Gene Therapy for BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: Current Evidence and Future Directions
Simple Summary
Abstract
1. Introduction
2. Considerations for Gene Therapy in Bladder Cancer
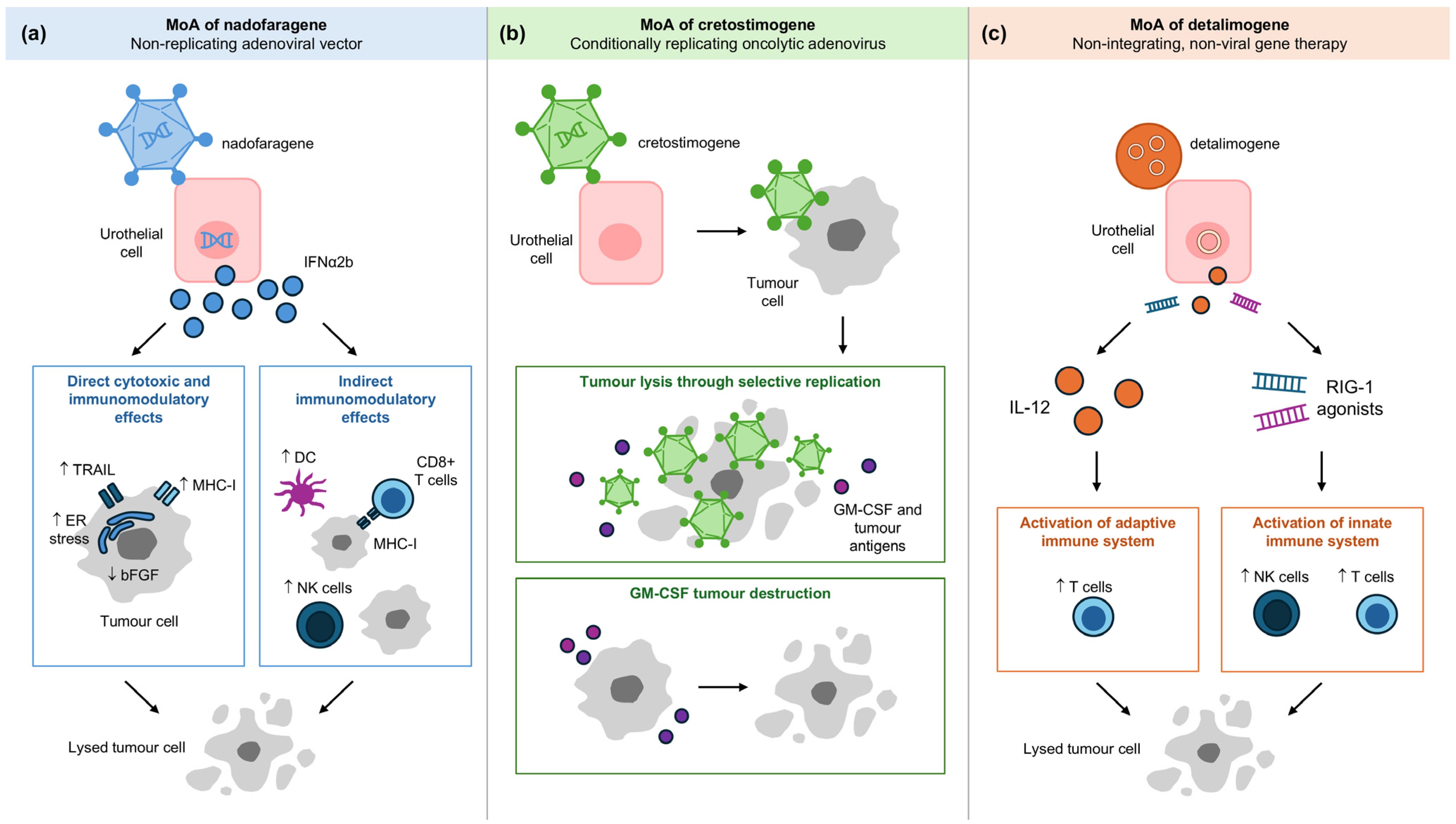
3. Nadofaragene Firadenovec
4. Cretostimogene Grenadenorepvec (CG0070)
5. Detalimogene Voraplasmid (EG-70)
6. Comparative Effectiveness
7. Future Perspectives
7.1. Patient Selection and Biomarker Development
7.2. Patient Perspective and QoL Considerations
7.3. Cost and Access Considerations
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder cancer, version 3.2020, nccn clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef]
- Deininger, S.; Torzsok, P.; Mitterberger, M.; Pallauf, M.; Oswald, D.; Deininger, C.; Lusuardi, L. From interferon to checkpoint inhibition therapy-a systematic review of new immune-modulating agents in bacillus calmette-guerin (bcg) refractory non-muscle-invasive bladder cancer (nmibc). Cancers 2022, 14, 694. [Google Scholar] [CrossRef]
- Leslie, S.W.; Soon-Sutton, T.L.; Aeddula, N.R. Bladder cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Martini, A.; Tholomier, C.; Mokkapati, S.; Dinney, C.P.N. Interferon gene therapy with nadofaragene firadenovec for bladder cancer: From bench to approval. Front. Immunol. 2023, 14, 1260498. [Google Scholar] [CrossRef]
