Design and Interim Recruitment Outcomes of a Multi-Modal, Multi-Level Patient Navigation Intervention for Lung Cancer Screening in the Southeast U.S.
Simple Summary
Abstract
1. Introduction
1.1. Lung Cancer Mortality Rates in the United States (U.S.) and in Virginia (V.A.), South Carolina (S.C.), and North Carolina (N.C.)
1.2. The Importance of Lung Cancer Screening in Reducing Lung Cancer Mortality Rates
1.3. Barriers to Lung Cancer Screening
1.4. Patient Navigation
2. Materials and Methods
2.1. Institutional Review Board Approval
2.2. Participant Inclusion Criteria
- Meets current USPSTF guidelines for lung cancer screening
- Adults aged 50 to 80 years
- 20 pack-year smoking history (Note: A pack-year is a way of calculating how much a person has smoked in their lifetime. One pack-year is the equivalent of smoking an average of 20 cigarettes—1 pack—per day for a year.)
- Currently smokes or has quit smoking within the past 15 years
- Identifies as Black or African American (Note: Both Hispanic/Latino and Non-Hispanic/Latino patients are eligible as long as they also identify as Black or African American [e.g., Afro-Latino]).
- Willing to complete all navigation-related study activities
- Able to understand and the willingness to sign a written informed consent document
2.3. Federally Qualified Health Center Partnerships
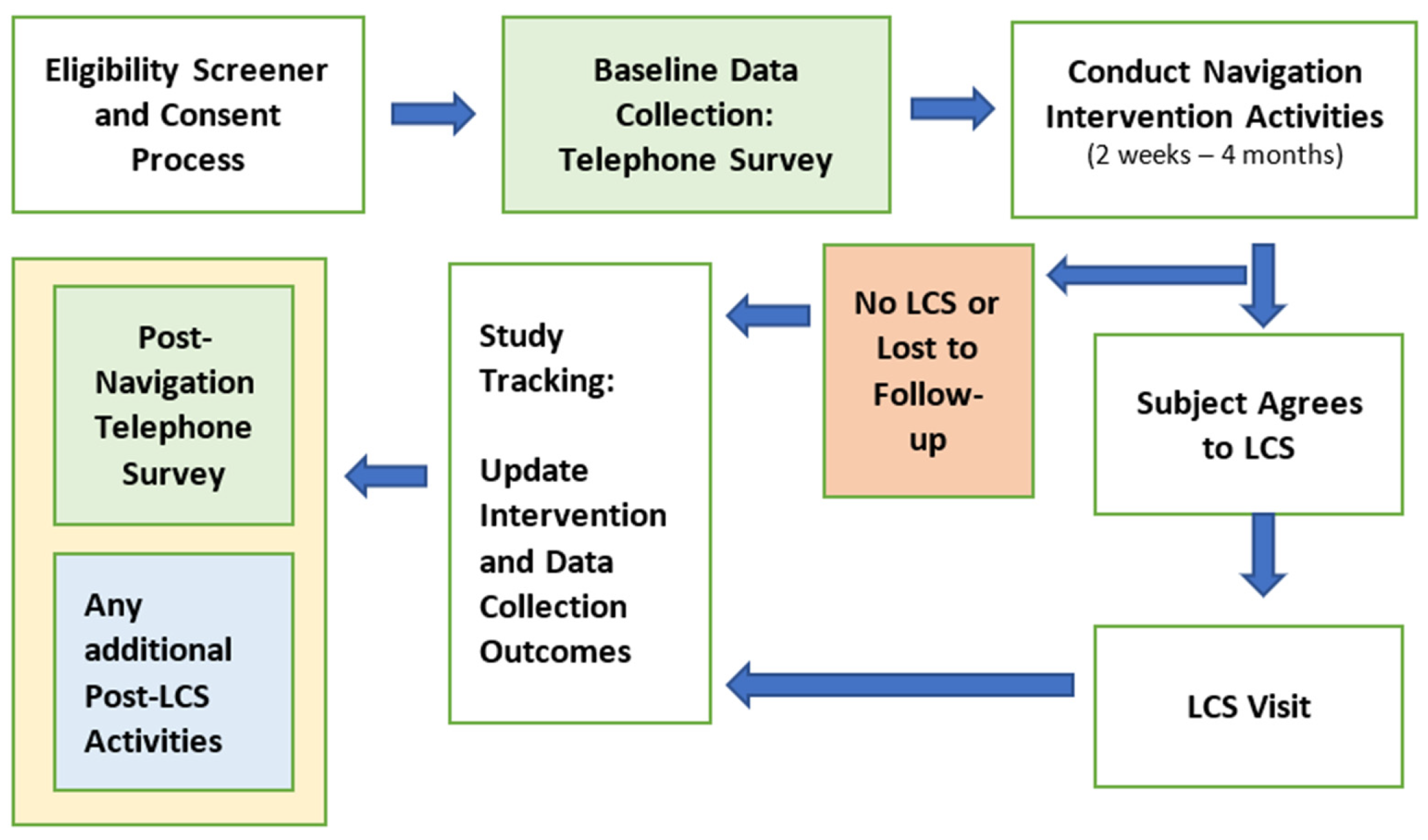
2.4. Centralized Patient Navigation Intervention
- Superb interpersonal skills
- The ability to engage in active listening with the participants
- Problem-solving skills
- Knowledge of available resources
- Familiarity with the study’s research processes
2.5. Conceptual Framework of the Patient Navigation Intervention
- Individual
- Organizational
- Economic
- Sociocultural
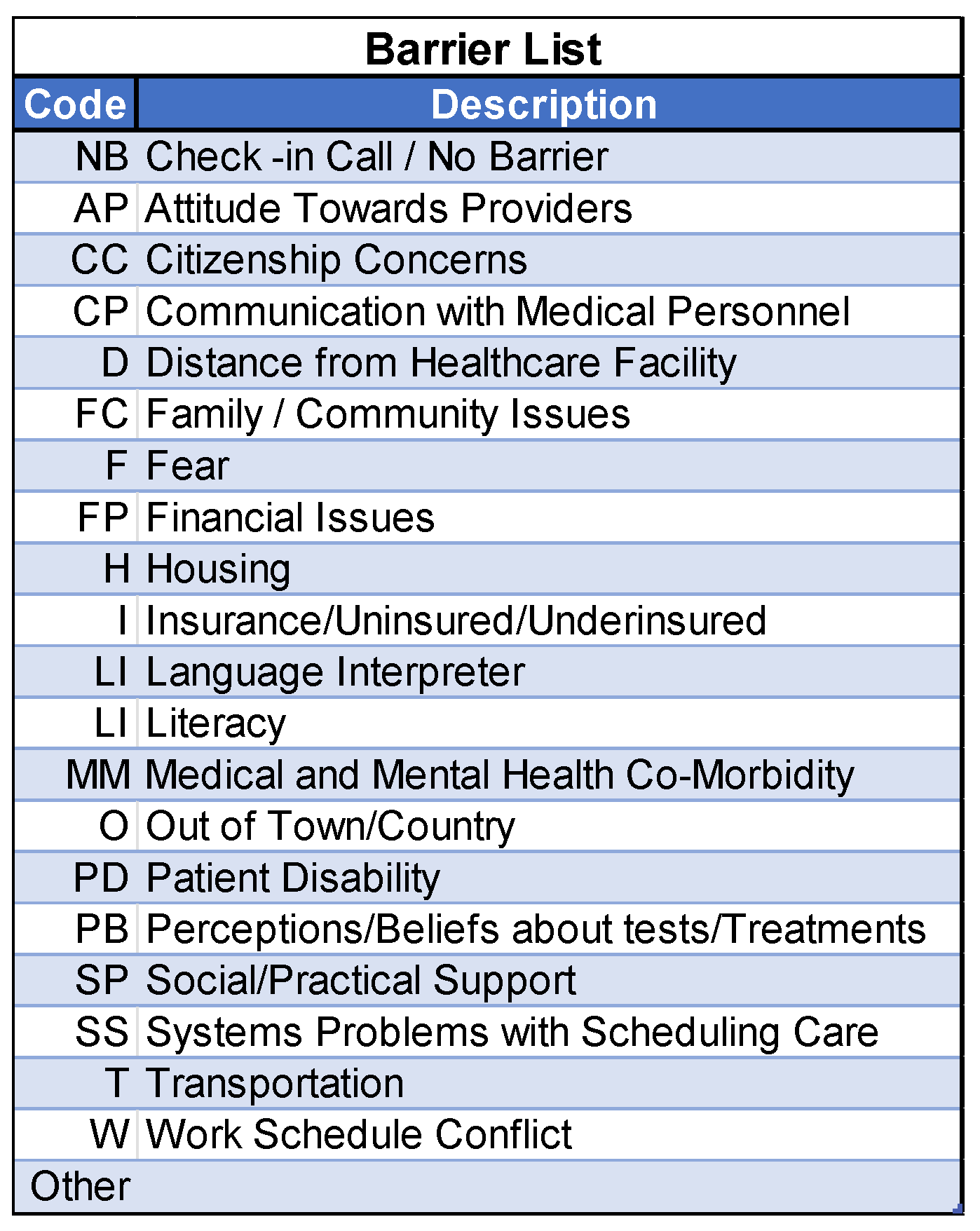
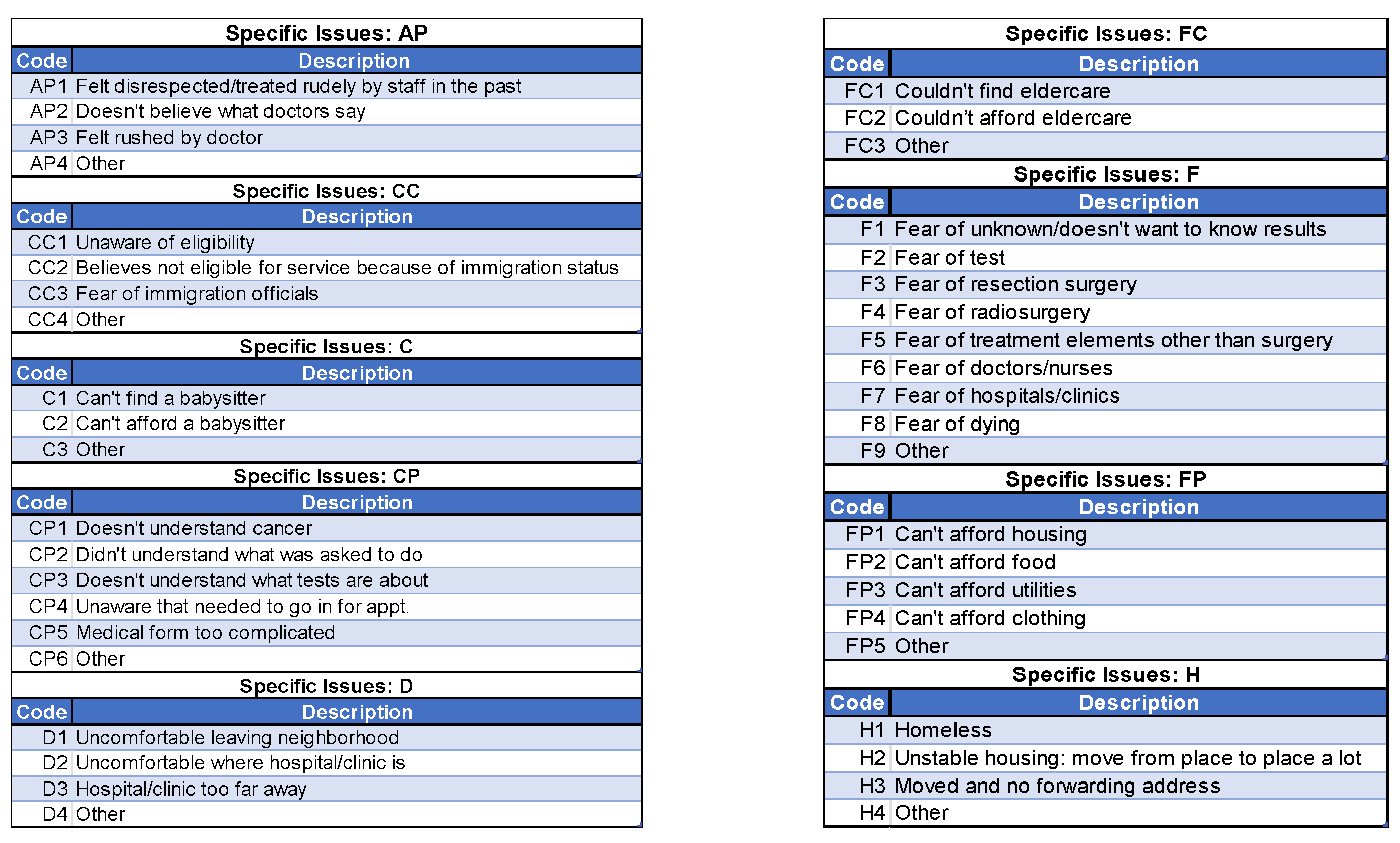
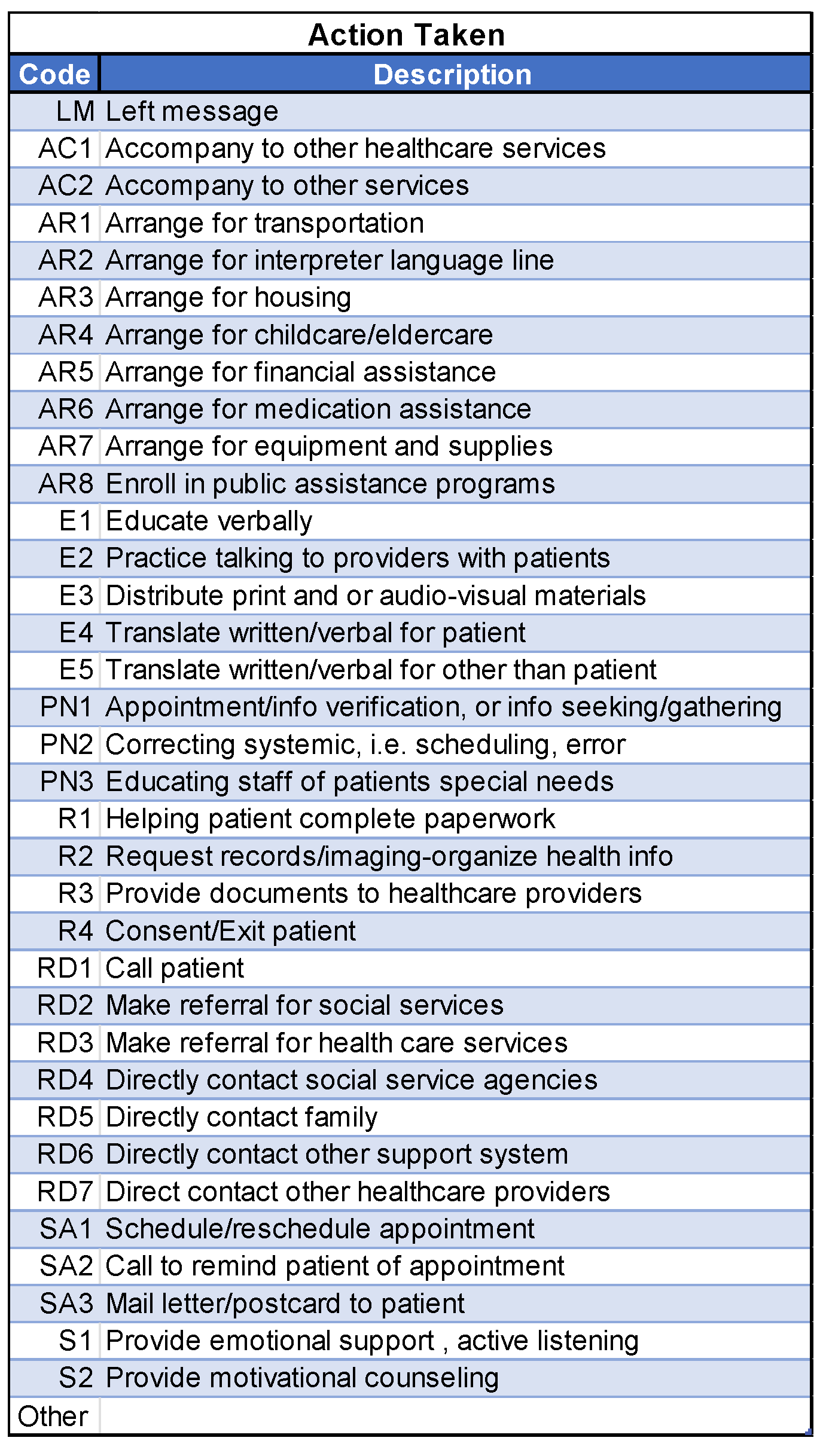
2.6. NCI Barrier Plan Form
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SC3 Study | Southeastern Consortium for Lung Cancer Screening (SC3) Study |
| FQHC | Federally qualified health center |
| LCS | Lung cancer screening |
| SU2C | Stand Up To Cancer |
| USPSTF | U.S. Preventive Services Task Force |
