Emerging Diagnostics and Therapies in Neuroendocrine Neoplasms: A Critical Review
Simple Summary
Abstract
1. Introduction
2. Diagnostic Advances: Molecular Markers, Liquid Biopsy, and Functional Imaging
3. Current Systemic Therapies for Neuroendocrine Tumors (NETs)
3.1. Hormonal Control and Symptom Management
3.2. Targeted Therapies: mTOR Inhibitors and Tyrosine Kinase Inhibitors
3.3. Cytotoxic Chemotherapy
3.4. Peptide Receptor Radionuclide Therapy (PRRT)
3.5. Treatment Sequencing, Access, and Special Populations
4. Emerging and Future Directions
4.1. Novel Radiopharmaceuticals and Personalized Dosimetry
4.2. Novel Systemic Therapies
- Belzutifan
- Immunotherapy
- CAR T-cell therapy
4.3. DNA Damage Response Inhibition as a Radiosensitizer
4.4. Adaptive and Biomarker-Enriched Clinical Trials
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chauhan, A.; Yu, Q.; Ray, N.; Farooqui, Z.; Huang, B.; Durbin, E.B.; Tucker, T.; Evers, M.; Arnold, S.; Anthony, L.B. Global burden of neuroendocrine tumors and changing incidence in Kentucky. Oncotarget 2018, 9, 19245–19254. [Google Scholar] [CrossRef]
- Dasari, A.; Wallace, K.; Halperin, D.M.; Maxwell, J.; Kunz, P.; Singh, S.; Chasen, B.; Yao, J.C. Epidemiology of Neuroendocrine Neoplasms in the US. JAMA Netw. Open 2025, 8, e2515798. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Cabozantinib for Adults and Pediatric Patients 12 Years of Age and Older with pNET and epNET; Food and Drug Administration (FDA): Silver Spring, MD, USA, 2025. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-cabozantinib-adults-and-pediatric-patients-12-years-age-and-older-pnet-and-epnet (accessed on 29 June 2025).
- FDA Approves Belzutifan for Pheochromocytoma or Paraganglioma; Food and Drug Administration (FDA): Silver Spring, MD, USA, 2025. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-belzutifan-pheochromocytoma-or-paraganglioma (accessed on 29 June 2025).
- Wang, Y.-H.; Yang, Q.-C.; Lin, Y.; Xue, L.; Chen, M.-H.; Chen, J. Chromogranin A as a marker for diagnosis, treatment, and survival in patients with gastroenteropancreatic neuroendocrine neoplasm. Medicine 2014, 93, e247. [Google Scholar] [CrossRef] [PubMed]
- Loree, J.M.; Chan, D.; Lim, J.; Stuart, H.; Fidelman, N.; Koea, J.; Posavad, J.; Cummins, M.; Doucette, S.; Myrehaug, S.; et al. Biomarkers to Inform Prognosis and Treatment for Unresectable or Metastatic GEP-NENs. JAMA Oncol. 2024, 10, 1707–1720. [Google Scholar] [CrossRef]
- Marotta, V.; Zatelli, M.C.; Sciammarella, C.; Ambrosio, M.R.; Bondanelli, M.; Colao, A.; Faggiano, A. Chromogranin A as circulating marker for diagnosis and management of neuroendocrine neoplasms: More flaws than fame. Endocr.-Relat. Cancer 2018, 25, R11–R29. [Google Scholar] [CrossRef] [PubMed]
- Bevere, M.; Masetto, F.; Carazzolo, M.E.; Bettega, A.; Gkountakos, A.; Scarpa, A.; Simbolo, M. An Overview of Circulating Biomarkers in Neuroendocrine Neoplasms: A Clinical Guide. Diagnostics 2023, 13, 2820. [Google Scholar] [CrossRef]
- Campana, D.; Nori, F.; Piscitelli, L.; Morselli-Labate, A.M.; Pezzilli, R.; Corinaldesi, R.; Tomassetti, P. Chromogranin A: Is it a useful marker of neuroendocrine tumors? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 1967–1973. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Y.; Long, J.; Yao, X.; Wang, J.; Zang, S.; Qu, W.; Wang, F. Serum chromogranin A for the diagnosis of gastroenteropancreatic neuroendocrine neoplasms and its association with tumour expression. Oncol. Lett. 2019, 17, 1497–1504. [Google Scholar] [CrossRef]
- Singh, S.; Bergsland, E.K.; Card, C.M.; Hope, T.A.; Kunz, P.L.; Laidley, D.T.; Lawrence, B.; Leyden, S.; Metz, D.C.; Michael, M.; et al. Commonwealth Neuroendocrine Tumour Research Collaboration and the North American Neuroendocrine Tumor Society Guidelines for the Diagnosis and Management of Patients with Lung Neuroendocrine Tumors: An International Collaborative Endorsement and Update of the 2015 European Neuroendocrine Tumor Society Expert Consensus Guidelines. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 1577–1598. [Google Scholar] [CrossRef]
- Öberg, K.; Califano, A.; Strosberg, J.R.; Ma, S.; Pape, U.; Bodei, L.; Kaltsas, G.; Toumpanakis, C.; Goldenring, J.R.; Frilling, A.; et al. A meta-analysis of the accuracy of a neuroendocrine tumor mRNA genomic biomarker (NETest) in blood. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 202–212. [Google Scholar] [CrossRef]
- Smolkova, B.; Kataki, A.; Earl, J.; Ruz-Caracuel, I.; Cihova, M.; Urbanova, M.; Buocikova, V.; Tamargo, S.; Rovite, V.; Niedra, H.; et al. Liquid biopsy and preclinical tools for advancing diagnosis and treatment of patients with pancreatic neuroendocrine neoplasms. Crit. Rev. Oncol. Hematol. 2022, 180, 103865. [Google Scholar] [CrossRef]
- Boons, G.; Vandamme, T.; Mariën, L.; Lybaert, W.; Roeyen, G.; Rondou, T.; Papadimitriou, K.; Janssens, K.; Op de Beeck, B.; Simoens, M.; et al. Longitudinal Copy-Number Alteration Analysis in Plasma Cell-Free DNA of Neuroendocrine Neoplasms is a Novel Specific Biomarker for Diagnosis, Prognosis, and Follow-up. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 338–349. [Google Scholar] [CrossRef]
- Gerard, L.; Garcia, J.; Gauthier, A.; Lopez, J.; Durand, A.; Hervieu, V.; Lemelin, A.; Chardon, L.; Landel, V.; Gibert, B.; et al. ctDNA in Neuroendocrine Carcinoma of Gastroenteropancreatic Origin or of Unknown Primary: The CIRCAN-NEC Pilot Study. Neuroendocrinology 2021, 111, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Padda, S.K.; Aggarwal, R.R.; Ashok, A.; Mauer, E.; Shirazi, M.; Bergsland, E.K. Prevalence of high tumor mutational burden (TMB-H) and microsatellite instability-high (MSI-H) status in neuroendocrine neoplasms. J. Clin. Oncol. 2022, 40, 2625. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Endocrine and Neuroendocrine Tumours; WHO: Geneva, Switzerland, 2025; Available online: http://publications.iarc.who.int/Book-And-Report-Series/Who-Classification-Of-Tumours/Endocrine-And-Neuroendocrine-Tumours-2025 (accessed on 1 July 2025).