- Duplisea, J.J.; Mokkapati, S.; Plote, D.; Schluns, K.S.; McConkey, D.J.; Yla-Herttuala, S.; Parker, N.R.; Dinney, C.P. The development of interferon-based gene therapy for bcg unresponsive bladder cancer: From bench to bedside. World J. Urol. 2019, 37, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Bjurlin, M.A.; Matulewicz, R.S. Comprehensive Diagnostic Approach to Bladder Cancer; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Herranz, R.; Oto, J.; Plana, E.; Fernandez-Pardo, A.; Cana, F.; Martinez-Sarmiento, M.; Vera-Donoso, C.D.; Espana, F.; Medina, P. Circulating cell-free DNA in liquid biopsies as potential biomarker for bladder cancer: A systematic review. Cancers 2021, 13, 1448. [Google Scholar] [CrossRef]
- Nerli, R.B.; Ghagane, S.C.; Shankar, K.; Sanikop, A.C.; Hiremath, M.B.; Dixit, N.S.; Magadum, L. Low-grade, multiple, ta non-muscle-invasive bladder tumors: Tumor recurrence and worsening progression. Indian J. Surg. Oncol. 2018, 9, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Bryce, R.; Tosone, C.; Sullivan, J.; Linback, T.; Steinberg, G. A phase 1/2 study of eg-70 (detalimogene voraplasmid) intravesical monotherapy for patients with bcg-unresponsive non-muscle invasive bladder cancer with carcinoma in situ. J. Clin. Oncol. 2024, 42, TPS4626. [Google Scholar] [CrossRef]
- Narayan, V.M.; Meeks, J.J.; Jakobsen, J.S.; Shore, N.D.; Sant, G.R.; Konety, B.R. Mechanism of action of nadofaragene firadenovec-vncg. Front. Oncol. 2024, 14, 1359725. [Google Scholar] [CrossRef]
- Food and Drug Administration. Bcg-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drug and Biological Products for Treatment. Available online: https://www.fda.gov/media/101468/download (accessed on 7 September 2024).
