al. IRE1α Cleaves Select microRNAs During ER Stress to Derepress Translation of Proapoptotic Caspase-2. Science 2012, 338, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Fu, N.; Yang, P. MiR-17 Downregulation by High Glucose Stabilizes Thioredoxin-Interacting Protein and Removes Thioredoxin Inhibition on ASK1 Leading to Apoptosis. Toxicol. Sci. 2016, 150, 84–96. [Google Scholar] [CrossRef]
- Lerner, A.G.; Upton, J.-P.; Praveen, P.V.K.; Ghosh, R.; Nakagawa, Y.; Igbaria, A.; Shen, S.; Nguyen, V.; Backes, B.J.; Heiman, M.; et al. IRE1α Induces Thioredoxin-Interacting Protein to Activate the NLRP3 Inflammasome and Promote Programmed Cell Death under Irremediable ER Stress. Cell Metab. 2012, 16, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Nishitoh, H.; Matsuzawa, A.; Tobiume, K.; Saegusa, K.; Takeda, K.; Inoue, K.; Hori, S.; Kakizuka, A.; Ichijo, H. ASK1 Is Essential for Endoplasmic Reticulum Stress-Induced Neuronal Cell Death Triggered by Expanded Polyglutamine Repeats. Genes Dev. 2002, 16, 1345–1355. [Google Scholar] [CrossRef]
- Urano, F.; Wang, X.; Bertolotti, A.; Zhang, Y.; Chung, P.; Harding, H.P.; Ron, D. Coupling of Stress in the ER to Activation of JNK Protein Kinases by Transmembrane Protein Kinase IRE1. Science 2000, 287, 664–666. [Google Scholar] [CrossRef]
- Halbleib, K.; Pesek, K.; Covino, R.; Hofbauer, H.F.; Wunnicke, D.; Hänelt, I.; Hummer, G.; Ernst, R. Activation of the Unfolded Protein Response by Lipid Bilayer Stress. Mol. Cell 2017, 67, 673–684.e8. [Google Scholar] [CrossRef]
- Kanekura, K.; Ma, X.; Murphy, J.T.; Zhu, L.J.; Diwan, A.; Urano, F. IRE1 Prevents Endoplasmic Reticulum Membrane Permeabilization and Cell Death under Pathological Conditions. Sci. Signal. 2015, 8, ra62. [Google Scholar] [CrossRef]
- Thuerauf, D.J.; Marcinko, M.; Belmont, P.J.; Glembotski, C.C. Effects of the Isoform-Specific Characteristics of ATF6α and ATF6β on Endoplasmic Reticulum Stress Response Gene Expression and Cell Viability *. J. Biol. Chem. 2007, 282, 22865–22878. [Google Scholar] [CrossRef]
- Nadanaka, S.; Okada, T.; Yoshida, H.; Mori, K. Role of Disulfide Bridges Formed in the Luminal Domain of ATF6 in Sensing Endoplasmic Reticulum Stress. Mol. Cell. Biol. 2007, 27, 1027–1043. [Google Scholar] [CrossRef]
- Hillary, R.F.; FitzGerald, U. A Lifetime of Stress: ATF6 in Development and Homeostasis. J. Biomed. Sci. 2018, 25, 48. [Google Scholar] [CrossRef]
- Shen, J.; Snapp, E.L.; Lippincott-Schwartz, J.; Prywes, R. Stable Binding of ATF6 to BiP in the Endoplasmic Reticulum Stress Response. Mol. Cell. Biol. 2005, 25, 921–932. [Google Scholar] [CrossRef]
- Lei, Y.; Yu, H.; Ding, S.; Liu, H.; Liu, C.; Fu, R. Molecular Mechanism of ATF6 in Unfolded Protein Response and Its Role in Disease. Heliyon 2024, 10, e25937. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-K.; Blackwood, E.A.; Azizi, K.; Thuerauf, D.J.; Fahem, A.G.; Hofmann, C.; Kaufman, R.J.; Doroudgar, S.; Glembotski, C.C. ATF6 Decreases Myocardial Ischemia/Reperfusion Damage and Links ER Stress and Oxidative Stress Signaling Pathways in the Heart. Circ. Res. 2017, 120, 862–875. [Google Scholar] [CrossRef]
- Shen, J.; Prywes, R. Dependence of Site-2 Protease Cleavage of ATF6 on Prior Site-1 Protease Digestion Is Determined by the Size of the Luminal Domain of ATF6. J. Biol. Chem. 2004, 279, 43046–43051. [Google Scholar] [CrossRef] [PubMed]
- Coleman, O.I.; Lobner, E.M.; Bierwirth, S.; Sorbie, A.; Waldschmitt, N.; Rath, E.; Berger, E.; Lagkouvardos, I.; Clavel, T.; McCoy, K.D.; et al. Activated ATF6 Induces Intestinal Dysbiosis and Innate Immune Response to Promote Colorectal Tumorigenesis. Gastroenterology 2018, 155, 1539–1552.e12. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, M.; Ishikawa, T.; Ishiguro, M.; Tokura, M.; Yamauchi, S.; Kikuchi, A.; Uetake, H.; Yasuno, M.; Kawano, T. Expression of ATF6 as a Marker of Pre-Cancerous Atypical Change in Ulcerative Colitis-Associated Colorectal Cancer: A Potential Role in the Management of Dysplasia. J. Gastroenterol. 2018, 53, 631–641. [Google Scholar] [CrossRef]
- Spaan, C.N.; Smit, W.L.; van Lidth de Jeude, J.F.; Meijer, B.J.; Muncan, V.; van den Brink, G.R.; Heijmans, J. Expression of UPR Effector Proteins ATF6 and XBP1 Reduce Colorectal Cancer Cell Proliferation and Stemness by Activating PERK Signaling. Cell Death Dis. 2019, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chang, L.; Hu, J. Activating Transcription Factor 6 Regulated Cell Growth, Migration and Inhibiteds Cell Apoptosis and Autophagy via MAPK Pathway in Cervical Cancer. J. Reprod. Immunol. 2020, 139, 103120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.B.; Snyder, J.T.; Alonso, L.C. Atf6α Impacts Cell Number by Influencing Survival, Death and Proliferation. Mol. Metab. 2019, 27, S69–S80. [Google Scholar] [CrossRef]
- Rodvold, J.J.; Chiu, K.T.; Hiramatsu, N.; Nussbacher, J.K.; Galimberti, V.; Mahadevan, N.R.; Willert, K.; Lin, J.H.; Zanetti, M. Intercellular Transmission of the Unfolded Protein Response Promotes Survival and Drug Resistance in Cancer Cells. Sci. Signal. 2017, 10, eaah7177. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, S.; Muhammad, S.; Ren, Q.; Sun, C. miR-103/107 Promote ER Stress-Mediated Apoptosis via Targeting the Wnt3a/β-Catenin/ATF6 Pathway in Preadipocytes. J. Lipid Res. 2018, 59, 843–853. [Google Scholar] [CrossRef]
- Morishima, N.; Nakanishi, K.; Nakano, A. Activating Transcription Factor-6 (ATF6) Mediates Apoptosis with Reduction of Myeloid Cell Leukemia Sequence 1 (Mcl-1) Protein via Induction of WW Domain Binding Protein 1 *. J. Biol. Chem. 2011, 286, 35227–35235. [Google Scholar] [CrossRef]
- Ma, Y.; Brewer, J.W.; Alan Diehl, J.; Hendershot, L.M. Two Distinct Stress Signaling Pathways Converge Upon the CHOP Promoter During the Mammalian Unfolded Protein Response. J. Mol. Biol. 2002, 318, 1351–1365. [Google Scholar] [CrossRef]
- Yang, H.; Niemeijer, M.; van de Water, B.; Beltman, J.B. ATF6 Is a Critical Determinant of CHOP Dynamics during the Unfolded Protein Response. iScience 2020, 23, 100860. [Google Scholar] [CrossRef]
- Bu, Y.; Diehl, J.A. PERK Integrates Oncogenic Signaling and Cell Survival During Cancer Development. J. Cell. Physiol. 2016, 231, 2088–2096. [Google Scholar] [CrossRef]
- Teske, B.F.; Wek, S.A.; Bunpo, P.; Cundiff, J.K.; McClintick, J.N.; Anthony, T.G.; Wek, R.C. The eIF2 Kinase PERK and the Integrated Stress Response Facilitate Activation of ATF6 during Endoplasmic Reticulum Stress. Mol. Biol. Cell 2011, 22, 4390–4405. [Google Scholar] [CrossRef] [PubMed]