- Liu, A.J.; Ueberroth, B.E.; McGarrah, P.W.; Buckner Petty, S.A.; Kendi, A.T.; Starr, J.; Hobday, T.J.; Halfdanarson, T.R.; Sonbol, M.B. Treatment Outcomes of Well-Differentiated High-Grade Neuroendocrine Tumors. Oncol. 2021, 26, 383–388. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, J.W.; Han, S.-W.; Oh, D.-Y.; Lee, S.-H.; Kim, D.-W.; Im, S.-A.; Kim, T.-Y.; Seog Heo, D.; Bang, Y.-J. Biological characteristics and treatment outcomes of metastatic or recurrent neuroendocrine tumors: Tumor grade and metastatic site are important for treatment strategy. BMC Cancer 2010, 10, 448. [Google Scholar] [CrossRef]
- Chung, Y.R.; Jang, M.H.; Park, S.Y.; Gong, G.; Jung, W.-H. Interobserver Variability of Ki-67 Measurement in Breast Cancer. J. Pathol. Transl. Med. 2016, 50, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Govind, D.; Jen, K.-Y.; Matsukuma, K.; Gao, G.; Olson, K.A.; Gui, D.; Wilding, G.E.; Border, S.P.; Sarder, P. Improving the accuracy of gastrointestinal neuroendocrine tumor grading with deep learning. Sci. Rep. 2020, 10, 11064. [Google Scholar] [CrossRef]
- van Velthuysen, M.-L.F.; Groen, E.J.; van der Noort, V.; van de Pol, A.; Tesselaar, M.E.T.; Korse, C.M. Grading of neuroendocrine neoplasms: Mitoses and Ki-67 are both essential. Neuroendocrinology 2014, 100, 221–227. [Google Scholar] [CrossRef]
- Panzuto, F.; Cicchese, N.; Partelli, S.; Rinzivillo, M.; Capurso, G.; Merola, E.; Manzoni, M.; Pucci, E.; Iannicelli, E.; Pilozzi, E.; et al. Impact of Ki67 re-assessment at time of disease progression in patients with pancreatic neuroendocrine neoplasms. PLoS ONE 2017, 12, e0179445. [Google Scholar] [CrossRef]
- Marinoni, I.; Kurrer, A.S.; Vassella, E.; Dettmer, M.; Rudolph, T.; Banz, V.; Hunger, F.; Pasquinelli, S.; Speel, E.-J.; Perren, A. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology 2014, 146, 453–460.e5. [Google Scholar] [CrossRef]
- Pulvirenti, A.; Pea, A.; Chang, D.K.; Jamieson, N.B. Clinical and Molecular Risk Factors for Recurrence Following Radical Surgery of Well-Differentiated Pancreatic Neuroendocrine Tumors. Front. Med. 2020, 7, 385. [Google Scholar] [CrossRef]
- Yasunaga, Y.; Tanaka, M.; Arita, J.; Hasegawa, K.; Ushiku, T. Loss of ATRX and DAXX in pancreatic neuroendocrine tumors: Association with recurrence risk, cellular phenotype, and heterogeneity. Hum. Pathol. 2024, 150, 51–57. [Google Scholar] [CrossRef] [PubMed]
- van ’t Veld, B.R.; Hackeng, W.M.; Luchini, C.; Brosens, L.A.A.; Dreijerink, K.M.A. Clinical Relevance of ATRX/DAXX Gene Mutations and ALT in Functioning Pancreatic Neuroendocrine Tumors. Endocr. Pathol. 2025, 36, 3. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Liu, T.-C.; Roncaioli, J.L.; Cao, D.; Zeh, H.J.; Zureikat, A.H.; Tsung, A.; Marsh, J.W.; Lee, K.K.; Hogg, M.E.; et al. Alternative Lengthening of Telomeres and Loss of DAXX/ATRX Expression Predicts Metastatic Disease and Poor Survival in Patients with Pancreatic Neuroendocrine Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 600–609. [Google Scholar] [CrossRef]
- Scarpa, A.; Chang, D.K.; Nones, K.; Corbo, V.; Patch, A.-M.; Bailey, P.; Lawlor, R.T.; Johns, A.L.; Miller, D.K.; Mafficini, A.; et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017, 543, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Riechelmann, R.P.; Torrezan, G.T.; Bergsland, E.; Raj, N.; Strosberg, J.; Moon, F.; Felismino, T.C.; Knappskog, S.; Trevizani, E.; Cingarlini, S.; et al. Pancreatic neuroendocrine tumors and MUTYH pathogenic variants: A multinational study. Ther. Adv. Med. Oncol. 2025, 17, 17588359251356335. [Google Scholar] [CrossRef]
- Hallqvist, A.; Brynjarsdóttir, E.; Krantz, T.; Sjögren, M.; Svensson, J.; Bernhardt, P. 177Lu-DOTATATE in Combination with PARP Inhibitor Olaparib Is Feasible in Patients with Somatostatin-Positive Tumors: Results from the LuPARP Phase I Trial. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2025, 66, 707–712. [Google Scholar] [CrossRef]
- Di Domenico, A.; Pipinikas, C.P.; Maire, R.S.; Bräutigam, K.; Simillion, C.; Dettmer, M.S.; Vassella, E.; Thirlwell, C.; Perren, A.; Marinoni, I. Epigenetic landscape of pancreatic neuroendocrine tumours reveals distinct cells of origin and means of tumour progression. Commun. Biol. 2020, 3, 740. [Google Scholar] [CrossRef]
- Kunz, P.L.; Graham, N.T.; Catalano, P.J.; Nimeiri, H.S.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Martin, B.A.; Yao, J.C.; Kulke, M.H.; et al. Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients with Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 1359–1369. [Google Scholar] [CrossRef]
- Evans, M.G.; Xiu, J.; Darabi, S.; Crymes, A.; Bedeir, A.; Bryant, D.A.; Oberley, M.J.; Demeure, M.J. Loss of O6--Methylguanine--DNA Methyltransferase Protein Expression by Immunohistochemistry Is Associated with Response to Capecitabine and Temozolomide in Neuroendocrine Neoplasms. World J. Surg. 2025, 49, 964. [Google Scholar] [CrossRef] [PubMed]
- Brighi, N.; Lamberti, G.; Andrini, E.; Mosconi, C.; Manuzzi, L.; Donati, G.; Lisotti, A.; Campana, D. Prospective Evaluation of MGMT-Promoter Methylation Status and Correlations with Outcomes to Temozolomide-Based Chemotherapy in Well-Differentiated Neuroendocrine Tumors. Curr. Oncol. 2023, 30, 1381–1394. [Google Scholar] [CrossRef]
- Walter, T.; Lecomte, T.; Hadoux, J.; Niccoli, P.; Saban-Roche, L.; Gaye, E.; Guimbaud, R.; Baconnier, M.; Hautefeuille, V.; Do Cao, C.; et al. Oxaliplatin-Based Versus Alkylating Agent in Neuroendocrine Tumors According to the O6-Methylguanine-DNA Methyltransferase Status: A Randomized Phase II Study (MGMT-NET). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2025, 43, 960–971. [Google Scholar] [CrossRef]
- Kapoor, M.; Kasi, A. Octreotide Scan. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK559330/ (accessed on 1 July 2025).