- Shore, N.D.; Boorjian, S.A.; Canter, D.J.; Ogan, K.; Karsh, L.I.; Downs, T.M.; Gomella, L.G.; Kamat, A.M.; Lotan, Y.; Svatek, R.S.; et al. Intravesical rad-ifnalpha/syn3 for patients with high-grade, bacillus calmette-guerin-refractory or relapsed non-muscle-invasive bladder cancer: A phase II randomized study. J. Clin. Oncol. 2017, 35, 3410–3416. [Google Scholar] [CrossRef]
- Babjuk, M.; Bohle, A.; Burger, M.; Comperat, E.; Kaasinen, E.; Palou Redorta, J.; van Rhijn, B.; Roupret, M.; Shariat, S.; Sylvester, R.; et al. Statement Concerning the Shortage of BCG Vaccine from the EAU Guidelines Panel on Non-Muscle Invasive Bladder Cancer. Available online: https://d56bochluxqnz.cloudfront.net/documents/guideline-publications/non-muscle-invasive-bladder-cancer/NMIBC-Guidelines-Panel-Statement-Concerning-Shortage-of-BCG-Vaccine.pdf (accessed on 7 August 2024).
- American Urological Association. Important Message About the BCG Shortage. Available online: https://www.auanet.org/about-us/bcg-shortage-info (accessed on 8 November 2025).
- Kokorovic, A.; Ory, J.; Saad, F. Emerging treatment options for bacillus calmette-guerin-unresponsive non-muscle invasive bladder cancer. Curr. Opin. Support. Palliat. Care 2022, 16, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Dinney, C.P.; Greenberg, R.E.; Steinberg, G.D. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus calmette-guérin. Urol. Oncol. 2013, 31, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Shiraishi, K.; Azuma, H.; Inoue, K.; Uemura, H.; Eto, M.; Ohyama, C.; Ogawa, O.; Kikuchi, E.; Kitamura, H.; et al. Clinical practice guidelines for bladder cancer 2019 update by the Japanese urological association: Summary of the revision. Int. J. Urol. 2020, 27, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Iida, K.; Nishimura, N.; Miyamoto, T.; Fujimoto, K.; Tomida, R.; Matsumoto, K.; Numakura, K.; Inokuchi, J.; Morizane, S.; et al. Non-maintenance intravesical bacillus calmette–guérin induction therapy with eight doses in patients with high- or highest-risk non-muscle invasive bladder cancer: A retrospective non-randomized comparative study. BMC Cancer 2021, 21, 266. [Google Scholar] [CrossRef]
- Iida, K.; Miyake, M.; Murakami, K.; Komiyama, M.; Okajima, E.; Sazuka, T.; Nishiyama, N.; Yasumoto, H.; Kimura, T.; Ito, A.; et al. Bacillus calmette-guérin-unresponsive non-muscle invasive bladder cancer outcomes in patients without radical cystectomy. Int. J. Clin. Oncol. 2021, 26, 2104–2112. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-first-adenoviral-vector-based-gene-therapy-high-risk-bacillus-calmette-Guerin (accessed on 7 August 2024).
- Narayan, V.M.; Boorjian, S.A.; Alemozaffar, M.; Konety, B.R.; Shore, N.D.; Gomella, L.G.; Kamat, A.M.; Bivalacqua, T.J.; Montgomery, J.S.; Lerner, S.P.; et al. Efficacy of intravesical nadofaragene firadenovec for patients with bacillus calmette-guérin-unresponsive nonmuscle-invasive bladder cancer: 5-year follow-up from a phase 3 trial. J. Urol. 2024, 212, 74–86. [Google Scholar] [CrossRef]
- Packiam, V.T.; Lamm, D.L.; Barocas, D.A.; Trainer, A.; Fand, B.; Davis, R.L., 3rd; Clark, W.; Kroeger, M.; Dumbadze, I.; Chamie, K.; et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol. Oncol. 2018, 36, 440–447. [Google Scholar] [CrossRef]