- Rojo De La Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Zhang, D.D.; Hannink, M. Distinct Cysteine Residues in Keap1 Are Required for Keap1-Dependent Ubiquitination of Nrf2 and for Stabilization of Nrf2 by Chemopreventive Agents and Oxidative Stress. Mol. Cell. Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Diehl, J.A. PERK-Dependent Activation of Nrf2 Contributes to Redox Homeostasis and Cell Survival Following Endoplasmic Reticulum Stress. J. Biol. Chem. 2004, 279, 20108–20117. [Google Scholar] [CrossRef]
- Jang, J.E.; Eom, J.-I.; Jeung, H.-K.; Chung, H.; Kim, Y.R.; Kim, J.S.; Cheong, J.-W.; Min, Y.H. PERK/NRF2 and Autophagy Form a Resistance Mechanism against G9a Inhibition in Leukemia Stem Cells. J. Exp. Clin. Cancer Res. 2020, 39, 66. [Google Scholar] [CrossRef] [PubMed]
- Küper, A.; Baumann, J.; Göpelt, K.; Baumann, M.; Sänger, C.; Metzen, E.; Kranz, P.; Brockmeier, U. Overcoming Hypoxia-Induced Resistance of Pancreatic and Lung Tumor Cells by Disrupting the PERK-NRF2-HIF-Axis. Cell Death Dis. 2021, 12, 82. [Google Scholar] [CrossRef]
- Ortmann, B.M. Hypoxia-Inducible Factor in Cancer: From Pathway Regulation to Therapeutic Opportunity. BMJ Oncol. 2024, 3, e000154. [Google Scholar] [CrossRef] [PubMed]
- Wek, R.C. Role of eIF2α Kinases in Translational Control and Adaptation to Cellular Stress. Cold Spring Harb. Perspect. Biol. 2018, 10, a032870. [Google Scholar] [CrossRef] [PubMed]
- Fels, D.R.; Koumenis, C. The PERK/eIF2α/ATF4 Module of the UPR in Hypoxia Resistance and Tumor Growth. Cancer Biol. Ther. 2006, 5, 723–728. [Google Scholar] [CrossRef]
- Kouroku, Y.; Fujita, E.; Tanida, I.; Ueno, T.; Isoai, A.; Kumagai, H.; Ogawa, S.; Kaufman, R.; Kominami, E.; Momoi, T. ER Stress (PERK/eIF2a Phosphorylation) Mediates the Polyglutamine-Induced LC3 Conversion, an Essential Step for Autophagy Formation. Cell Death Differ. 2007, 14, 230–239. [Google Scholar] [CrossRef]
- Humeau, J.; Leduc, M.; Cerrato, G.; Loos, F.; Kepp, O.; Kroemer, G. Phosphorylation of Eukaryotic Initiation Factor-2α (eIF2α) in Autophagy. Cell Death Dis. 2020, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Rouschop, K.M.; Dubois, L.J.; Keulers, T.G.; Van Den Beucken, T.; Lambin, P.; Bussink, J.; Van Der Kogel, A.J.; Koritzinsky, M.; Wouters, B.G. PERK/eIF2α Signaling Protects Therapy Resistant Hypoxic Cells through Induction of Glutathione Synthesis and Protection against ROS. Proc. Natl. Acad. Sci. USA 2013, 110, 4622–4627. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Li, H.; Hu, Z.; Zhang, W. eIF 2α Kinases PERK and GCN 2 Act on FOXO to Potentiate FOXO Activity. Genes Cells 2018, 23, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hietakangas, V.; Wee, S.; Lim, S.C.; Gunaratne, J.; Cohen, S.M. ER Stress Potentiates Insulin Resistance through PERK-Mediated FOXO Phosphorylation. Genes Dev. 2013, 27, 441–449. [Google Scholar] [CrossRef]
- Lin, A.; Yao, J.; Zhuang, L.; Wang, D.; Han, J.; Lam, E.W.-F.; TCGA Research Network; Gan, B. The FoxO–BNIP3 Axis Exerts a Unique Regulation of mTORC1 and Cell Survival under Energy Stress. Oncogene 2014, 33, 3183–3194. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, R.; Sun, X.; Guo, Q.; Zhang, Y.; Zhang, N.; Oh, Y.; Fan, L.; Wang, C.; Gu, N. Fatty Acid Palmitate Suppresses FoxO1 Expression via PERK and IRE1 Unfolded Protein Response in C2C12 Myotubes. Toxicol. In Vitro 2022, 85, 105459. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Yin, Y.; Li, M.; Wang, B.; Yang, L.; Jiang, Y. FOXO3-Mediated up-Regulation of Bim Contributes to Rhein-Induced Cancer Cell Apoptosis. Apoptosis 2015, 20, 399–409. [Google Scholar] [CrossRef]
- Pytel, D.; Majsterek, I.; Diehl, J.A. Tumor Progression and the Different Faces of the PERK Kinase. Oncogene 2016, 35, 1207–1215. [Google Scholar] [CrossRef]
- Zhao, C.; Du, G.; Skowronek, K.; Frohman, M.A.; Bar-Sagi, D. Phospholipase D2-Generated Phosphatidic Acid Couples EGFR Stimulation to Ras Activation by Sos. Nat. Cell Biol. 2007, 9, 707–712. [Google Scholar] [CrossRef]
- Bobrovnikova-Marjon, E.; Pytel, D.; Riese, M.J.; Vaites, L.P.; Singh, N.; Koretzky, G.A.; Witze, E.S.; Diehl, J.A. PERK Utilizes Intrinsic Lipid Kinase Activity To Generate Phosphatidic Acid, Mediate Akt Activation, and Promote Adipocyte Differentiation. Mol. Cell. Biol. 2012, 32, 2268–2278. [Google Scholar] [CrossRef]
- Perea, V.; Cole, C.; Lebeau, J.; Dolina, V.; Baron, K.R.; Madhavan, A.; Kelly, J.W.; Grotjahn, D.A.; Wiseman, R.L. PERK Signaling Promotes Mitochondrial Elongation by Remodeling Membrane Phosphatidic Acid. EMBO J. 2023, 42, e113908. [Google Scholar] [CrossRef]
- Dubois, C.; Puthooru, D.K.; Abeele, F.V. The PERK/Akt Pathway Mediates Apoptosis Resistance to ER Ca2+ Stress in LNCaP Prostate Cancer Cells. J. Cancer Sci. Clin. Ther. 2023, 7, 100–107. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Cheng, J.; Elfiky, A.A.; Wei, C.; Fu, J. New Progresses on Cell Surface Protein HSPA5/BiP/GRP78 in Cancers and COVID-19. Front. Immunol. 2023, 14, 1166680. [Google Scholar] [CrossRef]
- Silva, N.S.M.; Siebeneichler, B.; Oliveira, C.S.; Dores-Silva, P.R.; Borges, J.C. The Regulation of the Thermal Stability and Affinity of the HSPA5 (Grp78/BiP) by Clients and Nucleotides Is Modulated by Domains Coupling. Biochim. Biophys. Acta BBA—Proteins Proteom. 2024, 1872, 141034. [Google Scholar] [CrossRef] [PubMed]
- Behnke, J.; Feige, M.J.; Hendershot, L.M. BiP and Its Nucleotide Exchange Factors Grp170 and Sil1: Mechanisms of Action and Biological Functions. J. Mol. Biol. 2015, 427, 1589–1608. [Google Scholar] [CrossRef]
- Simons, J.F.; Ferro-Novick, S.; Rose, M.D.; Helenius, A. BiP/Kar2p Serves as a Molecular Chaperone during Carboxypeptidase Y Folding in Yeast. J. Cell Biol. 1995, 130, 41–49. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Law, D.T.; Williams, D.B. Binding Protein BiP Is Required for Translocation of Secretory Proteins into the Endoplasmic Reticulum in Saccharomyces Cerevisiae. Proc. Natl. Acad. Sci. USA 1991, 88, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, G.; Ha, D.P.; Wang, J.; Xiong, M.; Lee, A.S. ER Chaperone GRP78/BiP Translocates to the Nucleus under Stress and Acts as a Transcriptional Regulator. Proc. Natl. Acad. Sci. USA 2023, 120, e2303448120. [Google Scholar] [CrossRef]
- Jiang, D.; Niwa, M.; Koong, A.C. Targeting the IRE1α–XBP1 Branch of the Unfolded Protein Response in Human Diseases. Semin. Cancer Biol. 2015, 33, 48–56. [Google Scholar] [CrossRef]
- Cabrera, M.; Muñiz, M.; Hidalgo, J.; Vega, L.; Martín, M.E.; Velasco, A. The Retrieval Function of the KDEL Receptor Requires PKA Phosphorylation of Its C-Terminus. Mol. Biol. Cell 2003, 14, 4114–4125. [Google Scholar] [CrossRef]
- Jin, H.; Komita, M.; Aoe, T. The Role of BiP Retrieval by the KDEL Receptor in the Early Secretory Pathway and Its Effect on Protein Quality Control and Neurodegeneration. Front. Mol. Neurosci. 2017, 10, 222. [Google Scholar] [CrossRef]
- Aoe, T. The KDEL Receptor, ERD2, Regulates Intracellular Traffic by Recruiting a GTPase-Activating Protein for ARF1. EMBO J. 1997, 16, 7305–7316. [Google Scholar] [CrossRef] [PubMed]