- Deppen, S.A.; Blume, J.; Bobbey, A.J.; Shah, C.; Graham, M.M.; Lee, P.; Delbeke, D.; Walker, R.C. 68Ga-DOTATATE Compared with 111In-DTPA-Octreotide and Conventional Imaging for Pulmonary and Gastroenteropancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 872–878. [Google Scholar] [CrossRef]
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovacs, P.; Von Guggenberg, E.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2007, 48, 508–518. [Google Scholar] [CrossRef]
- Krausz, Y.; Freedman, N.; Rubinstein, R.; Lavie, E.; Orevi, M.; Tshori, S.; Salmon, A.; Glaser, B.; Chisin, R.; Mishani, E.; et al. 68Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: Comparison with 111In-DTPA-octreotide (OctreoScan®). Mol. Imaging Biol. 2011, 13, 583–593. [Google Scholar] [CrossRef]
- Hope, T.A.; Allen-Auerbach, M.; Bodei, L.; Calais, J.; Dahlbom, M.; Dunnwald, L.K.; Graham, M.M.; Jacene, H.A.; Heath, C.L.; Mittra, E.S.; et al. SNMMI Procedure Standard/EANM Practice Guideline for SSTR PET: Imaging Neuroendocrine Tumors. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2023, 64, 204–210. [Google Scholar] [CrossRef]
- Mallak, N.; O’Brien, S.R.; Pryma, D.A.; Mittra, E. Theranostics in Neuroendocrine Tumors. Cancer J. Sudbury Mass 2024, 30, 185–193. [Google Scholar] [CrossRef]
- Singh, S.; Halperin, D.; Myrehaug, S.; Herrmann, K.; Pavel, M.; Kunz, P.L.; Chasen, B.; Tafuto, S.; Lastoria, S.; Capdevila, J.; et al. [177Lu]Lu-DOTA-TATE plus long-acting octreotide versus high-dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2–3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): An open-label, randomised, phase 3 study. Lancet 2024, 403, 2807–2817. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Bergsland, E.K.; Bozkurt, M.F.; Graham, M.; Heaney, A.P.; Herrmann, K.; Howe, J.R.; Kulke, M.H.; Kunz, P.L.; Mailman, J.; et al. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Asa, S.L.; Lee, Z.; Tirumani, S.H.; Li, Q.; Avril, N.; Bajor, D.; Mahipal, A.; Chakrabarti, S.; Selfridge, J.E.; et al. The predictive impact of dual somatostatin receptor/fluorodeoxyglucose (FDG) positron emission tomography (PET) in metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs): Review of literature and a single institution experience. J. Gastrointest. Oncol. 2023, 14, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yu, J.; Li, J.; Shen, L.; Li, N.; Zhu, H.; Zhai, S.; Zhang, Y.; Yang, Z.; Lu, M. Clinical and Prognostic Value of PET/CT Imaging with Combination of 68Ga-DOTATATE and 18F-FDG in Gastroenteropancreatic Neuroendocrine Neoplasms. Contrast Media Mol. Imaging 2018, 2018, 2340389. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, X.; Li, Q.; Wang, R. Comparison of 18F-FDG PET/CT and 18F-DOTATATE PET/CT in the diagnosis of multiple metastases in rectal neuroendocrine neoplasms. Radiol. Case Rep. 2024, 19, 3757–3762. [Google Scholar] [CrossRef]
- Chan, D.L.; Hayes, A.R.; Karfis, I.; Conner, A.; Furtado O’Mahony, L.; Mileva, M.; Bernard, E.; Roach, P.; Marin, G.; Pavlakis, N.; et al. Dual [68Ga]DOTATATE and [18F]FDG PET/CT in patients with metastatic gastroenteropancreatic neuroendocrine neoplasms: A multicentre validation of the NETPET score. Br. J. Cancer 2023, 128, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in Metastatic Enteropancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.-F.; Bläker, M.; et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors: A Report From the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Müller, H.-H.; Arnold, R.; PROMID Study Group. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef]
- Yau, H.; Kinaan, M.; Quinn, S.L.; Moraitis, A.G. Octreotide long-acting repeatable in the treatment of neuroendocrine tumors: Patient selection and perspectives. Biol. Targets Ther. 2017, 11, 115–122. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Marginean, H.; Leung, M.; Asmis, T.; Vickers, M.; Goodwin, R. Real world use of lanreotide in neuroendocrine tumors. J. Gastrointest. Oncol. 2023, 14, 1488–1495. [Google Scholar] [CrossRef]
- Del Rivero, J.; Perez, K.; Kennedy, E.B.; Mittra, E.S.; Vijayvergia, N.; Arshad, J.; Basu, S.; Chauhan, A.; Dasari, A.N.; Bellizzi, A.M.; et al. Systemic Therapy for Tumor Control in Metastatic Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors: ASCO Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 5049–5067. [Google Scholar] [CrossRef]
- Pavel, M.; Gross, D.J.; Benavent, M.; Perros, P.; Srirajaskanthan, R.; Warner, R.R.P.; Kulke, M.H.; Anthony, L.B.; Kunz, P.L.; Hörsch, D.; et al. Telotristat ethyl in carcinoid syndrome: Safety and efficacy in the TELECAST phase 3 trial. Endocr. Relat. Cancer 2018, 25, 309–322. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Markison, S.; Kim, S.H.; Han, S.; Chen, M.; Kusnetzow, A.K.; Rico-Bautista, E.; Johns, M.; Luo, R.; et al. Discovery of Paltusotine (CRN00808), a Potent, Selective, and Orally Bioavailable Non-peptide SST2 Agonist. ACS Med. Chem. Lett. 2023, 14, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Shaheen, S.; Usiskin, K.; Odueyungbo, A.; Mui, B.C.; Dillon, J. Phase 2 Study to Evaluate the Safety, Pharmacokinetics, and Dose Response of Paltusotine Carcinoid Syndrome. Endocr. Abstr. 2023, 89, 21388. [Google Scholar] [CrossRef]
- Chauhan, A.; Mohamed, A.; Usiskin, K.; Mui, B.C.; Dillon, J.; Zhou, D.; Quock, T.P.; Sharafali, Z.; Shaheen, S.; O’COnnor, M.; et al. Once-daily oral paltusotine in the treatment of patients with carcinoid syndrome: Results from a phase 2, randomized, parallel-group study. Endocr. Abstr. 2025, 108, C5. [Google Scholar] [CrossRef]
- CAREFNDR: A Phase III, Randomised, Parallel Group, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Paltusotine in Adults with Carcinoid Syndrome Due to Well-Differentiated Neuroendocrine Tumours—Enets.org. Available online: https://www.enets.org/abstract/carefndr-a-phase-iii-randomised-parallel-group-placebo-controlled-study-to-evaluate-the-efficacy-and-safety-of-paltusotine-in-adults-with-carcinoid-syndrome-due-to-well-differentiated-neuroendocrine-tumours.html (accessed on 16 July 2025).
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O’Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 863–881. [Google Scholar] [CrossRef]
- Osataphan, S.; Vamvini, M.; Rosen, E.D.; Pei, L.; Erlikh, N.; Singh, G.; Dhorajiya, P.; Parker, J.A.; Dreyfuss, J.M.; Rattani, A.; et al. Anti–Insulin Receptor Antibody for Malignant Insulinoma and Refractory Hypoglycemia. N. Engl. J. Med. 2023, 389, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Patti, M.-E.; Tan, M.; Shaheen, S.; Norena, J.A.; Yerevanian, A.; Wilson, L.M.; Lester, L.; Sidhu, J.; Hood, D.E.; et al. RZ358 (ersodetug) as a novel therapy for hypoglycemia due to tumor hyperinsulinism: Outcomes from an expanded access program for compassionate use. Endocr. Abstr. 2025, 108, C10. [Google Scholar] [CrossRef]
- Pusceddu, S.; De Braud, F.; Russo, G.L.; Concas, L.; Femia, D.; Vernieri, C.; Indini, A.; Formisano, B.; Buzzoni, R. How do the results of the RADIANT trials impact on the management of NET patients? A systematic review of published studies. Oncotarget 2016, 7, 44841–44847. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- AFINITOR (Everolimus) Tablets for Oral Administration. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=022334 (accessed on 5 July 2025).
- Taboada, R.G.; Brito, A.B.; Silva, A.L.; Weschenfelder, R.F.; Riechelmann, R.P. The Efficacy of a Lower Dose of Everolimus in Patients with Advanced Neuroendocrine Tumors. Cancers 2024, 16, 3773. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Chan, J.A.; Geyer, S.; Zemla, T.; Knopp, M.V.; Behr, S.; Pulsipher, S.; Ou, F.-S.; Dueck, A.C.; Acoba, J.; Shergill, A.; et al. Phase 3 Trial of Cabozantinib to Treat Advanced Neuroendocrine Tumors. N. Engl. J. Med. 2025, 392, 653–665. [Google Scholar] [CrossRef]
- Müller, C.; Kreissl, M.C.; Klose, S.; Krause, A.; Keitel, V.; Venerito, M. Long-term treatment with streptozocin/5-fluorouracil chemotherapy in patients with metastatic pancreatic neuroendocrine tumors: Case series. Medicine 2022, 101, e28610. [Google Scholar] [CrossRef] [PubMed]
- Management of Advanced Gastroenteropancreatic Neuroendocrine Tumors. J. Natl. Compr. Cancer Netw. 2025, 23, 1–3. [CrossRef]
- Bergsland, E.; Ganti, A.K.P.; Lieu, C.; Trikalinos, N.A. NCCN Guidelines Index Table of Contents Discussion. 2025. Available online: https://www.nccn.org/guidelines/guidelines-detail?id=1448 (accessed on 5 July 2025).
