Microplastics Exposure Impact on Lung Cancer—Literature Review
Simple Summary
Abstract
1. Methods
2. Epidemiology of Lung Cancer and the Issue of Microplastics
- (1)
- Lung adenocarcinoma, which represents approximately 40% of cases and 55% of non-small cell lung cancers, arises from the bronchial epithelium and is typically classified as a peripheral lung cancer. It usually presents as pulmonary nodules; however, its course may be insidious because of the tendency for early metastasis.
- (2)
- Squamous cell carcinoma, accounting for 30–40% of cases, also originates from the alveolar epithelium. Approximately two-thirds of cases are located centrally, while one-third are peripheral. This subtype generally develops slowly but is frequently associated with bronchial obstruction and obstructive pulmonary disease.
- (3)
- Large-cell carcinoma—a relatively rare variant of lung cancer, accounting for approximately 9% of cases. It is strongly associated with smoking and other carcinogenic exposures, often occupational in nature, and occurs more frequently in men. This subtype is characterized by a low survival rate, a high propensity for chest wall invasion, and early metastasis [4].
3. Lung Cancer Diagnosis
4. Lung Cancer Therapies
5. Global Burden of Microplastics and Perspectives on Its Reduction
6. Methods for Detecting Plastic in the Environment and Organisms
7. Microplastics in Organisms and Tissue—The Molecular Basis of Plastic Toxicity
7.1. Mechanisms of Microplastics-Induced Toxicity
7.2. Microplastic-Induced Damage Cell Coping Mechanisms
7.3. Role of the Microplastics Modification and Additives
7.4. Biofilm on Microplastic Particles
8. The Impact of Plastic on the Risk and Course of Lung Cancer
9. The Impact of Plastic on Cellular Pathways in Lung Cancer
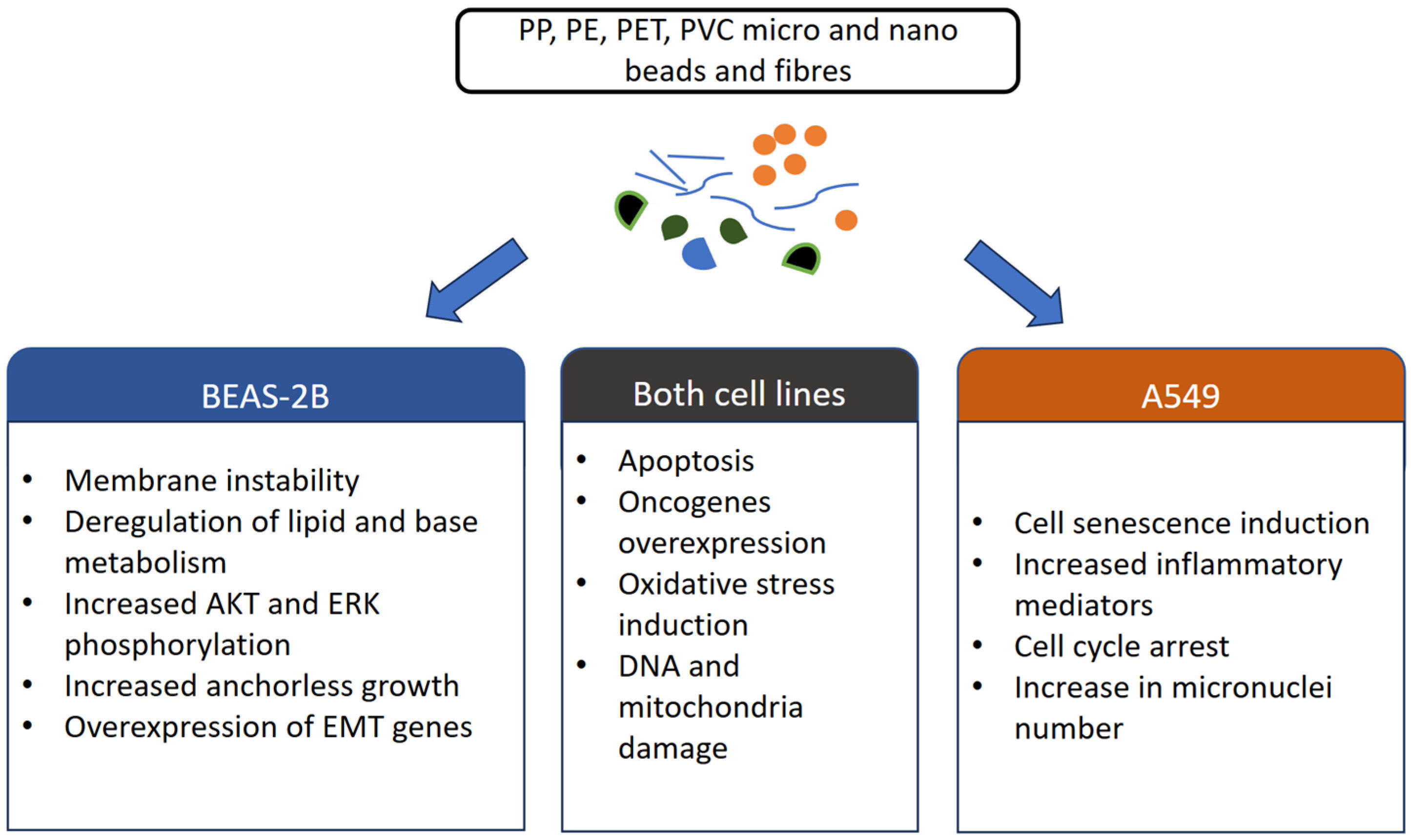
9.1. mP Impact on BEAS-2B Cells
9.2. MP Impact on A549 and Calu 3 Carcinoma Cells
9.3. MP Impact on In Vivo Models
9.4. MP Increases Probability of Asthma Occurrence
10. Conclusions and Potential Clinical Applications
Author Contributions
Funding
Conflicts of Interest
References
- Healthdata—Tracheal, Bronchus and Lung Cancer Level 3. Available online: https://www.healthdata.org/research-analysis/diseases-injuries-risks/factsheets/2021-tracheal-bronchus-and-lung-cancer-level-3 (accessed on 1 September 2025).