- Kelber, J.A.; Panopoulos, A.D.; Shani, G.; Booker, E.C.; Belmonte, J.C.; Vale, W.W.; Gray, P.C. Blockade of Cripto Binding to Cell Surface GRP78 Inhibits Oncogenic Cripto Signaling via MAPK/PI3K and Smad2/3 Pathways. Oncogene 2009, 28, 2324–2336. [Google Scholar] [CrossRef]
- Rangel, M.C.; Karasawa, H.; Castro, N.P.; Nagaoka, T.; Salomon, D.S.; Bianco, C. Role of Cripto-1 during Epithelial-to-Mesenchymal Transition in Development and Cancer. Am. J. Pathol. 2012, 180, 2188–2200. [Google Scholar] [CrossRef]
- Shani, G.; Fischer, W.H.; Justice, N.J.; Kelber, J.A.; Vale, W.; Gray, P.C. GRP78 and Cripto Form a Complex at the Cell Surface and Collaborate To Inhibit Transforming Growth Factor β Signaling and Enhance Cell Growth. Mol. Cell. Biol. 2008, 28, 666–677. [Google Scholar] [CrossRef]
- Misra, U.K.; Deedwania, R.; Pizzo, S.V. Binding of Activated A2-Macroglobulin to Its Cell Surface Receptor GRP78 in 1-LN Prostate Cancer Cells Regulates PAK-2-Dependent Activation of LIMK. J. Biol. Chem. 2005, 280, 26278–26286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Liu, Y.; Wang, X.; Ren, M. Multidimensional Pan-Cancer Analysis of HSPA5 and Its Validation in the Prognostic Value of Bladder Cancer. Heliyon 2024, 10, e27184. [Google Scholar] [CrossRef]
- Fu, J.; Wei, C.; He, J.; Zhang, L.; Zhou, J.; Balaji, K.S.; Shen, S.; Peng, J.; Sharma, A.; Fu, J. Evaluation and Characterization of HSPA5 (GRP78) Expression Profiles in Normal Individuals and Cancer Patients with COVID-19. Int. J. Biol. Sci. 2021, 17, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef]
- Ghazi, N.; Aali, N.; Shahrokhi, V.-R.; Mohajertehran, F. Relative Expression of SOX2 and OCT4 in Oral Squamous Cell Carcinoma and Oral Epithelial Dysplasia. Rep. Biochem. Mol. Biol. 2020, 9, 171–179. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.W.; Wong, G.; Earle, C.; Chen, L. Hyaluronan-CD44v3 Interaction with Oct4-Sox2-Nanog Promotes miR-302 Expression Leading to Self-Renewal, Clonal Formation, and Cisplatin Resistance in Cancer Stem Cells from Head and Neck Squamous Cell Carcinoma. J. Biol. Chem. 2012, 287, 32800–32824. [Google Scholar] [CrossRef]
- Chen, J.-L.; Tai, Y.-S.; Tsai, H.-Y.; Hsieh, C.-Y.; Chen, C.-L.; Liu, C.-J.; Wu, D.-C.; Huang, Y.-B.; Lin, M.-W. Betulinic Acid Inhibits the Stemness of Gastric Cancer Cells by Regulating the GRP78-TGF-Β1 Signaling Pathway and Macrophage Polarization. Molecules 2023, 28, 1725. [Google Scholar] [CrossRef]
- Cha-Molstad, H.; Sung, K.S.; Hwang, J.; Kim, K.A.; Yu, J.E.; Yoo, Y.D.; Jang, J.M.; Han, D.H.; Molstad, M.; Kim, J.G.; et al. Amino-Terminal Arginylation Targets Endoplasmic Reticulum Chaperone BiP for Autophagy through P62 Binding. Nat. Cell Biol. 2015, 17, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Miao, Y.; Wang, J.; Gan, J.; Feng, J. Predictive Value and Immunological Role of the HSPA5 Gene in Cervical Cancer. Biochem. Genet. 2025, 63, 1566–1583. [Google Scholar] [CrossRef]
- Yuxiong, W.; Faping, L.; Bin, L.; Yanghe, Z.; Yao, L.; Yunkuo, L.; Yishu, W.; Honglan, Z. Regulatory Mechanisms of the cAMP-Responsive Element Binding Protein 3 (CREB3) Family in Cancers. Biomed. Pharmacother. 2023, 166, 115335. [Google Scholar] [CrossRef] [PubMed]
- Oh-hashi, K.; Yamamoto, A.; Murase, R.; Hirata, Y. Comparative Analysis of CREB3 and CREB3L2 Protein Expression in HEK293 Cells. Int. J. Mol. Sci. 2021, 22, 2767. [Google Scholar] [CrossRef]
- Lv, B.; Zhang, D. Targeting CREBRF in Cancer: Mechanistic Insights and Future Directions. Biol. Targets Ther. 2025, 19, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Audas, T.E.; Li, Y.; Liang, G.; Lu, R. A Novel Protein, Luman/CREB3 Reruitment Factor, Inhibits Luman Activation of the Unfolded Protein Response. Mol. Cell. Biol. 2008, 28, 3952–3966. [Google Scholar] [CrossRef]
- Oh-hashi, K.; Hasegawa, T.; Naruse, Y.; Hirata, Y. Molecular Characterization of Mouse CREB3 Regulatory Factor in Neuro2a Cells. Mol. Biol. Rep. 2021, 48, 5411–5420. [Google Scholar] [CrossRef]
- Mirabelli, C.; Pelletier, I.; Téoulé, F.; Vidalain, P.-O.; Brisac, C.; Tangy, F.; Delpeyroux, F.; Blondel, B. The CREB3-Herp Signalling Module Limits the Cytosolic Calcium Concentration Increase and Apoptosis Induced by Poliovirus. J. Gen. Virol. 2016, 97, 2194–2200. [Google Scholar] [CrossRef]
- Schmitt, B.M.; Ampofo, E.; Stumpf, H.; Montenarh, M.; Götz, C. The Stability of CREB3/Luman Is Regulated by Protein Kinase CK2 Phosphorylation. Biochem. Biophys. Res. Commun. 2020, 523, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-P.; Mak, T.-Y.; Chin, K.-T.; Ng, I.O.-L.; Jin, D.-Y. N-Linked Glycosylation Is Required for Optimal Proteolytic Activation of Membrane-Bound Transcription Factor CREB-H. J. Cell Sci. 2010, 123, 1438–1448. [Google Scholar] [CrossRef]
- Oh-hashi, K.; Hasegawa, T.; Mizutani, Y.; Takahashi, K.; Hirata, Y. Elucidation of Brefeldin A-Induced ER and Golgi Stress Responses in Neuro2a Cells. Mol. Cell. Biochem. 2021, 476, 3869–3877. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, X.; Wu, J.; Sakaki, K.; Saunders, T.; Rutkowski, D.T.; Back, S.H.; Kaufman, R.J. Endoplasmic Reticulum Stress Activates Cleavage of CREBH to Induce a Systemic Inflammatory Response. Cell 2006, 124, 587–599. [Google Scholar] [CrossRef]
- Bailey, D.; Barreca, C.; O’Hare, P. Trafficking of the bZIP Transmembrane Transcription Factor CREB-H into Alternate Pathways of ERAD and Stress-Regulated Intramembrane Proteolysis. Traffic 2007, 8, 1796–1814. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Audas, T.E.; Li, Y.; Cockram, G.P.; Dean, J.D.; Martyn, A.C.; Kokame, K.; Lu, R. Luman/CREB3 Induces Transcription of the Endoplasmic Reticulum (ER) Stress Response Protein Herp through an ER Stress Response Element. Mol. Cell. Biol. 2006, 26, 7999–8010. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-Y.; Kim, E.; Yoon, S.K.; Yoon, J.-B. Herp Enhances ER-Associated Protein Degradation by Recruiting Ubiquilins. Biochem. Biophys. Res. Commun. 2008, 369, 741–746. [Google Scholar] [CrossRef]
- DenBoer, L.M.; Hardy-Smith, P.W.; Hogan, M.R.; Cockram, G.P.; Audas, T.E.; Lu, R. Luman Is Capable of Binding and Activating Transcription from the Unfolded Protein Response Element. Biochem. Biophys. Res. Commun. 2005, 331, 113–119. [Google Scholar] [CrossRef]
- Wang, X.; Fan, A.; Hong, L. CREB3-Mediated Upregulation of MIR210HG Transcription Enhances Proliferation in Colon Cancer Cells. Transl. Cancer Res. 2025, 14, 2874–2884. [Google Scholar] [CrossRef]
- Hu, Y.; Chu, L.; Liu, J.; Yu, L.; Song, S.; Yang, H.; Han, F. Knockdown of CREB3 Activates Endoplasmic Reticulum Stress and Induces Apoptosis in Glioblastoma. Aging 2019, 11, 8156–8168. [Google Scholar] [CrossRef]