- Li, R.; Shah, P.H.; Stewart, T.F.; Bivalacqua, T.; Lamm, D.L.; Geynisman, D.M.; Meeks, J.J.; Uchio, E.M.; Jacob, J.M.; Dickstein, R.J.; et al. Final results of CORE-001: A phase-2, single arm study of cretostimogene grenadenorepvec in combination with pembrolizumab in patients with BCG-unresponsive, non-muscle invasive bladder cancer with carcinoma in situ. J. Clin. Oncol. 2024, 42, 4601. [Google Scholar] [CrossRef]
- Tyson, M.D.; Uchio, E.; Nam, J.K.; Lamm, D.L.; Bivalacqua, T.J.; Shore, N.D.; Kassouf, W.; Steinberg, G.D.; Black, P.C.; Kamat, A.M.; et al. Pivotal results from BOND-003: A phase 3, single-arm study of intravesical crestotimogene grenadenorepvec for the treatment of high risk, BCG-unresponsive non-muscle invasive bladder cancer with carcinoma in situ. J. Urol. 2024, 211, e1. [Google Scholar] [CrossRef]
- Steinberg, G.D.; Kolata, S.; Lotan, Y.; Tosone, C.; Mazanet, R. Legend, a phase 1/2 study of detalimogene voraplasmid (EG-70) intravesical monotherapy demonstrated clinical responses in BCG-unresponsive NMIBC patients with CIS. Urol. Oncol. Semin. Orig. Investig. 2024, 42, S48. [Google Scholar] [CrossRef]
- Kalota, S.; Joshi, S.; Bui, M.; Dickstein, R.; Liu, J.J.; Lotan, Y.; Johnson, S.; Taylor, J.; Bryce, R.; Tosone, C.; et al. Legend: A phase 1/2 study of EG-70 (detalimogene voraplasmid), a novel, non-viral intravesical gene therapy for patients with BCG-unresponsive non-muscle invasive bladder cancer with carcinoma in situ (CIS). J. Urol. 2024, 211, e5. [Google Scholar] [CrossRef]
- Goncalves, G.A.R.; Paiva, R.M.A. Gene therapy: Advances, challenges and perspectives. Einstein 2017, 15, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Highlights of Prescribing Information: Adstiladrin (Nadofaragene Firadenovec-Vncg). Available online: https://www.fda.gov/media/164029/download?attachment (accessed on 8 November 2025).
- Benedict, W.F.; Tao, Z.; Kim, C.S.; Zhang, X.; Zhou, J.H.; Adam, L.; McConkey, D.J.; Papageorgiou, A.; Munsell, M.; Philopena, J.; et al. Intravesical Ad-IFNα causes marked regression of human bladder cancer growing orthotopically in nude mice and overcomes resistance to IFN-α protein. Mol. Ther. 2004, 10, 525–532. [Google Scholar] [CrossRef]
- Tao, Z.; Connor, R.J.; Ashoori, F.; Dinney, C.P.; Munsell, M.; Philopena, J.A.; Benedict, W.F. Efficacy of a single intravesical treatment with Ad-IFN/Syn 3 is dependent on dose and urine IFN concentration obtained: Implications for clinical investigation. Cancer Gene Ther. 2006, 13, 125–130. [Google Scholar] [CrossRef]
- Iqbal Ahmed, C.M.; Johnson, D.E.; Demers, G.W.; Engler, H.; Howe, J.A.; Wills, K.N.; Wen, S.F.; Shinoda, J.; Beltran, J.; Nodelman, M.; et al. Interferon α2b gene delivery using adenoviral vector causes inhibition of tumor growth in xenograft models from a variety of cancers. Cancer Gene Ther. 2001, 8, 788–795. [Google Scholar] [CrossRef]
- Dinney, C.P.; Fisher, M.B.; Navai, N.; O’Donnell, M.A.; Cutler, D.; Abraham, A.; Young, S.; Hutchins, B.; Caceres, M.; Kishnani, N.; et al. Phase I trial of intravesical recombinant adenovirus mediated interferon-α2b formulated in Syn3 for Bacillus Calmette-Guerin failures in nonmuscle invasive bladder cancer. J. Urol. 2013, 190, 850–856. [Google Scholar] [CrossRef]
- Navai, N.; Benedict, W.F.; Zhang, G.; Abraham, A.; Ainslie, N.; Shah, J.B.; Grossman, H.B.; Kamat, A.M.; Dinney, C.P. Phase 1b trial to evaluate tissue response to a second dose of intravesical recombinant adenoviral interferon α2b formulated in Syn3 for failures of bacillus calmette-guerin (BCG) therapy in nonmuscle invasive bladder cancer. Ann. Surg. Oncol. 2016, 23, 4110–4114. [Google Scholar] [CrossRef] [PubMed]