- Kenmotsu, H.; Niho, S.; Tsuboi, M.; Wakabayashi, M.; Ishii, G.; Nakagawa, K.; Daga, H.; Tanaka, H.; Saito, H.; Aokage, K.; et al. Randomized Phase III Study of Irinotecan Plus Cisplatin Versus Etoposide Plus Cisplatin for Completely Resected High-Grade Neuroendocrine Carcinoma of the Lung: JCOG1205/1206. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 4292–4301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, J.; Li, J.; Zhang, X.; Zhou, J.; Wang, X.; Peng, Z.; Shen, L.; Lu, M. Etoposide and cisplatin versus irinotecan and cisp latin as the first-line therapy for patients with advanced, poorly differentiated gastroenteropancreatic neuroendocrine carcinoma: A randomized phase 2 study. Cancer 2020, 126 (Suppl. 9), 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Frizziero, M.; Spada, F.; Lamarca, A.; Kordatou, Z.; Barriuso, J.; Nuttall, C.; McNamara, M.G.; Hubner, R.A.; Mansoor, W.; Manoharan, P.; et al. Carboplatin in Combination with Oral or Intravenous Etoposide for Extra-Pulmonary, Poorly-Differentiated Neuroendocrine Carcinomas. Neuroendocrinology 2019, 109, 100–112. [Google Scholar] [CrossRef]
- Sorbye, H.; Hjortland, G.O.; Vestermark, L.W.; Ladekarl, M.; Svensson, J.; Sundlöv, A.; Janson, E.T.; Garresori, H.; Hofsli, E.; Kersten, C.; et al. Characteristics and treatment outcome in a prospective cohort of 639 advanced high-grade digestive neuroendocrine neoplasms (NET G3 and NEC). The NORDIC NEC 2 study. Br. J. Cancer 2025, 133, 316–324. [Google Scholar] [CrossRef]
- Hadoux, J.; Afchain, P.; Walter, T.; Tougeron, D.; Hautefeuille, V.; Monterymard, C.; Lorgis, V.; Thuillier, F.; Baudin, E.; Scoazec, J.Y.; et al. FOLFIRINEC: A randomized phase II trial of mFOLFIRINOX vs platinum-etoposide for metastatic neuroendocrine carcinoma of gastroenteropancreatic or unknown origin. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2021, 53, 824–829. [Google Scholar] [CrossRef]
- Grupo Espanol de Tumores Neuroendocrinos. Randomized Open Label Study to Compare the Efficacy and Safety of Everolimus Followed by Chemotherapy with Streptozotocin- Fluorouracilo (STZ-5FU) Upon Progres-Sion or the Reverse Sequence, in Advanced Progressive Pancreatic NETs (pNETs). Clinicaltrials.gov, Clinical tri-al Registration NCT02246127. 2025. Available online: https://clinicaltrials.gov/study/NCT02246127 (accessed on 25 July 2025).
- Salazar, R.; Tafuto, S.; Krogh, M.; Teule, A.; Garcia-Carbonero, R.; Klumpen, H.J.; Cremer, B.; Sevilla, I.; Eriksson, B.; Tabaksblat, E.; et al. LBA45 Randomized open label phase III study comparing the efficacy and safety of everolimus followed by chemotherapy (CT) with streptozotocin (STZ)-5FU upon progression or the reverse sequence, in advanced progressive panNETs: The SEQTOR study (GETNE 1206). Ann. Oncol. 2022, 33, S1412. [Google Scholar] [CrossRef]
- Castillon, J.C.; Tafuto, S.; Krogh, M.; Teule, A.; Garcia-Carbonero, R.; Klumpen, H.J.; Cremer, B.; Sevilla, I.; Eriksson, B.K.; Tabaksblat, E.M.; et al. 1142O Multivariable analysis of streptozotocin plus 5-fluorouracil and everolimus sequences in advanced pancreatic neuroendocrine tumor patients: The SEQTOR trial (GETNE-1206). Ann. Oncol. 2024, 35, S750. [Google Scholar] [CrossRef]
- Efficacy and Safety of [177Lu]Lu-Edotreotide vs. Everolimus in Patients with Grade 1 or Grade 2 Gastroenteropancreatic Neuroendocrine Tumours: COMPETE Phase 3 Trial—Enets.org. Available online: https://www.enets.org/abstract/efficacy-and-safety-of-177lu-lu-edotreotide-vs-everolimus-in-patients-with-grade-1-or-grade-2-gastroenteropancreatic-neuroendocrine-tumours-compete-phase-3-trial.html (accessed on 19 July 2025).
- Opalinska, M.; Kamiński, G.; Dedecjus, M.; Kowalska, A.; Kolodziej, M.; Saracyn, M.; Garnuszek, P.; Lenda-Tracz, W.; Gąsior-Perczak, D.; Borkowska, A.; et al. Personalized dosimetry as a key for optimizing radioligand therapy (RLT) with 177Lu- or 177Lu/90Y-DOTA-TATE in patients with well-differentiated neuroendocrine tumors—An update on the initial results of the DUONEN multicenter study. Endocr. Abstr. 2024, 99, EP601. [Google Scholar] [CrossRef]
- Secondary Endpoint Results of the First Multicentric Randomised Phase II Trial Investigating the Antitumour Efficacy of 177Lutetium-DOTA-Octreotate (OCLU) in Advanced Progressive Neuroendocrine Pancreatic Tumour: The OCLURANDOM Trial—Enets.org. Available online: https://www.enets.org/abstract/secondary-endpoint-results-of-the-first-multicentric-randomised-phase-ii-trial-investigating-the-antitumour-efficacy-of-177lutetium-dota-octreotate-oclu-in-advanced-progressive-neuroendocrine-pancreatic-tumour-the-oclurandom-trial.html (accessed on 20 July 2025).
- A phase III Study of Combination Therapy with Everolimus Plus Lanreotide Versus Everolimus Monotherapy for Unresectable or Recurrent Gastroenteropancreatic Neuroendocrine Tumor (JCOG1901, STARTER-NET). J. Clin. Oncology. 2025, 43, 652. Available online: https://ascopubs.org/doi/10.1200/JCO.2025.43.4_suppl.652 (accessed on 21 July 2025). [CrossRef]
- Schmitt, M.; Bohnenberger, H.; Bartsch, D.K.; Wagner, D.-C.; Litmeyer, A.-S.; Grass, A.; Rinke, A.; Koch, C.; Kremer, M.; Evert, M.; et al. DLL3 Expression in Neuroendocrine Carcinomas and Neuroendocrine Tumours: Insights From a Multicentric Cohort of 1294 Pulmonary and Extrapulmonary Neuroendocrine Neoplasms. Endocr. Pathol. 2025, 36, 9. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, V.; Greystoke, A.; Reck, M.; Liu, M.; Mueller, M.; Duenzinger, U.; Bergsland, E.B.; Owonikoko, T. Abstract CT279: DareonTM-5: An open-label phase 2 trial of BI 764532, a DLL3-targeting T-cell engager, in patients (pts) with relapsed/refractory small cell lung cancer or other neuroendocrine carcinomas. Cancer Res. 2024, 84, CT279. [Google Scholar] [CrossRef]
- DAREONTM-7: A Phase I, Open-Label, Dose Escalation and Expansion Cohort Trial of the Delta-Like Ligand (DLL3)-Targeting T-Cell Engager BI 764532, Plus First-Line Platinum-Based Chemotherapy in Patients with DLL3-Positive Neuroendocrine Carcinomas—Enets.org. Available online: https://www.enets.org/abstract/dareonc-7-a-phase-i-open-label-dose-escalation-and-expansion-cohort-trial-of-the-delta-like-ligand-dll3-targeting-t-cell-engager-bi-764532-plus-first-line-platinum-based-chemotherapy-in-patients-with-dll3-positive-neuroendocrine-carcinomas1.html?utm_ (accessed on 7 October 2025).