- He, Y.; Han, S.; Li, H.; Wu, Y.; Jia, W.; Chen, Z.; Pan, Y.; Cai, N.; Wen, J.; Li, G.; et al. CREB3 Suppresses Hepatocellular Carcinoma Progression by Depressing AKT Signaling through Competitively Binding with Insulin Receptor and Transcriptionally Activating RNA-binding Motif Protein 38. MedComm 2024, 5, e633. [Google Scholar] [CrossRef]
- Mellor, P.; Deibert, L.; Calvert, B.; Bonham, K.; Carlsen, S.A.; Anderson, D.H. CREB3L1 Is a Metastasis Suppressor That Represses Expression of Genes Regulating Metastasis, Invasion, and Angiogenesis. Mol. Cell. Biol. 2013, 33, 4985–4995. [Google Scholar] [CrossRef]
- Sheng, Z.; Li, L.; Zhu, L.J.; Smith, T.W.; Demers, A.; Ross, A.H.; Moser, R.P.; Green, M.R. A Genome-Wide RNA Interference Screen Reveals an Essential CREB3L2-ATF5-MCL1 Survival Pathway in Malignant Glioma with Therapeutic Implications. Nat. Med. 2010, 16, 671–677. [Google Scholar] [CrossRef]
- Cao, Z.-J.; You, J.; Fan, Y.-M.; Yang, J.-Y.; Sun, J.; Ma, X.; Zhang, J.; Li, Z.; Wang, X.; Feng, Y.-X. Noncanonical UPR Factor CREB3L2 Drives Immune Evasion of Triple-Negative Breast Cancer through Hedgehog Pathway Modulation in T Cells. Sci. Adv. 2025, 11, eads5434. [Google Scholar] [CrossRef] [PubMed]
- Lui, W.-O.; Zeng, L.; Rehrmann, V.; Deshpande, S.; Tretiakova, M.; Kaplan, E.L.; Leibiger, I.; Leibiger, B.; Enberg, U.; Höög, A.; et al. CREB3L2-PPARγ Fusion Mutation Identifies a Thyroid Signaling Pathway Regulated by Intramembrane Proteolysis. Cancer Res. 2008, 68, 7156–7164. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-T. The Liver-Enriched Transcription Factor CREB-H Is a Growth Suppressor Protein Underexpressed in Hepatocellular Carcinoma. Nucleic Acids Res. 2005, 33, 1859–1873. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.K.; Kim, S.Y.; Kyaw, Y.Y.; Win, A.A.; Koo, S.-H.; Kim, H.-H.; Cheong, J. HBx Induces the Proliferation of Hepatocellular Carcinoma Cells via AP1 Over-Expressed as a Result of ER Stress. Biochem. J. 2015, 466, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.; Lu, L.; Dong, K.; Geng, W.; Lv, Y.; Gao, H. The Novel Transcription Factor CREB3L4 Contributes to the Progression of Human Breast Carcinoma. J. Mammary Gland Biol. Neoplasia 2020, 25, 37–50. [Google Scholar] [CrossRef]
- Wang, P.; Han, L.; Yu, M.; Cao, Z.; Li, X.; Shao, Y.; Zhu, G. The Prognostic Value of PERK in Cancer and Its Relationship With Immune Cell Infiltration. Front. Mol. Biosci. 2021, 8, 648752. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, H.; Song, Z.; Jiang, Y.; Kim, H.; Samavati, L.; Nguyen, H.M.; Yang, Z.-Q. The UPR Transducer IRE1 Promotes Breast Cancer Malignancy by Degrading Tumor Suppressor microRNAs. iScience 2020, 23, 101503. [Google Scholar] [CrossRef]
- Martinez-Turtos, A.; Paul, R.; Grima-Reyes, M.; Issaoui, H.; Krug, A.; Mhaidly, R.; Bossowski, J.P.; Chiche, J.; Marchetti, S.; Verhoeyen, E.; et al. IRE1α Overexpression in Malignant Cells Limits Tumor Progression by Inducing an Anti-Cancer Immune Response. OncoImmunology 2022, 11, 2116844. [Google Scholar] [CrossRef]
- Pramanik, J.; Chen, X.; Kar, G.; Henriksson, J.; Gomes, T.; Park, J.-E.; Natarajan, K.; Meyer, K.B.; Miao, Z.; McKenzie, A.N.J.; et al. Genome-Wide Analyses Reveal the IRE1a-XBP1 Pathway Promotes T Helper Cell Differentiation by Resolving Secretory Stress and Accelerating Proliferation. Genome Med. 2018, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, K.; Onodera, A.; Kiuchi, M.; Tsuji, K.; Hirahara, K.; Nakayama, T. Conventional and Pathogenic Th2 Cells in Inflammation, Tissue Repair, and Fibrosis. Front. Immunol. 2022, 13, 945063. [Google Scholar] [CrossRef]
- Zaynagetdinov, R.; Sherrill, T.P.; Gleaves, L.A.; McLoed, A.G.; Saxon, J.A.; Habermann, A.C.; Connelly, L.; Dulek, D.; Peebles, R.S.; Fingleton, B.; et al. Interleukin-5 Facilitates Lung Metastasis by Modulating the Immune Microenvironment. Cancer Res. 2015, 75, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, R.A.; Martín-Regalado, Á.; Pintado-Berninches, L.; Robles, J.; Ramírez-González, M.Á.; Boukich, I.; Sanchez-Gómez, P.; Balyasnikova, I.V.; Casal, J.I. Schnurri-3 Drives Tumor Growth and Invasion in Cancer Cells Expressing Interleukin-13 Receptor Alpha 2. Cell Death Dis. 2023, 14, 742. [Google Scholar] [CrossRef]
- Protti, M.P.; De Monte, L. Cross-Talk within the Tumor Microenvironment Mediates Th2-Type Inflammation in Pancreatic Cancer. OncoImmunology 2012, 1, 89–91. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Luo, Y.; Liu, J.; Wang, X.; Feng, R.; Huang, J.; Du, H.; Li, Q.; Tan, J.; et al. Pharmaceutical Targeting Th2-Mediated Immunity Enhances Immunotherapy Response in Breast Cancer. J. Transl. Med. 2022, 20, 615. [Google Scholar] [CrossRef]
- Shan, B.; Wang, X.; Wu, Y.; Xu, C.; Xia, Z.; Dai, J.; Shao, M.; Zhao, F.; He, S.; Yang, L.; et al. The Metabolic ER Stress Sensor IRE1α Suppresses Alternative Activation of Macrophages and Impairs Energy Expenditure in Obesity. Nat. Immunol. 2017, 18, 519–529. [Google Scholar] [CrossRef]
- Batista, A.; Rodvold, J.J.; Xian, S.; Searles, S.C.; Lew, A.; Iwawaki, T.; Almanza, G.; Waller, T.C.; Lin, J.; Jepsen, K.; et al. IRE1α Regulates Macrophage Polarization, PD-L1 Expression, and Tumor Survival. PLOS Biol. 2020, 18, e3000687. [Google Scholar] [CrossRef]
- Dong, H.; Adams, N.M.; Xu, Y.; Cao, J.; Allan, D.S.J.; Carlyle, J.R.; Chen, X.; Sun, J.C.; Glimcher, L.H. The IRE1 Endoplasmic Reticulum Stress Sensor Activates Natural Killer Cell Immunity in Part by Regulating C-Myc. Nat. Immunol. 2019, 20, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Cao, J.; Xu, L.; Tang, Q.; Dobrolecki, L.E.; Lv, X.; Talukdar, M.; Lu, Y.; Wang, X.; Hu, D.Z.; et al. Pharmacological Targeting of MYC-Regulated IRE1/XBP1 Pathway Suppresses MYC-Driven Breast Cancer. J. Clin. Investig. 2018, 128, 1283–1299. [Google Scholar] [CrossRef]
- Unal, B.; Kuzu, O.F.; Jin, Y.; Osorio, D.; Kildal, W.; Pradhan, M.; Kung, S.H.Y.; Oo, H.Z.; Daugaard, M.; Vendelbo, M.; et al. Targeting IRE1α Reprograms the Tumor Microenvironment and Enhances Anti-Tumor Immunity in Prostate Cancer. Nat. Commun. 2024, 15, 8895. [Google Scholar] [CrossRef]
- Crowley, M.J.P.; Bhinder, B.; Markowitz, G.J.; Martin, M.; Verma, A.; Sandoval, T.A.; Chae, C.-S.; Yomtoubian, S.; Hu, Y.; Chopra, S.; et al. Tumor-Intrinsic IRE1α Signaling Controls Protective Immunity in Lung Cancer. Nat. Commun. 2023, 14, 120. [Google Scholar] [CrossRef]
- Sharma, S.; Yang, S.-C.; Zhu, L.; Reckamp, K.; Gardner, B.; Baratelli, F.; Huang, M.; Batra, R.K.; Dubinett, S.M. Tumor Cyclooxygenase-2/Prostaglandin E2–Dependent Promotion of FOXP3 Expression and CD4+CD25+ T Regulatory Cell Activities in Lung Cancer. Cancer Res. 2005, 65, 5211–5220. [Google Scholar] [CrossRef]