| NIMHD | National Institute on Minority Health and Health Disparities |
| NLST | National Lung Screening Trial |
References
- American Cancer Society. Cancer Facts & Figures. 2025. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2025-cancer-facts-figures.html (accessed on 1 August 2025).
- Barker, A.M.; Wiener, R.S.; Reisman, J.; Kearney, L.; Dones, M.; Fix, G.M. Black US military veterans respond favourably to a booklet using narratives to normalise shared decision-making. Public Health Pract. 2025, 9, 100606. [Google Scholar] [CrossRef]
- Jaferian, S.; Love, T.; Singh, S.K.; Xie, Y.; Hill, E.; Wolf, J.R. Racial and urban-rural disparities in lung cancer care: Insight from a Latent Class Growth Analysis. J. Cancer Policy 2025, 44, 100585. [Google Scholar] [CrossRef]
- Butler, D.C.; Petterson, S.; Phillips, R.L.; Bazemore, A.W. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv. Res. 2013, 48 Pt 1, 539–559. [Google Scholar] [CrossRef]
- Moyer, V.A.; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 330–338. [Google Scholar] [CrossRef]
- Rivera, M.P.; Katki, H.A.; Tanner, N.T.; Triplette, M.; Sakoda, L.C.; Wiener, R.S.; Cardarelli, R.; Carter-Harris, L.; Crothers, K.; Fathi, J.T.; et al. Addressing Disparities in Lung Cancer Screening Eligibility and Healthcare Access. An Official American Thoracic Society Statement. Am. J. Respir. Crit. Care Med. 2020, 202, e95–e112. [Google Scholar] [CrossRef]
- Korn, A.R.; Walsh-Bailey, C.; Correa-Mendez, M.; DelNero, P.; Pilar, M.; Sandler, B.; Brownson, R.C.; Emmons, K.M.; Oh, A.Y. Social determinants of health and US cancer screening interventions: A systematic review. CA Cancer J. Clin. 2023, 73, 461–479. [Google Scholar] [CrossRef]
- Ferguson, M.K.; Demchuk, C.; Wroblewski, K.; Huisingh-Scheetz, M.; Thompson, K.; Farnan, J.; Acevedo, J. Does Race Influence Risk Assessment and Recommendations for Lung Resection? A Randomized Trial. Ann. Thorac. Surg. 2018, 106, 1013–1017. [Google Scholar] [CrossRef]
- Henley, S.J.; Anderson, R.N.; Thomas, C.C.; Massetti, G.M.; Peaker, B.; Richardson, L.C. Invasive Cancer Incidence, 2004–2013, and Deaths, 2006–2015, in Nonmetropolitan and Metropolitan Counties—United States. MMWR Surveill. Summ. 2017, 66, 1–13. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Dwyer-Lindgren, L.; Fitzmaurice, C.; Stubbs, R.W.; Bertozzi-Villa, A.; Morozoff, C.; Charara, R.; Allen, C.; Naghavi, M.; Murray, C.J.L. Trends and Patterns of Disparities in Cancer Mortality Among US Counties, 1980–2014. JAMA 2017, 317, 388–406. [Google Scholar] [CrossRef]
- Zafar, S.Y.; Abernethy, A.P. Financial toxicity, Part I: A new name for a growing problem. Oncology 2013, 27, 80–149. [Google Scholar]
- Zafar, S.Y.; McNeil, R.B.; Thomas, C.M.; Lathan, C.S.; Ayanian, J.Z.; Provenzale, D. Population-based assessment of cancer survivors’ financial burden and quality of life: A prospective cohort study. J. Oncol. Pract. 2015, 11, 145–150. [Google Scholar] [CrossRef]
- Turrini, G.; Branham, D.K.; Chen, L.; Conmy, A.B.; Chappel, A.R.; De Lew, N.; Sommers, B.D. Access to Affordable Care in Rural America: Current Trends and Key Challenges(Research Report No. HP-2021-16). July 2021. Available online: https://aspe.hhs.gov/sites/default/files/documents/09e40880648376a13756c59028a56bb4/rural-health-rr.pdf (accessed on 1 August 2025).