- Wermke, M.; Felip, E.; Gambardella, V.; Kuboki, Y.; Morgensztern, D.; Oum’Hamed, Z.; Geng, J.; Studeny, M.; Owonikoko, T.K. A phase I, open-label, dose-escalation trial of BI 764532, a DLL3/CD3 bispecific antibody, in patients (pts) with small cell lung carcinoma (SCLC) or other neuroendocrine neoplasms expressing DLL3. J. Clin. Oncol. 2021, 39, TPS8588. [Google Scholar] [CrossRef]
- Lutathera|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lutathera (accessed on 19 July 2025).
- Mph, T.A.-T.; Md, E.P.; Md, J.S. Risk of Myelodysplastic Syndrome/Acute Leukemia with Sequential Capecitabine/Temozolomide and 177Lu-Dotatate. Endocr. Abstr. 2023, 89, 513. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Baudin, E.; Walter, T.A.; Beron, A.; Smith, D.; Hadoux, J.; Lachachi, C.; Taieb, D.; Ansquer, C.; Dierickx, L.O.; Bourg, L.d.M.d.; et al. 887O First multicentric randomized phase II trial investigating the antitumor efficacy of peptide receptor radionucleide therapy with 177Lutetium-Octreotate (OCLU) in unresectable progressive neuroendocrine pancreatic tumor: Results of the OCLURANDOM trial. Ann. Oncol. 2022, 33, S954. [Google Scholar] [CrossRef]
- Fata, C.R.; Gonzalez, R.S.; Liu, E.; Cates, J.M.; Shi, C. Mesenteric Tumor Deposits in Midgut Small Intestinal Neuroendocrine Tumors Are a Stronger Indicator Than Lymph Node Metastasis for Liver Metastasis and Poor Prognosis. Am. J. Surg. Pathol. 2017, 41, 128–133. [Google Scholar] [CrossRef]
- Baudin, E.; Capdevila, J.; Hörsch, D.; Singh, S.; Caplin, M.E.; Wolin, E.M.; Buikhuisen, W.; Raderer, M.; Dansin, E.; Grohe, C.; et al. Treatment of advanced BP-NETS with lanreotide autogel/depot vs placebo: The phase III SPINET study. Endocr. Relat. Cancer 2024, 31, e230337. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Montilla-Soler, J.; El-Haddad, G.; Haider, M.; Strosberg, J. Somatostatin Receptor Expression in Lung Neuroendocrine Tumors: An Analysis of DOTATATE PET Scans. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2023, 64, 1895–1898. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Wei, X.; Yang, K.; Tan, W.; Qiu, Z.; Li, S.; Chen, Q.; Song, Y.; Gao, S. Racial disparities in pancreatic neuroendocrine tumors survival: A SEER study. Cancer Med. 2017, 6, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Naqvi, S.; Cohn, A.L.; Delpassand, E.S.; Wagner, V.J.; Tworowska, I.; Torgue, J.; Woloski, R.; Manuel, A.; Maluccio, M.A. Safety, tolerability and efficacy of 212Pb-DOTAMTATE as a targeted alpha therapy for subjects with unresectable or metastatic somatostatin receptor-expressing gastroenteropancreatic neuroendocrine tumors (SSTR+ GEP-NETs): A phase 2 study. J. Clin. Oncol. 2024, 42, 4020. [Google Scholar] [CrossRef]
- Halperin, D.M.; Morris, M.; Ulaner, G.A.; Strosberg, J.R.; Mehr, S.H.; Li, D.; Soares, H.P.; Anthony, L.B.; Kotiah, S.D.; Jacene, H.; et al. Phase Ib portion of the ACTION-1 phase Ib/3 trial of RYZ101 in gastroenteropancreatic neuroendocrine tumors (GEP-NET) progressing after 177Lu somatostatin analogue (SSA) therapy: Safety and efficacy findings. J. Clin. Oncol. 2024, 42, 3091. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Morris, M.; Ulaner, G.A.; Halperin, D.M.; Mehr, S.H.; Li, D.; Soares, H.P.; Anthony, L.B.; Kotiah, S.D.; Jacene, H.; et al. Phase 1b portion of the ACTION-1 phase 1b/3 trial of RYZ101 in gastroenteropancreatic neuroendocrine tumors (GEP-NET) progressing after 177Lu somatostatin analogue (SSA) therapy: Safety and efficacy findings. J. Clin. Oncol. 2025, 43, 661. [Google Scholar] [CrossRef]
- Perspective Therapeutics A Phase I/IIa First-in-Human Study of [212Pb]VMT-α-NET Targeted Alpha-Particle Therapy for Advanced SSTR2 Positive Neuroendocrine Tumors; Clinicaltrials.gov, Clinical Trial Registration NCT05636618. 2025. Available online: https://clinicaltrials.gov/study/NCT05636618 (accessed on 20 July 2025).
- Chandekar, K.R.; Bal, C. Advances with 225Ac-DOTATATE Targeted Alpha Therapy in Somatostatin Receptor Positive Neuroendocrine Tumors. Semin. Nucl. Med. 2025, 55, 975–987. [Google Scholar] [CrossRef]
- Danieli, R.; Mileva, M.; Marin, G.; Kristanto, P.; Delbart, W.; Vanderlinden, B.; Wimana, Z.; Hendlisz, A.; Levillain, H.; Reynaert, N.; et al. Evolution of dosimetric parameters through PRRT and potential impact on clinical practice: Data from the prospective phase II LUMEN study. EJNMMI Res. 2024, 14, 110. [Google Scholar] [CrossRef]
- Opalinska, M.; Kamiński, G.; Dedecjus, M.; Kowalska, A.; Kolodziej, M.; Saracyn, M.; Garnuszek, P.; Lenda-Tracz, W.; Borkowska, A.; Gąsior-Perczak, D.; et al. DUONEN multicenter study—Personalized PRRT treatment with 177Lu- or 177Lu/90Y-DOTA-TATE in patients with neuroendocrine tumors based on individual dosimetry. Endocr. Abstr. 2023, 90, P440. [Google Scholar] [CrossRef]
- Deshayes, E.; Karfis, I.; Santoro, L.; Mileva, M.; Danieli, R.; Hebert, K.; Maccauro, M.; Chiesa, C.; Bardiès, M. Patient-Specific Dosimetry-Driven PRRT: Time to Move Forward! J. Nucl. Med. 2025, 25, 6939–6947. [Google Scholar] [CrossRef]
- Bednarz, B. Theranostics and Patient-Specific Dosimetry. Semin. Radiat. Oncol. 2023, 33, 317–326. [Google Scholar] [CrossRef]
- Trautwein, N.F.; Hinterleitner, C.; Kiefer, L.S.; Singer, S.; Mattern, S.; Schwenck, J.; Reischl, G.; Sipos, B.; Lauer, U.M.; Dittmann, H.; et al. Radiosensitizing Favors Response to Peptide Receptor Radionuclide Therapy in Patients with Highly Proliferative Neuroendocrine Malignancies: Preliminary Evidence From a Clinical Pilot Study. Clin. Nucl. Med. 2024, 49, 207–214. [Google Scholar] [CrossRef] [PubMed]
- di Santo, G.; Santo, G.; Sviridenko, A.; Virgolini, I. Peptide receptor radionuclide therapy combinations for neuroendocrine tumours in ongoing clinical trials: Status 2023. Theranostics 2024, 14, 940–953. [Google Scholar] [CrossRef]
- Borghesani, M.; Gervaso, L.; Cella, C.A.; Benini, L.; Ciardiello, D.; Algeri, L.; Ferrero, A.; Valenza, C.; Guidi, L.; Zampino, M.G.; et al. Promising targetable biomarkers in pancreatic neuroendocrine tumours. Expert Rev. Endocrinol. Metab. 2023, 18, 387–398. [Google Scholar] [CrossRef]