- Tang, C.-H.A.; Zundell, J.A.; Ranatunga, S.; Lin, C.; Nefedova, Y.; Del Valle, J.R.; Hu, C.-C.A. Agonist-Mediated Activation of STING Induces Apoptosis in Malignant B Cells. Cancer Res. 2016, 76, 2137–2152. [Google Scholar] [CrossRef]
- Yang, Z.; Huo, Y.; Zhou, S.; Guo, J.; Ma, X.; Li, T.; Fan, C.; Wang, L. Cancer Cell-Intrinsic XBP1 Drives Immunosuppressive Reprogramming of Intratumoral Myeloid Cells by Promoting Cholesterol Production. Cell Metab. 2022, 34, 2018–2035.e8. [Google Scholar] [CrossRef] [PubMed]
- Di Conza, G.; Tsai, C.-H.; Gallart-Ayala, H.; Yu, Y.-R.; Franco, F.; Zaffalon, L.; Xie, X.; Li, X.; Xiao, Z.; Raines, L.N.; et al. Tumor-Induced Reshuffling of Lipid Composition on the Endoplasmic Reticulum Membrane Sustains Macrophage Survival and pro-Tumorigenic Activity. Nat. Immunol. 2021, 22, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Ruiz, J.R.; Silberman, P.C.; Rutkowski, M.R.; Chopra, S.; Perales-Puchalt, A.; Song, M.; Zhang, S.; Bettigole, S.E.; Gupta, D.; Holcomb, K.; et al. ER Stress Sensor XBP1 Controls Anti-Tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell 2015, 161, 1527–1538. [Google Scholar] [CrossRef]
- Veglia, F.; Tyurin, V.A.; Mohammadyani, D.; Blasi, M.; Duperret, E.K.; Donthireddy, L.; Hashimoto, A.; Kapralov, A.; Amoscato, A.; Angelini, R.; et al. Lipid Bodies Containing Oxidatively Truncated Lipids Block Antigen Cross-Presentation by Dendritic Cells in Cancer. Nat. Commun. 2017, 8, 2122. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.; Sierra, R.A.; Trillo-Tinoco, J.; Cao, Y.; Innamarato, P.; Payne, K.K.; De Mingo Pulido, A.; Mandula, J.; Zhang, S.; Thevenot, P.; et al. The Unfolded Protein Response Mediator PERK Governs Myeloid Cell-Driven Immunosuppression in Tumors through Inhibition of STING Signaling. Immunity 2020, 52, 668–682.e7. [Google Scholar] [CrossRef]
- Sarcinelli, C.; Dragic, H.; Piecyk, M.; Barbet, V.; Duret, C.; Barthelaix, A.; Ferraro-Peyret, C.; Fauvre, J.; Renno, T.; Chaveroux, C.; et al. ATF4-Dependent NRF2 Transcriptional Regulation Promotes Antioxidant Protection during Endoplasmic Reticulum Stress. Cancers 2020, 12, 569. [Google Scholar] [CrossRef]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.-R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef]
- Sheshadri, N.; Poria, D.K.; Sharan, S.; Hu, Y.; Yan, C.; Koparde, V.N.; Balamurugan, K.; Sterneck, E. PERK Signaling through C/EBPδ Contributes to ER Stress-Induced Expression of Immunomodulatory and Tumor Promoting Chemokines by Cancer Cells. Cell Death Dis. 2021, 12, 1038. [Google Scholar] [CrossRef]
- Wang, D.; Yang, L.; Yu, W.; Wu, Q.; Lian, J.; Li, F.; Liu, S.; Li, A.; He, Z.; Liu, J.; et al. Colorectal Cancer Cell-Derived CCL20 Recruits Regulatory T Cells to Promote Chemoresistance via FOXO1/CEBPB/NF-κB Signaling. J. Immunother. Cancer 2019, 7, 215. [Google Scholar] [CrossRef]
- Kadomoto, S.; Izumi, K.; Mizokami, A. The CCL20-CCR6 Axis in Cancer Progression. Int. J. Mol. Sci. 2020, 21, 5186. [Google Scholar] [CrossRef]
- Deng, J.; Lu, P.D.; Zhang, Y.; Scheuner, D.; Kaufman, R.J.; Sonenberg, N.; Harding, H.P.; Ron, D. Translational Repression Mediates Activation of Nuclear Factor Kappa B by Phosphorylated Translation Initiation Factor 2. Mol. Cell. Biol. 2004, 24, 10161–10168. [Google Scholar] [CrossRef] [PubMed]
- Raines, L.N.; Zhao, H.; Wang, Y.; Chen, H.-Y.; Gallart-Ayala, H.; Hsueh, P.-C.; Cao, W.; Koh, Y.; Alamonte-Loya, A.; Liu, P.-S.; et al. PERK Is a Critical Metabolic Hub for Immunosuppressive Function in Macrophages. Nat. Immunol. 2022, 23, 431–445. [Google Scholar] [CrossRef]
- Moncan, M.; Mnich, K.; Blomme, A.; Almanza, A.; Samali, A.; Gorman, A.M. Regulation of Lipid Metabolism by the Unfolded Protein Response. J. Cell. Mol. Med. 2021, 25, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gui, F.; Zhang, Z.; Chen, Z.; Zhang, T.; Hu, Y.; Wei, H.; Fu, Y.; Chen, X.; Wu, Z. IRE1α-XBP1s Axis Regulates SREBP1-Dependent MRP1 Expression to Promote Chemoresistance in Non-Small Cell Lung Cancer Cells. Thorac. Cancer 2024, 15, 2116–2127. [Google Scholar] [CrossRef] [PubMed]
- Almanza, A.; Mnich, K.; Blomme, A.; Robinson, C.M.; Rodriguez-Blanco, G.; Kierszniowska, S.; McGrath, E.P.; Le Gallo, M.; Pilalis, E.; Swinnen, J.V.; et al. Regulated IRE1α-Dependent Decay (RIDD)-Mediated Reprograming of Lipid Metabolism in Cancer. Nat. Commun. 2022, 13, 2493. [Google Scholar] [CrossRef]
- Rysman, E.; Brusselmans, K.; Scheys, K.; Timmermans, L. De Novo Lipogenesis Protects Cancer Cells from Free Radicals and Chemotherapeutics by Promoting Membrane Lipid Saturation. Cancer Res. 2010, 70, 8117–8126. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Tang, C.-H.A.; Song, J.H.; Mancuso, A.; Del Valle, J.R.; Cao, J.; Xiang, Y.; Dang, C.V.; Lan, R.; Sanchez, D.J.; et al. IRE1α RNase–Dependent Lipid Homeostasis Promotes Survival in Myc-Transformed Cancers. J. Clin. Investig. 2018, 128, 1300–1316. [Google Scholar] [CrossRef]
- Zhao, J.; Zhi, Z.; Wang, C.; Xing, H.; Song, G.; Yu, X.; Zhu, Y.; Wang, X.; Zhang, X.; Di, Y. Exogenous Lipids Promote the Growth of Breast Cancer Cells via CD36. Oncol. Rep. 2017, 38, 2105–2115. [Google Scholar] [CrossRef]
- Tadros, S.; Shukla, S.K.; King, R.J.; Gunda, V.; Vernucci, E.; Abrego, J.; Chaika, N.V.; Yu, F.; Lazenby, A.J.; Berim, L.; et al. De Novo Lipid Synthesis Facilitates Gemcitabine Resistance through Endoplasmic Reticulum Stress in Pancreatic Cancer. Cancer Res. 2017, 77, 5503–5517. [Google Scholar] [CrossRef]
- Magtanong, L.; Ko, P.-J.; To, M.; Cao, J.Y.; Forcina, G.C.; Tarangelo, A.; Ward, C.C.; Cho, K.; Patti, G.J.; Nomura, D.K.; et al. Exogenous Monounsaturated Fatty Acids Promote a Ferroptosis-Resistant Cell State. Cell Chem. Biol. 2019, 26, 420–432.e9. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, J.; Calvisi, D.F.; Chen, X. Role of Lipogenesis Rewiring in Hepatocellular Carcinoma. Semin. Liver Dis. 2022, 42, 077–086. [Google Scholar] [CrossRef] [PubMed]
- Paternostro, R.; Sieghart, W.; Trauner, M.; Pinter, M. Cancer and Hepatic Steatosis. ESMO Open 2021, 6, 100185. [Google Scholar] [CrossRef]
- Bobrovnikova-Marjon, E.; Hatzivassiliou, G.; Grigoriadou, C.; Romero, M.; Cavener, D.R.; Thompson, C.B.; Diehl, J.A. PERK-Dependent Regulation of Lipogenesis during Mouse Mammary Gland Development and Adipocyte Differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 16314–16319. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of Fatty Acid Metabolism in Cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, W.; Liu, Y.; Wang, W.; Deng, W. Role of ATP Citrate Lyase and Its Complementary Partner on Fatty Acid Synthesis in Gastric Cancer. Sci. Rep. 2024, 14, 30043. [Google Scholar] [CrossRef]
- Oyadomari, S.; Harding, H.P.; Zhang, Y.; Oyadomari, M.; Ron, D. Dephosphorylation of Translation Initiation Factor 2α Enhances Glucose Tolerance and Attenuates Hepatosteatosis in Mice. Cell Metab. 2008, 7, 520–532. [Google Scholar] [CrossRef]