- Mitchell, A.; Muluneh, B.; Patel, R.; Basch, E. Pharmaceutical assistance programs for cancer patients in the era of orally administered chemotherapeutics. J. Oncol. Pharm. Pract. 2018, 24, 424–432. [Google Scholar] [CrossRef]
- Spencer, J.C.; Samuel, C.A.; Rosenstein, D.L.; Reeder-Hayes, K.E.; Manning, M.L.; Sellers, J.B.; Wheeler, S.B. Oncology navigators’ perceptions of cancer-related financial burden and financial assistance resources. Support. Care Cancer 2018, 26, 1315–1321. [Google Scholar] [CrossRef]
- Biddell, C.B.; Spees, L.P.; Petermann, V.; Rosenstein, D.L.; Manning, M.; Gellin, M.; Padilla, N.; Samuel-Ryals, C.A.; Birken, S.A.; Reeder-Hayes, K.; et al. Financial Assistance Processes and Mechanisms in Rural and Nonrural Oncology Care Settings. JCO Oncol. Pract. 2022, 18, e1392–e1406. [Google Scholar] [CrossRef]
- Bach, P.B.; Cramer, L.D.; Warren, J.L.; Begg, C.B. Racial differences in the treatment of early-stage lung cancer. N. Engl. J. Med. 1999, 341, 1198–1205. [Google Scholar] [CrossRef]
- Wells, K.J.; Battaglia, T.A.; Dudley, D.J.; Garcia, R.; Greene, A.; Calhoun, E.; Mandelblatt, J.S.; Paskett, E.D.; Raich, P.C.; The Patient Navigation Research Program. Patient navigation: State of the art or is it science? Cancer 2008, 113, 1999–2010. [Google Scholar] [CrossRef]
- Sherman, D.E. Transforming Practices Through the Oncology Care Model: Financial Toxicity and Counseling. J. Oncol. Pract. 2017, 13, 519–522. [Google Scholar] [CrossRef]
- Reeder-Hayes, K.E.; Biddell, C.B.; Manning, M.L.; Rosenstein, D.L.; Samuel-Ryals, C.A.; Spencer, J.C.; Smith, S.; Deal, A.; Gellin, M.; Wheeler, S.B. Knowledge, Attitudes, and Resources of Frontline Oncology Support Personnel Regarding Financial Burden in Patients With Cancer. JCO Oncol. Pract. 2023, 19, 654–661. [Google Scholar] [CrossRef]
- Alvidrez, J.; Castille, D.; Laude-Sharp, M.; Rosario, A.; Tabor, D. The National Institute on Minority Health and Health Disparities Research Framework. Am. J. Public Health 2019, 109 (Suppl. S1), S16–S20. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.P.; Muth, B.J.; Kerner, J.F. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract. 1995, 3, 19–30. [Google Scholar] [PubMed]
- Vargas, R.B.; Ryan, G.W.; Jackson, C.A.; Rodriguez, R.; Freeman, H.P. Characteristics of the original patient navigation programs to reduce disparities in the diagnosis and treatment of breast cancer. Cancer 2008, 113, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.M.; Ward, A.J. Recruiting minorities into clinical trials: Toward a participant-friendly system. J. Natl. Cancer Inst. 1995, 87, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Biddell, C.B.; Waters, A.R.; Angove, R.S.M.; Gallagher, K.D.; Rosenstein, D.L.; Spees, L.P.; Kent, E.E.; Planey, A.M.; Wheeler, S.B. Facing financial barriers to healthcare: Patient-informed adaptation of a conceptual framework for adults with a history of cancer. Front. Psychol. 2023, 14, 1178517. [Google Scholar] [CrossRef]
- Hsieh, H.F.; Shannon, S.E. Three approaches to qualitative content analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef]
- NPIdb. Federally Qualified Health Center (FQHC)-261QF0400X—South Carolina. 2025. Available online: https://npidb.org/organizations/ambulatory_health_care/federally-qualified-health-center-fqhc_261qf0400x/sc/ (accessed on 7 June 2025).