- Boorjian, S.A.; Alemozaffar, M.; Konety, B.R.; Shore, N.D.; Gomella, L.G.; Kamat, A.M.; Bivalacqua, T.J.; Montgomery, J.S.; Lerner, S.P.; Busby, J.E.; et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021, 22, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Boorjian, S.A.; Narayan, V.M.; Konety, B.R.; Master, V.A.; Shore, N.D.; Kamat, A.M.; Bivalacqua, T.J.; Kates, M.R.; Montgomery, J.S.; Crispen, P.L.; et al. A0583—Efficacy of intravesical nadofaragene firadenovec-vncg for patients with Bacillus Calmette-Guérin-unresponsive non-muscle-invasive bladder cancer: 36-month follow-up from a phase 3 trial. Eur. Urol. 2024, 85, S1492–S1493. [Google Scholar] [CrossRef]
- Tyson, M.D.; Uchio, E.M.; Nam, J.K.; Joshi, S.S.; Bivalacqua, T.J.; Steinberg, G.D.; Kitamura, H.; Tran, B.; Li, R. Final results: Bond-003 cohort c- phase 3, single-arm study of intravesical cretostimogene grenadenorepvec for high-risk bcg-unresponsive non-muscle invasive bladder cancer with carcinoma in situ. J. Urol. 2025, 213, e1. [Google Scholar] [CrossRef]
- Taylor, J.A.; Joshi, S.; Satkunasivam, R.; Dickstein, R.J.; Salmasi, A.; Lotan, Y.; Johnson, S.; Pruthi, R.; Tosone, C.; Schuckman, A.K.; et al. Preliminary results from LEGEND: A phase 2 study of detalimogene voraplasmid (EG-70), a novel, non-viral intravesical gene therapy for patients with BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS). J. Clin. Oncol. 2025, 43, 802. [Google Scholar] [CrossRef]
- Moyer, J.A.; Durant, A.; Nguyen, M.; Mi, L.; Zganjar, A.; Lyon, T.D.; Shah, P.H.; Boorjian, S.A.; Tyson, M. Real-world outcomes of nadofaragene firadenovec in BCG-unresponsive non-muscle invasive bladder cancer. J. Clin. Oncol. 2025, 43, 716. [Google Scholar] [CrossRef]
- Daneshmand, S.; Canter, D.; Krupski, T.L.; Shoskes, D.A.; Armandi, D.; Shore, N.D. ABLE-41, a real-world evidence study for bladder cancer patients treated with nadofaragene firadenovec: Baseline patient characteristics and demographics. J. Clin. Oncol. 2025, 43, e16608. [Google Scholar] [CrossRef]
- Inoue, K.; Kikuchi, E.; Nishiyama, H.; Nasu, Y. Efficacy and safety of nadofaragene firadenovec for BCG-Unresponsive non–muscle-invasive bladder cancer: Initial results From an ongoing Japanese phase 3 trial. In Proceedings of the 112th Annual Meeting of the Japanese Urological Association, Fukuoka, Japan, 19 April 2025. [Google Scholar]
- Daneshmand, S.; Boorjian, S.A.; Meeks, J.J.; Bivalacqua, T.; Lotan, Y.; Kassouf, W.; Kulkarni, G.S.; Gontero, P.; Albers, P.; Jakobsen, J.S.; et al. ABLE-22: Safety and efficacy evaluation of nadofaragene firadenovec alone or in combination with chemotherapy or immunotherapy—A randomized, open-label, phase 2 study. J. Clin. Oncol. 2025, 43, TPS891. [Google Scholar] [CrossRef]
- Shore, N.D.; Dickstein, R.J.; Tyson, M.; Narayan, V.M.; Agarwal, G.; Goldfischer, E.R.; Williams, M.B.; Baayen, C.; Juul, K.; Sandstrom, P.; et al. ABLE-32: A randomized, controlled, phase 3b clinical trial of nadofaragene firadenovec-vncg versus observation in patients with intermediate-risk non–muscle-invasive bladder cancer. J. Clin. Oncol. 2025, 43, TPS888. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Low-Grade UTUC Treated with Nadofaragene Firadenovec Administered to Renal Pelvis (LUNAR). Available online: https://www.clinicaltrials.gov/study/NCT06668493?term=AREA%5BBasicSearch%5D(AREA%5BLeadSponsorName%5D(ferring))&rank=2 (accessed on 4 July 2025).