- Fang, D.; Wan, Y.; Shen, W.; Cao, J.; Sun, Z.; Yu, H.; Zhang, Q.; Cheng, W.; Chen, J.; Ning, B. Endoplasmic Reticulum Stress Leads to Lipid Accumulation through Upregulation of SREBP-1c in Normal Hepatic and Hepatoma Cells. Mol. Cell. Biochem. 2013, 381, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.T.; Wu, J.; Back, S.-H.; Callaghan, M.U.; Ferris, S.P.; Iqbal, J.; Clark, R.; Miao, H.; Hassler, J.R.; Fornek, J.; et al. UPR Pathways Combine to Prevent Hepatic Steatosis Caused by ER Stress-Mediated Suppression of Transcriptional Master Regulators. Dev. Cell 2008, 15, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, S.; Wang, J.; Dai, J.; Cai, J.; Yan, S.; Huang, Z.; He, S.; Wang, P.; Liu, J.; et al. Phosphorylation at Ser724 of the ER Stress Sensor IRE1α Governs Its Activation State and Limits ER Stress–Induced Hepatosteatosis. J. Biol. Chem. 2022, 298, 101997. [Google Scholar] [CrossRef]
- Coleman, O.I.; Sorbie, A.; Riva, A.; Von Stern, M.; Kuhls, S.; Selegato, D.M.; Siegert, L.; Keidel, I.; Köhler, N.; Wirbel, J.; et al. ATF6 Activation Alters Colonic Lipid Metabolism Causing Tumour-Associated Microbial Adaptation. Nat. Metab. 2025, 7, 1830–1850. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Liu, Y.; Qiao, Z.; Bai, T.; Yang, L.; Liu, B. Dihydroartemisinin Triggers Ferroptosis in Primary Liver Cancer Cells by Promoting and Unfolded Protein Response-induced Upregulation of CHAC1 Expression. Oncol. Rep. 2021, 46, 240. [Google Scholar] [CrossRef]
- Cherubini, A.; Zito, E. ER Stress as a Trigger of UPR and ER-Phagy in Cancer Growth and Spread. Front. Oncol. 2022, 12, 997235. [Google Scholar] [CrossRef]
- Smith, M.D.; Harley, M.E.; Kemp, A.J.; Wills, J.; Lee, M.; Arends, M.; Von Kriegsheim, A.; Behrends, C.; Wilkinson, S. CCPG1 Is a Non-Canonical Autophagy Cargo Receptor Essential for ER-Phagy and Pancreatic ER Proteostasis. Dev. Cell 2018, 44, 217–232.e11. [Google Scholar] [CrossRef]
- Chipurupalli, S.; Ganesan, R.; Martini, G.; Mele, L.; Reggio, A.; Esposito, M.; Kannan, E.; Namasivayam, V.; Grumati, P.; Desiderio, V.; et al. Cancer Cells Adapt FAM134B/BiP Mediated ER-Phagy to Survive Hypoxic Stress. Cell Death Dis. 2022, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Duan, B.; Zhang, Y.; Zhang, X.; Xia, B. Excessive ER-Phagy Mediated by the Autophagy Receptor FAM134B Results in ER Stress, the Unfolded Protein Response, and Cell Death in HeLa Cells. J. Biol. Chem. 2019, 294, 20009–20023. [Google Scholar] [CrossRef]
- Zhao, D.; Zou, C.-X.; Liu, X.-M.; Jiang, Z.-D.; Yu, Z.-Q.; Suo, F.; Du, T.-Y.; Dong, M.-Q.; He, W.; Du, L.-L. A UPR-Induced Soluble ER-Phagy Receptor Acts with VAPs to Confer ER Stress Resistance. Mol. Cell 2020, 79, 963–977.e3. [Google Scholar] [CrossRef] [PubMed]
- Corazzari, M.; Gagliardi, M.; Fimia, G.M.; Piacentini, M. Endoplasmic Reticulum Stress, Unfolded Protein Response, and Cancer Cell Fate. Front. Oncol. 2017, 7, 78. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y.; Hua, H.; Li, M.; Luo, T.; Xu, L.; Wang, R.; Liu, D.; Zhang, Y.; Jiang, Y. Blockade of GRP78 Sensitizes Breast Cancer Cells to Microtubules-interfering Agents That Induce the Unfolded Protein Response. J. Cell. Mol. Med. 2009, 13, 3888–3897. [Google Scholar] [CrossRef]
- Dastghaib, S.; Shafiee, S.M.; Ramezani, F.; Ashtari, N.; Tabasi, F.; Saffari-Chaleshtori, J.; Siri, M.; Vakili, O.; Igder, S.; Zamani, M.; et al. NRF-Mediated Autophagy and UPR: Exploring New Avenues to Overcome Cancer Chemo-Resistance. Eur. J. Pharmacol. 2025, 988, 177210. [Google Scholar] [CrossRef]
- Chen, F.; Wang, H.; Zhu, J.; Zhao, R.; Xue, P.; Zhang, Q.; Bud Nelson, M.; Qu, W.; Feng, B.; Pi, J. Camptothecin Suppresses NRF2–ARE Activity and Sensitises Hepatocellular Carcinoma Cells to Anticancer Drugs. Br. J. Cancer 2017, 117, 1495–1506. [Google Scholar] [CrossRef]
- Muggia, F.M.; Creaven, P.J.; Hansen, H.H.; Cohen, M.H.; Selawry, O.S. Phase I Clinical Trial of Weekly and Daily Treatment with Camptothecin (NSC-100880): Correlation with Preclinical Studies. Cancer Chemother. Rep. 1972, 56, 515–521. [Google Scholar]
- Gottlieb, J.A.; Luce, J.K. Treatment of Malignant Melanoma with Camptothecin (NSC-100880). Cancer Chemother. Rep. 1972, 56, 103–105. [Google Scholar]
- Palam, L.R.; Gore, J.; Craven, K.E.; Wilson, J.L.; Korc, M. Integrated Stress Response Is Critical for Gemcitabine Resistance in Pancreatic Ductal Adenocarcinoma. Cell Death Dis. 2015, 6, e1913. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lin, D.-C.; Guo, X.; Masouleh, B.K.; Gery, S.; Cao, Q.; Alkan, S.; Ikezoe, T.; Akiba, C.; Paquette, R.; et al. Inhibition of IRE1a-Driven pro-Survival Pathways Is a Promising Therapeutic Application in Acute Myeloid Leukemia. Oncotarget 2016, 7, 18736–18749. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Devi, A.; Mishra, S. Molecular Docking and Molecular Dynamics Studies Reveal Structural Basis of Inhibition and Selectivity of Inhibitors EGCG and OSU-03012 toward Glucose Regulated Protein-78 (GRP78) Overexpressed in Glioblastoma. J. Mol. Model. 2015, 21, 272. [Google Scholar] [CrossRef] [PubMed]
- Pyrko, P.; Schönthal, A.H.; Hofman, F.M.; Chen, T.C.; Lee, A.S. The Unfolded Protein Response Regulator GRP78/BiP as a Novel Target for Increasing Chemosensitivity in Malignant Gliomas. Cancer Res. 2007, 67, 9809–9816. [Google Scholar] [CrossRef] [PubMed]
- Mimura, N.; Fulciniti, M.; Gorgun, G.; Tai, Y.-T.; Cirstea, D.; Santo, L.; Hu, Y.; Fabre, C.; Minami, J.; Ohguchi, H.; et al. Blockade of XBP1 Splicing by Inhibition of IRE1α Is a Promising Therapeutic Option in Multiple Myeloma. Blood 2012, 119, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shen, C.; Estrada-Bernal, A.; Robb, R.; Chatterjee, M.; Sebastian, N.; Webb, A.; Mo, X.; Chen, W.; Krishnan, S.; et al. Oncogenic KRAS Drives Radioresistance through Upregulation of NRF2-53BP1-Mediated Non-Homologous End-Joining Repair. Nucleic Acids Res. 2021, 49, 11067–11082. [Google Scholar] [CrossRef]
- Tao, J.; Mao, M.; Lu, Y.; Deng, L.; Yu, S.; Zeng, X.; Jia, W.; Wu, Z.; Li, C.; Ma, R.; et al. ΔNp63α Promotes Radioresistance in Esophageal Squamous Cell Carcinoma through the PLEC-KEAP1-NRF2 Feedback Loop. Cell Death Dis. 2024, 15, 793. [Google Scholar] [CrossRef]
- Wang, W.; Oguz, G.; Lee, P.L.; Bao, Y.; Wang, P.; Terp, M.G.; Ditzel, H.J.; Yu, Q. KDM4B-Regulated Unfolded Protein Response as a Therapeutic Vulnerability in PTEN-Deficient Breast Cancer. J. Exp. Med. 2018, 215, 2833–2849. [Google Scholar] [CrossRef]
- Moffitt Cancer Center. ER Stress Pathway Inhibitors Potentiate Cancer Immunotherapy. 2020. Available online: https://www.moffitt.org/contentassets/c2e65cb5c1844d0e8102c9bc4cb19530/20ma005-perk-ire1-inhibitors-tom.pdf (accessed on 10 November 2025).