- Health Resources & Services Administration. Health Center Program Uniform Data System (UDS) Data Overview. 2025. Available online: https://data.hrsa.gov/topics/healthcenters/uds/overview?grantNum=H80CS00293 (accessed on 8 August 2025).
- NCDHHS Division of Public Health. NC State Center for Health Statistics. SCHS: Cancer Incidence Data Visualization Tools. Cancer Incidence Rates by County. 2024. Available online: https://schs.dph.ncdhhs.gov/data/cancer/county_yearly_incidence.html (accessed on 1 August 2025).
- NCIOM. North Carolina Health Data. Available online: https://nciom.org/nc-health-data/ (accessed on 1 August 2025).
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Clapp, J.D.; Clingan, K.L.; Gareen, I.F.; Lynch, D.A.; Marcus, P.M.; Pinsky, P.F. Baseline characteristics of participants in the randomized national lung screening trial. J. Natl. Cancer Inst. 2010, 102, 1771–1779. [Google Scholar]
- Lee, S.J.C.; Lee, J.; Zhu, H.; Chen, P.M.; Wahid, U.; Hamann, H.A.; Bhalla, S.; Cardenas, R.C.; Natchimuthu, V.S.; Johnson, D.H.; et al. Assessing Barriers and Facilitators to Lung Cancer Screening: Initial Findings from a Patient Navigation Intervention. Popul. Health Manag. 2023, 26, 177–184. [Google Scholar] [CrossRef]
- Percac-Lima, S.; Ashburner, J.M.; Rigotti, N.A.; Park, E.R.; Chang, Y.; Kuchukhidze, S.; Atlas, S.J. Patient navigation for lung cancer screening among current smokers in community health centers a randomized controlled trial. Cancer Med. 2018, 7, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, S.; Natchimuthu, V.; Lee, J.L.; Wahid, U.; Zhu, H.; Santini, N.O.; Browning, T.; Hamann, H.A.; Johnson, D.H.; Chiu, H.; et al. Effect of Patient Navigation on Completion of Lung Cancer Screening in Vulnerable Populations. J. Natl. Compr. Cancer Netw. 2024, 22, 151–157. [Google Scholar] [CrossRef]
| Module 1: Research 101 | Objectives
|
| Module 1.2: Overview of Health Disparities in Lung Cancer Screening and Treatment | Objectives
|
| Module 2: Patient Navigators: Roles and Responsibilities | Objectives
|
| Module 3: Overcoming Barriers to Care | Objectives
|
| Module 4: Health Literacy | Objectives
|
| Module 5: Communication Exercise Tool | Objectives
|
| Module 5.1: Effective Communication | Objective
|
| Module 5.2: Communication and Patient Navigation | Objectives
|
| Module 6: Developing Cultural Competency/Cultural Humility in Cancer Clinical Trials Research | Objectives
|
| Module 6.1: Developing Cultural Humility in Health Care | Objectives
|
| Individual | Economic |
|
|
| Organizational | Sociocultural |
|
|
| Characteristic | SC3 Study Cohort (N = 170) | NLST LDCT Arm [32] (N = 26,723) |
|---|---|---|
| Age Group, years | ||
| 50–54 | 7.65% | 0.01% |
| 55–64 | 44.12% | 73.38% |
| 65–74 | 34.70% | 26.60% |
| 75–79 | 8.82% | 0.00% |
| Missing | 4.71% | 0.01% |
| Sex | ||
| Female | 37.06% | 40.99% |