- CG Oncology. CG Oncology Receives Both FDA Fast Track and Breakthrough Therapy Designation for Cretostimogene Grenadenorepvec in High-Risk BCG-Unresponsive Non-Muscle Invasive Bladder Cancer. Available online: https://cgoncology.com/cg-oncology-receives-both-fda-fast-track-and-breakthrough-therapy-designation-for-cretostimogene-grenadenorepvec-in-high-risk-bcg-unresponsive-non-muscle-invasive-bladder-cancer/ (accessed on 2 September 2024).
- Burke, J.M.; Lamm, D.L.; Meng, M.V.; Nemunaitis, J.J.; Stephenson, J.J.; Arseneau, J.C.; Aimi, J.; Lerner, S.; Yeung, A.W.; Kazarian, T.; et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J. Urol. 2012, 188, 2391–2397. [Google Scholar] [CrossRef]
- Svatek, R.S.; Bivalacqua, T.; Keegan, K.A.; Daneshmand, S. PIVOT-006: A phase 3, randomized study of cretostimogene grenadenorepvec versus observation for the treatment of intermediate risk non-muscle invasive bladder cancer (IR-NMIBC) following transurethral resection of bladder tumor (TURBT). J. Clin. Oncol. 2024, 42, TPS715. [Google Scholar] [CrossRef]
- Svatek, R.S.; Bivalacqua, T.J.; Shore, N.D.; Jayram, G.; Josephson, D.Y.; Daneshmand, S. PIVOT-006: A phase 3, randomized study of adjuvant intravesical cretostimogene grenadenorepvec versus surveillance for the treatment of intermediate-risk non-muscle invasive bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2025, 43, 51. [Google Scholar] [CrossRef]
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 919–930. [Google Scholar] [CrossRef]
- Bivalacqua, T.; Daneshmand, S.; Shore, N.D.; Joshi, S.; Dinney, C.P.N. CORE-008: A phase 2, multi-arm, multi-cohort, open-label study to evaluate intravesical cretostimogene grenadenorepvec in participants with high-risk non-muscle invasive bladder cancer. J. Clin. Oncol. 2025, 43, TPS901. [Google Scholar] [CrossRef]
- Psutka, S.P.; Porten, S.P.; Scarpato, K.R.; Westerman, M.E.; Merrill, S.B.; Schuckman, A.K. Expanded access program of cretostimogene grenadenorepvec in patients with non-muscle invasive bladder cancer unresponsive to bacillus Calmette-Guerin. J. Clin. Oncol. 2025, 43, TPS902. [Google Scholar] [CrossRef]
- Mitra, A.P.; Narayan, V.M.; Mokkapati, S.; Miest, T.; Boorjian, S.A.; Alemozaffar, M.; Konety, B.R.; Shore, N.D.; Gomella, L.G.; Kamat, A.M.; et al. Antiadenovirus antibodies predict response durability to nadofaragene firadenovec therapy in BCG-unresponsive non-muscle-invasive bladder cancer: Secondary analysis of a Phase 3 clinical trial. Eur. Urol. 2022, 81, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Zehra, M.; Fatima, T.; Hanif, A.; Raufi, N.; Khan, A. Nadofaragene: A new era of precision medicine for bladder cancer. Ann. Med. Surg. 2024, 86, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Rieger, C.; Schlüchtermann, J.; Storz, E.; Kastner, L.; Pfister, D.; Heidenreich, A. Cost-effectiveness analysis of different treatment modalities in BCG-unresponsive NMIBC. BJU Int. 2024, 134, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Thorlund, K.; Dron, L.; Park, J.J.H.; Mills, E.J. Synthetic and external controls in clinical trials—A primer for researchers. Clin. Epidemiol. 2020, 12, 457–467. [Google Scholar] [CrossRef]