- Xu, L.; Peng, F.; Luo, Q.; Ding, Y.; Yuan, F.; Zheng, L.; He, W.; Zhang, S.S.; Fu, X.; Liu, J.; et al. IRE1α Silences dsRNA to Prevent Taxane-Induced Pyroptosis in Triple-Negative Breast Cancer. Cell 2024, 187, 7248–7266.e34. [Google Scholar] [CrossRef]
- Harnoss, J.M.; Le Thomas, A.; Reichelt, M.; Guttman, O.; Wu, T.D.; Marsters, S.A.; Shemorry, A.; Lawrence, D.A.; Kan, D.; Segal, E.; et al. IRE1α Disruption in Triple-Negative Breast Cancer Cooperates with Antiangiogenic Therapy by Reversing ER Stress Adaptation and Remodeling the Tumor Microenvironment. Cancer Res. 2020, 80, 2368–2379. [Google Scholar] [CrossRef]
- Raymundo, D.P.; Doultsinos, D.; Guillory, X.; Carlesso, A.; Eriksson, L.A.; Chevet, E. Pharmacological Targeting of IRE1 in Cancer. Trends Cancer 2020, 6, 1018–1030. [Google Scholar] [CrossRef]
- Gabrail, N.Y.; Erika, P.H.; Anthony, D.E.; Mothaffar, F.R. A Phase 1/2 Trial of ORIN1001, a First-in-Class IRE1 Inhibitor, in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2021, 39, 3080. [Google Scholar] [CrossRef]
- Wang, C.; Bai, M.; Wang, X.; Tan, C.; Zhang, D.; Chang, L.; Li, G.; Xie, L.; Su, J.; Wen, Y. Estrogen Receptor Antagonist Fulvestrant Inhibits Proliferation and Promotes Apoptosis of Prolactinoma Cells by Regulating the IRE1/XBP1 Signaling Pathway. Mol. Med. Rep. 2018, 18, 4037–4041. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Zhao, F.; Xuan, Q.; Shen, Z.; Xiao, J.; Shen, Q. Fenofibrate Inhibits the Growth of Prostate Cancer through Regulating Autophagy and Endoplasmic Reticulum Stress. Biochem. Biophys. Res. Commun. 2018, 503, 2685–2689. [Google Scholar] [CrossRef]
- Wiese, W.; Siwecka, N.; Wawrzynkiewicz, A.; Rozpędek-Kamińska, W.; Kucharska, E.; Majsterek, I. IRE1α Inhibitors as a Promising Therapeutic Strategy in Blood Malignancies. Cancers 2022, 14, 2526. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, F.; Mogre, S.; Son, J.; Blazanin, N.; Glick, A.B. The Multiple Roles of the Unfolded Protein Response Regulator IRE1α in Cancer. Mol. Carcinog. 2019, 58, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, J.J.; Xian, S.; Nussbacher, J.; Tsui, B.; Cameron Waller, T.; Searles, S.C.; Lew, A.; Jiang, P.; Babic, I.; Nomura, N.; et al. IRE1α and IGF Signaling Predict Resistance to an Endoplasmic Reticulum Stress-Inducing Drug in Glioblastoma Cells. Sci. Rep. 2020, 10, 8348. [Google Scholar] [CrossRef] [PubMed]
- Calvo, V.; Zheng, W.; Adam-Artigues, A.; Staschke, K.A.; Huang, X.; Cheung, J.F.; Nobre, A.R.; Fujisawa, S.; Liu, D.; Fumagalli, M.; et al. A PERK-Specific Inhibitor Blocks Metastatic Progression by Limiting Integrated Stress Response–Dependent Survival of Quiescent Cancer Cells. Clin. Cancer Res. 2023, 29, 5155–5172. [Google Scholar] [CrossRef]
- Feng, Y.-X.; Jin, D.X.; Sokol, E.S.; Reinhardt, F.; Miller, D.H.; Gupta, P.B. Cancer-Specific PERK Signaling Drives Invasion and Metastasis through CREB3L1. Nat. Commun. 2017, 8, 1079. [Google Scholar] [CrossRef]
- Denard, B.; Lee, C.; Ye, J. Doxorubicin Blocks Proliferation of Cancer Cells through Proteolytic Activation of CREB3L1. eLife 2012, 1, e00090. [Google Scholar] [CrossRef]
- Smith, A.L.; Andrews, K.L.; Beckmann, H.; Bellon, S.F.; Beltran, P.J.; Booker, S.; Chen, H.; Chung, Y.-A.; D’Angelo, N.D.; Dao, J.; et al. Discovery of 1 H -Pyrazol-3(2 H )-Ones as Potent and Selective Inhibitors of Protein Kinase R-like Endoplasmic Reticulum Kinase (PERK). J. Med. Chem. 2015, 58, 1426–1441. [Google Scholar] [CrossRef]
- Axten, J.M.; Romeril, S.P.; Shu, A.; Ralph, J.; Medina, J.R.; Feng, Y.; Li, W.H.H.; Grant, S.W.; Heerding, D.A.; Minthorn, E.; et al. Discovery of GSK2656157: An Optimized PERK Inhibitor Selected for Preclinical Development. ACS Med. Chem. Lett. 2013, 4, 964–968. [Google Scholar] [CrossRef]
- McLaughlin, M.; Pedersen, M.; Roulstone, V.; Bergerhoff, K.F.; Smith, H.G.; Whittock, H.; Kyula, J.N.; Dillon, M.T.; Pandha, H.S.; Vile, R.; et al. The PERK Inhibitor GSK2606414 Enhances Reovirus Infection in Head and Neck Squamous Cell Carcinoma via an ATF4-Dependent Mechanism. Mol. Ther.-Oncolytics 2020, 16, 238–249. [Google Scholar] [CrossRef]
- Atkins, C.; Liu, Q.; Minthorn, E.; Zhang, S.-Y.; Figueroa, D.J.; Moss, K.; Stanley, T.B.; Sanders, B.; Goetz, A.; Gaul, N.; et al. Characterization of a Novel PERK Kinase Inhibitor with Antitumor and Antiangiogenic Activity. Cancer Res. 2013, 73, 1993–2002. [Google Scholar] [CrossRef]
- Maas, N.L.; Diehl, J.A. Molecular Pathways: The PERKs and Pitfalls of Targeting the Unfolded Protein Response in Cancer. Clin. Cancer Res. 2015, 21, 675–679. [Google Scholar] [CrossRef]
- Mafi, S.; Dehghani, M.; Khalvati, B.; Abidi, H.; Ghorbani, M.; Jalali, P.; Whichelo, R.; Salehi, Z.; Markowska, A.; Reyes, A.; et al. Targeting PERK and GRP78 in Colorectal Cancer: Genetic Insights and Novel Therapeutic Approaches. Eur. J. Pharmacol. 2024, 982, 176899. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Rong, D.; Ding, J.; Zhang, X.; Wang, Y.; Fang, Y.; Xiao, J.; Yang, S.; Wang, H. Activation of the PERK/eIF2α Axis Is a Pivotal Prerequisite of Taxanes to Cancer Cell Apoptosis and Renders Synergism to Overcome Paclitaxel Resistance in Breast Cancer Cells. Cancer Cell Int. 2024, 24, 249. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.-S.; Yu, P.-P.; Sun, Y.; Wang, D.-F.; Deng, X.-F.; Bao, Y.-L.; Song, J.; Sun, L.-G.; Song, Z.-B.; Li, Y.-X. Paclitaxel Inhibits Selenoprotein S Expression and Attenuates Endoplasmic Reticulum Stress. Mol. Med. Rep. 2016, 13, 5118–5124. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Liu, X.; He, T.; Yang, C.; Jiang, J.; Fang, Y.; Fu, Z.; Yuan, Y.; Bai, S.; Qiu, T.; et al. Excipient of Paclitaxel Induces Metabolic Dysregulation and Unfolded Protein Response. iScience 2021, 24, 103170. [Google Scholar] [CrossRef]
- Chen, L.; He, J.; Zhou, J.; Xiao, Z.; Ding, N.; Duan, Y.; Li, W.; Sun, L. EIF2A Promotes Cell Survival during Paclitaxel Treatment in Vitro and in Vivo. J. Cell. Mol. Med. 2019, 23, 6060–6071. [Google Scholar] [CrossRef]
- Mhaidat, N.M.; Thorne, R.; Zhang, X.D.; Hersey, P. Involvement of Endoplasmic Reticulum Stress in Docetaxel-Induced JNK-Dependent Apoptosis of Human Melanoma. Apoptosis 2008, 13, 1505–1512. [Google Scholar] [CrossRef]
- National Cancer Institute Cabazitaxel. NCI Dictionaries. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/cabazitaxel (accessed on 10 November 2025).