- Fallah, J.; Brave, M.H.; Weinstock, C.; Mehta, G.U.; Bradford, D.; Gittleman, H.; Bloomquist, E.W.; Charlab, R.; Hamed, S.S.; Miller, C.P.; et al. FDA Approval Summary: Belzutifan for von Hippel-Lindau Disease-Associated Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 4843–4848. [Google Scholar] [CrossRef]
- Qiu, J.; Zhou, J.; Cai, L.; Kong, W.; Xue, W.; Zhang, J.; Dong, P.; Liu, J.; Li, W.; Li, N.; et al. Belzutifan monotherapy in Chinese patients (pts) with von Hippel-Lindau (VHL) disease–associated tumors: Results of LITESPARK-015 study. J. Clin. Oncol. 2025, 43, 534. [Google Scholar] [CrossRef]
- Albiges, L.; Rini, B.I.; Peltola, K.; Oria, G.A.D.V.; Burotto, M.; Rodriguez, C.S.; Ghatalia, P.; Iacovelli, R.; Lam, E.T.; Verzoni, E.; et al. LBA88 Belzutifan versus everolimus in participants (pts) with previously treated advanced clear cell renal cell carcinoma (ccRCC): Randomized open-label phase III LITESPARK-005 study. Ann. Oncol. 2023, 34, S1329–S1330. [Google Scholar] [CrossRef]
- Iliopoulos, O.; Iversen, A.B.; Narayan, V.; Maughan, B.L.; Beckermann, K.E.; Oudard, S.; Else, T.; Maranchie, J.K.; Goldberg, C.M.; Fu, W.; et al. Belzutifan for patients with von Hippel-Lindau disease-associated CNS haemangioblastomas (LITESPARK-004): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2024, 25, 1325–1336. [Google Scholar] [CrossRef]
- Tanno, L.; Naheed, S.; Dunbar, J.; Tod, J.; Lopez, M.A.; Taylor, J.; Machado, M.; Green, B.; Ashton-Key, M.; Chee, S.J.; et al. Analysis of Immune Landscape in Pancreatic and Ileal Neuroendocrine Tumours Demonstrates an Immune Cold Tumour Microenvironment. Neuroendocrinology 2022, 112, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Puccini, A.; Poorman, K.; Salem, M.E.; Soldato, D.; Seeber, A.; Goldberg, R.M.; Shields, A.F.; Xiu, J.; Battaglin, F.; Berger, M.D.; et al. Comprehensive genomic profiling of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 5943–5951. [Google Scholar] [CrossRef]
- Strosberg, J.; Mizuno, N.; Doi, T.; Grande, E.; Delord, J.-P.; Shapira-Frommer, R.; Bergsland, E.; Shah, M.; Fakih, M.; Takahashi, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, E.; Cingarlini, S.; Taboada, R.G.; Borghesani, M.; Felismino, T.C.; Silva, V.S.E.; Reni, A.; Luchini, C.; Torrezan, G.; Mafficini, A.; et al. 1154P Prospective multinational evaluation of alkylating-induced hypermutation in neuroendocrine neoplasms (NEN): Clinical and molecular profiles associated with response to immune checkpoint inhibitors (CPI). Ann. Oncol. 2024, 35, S755. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Koumarianou, A.; Riechelmann, R.; Hernando, J.; Cingarlini, S.; Crona, J.; Al-Toubah, T.E.; Apostolidis, L.; Bergsland, E.K.; Halfdanarson, T.R.; et al. Efficacy of immune checkpoint inhibitors in patients with advanced pancreatic NETs displaying high TMB and MMR alterations following treatment with alkylating agents. J. Clin. Oncol. 2025, 43, 662. [Google Scholar] [CrossRef]
- Patel, S.P.; Mayerson, E.; Chae, Y.K.; Strosberg, J.; Wang, J.; Konda, B.; Hayward, J.; McLeod, C.M.; Chen, H.X.; Sharon, E.; et al. A phase II basket trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART) SWOG S1609: High-grade neuroendocrine neoplasm cohort. Cancer 2021, 127, 3194–3201. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Baghdadi, T.A.; et al. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef]
- Riesco-Martinez, M.C.; Capdevila, J.; Alonso, V.; Jimenez-Fonseca, P.; Teule, A.; Grande, E.; Sevilla, I.; Benavent, M.; Alonso-Gordoa, T.; Custodio, A.; et al. Nivolumab plus platinum-doublet chemotherapy in treatment-naive patients with advanced grade 3 Neuroendocrine Neoplasms of gastroenteropancreatic or unknown origin: The multicenter phase 2 NICE-NEC trial (GETNE-T1913). Nat. Commun. 2024, 15, 6753. [Google Scholar] [CrossRef]
- Mohamed, A.; Vijayvergia, N.; Kurian, M.; Liu, L.; Fu, P.; Das, S. Exploring Real World Outcomes with Nivolumab Plus Ipilimumab in Patients with Metastatic Extra-Pulmonary Neuroendocrine Carcinoma (EP-NEC). Cancers 2022, 14, 2695. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Schell, M.J.; Morse, B.; Haider, M.; Valone, T.; Strosberg, J. Phase II study of pembrolizumab and lenvatinib in advanced well-differentiated neuroendocrine tumors. ESMO Open 2024, 9, 102386. [Google Scholar] [CrossRef]
- Yellapragada, S.V.; Forsythe, S.D.; Madigan, J.P.; Sadowski, S.M. The Role of the Tumor Microenvironment in Gastroenteropancreatic Neuroendocrine Tumors. Int. J. Mol. Sci. 2025, 26, 5635. [Google Scholar] [CrossRef]
- Duong, B.; Banskota, P.; Falchook, G.S. T cell Immunoglobulin and Mucin Domain Containing Protein 3 (TIM-3) Inhibitors in Oncology Clinical Trials: A Review. J. Immunother. Precis. Oncol. 2024, 7, 89–96. [Google Scholar] [CrossRef]
- Kirkwood, J. A Phase II Study of Anti-PD1 Monoclonal Antibody (Nivolumab, BMS-936558) Administered in Combination with Anti-LAG3 Monoclonal Antibody (Relatlimab, BMS-986016) in Patients with Metastatic Melanoma Naïve to Prior Immunotherapy in the Metastatic Setting. Clinicaltrials.gov, Clinical Trial Registration NCT03743766. 2024. Available online: https://clinicaltrials.gov/study/NCT03743766 (accessed on 20 July 2025).
- Chauhan, A. A Phase 1 Trial of the Oncolytic Virus SVV-001 in Combination with Nivolumab and Ipilimumab in Patients with Poorly Differentiated Neuroendocrine Carcinomas or Well-Differentiated High-Grade (Grade 3) Neuroen-Docrine Tumors. Clinicaltrials.gov, Clinical Trial Registration NCT06889493. 2025. Available online: https://clinicaltrials.gov/study/NCT06889493 (accessed on 1 July 2025).
- Chen, Q.; Lu, L.; Ma, W. Efficacy, Safety, and Challenges of CAR T-Cells in the Treatment of Solid Tumors. Cancers 2022, 14, 5983. [Google Scholar] [CrossRef]
- Kronig, M.-N.; Wehrli, M.; Salas-Benito, D.; Maus, M.V. Hurdles race for CAR-T cell therapy in digestive tract cancer. Immunol. Rev. 2023, 320, 100–119. [Google Scholar] [CrossRef] [PubMed]
- Chimeric Therapeutics. A Phase 1/2 Study to Evaluate CHM-2101, an Autologous Cadherin 17 (CDH17) Chimeric Antigen Receptor (CAR) T Cell Therapy for the Treatment of Relapsed or Refractory Gastrointestinal Cancers. Clinicaltrials.gov, Clinical Trial Registration NCT06055439. 2025. Available online: https://clinicaltrials.gov/study/NCT06055439 (accessed on 20 July 2025).