| Male | 62.94% | 59.01% |
| Race | ||
| Black | 100.00% | 4.48% |
| Marital status | ||
| Married/living with partner | 15.29% | 66.66% |
| Divorced | 11.18% | 19.44% |
| Separated | 5.29% | 1.26% |
| Widowed | 11.76% | 7.43% |
| Single | 30.59% | 4.70% |
| Don’t know/unsure | 25.89% | 0.51% |
| Education | ||
| Less than high school | 40.00% | 6.14% |
| High school or GED | 25.29% | 23.48% |
| Post-high school training (No college) | 0.00% | 14.10% |
| Some College/Associate’s degree | 18.82% | 23.43% |
| Bachelor degree | 5.89% | 16.86% |
| Graduate School | 0.00% | 14.70% |
| Other | 0.00% | 0.85% |
| Don’t know/unsure/missing | 10.00% | 0.44% |
| Smoking status | ||
| Current | 83.53% | 48.16% |
| Former | 16.47% | 51.84% |
| Among those who quit, time (years) since quitting smoking | ||
| Within 4 years | 6.47% | 14.75% |
| 4–9.9 years | 4.11% | 17.21% |
| 10–15 years | 3.53% | 19.67% |
| Missing | 2.36% | 0.21% |
| Median pack years | 28 | 48 |
| Common Reasons for Declining Participation | Common Concerns/Obstacles to Engaging in Lung Cancer Screening | Satisfaction with the Multimodal Navigation Approach |
|---|---|---|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ford, M.E.; Henderson, L.; Brenner, A.; Sheppard, V.B.; Wheeler, S.B.; Collins, T.; Williams, M.; Vélez Acevedo, R.; Lyu, C.; Summers, C.; et al. Design and Interim Recruitment Outcomes of a Multi-Modal, Multi-Level Patient Navigation Intervention for Lung Cancer Screening in the Southeast U.S. Cancers 2025, 17, 3633. https://doi.org/10.3390/cancers17223633
Ford ME, Henderson L, Brenner A, Sheppard VB, Wheeler SB, Collins T, Williams M, Vélez Acevedo R, Lyu C, Summers C, et al. Design and Interim Recruitment Outcomes of a Multi-Modal, Multi-Level Patient Navigation Intervention for Lung Cancer Screening in the Southeast U.S. Cancers. 2025; 17(22):3633. https://doi.org/10.3390/cancers17223633
Chicago/Turabian StyleFord, Marvella E., Louise Henderson, Alison Brenner, Vanessa B. Sheppard, Stephanie B. Wheeler, Tiffani Collins, Monique Williams, Rosuany Vélez Acevedo, Christopher Lyu, Chyanne Summers, and et al. 2025. "Design and Interim Recruitment Outcomes of a Multi-Modal, Multi-Level Patient Navigation Intervention for Lung Cancer Screening in the Southeast U.S." Cancers 17, no. 22: 3633. https://doi.org/10.3390/cancers17223633
APA StyleFord, M. E., Henderson, L., Brenner, A., Sheppard, V. B., Wheeler, S. B., Collins, T., Williams, M., Vélez Acevedo, R., Lyu, C., Summers, C., Scott, C., Polite-Powers, A. R., Slaughter, S. J., LaForte, D., King, D., McCoy, A. S., Zserai, J., Hill, S. S., Slan, M., ... Winn, R. A. (2025). Design and Interim Recruitment Outcomes of a Multi-Modal, Multi-Level Patient Navigation Intervention for Lung Cancer Screening in the Southeast U.S. Cancers, 17(22), 3633. https://doi.org/10.3390/cancers17223633