- Cevatemre, B.; Bulut, I.; Dedeoglu, B.; Isiklar, A.; Syed, H.; Bayram, O.Y.; Bagci-Onder, T.; Acilan, C. Exploiting Epigenetic Targets to Overcome Taxane Resistance in Prostate Cancer. Cell Death Dis. 2024, 15, 132. [Google Scholar] [CrossRef]
- Jin, Y.; Saatcioglu, F. Targeting the Unfolded Protein Response in Hormone-Regulated Cancers. Trends Cancer 2020, 6, 160–171. [Google Scholar] [CrossRef]
- Burwick, N.; Aktas, B.H. The eIF2-Alpha Kinase HRI: A Potential Target beyond the Red Blood Cell. Expert Opin. Ther. Targets 2017, 21, 1171–1177. [Google Scholar] [CrossRef]
- Robert, F.; Williams, C.; Yan, Y.; Donohue, E.; Cencic, R.; Burley, S.K.; Pelletier, J. Blocking UV-Induced eIF2α Phosphorylation with Small Molecule Inhibitors of GCN2. Chem. Biol. Drug Des. 2009, 74, 57–67. [Google Scholar] [CrossRef]
- Kato, Y.; Kunimasa, K.; Sugimoto, Y.; Tomida, A. BCR-ABL Tyrosine Kinase Inhibition Induces Metabolic Vulnerability by Preventing the Integrated Stress Response in K562 Cells. Biochem. Biophys. Res. Commun. 2018, 504, 721–726. [Google Scholar] [CrossRef]
- Jammi, N.V.; Whitby, L.R.; Beal, P.A. Small Molecule Inhibitors of the RNA-Dependent Protein Kinase. Biochem. Biophys. Res. Commun. 2003, 308, 50–57. [Google Scholar] [CrossRef]
- Singleton, D.C.; Harris, A.L. Targeting the ATF4 Pathway in Cancer Therapy. Expert Opin. Ther. Targets 2012, 16, 1189–1202. [Google Scholar] [CrossRef]
- Guo, W.; Wang, M.; Yang, Z.; Liu, D.; Ma, B.; Zhao, Y.; Chen, Y.; Hu, Y. Recent Advances in Small Molecule and Peptide Inhibitors of Glucose-Regulated Protein 78 for Cancer Therapy. Eur. J. Med. Chem. 2023, 261, 115792. [Google Scholar] [CrossRef] [PubMed]
- Gopal, U.; Mowery, Y.; Young, K.; Pizzo, S.V. Targeting Cell Surface GRP78 Enhances Pancreatic Cancer Radiosensitivity through YAP/TAZ Protein Signaling. J. Biol. Chem. 2019, 294, 13939–13952. [Google Scholar] [CrossRef] [PubMed]
- Sriratanasak, N.; Chunhacha, P.; Ei, Z.Z.; Chanvorachote, P. Cisplatin Induces Senescent Lung Cancer Cell-Mediated Stemness Induction via GRP78/Akt-Dependent Mechanism. Biomedicines 2022, 10, 2703. [Google Scholar] [CrossRef] [PubMed]
- Rauschert, N.; Brändlein, S.; Holzinger, E.; Hensel, F.; Müller-Hermelink, H.-K.; Vollmers, H.P. A New Tumor-Specific Variant of GRP78 as Target for Antibody-Based Therapy. Lab. Investig. 2008, 88, 375–386. [Google Scholar] [CrossRef]
- Cai, B.; Tomida, A.; Mikami, K.; Nagata, K.; Tsuruo, T. Down-Regulation of Epidermal Growth Factor Receptor-Signaling Pathway by Binding of GRP78/BiP to the Receptor under Glucose-Starved Stress Conditions. J. Cell. Physiol. 1998, 177, 282–288. [Google Scholar] [CrossRef]
- Dadey, D.Y.A.; Kapoor, V.; Hoye, K.; Khudanyan, A.; Collins, A.; Thotala, D.; Hallahan, D.E. Antibody Targeting GRP78 Enhances the Efficacy of Radiation Therapy in Human Glioblastoma and Non–Small Cell Lung Cancer Cell Lines and Tumor Models. Clin. Cancer Res. 2017, 23, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.E.; Gallagher, C.M.; Plate, L.; Gupta, M.; Liem, C.R.; Guo, X.; Tian, R.; Stroud, R.M.; Kampmann, M.; Weissman, J.S.; et al. Ceapins Block the Unfolded Protein Response Sensor ATF6α by Inducing a Neomorphic Inter-Organelle Tether. eLife 2019, 8, e46595. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.M.; Garri, C.; Cain, E.L.; Ang, K.K.-H.; Wilson, C.G.; Chen, S.; Hearn, B.R.; Jaishankar, P.; Aranda-Diaz, A.; Arkin, M.R.; et al. Ceapins Are a New Class of Unfolded Protein Response Inhibitors, Selectively Targeting the ATF6α Branch. eLife 2016, 5, e11878. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, J.; Cho, S.-Y.; Kang, Y.J.; Lee, J.; Kwon, J.; Rhee, H.; Bauer, S.; Kim, H.-S.; Lee, E.; et al. Identification of Novel Pathogenic Roles of BLZF1/ATF6 in Tumorigenesis of Gastrointestinal Stromal Tumor Showing Golgi-Localized Mutant KIT. Cell Death Differ. 2023, 30, 2309–2321. [Google Scholar] [CrossRef]
- Benedetti, R.; Romeo, M.A.; Arena, A.; Gilardini Montani, M.S.; Di Renzo, L.; D’Orazi, G.; Cirone, M. ATF6 Prevents DNA Damage and Cell Death in Colon Cancer Cells Undergoing ER Stress. Cell Death Discov. 2022, 8, 295. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Zhang, X.; Yuan, Z.; Zhang, L.; Miao, P. ATF Family Members as Therapeutic Targets in Cancer: From Mechanisms to Pharmacological Interventions. Pharmacol. Res. 2024, 208, 107355. [Google Scholar] [CrossRef]
- Kolb, P.S.; Ayaub, E.A.; Zhou, W.; Yum, V.; Dickhout, J.G.; Ask, K. The Therapeutic Effects of 4-Phenylbutyric Acid in Maintaining Proteostasis. Int. J. Biochem. Cell Biol. 2015, 61, 45–52. [Google Scholar] [CrossRef]
- Verma, J.; Warsame, C.; Seenivasagam, R.K.; Katiyar, N.K.; Aleem, E.; Goel, S. Nanoparticle-Mediated Cancer Cell Therapy: Basic Science to Clinical Applications. Cancer Metastasis Rev. 2023, 42, 601–627. [Google Scholar] [CrossRef]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in Cancer Theragnostic and Drug Delivery: A Comprehensive Review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef]
- Pandey, S.; Nandi, A.; Basu, S.; Ballav, N. Inducing Endoplasmic Reticulum Stress in Cancer Cells Using Graphene Oxide-Based Nanoparticles. Nanoscale Adv. 2020, 2, 4887–4894. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Allemailem, K.S.; Almatroudi, A.; Almatroodi, S.A.; Mahzari, A.; Alsahli, M.A.; Rahmani, A.H. Endoplasmic Reticulum Stress Provocation by Different Nanoparticles: An Innovative Approach to Manage the Cancer and Other Common Diseases. Molecules 2020, 25, 5336. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. (Chezy) Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Gianni, L.; Mansutti, M.; Anton, A.; Calvo, L.; Bisagni, G.; Bermejo, B.; Semiglazov, V.; Thill, M.; Chacon, J.I.; Chan, A.; et al. Comparing Neoadjuvant Nab-Paclitaxel vs Paclitaxel Both Followed by Anthracycline Regimens in Women With ERBB2/HER2 -Negative Breast Cancer—The Evaluating Treatment With Neoadjuvant Abraxane (ETNA) Trial: A Randomized Phase 3 Clinical Trial. JAMA Oncol. 2018, 4, 302. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Melisi, D.; Macarulla, T.; Pazo Cid, R.; Chandana, S.R.; De La Fouchardière, C.; Dean, A.; Kiss, I.; Lee, W.J.; Goetze, T.O.; et al. NALIRIFOX versus Nab-Paclitaxel and Gemcitabine in Treatment-Naive Patients with Metastatic Pancreatic Ductal Adenocarcinoma (NAPOLI 3): A Randomised, Open-Label, Phase 3 Trial. Lancet 2023, 402, 1272–1281. [Google Scholar] [CrossRef]
- Rasche, L.; Duell, J.; Castro, I.C.; Dubljevic, V.; Chatterjee, M.; Knop, S.; Hensel, F.; Rosenwald, A.; Einsele, H.; Topp, M.S.; et al. GRP78-Directed Immunotherapy in Relapsed or Refractory Multiple Myeloma-Results from a Phase 1 Trial with the Monoclonal Immunoglobulin M Antibody PAT-SM6. Haematologica 2015, 100, 377–384. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, B.; Gui, J.; Katlinski, K.V.; Brice, A.; Gao, Y.; Li, C.; Kushner, J.A.; Koumenis, C.; Diehl, J.A.; et al. Type I Interferons Mediate Pancreatic Toxicities of PERK Inhibition. Proc. Natl. Acad. Sci. USA 2015, 112, 15420–15425. [Google Scholar] [CrossRef]
- Rojas-Rivera, D.; Delvaeye, T.; Roelandt, R.; Nerinckx, W.; Augustyns, K.; Vandenabeele, P.; Bertrand, M.J.M. When PERK Inhibitors Turn out to Be New Potent RIPK1 Inhibitors: Critical Issues on the Specificity and Use of GSK2606414 and GSK2656157. Cell Death Differ. 2017, 24, 1100–1110. [Google Scholar] [CrossRef]
- Rimawi, M.F.; Hamilton, E.P.; Hurvitz, S.A.; Marks, D.K.; Elias, A.D.; Pluard, T.J.; Gabrail, N.Y.; Patterson, J.; Greene, S.; Zeng, Q. Early Efficacy Evaluation of ORIN1001, a First in Class IRE1 Alpha Inhibitor, in Advanced Solid Tumors. J. Clin. Oncol. 2023, 41, 1092. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, P.M.Q.; Truong, T.-A.; Samala, S.K.; Lakshmisha, B.M.; Biswal, P.; Koushki, K.; Mallepaddi, P.C.; Vijay, G.; Krishnan, S. The Unfolded Protein Response—Novel Mechanisms, Challenges, and Key Considerations for Therapeutic Intervention. Cancers 2025, 17, 3639. https://doi.org/10.3390/cancers17223639
Mai PMQ, Truong T-A, Samala SK, Lakshmisha BM, Biswal P, Koushki K, Mallepaddi PC, Vijay G, Krishnan S. The Unfolded Protein Response—Novel Mechanisms, Challenges, and Key Considerations for Therapeutic Intervention. Cancers. 2025; 17(22):3639. https://doi.org/10.3390/cancers17223639
Chicago/Turabian StyleMai, P. M. Quan, Tam-Anh Truong, Sai Kumar Samala, Bhoomika Muruvekere Lakshmisha, Prapannajeet Biswal, Khadijeh Koushki, Prudhvi Chand Mallepaddi, Geraldine Vijay, and Sunil Krishnan. 2025. "The Unfolded Protein Response—Novel Mechanisms, Challenges, and Key Considerations for Therapeutic Intervention" Cancers 17, no. 22: 3639. https://doi.org/10.3390/cancers17223639
APA StyleMai, P. M. Q., Truong, T.-A., Samala, S. K., Lakshmisha, B. M., Biswal, P., Koushki, K., Mallepaddi, P. C., Vijay, G., & Krishnan, S. (2025). The Unfolded Protein Response—Novel Mechanisms, Challenges, and Key Considerations for Therapeutic Intervention. Cancers, 17(22), 3639. https://doi.org/10.3390/cancers17223639