- Jonsson Comprehensive Cancer Center. Phase 1 Dose Escalation Study of Systemically Administered IL13Ra2 Chimeric Antigen Receptor (CAR) T Cells After a Nonmyeloablative Conditioning Regimen in Patients with Metastatic Melanoma and Other Solid Tumors. Clinicaltrials.gov, Clinical Trial Registration NCT04119024. 2025. Available online: https://clinicaltrials.gov/study/NCT04119024 (accessed on 20 July 2025).
- Si, Y.; Kim, S.; Ou, J.; Lu, Y.; Ernst, P.; Chen, K.; Whitt, J.; Carter, A.M.; Markert, J.M.; Bibb, J.A.; et al. Anti-SSTR2 antibody-drug conjugate for neuroendocrine tumor therapy. Cancer Gene Ther. 2021, 28, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Pelle, E.; Cives, M.; Chaoul, N.; D’ANgelo, G.; Medina, E.; Mason, C.C.; Snedal, S.A.; Bustos-Perez, X.E.; Maiorano, G.; Luca, V.C.; et al. A novel hormone based anti-SSTR bispecific T-cell engager for the treatment of neuroendocrine tumorrs. Endocr. Abstr. 2025, 108, B6. [Google Scholar] [CrossRef]
- Mohamed, A.; Elhawi, M.; Trybula, M.; Elshawy, M.; Chakrabarti, S.; Selfridge, E.; Asa, S.L. Role of Bispecific Antibodies in Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NENs): Review of Literature. Clin. Med. Insights Oncol. 2024, 18, 11795549241285213. [Google Scholar] [CrossRef] [PubMed]
- A Phase II Randomized Control Trial of Triapine Plus Lutetium Lu 177 Dotatate Versus Lutetium Lu 177 Dota-Tate Alone for Well-Differentiated Somatostatin Receptor-Positive Neuroendocrine Tumors|Joint Clinical Trials Office. Available online: https://jcto.weill.cornell.edu/open_clinical_trials/a-phase-ii-randomized-control-trial-of-triapine-plus-lutetium-lu-177-dotatate-versus-lutetium-lu-177-dotatate-alone-for-well-differentiated-somatostatin-receptor-positive-neuroendocrine-tumors (accessed on 20 July 2025).
- Gujarathi, R.; Tobias, J.; Abou Azar, S.; Keutgen, X.M.; Liao, C.-Y. Peptide Receptor Radionuclide Therapy versus Capecitabine/Temozolomide for the Treatment of Metastatic Pancreatic Neuroendocrine Tumors. Cancers 2024, 16, 2993. [Google Scholar] [CrossRef]
- Moser, E.; Ura, A.; Vogel, L.; Steiger, K.; Mogler, C.; Evert, M.; Märkl, B.; Scheidhauer, K.; Martignoni, M.; Friess, H.; et al. ARX, PDX1, ISL1, and CDX2 Expression Distinguishes 5 Subgroups of Pancreatic Neuroendocrine Tumors with Correlations to Histology, Hormone Expression, and Outcome. Mod. Pathol. 2024, 37, 100595. [Google Scholar] [CrossRef]
- Alcala, N.; Voegele, C.; Mangiante, L.; Sexton-Oates, A.; Clevers, H.; Fernandez-Cuesta, L.; Dayton, T.L.; Foll, M. Multi-omic dataset of patient-derived tumor organoids of neuroendocrine neoplasms. GigaScience 2024, 13, giae008. [Google Scholar] [CrossRef]
- Soler, R.S.; Scarpa, A.; Lawlor, R.T.; Sadanandam, A.; Tafuto, S.; Krogh, M.; Teule, A.; Garcia-Carbonero, R.; Klumpen, H.J.; Cremer, B.; et al. 1150P Search for biomarkers to personalize treatment with streptozotocin plus 5-fluorouracil or everolimus in patients with advanced pancreatic neuroendocrine tumors: The randomized phase III SEQTOR trial (GETNE-1206). Ann. Oncol. 2024, 35, S754. [Google Scholar] [CrossRef]
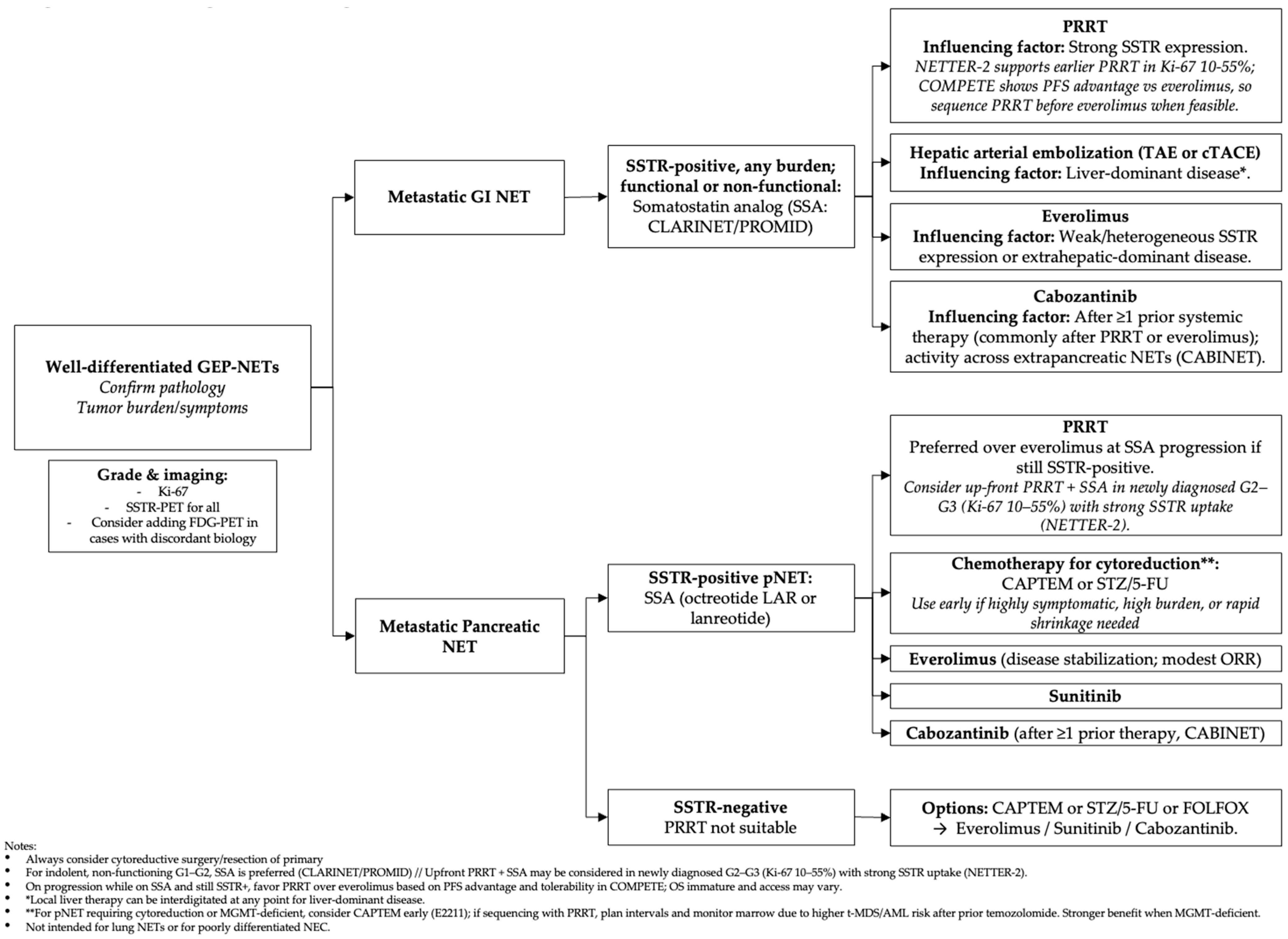
| Therapy (Mechanism) | Indication (NET Subtype/Line) | Supporting Trial (s) (N) | Efficacy Outcomes | Critical Commentary |
|---|---|---|---|---|
| Octreotide LAR/Lanreotide (SSTR agonists) | Well-differentiated G1–G2 GEP-NETs; first-line for tumor control and symptom control | PROMID (midgut) N = 85; CLARINET (non-functioning enteropancreatic, Ki-67 ≤10%) N = 204. | PROMID: TTP 14.3 vs. 6.0 mo (HR 0.34). CLARINET: 24-mo PFS 65% vs. 33%; median PFS NR vs. 18.0 mo. | Antiproliferative effect strongest in low-volume, low-Ki-67 disease; limited tumor shrinkage; OS effect confounded by crossover. Not studied for G3 WD disease. |
| Everolimus (mTOR inhibitor) | Progressive G1–G2 pNET; non-functioning GI/lung NET after SSA or upon progression | RADIANT-3 (pNET) N = 410; RADIANT-4 (GI/lung NET) N = 302. | RADIANT-3: PFS 11.0 vs. 4.6 mo (central). RADIANT-4: PFS 11.0 vs. 3.9 mo; HR ~0.48–0.59 across analyses; ORR low. | Reliable PFS benefit across prior-therapy strata; ORR modest; OS neutral due to crossover. Mucocutaneous and metabolic AEs require monitoring. |
| Sunitinib (VEGFR/PDGFR inhibitor) | Progressive, well-differentiated pNET | Phase III SUN 1111 (pNET) (2011) N = 171 | PFS: 11.4 mo vs. 5.5 mo on placebo (HR 0.42, p < 0.001). | pNET-specific evidence; trial halted early, but effect size and consistency support use. Hypertension, fatigue, diarrhea common. |
| Cabozantinib (Multi-kinase inhibitor: VEGFR2, MET, AXL) | pNET and extra-pancreatic NET after prior therapy | CABINET (Phase III, 2025) N = 298 | pNET: PFS 13.8 vs. 4.4 mo (HR 0.23); ORR 19%. epNET: PFS 8.4 vs. 3.9 mo (HR 0.38); ORR 5%. | Expands kinase options in both pNET and epNET; clinically meaningful PFS with low ORR typical of antiangiogenic TKIs; grade ≥ 3 AEs ~60% require dose adjustment. OS not yet clearly improved (crossover). |