- Wang, X.; Dormont, F.; Lorenzato, C.; Latouche, A.; Hernandez, R.; Rouzier, R. Current perspectives for external control arms in oncology clinical trials: Analysis of EMA approvals 2016-2021. J. Cancer Policy 2023, 35, 100403. [Google Scholar] [CrossRef]
- Narayan, V.M.; Dinney, C.P.N. Intravesical gene therapy. Urol. Clin. N. Am. 2020, 47, 93–101. [Google Scholar] [CrossRef]
- Jung, A.; Nielsen, M.E.; Crandell, J.L.; Palmer, M.H.; Bryant, A.L.; Smith, S.K.; Mayer, D.K. Quality of life in non-muscle-invasive bladder cancer survivors: A systematic review. Cancer Nurs. 2019, 42, E21–E33. [Google Scholar] [CrossRef]
- Svatek, R.S.; Hollenbeck, B.K.; Holmäng, S.; Lee, R.; Kim, S.P.; Stenzl, A.; Lotan, Y. The economics of bladder cancer: Costs and considerations of caring for this disease. Eur. Urol. 2014, 66, 253–262. [Google Scholar] [CrossRef]


| Agent | Delivery Platform | Therapeutic Gene (s) Delivered | Primary Immunologic Goal | Dosing |
|---|---|---|---|---|
| Nadofaragene firadenovec | Non-replicating adenovirus [11,22] | IFNα2b | Enhance innate and adaptive immunity |
|
| Cretostimogene grenadenorepvec (CG0070) | Replicating adenovirus [23,24,25] | GM-CSF | Induce immunogenic cell death |
|
| Detalimogene voraplasmid (EG-70) | Non-viral plasmid delivery [10,26,27] | IL-12 RIG-I | Prime and expand T-cell and NK-cell response |
|
| Agent | Regulatory Status | Study Phase | Number of Patients | Efficacy | Safety | ||
|---|---|---|---|---|---|---|---|
| Median Follow-Up | CR | Median DoR | |||||
| Nadofaragene firadenovec | FDA-approved | Phase III [22,35] | 157 a | 19.7 mo |
| 9.69 mo |
|
| Cretostimogene grenadenorepvec (CG0070) | FDA Fast Track and Breakthrough Therapy Designation | Phase III [37] | 112 | 22.3 mo |
| 27.9 mo |
|
| Phase II (cretostimogene + pembrolizumab) [24] | 35 | NR |
| Has not been reached (>21 mo) |
| ||
| Detalimogene voraplasmid (EG-70) | Investigational, not yet approved | Phase I/II (ongoing) [38] | 42 | NR | Preliminary results
| NR |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinton, P. Gene Therapy for BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: Current Evidence and Future Directions. Cancers 2025, 17, 3631. https://doi.org/10.3390/cancers17223631
Pinton P. Gene Therapy for BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: Current Evidence and Future Directions. Cancers. 2025; 17(22):3631. https://doi.org/10.3390/cancers17223631
Chicago/Turabian StylePinton, Philippe. 2025. "Gene Therapy for BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: Current Evidence and Future Directions" Cancers 17, no. 22: 3631. https://doi.org/10.3390/cancers17223631
APA StylePinton, P. (2025). Gene Therapy for BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: Current Evidence and Future Directions. Cancers, 17(22), 3631. https://doi.org/10.3390/cancers17223631