| Capecitabine + Temozolomide (CAPTEM) (Cytotoxic chemotherapy) | Advanced pNET (often used second-line or later) | ECOG-ACRIN E2211 (Phase II randomized) N = 144 | PFS 22.7 vs. 14.4 mo (HR 0.58); OS 58.7 vs. 53.8 mo (NS). MGMT deficiency associated with higher response. | Only randomized evidence is phase II; nevertheless, robust PFS and response support use. Consider MGMT testing to enrich benefit; myelosuppression and nausea manageable with standard prophylaxis. |
| 177Lu-DOTATATE (PRRT) (beta-emitting radioligand) | SSTR-positive WD GEP-NETs: (A) midgut after progression; (B) first-line for higher-grade WD (G2–G3) per NETTER-2 | NETTER-1 (midgut) N = 229; NETTER-2 (first-line G2–G3 WD GEP-NETs) N = 226. | NETTER-1: median PFS NR vs. 8.4 mo; HR 0.21; ORR 18% vs. 3%. NETTER-2: PFS 22.8 vs. 8.5 mo; HR 0.28. | Strongest randomized PFS evidence in SSTR-positive disease; hematologic/renal toxicity usually low-grade with amino-acid protection. OS interpretation limited by crossover. First-line adoption for G2–G3 will depend on guideline updates and access. |
| Trial (Phase)—Intervention | Population | Design/Primary Endpoint | Results or Status (Mid 2025) |
|---|---|---|---|
| COMPETE (Phase III)—177Lu-edotreotide vs. Everolimus [81] | Metastatic SSTR-positive G1–G2 GEP-NETs; no prior PRRT (pNET and non-pNET) | Open-label RCT; primary: PFS (central review) | Positive. PFS 23.9 vs. 14.1 mo, HR 0.67, p = 0.022; ORR 16% vs. 5%; fewer grade 3–4 AEs with PRRT. |
| DUONEN (Phase III)—standard 177Lu-DOTATATE vs. dosimetry-guided 177Lu/90Y tandem regimens [82] | Advanced WD NETs (G1–G2), SSTR-positive, progressed on SSA | Multi-center, 4-arm trial; standard 7.4 GBq 177Lu × 4 vs. mixed 177Lu/90Y (increasing 90Y by dosimetry); primary: PFS | Ongoing. Interim safety acceptable; tests whether personalization (incl. 90Y for larger lesions) improves tumor control/PFS. |
| OCLURANDUM (Phase III)— 177Lu-DOTATATE vs. Sunitinib [83] | Advanced pancreatic NET (G1–G2), progressed on SSA | Open-label RCT; primary: 12-mo PFS (non-inferiority → superiority); crossover allowed at progression | Efficacy signal for PRRT. ORR 63% vs. 30%; median PFS 20.7 vs. 11.0 mo. OS numerically longer with sunitinib (64.4 vs. 55.8 mo) likely from crossover; PRRT better tolerated. |
| STARTER-NET/JCOG1901 (Phase III)—Everolimus + Lanreotide vs. Everolimus [84] | Unresectable GEP-NET (G1–G2), first-line systemic therapy | Randomized 2-arm; primary: PFS; stratified by primary site | Interim positive. PFS 29.7 vs. 11.5 mo, HR 0.38, p < 0.001; ORR higher with combo (~18% vs. 5%); OS immature; toxicity manageable. |
| SEQTOR (GETNE-1206)—Phase III Everolimus → STZ/5-FU vs. STZ/5-FU → Everolimus [79] | Advanced, progressive pNET (G1–G2); systemic-therapy-naïve | Open-label 2-arm sequence study; primary (amended): 12-mo PFS after first-line (PFS1); key secondary: PFS1 + PFS2, ORR, OS, safety | Similar disease control either way (PFS1 21.5 vs. 23.8 mo). Higher ORR when STZ/5-FU first (30% vs. 11%). |
| STELLAR-311 (Phase II/III)—Zanzalintinib (XL092) vs. Everolimus [NCT06943755] | Unresectable/metastatic WD (G1–G3) pNET/epNET | Randomized phase II/III; primary: PFS (central review); key secondary: ORR, OS, safety. | Ongoing. |
| DAREON-5 (Phase II)—Obrixtamig (BI 764532) [85,86,87,88] | DLL3-positive extrapulmonary high-grade NEC (incl. LCNEC/GEP-NEC), relapsed/refractory | Open-label, multicenter dose-selection; primary: ORR (RECIST v1.1); secondary: DOR, PFS, safety | Ongoing. Early-phase data show confirmed responses; no randomized data yet. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez-Felix, J.H.; Meneses-Medina, M.I.; Riechelmann, R.; Strosberg, J.; Garcia-Carbonero, R.; Rivero, J.d. Emerging Diagnostics and Therapies in Neuroendocrine Neoplasms: A Critical Review. Cancers 2025, 17, 3632. https://doi.org/10.3390/cancers17223632
Hernandez-Felix JH, Meneses-Medina MI, Riechelmann R, Strosberg J, Garcia-Carbonero R, Rivero Jd. Emerging Diagnostics and Therapies in Neuroendocrine Neoplasms: A Critical Review. Cancers. 2025; 17(22):3632. https://doi.org/10.3390/cancers17223632
Chicago/Turabian StyleHernandez-Felix, Jorge H., Monica Isabel Meneses-Medina, Rachel Riechelmann, Jonathan Strosberg, Rocio Garcia-Carbonero, and Jaydira del Rivero. 2025. "Emerging Diagnostics and Therapies in Neuroendocrine Neoplasms: A Critical Review" Cancers 17, no. 22: 3632. https://doi.org/10.3390/cancers17223632
APA StyleHernandez-Felix, J. H., Meneses-Medina, M. I., Riechelmann, R., Strosberg, J., Garcia-Carbonero, R., & Rivero, J. d. (2025). Emerging Diagnostics and Therapies in Neuroendocrine Neoplasms: A Critical Review. Cancers, 17(22), 3632. https://doi.org/10.3390/cancers17223632