- Han, Z.; Zhu, Z.; Zhang, Z.; Dong, J.; Yang, X.; Feng, J. Global Burden of Lung Cancer in Adolescents and Adults Aged 15–45: Analysis of the Global Burden of Disease Study (1990–2021). Front. Med. 2025, 12, 1600662. [Google Scholar] [CrossRef]
- Fan, X.; Yin, L.; Hou, X.; Zhou, Q. Temporal Trends in the Burden of Tracheal, Bronchial, and Lung Cancer in China and Globally: A Comprehensive Analysis from 1990 to 2021. Chin. Med. J. Pulm. Crit. Care Med. 2025, 3, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Tai, Q.; Zhang, L.; Hu, X. Clinical Characteristics and Treatments of Large Cell Lung Carcinoma: A Retrospective Study Using SEER Data. Transl. Cancer Res. 2020, 9, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Types of Lung Cancer. Available online: https://www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/basics/lung-cancer-types (accessed on 1 September 2025).
- Zhang, H.; Wei, H.; Han, S.; Zheng, L.; Chen, X.; Li, Z.; Wang, L. A Comprehensive Examination of the Impact of Environmental Pollution on Lung Cancer: A Review. J. Adv. Res. 2025, in press. [CrossRef] [PubMed]
- Global Plastic Demand Shows No Signs of Slowing. Available online: https://finance.yahoo.com/news/global-plastic-demand-shows-no-090455294.html?guccounter=1&guce_referrer=aHR0cHM6Ly93d3cuZ29vZ2xlLmNvbS8&guce_referrer_sig=AQAAAFf5fHIMBHxLU_oh1CeRvfca4ibtyPf7IJXrOUl7IButvdPl-SgqYmKf98BrbSjjnAi49zkU6cEWfhAVDAsON2d-5Eo0Zr3sepLoNRlm6r4gf5c58k7H-s9B4jaNHJHHJxnV2z3zddW8gbklvnhS7DXX02TS9tBogKpzJbjc0le- (accessed on 30 August 2025).
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic Contamination in an Urban Area: A Case Study in Greater Paris. Environ. Chem. 2015, 12, 592. [Google Scholar] [CrossRef]
- Wieland, S.; Balmes, A.; Bender, J.; Kitzinger, J.; Meyer, F.; Ramsperger, A.F.; Roeder, F.; Tengelmann, C.; Wimmer, B.H.; Laforsch, C.; et al. From Properties to Toxicity: Comparing Microplastics to Other Airborne Microparticles. J. Hazard. Mater. 2022, 428, 128151. [Google Scholar] [CrossRef]
- Wang, M.; Kim, R.Y.; Kohonen-Corish, M.R.J.; Chen, H.; Donovan, C.; Oliver, B.G. Particulate Matter Air Pollution as a Cause of Lung Cancer: Epidemiological and Experimental Evidence. Br. J. Cancer 2025, 132, 986–996. [Google Scholar] [CrossRef]
- Lynch, H.N.; Loftus, C.T.; Cohen, J.M.; Kerper, L.E.; Kennedy, E.M.; Goodman, J.E. Weight-of-Evidence Evaluation of Associations Between Particulate Matter Exposure and Biomarkers of Lung Cancer. Regul. Toxicol. Pharmacol. 2016, 82, 53–93. [Google Scholar] [CrossRef]
- Tomonaga, T.; Higashi, H.; Izumi, H.; Nishida, C.; Kawai, N.; Sato, K.; Morimoto, T.; Higashi, Y.; Yatera, K.; Morimoto, Y. Investigation of Pulmonary Inflammatory Responses Following Intratracheal Instillation of and Inhalation Exposure to Polypropylene Microplastics. Part. Fibre Toxicol. 2024, 21, 29. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Huang, K.-Y.; Chen, C.-C.; Chang, Y.-H.; Li, H.-J.; Wang, T.-H.; Yang, P.-C. The Role of PM2.5 Exposure in Lung Cancer: Mechanisms, Genetic Factors, and Clinical Implications. EMBO Mol. Med. 2024, 17, 31–40. [Google Scholar] [CrossRef]
- Monoson, A.; Schott, E.; Ard, K.; Kilburg-Basnyat, B.; Tighe, R.M.; Pannu, S.; Gowdy, K.M. Air Pollution and Respiratory Infections: The Past, Present, and Future. Toxicol. Sci. 2023, 192, 3–14. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, J.; Yu, H.; Su, H.; Yang, Y.; Cao, Y.; Zhang, Q.; Ren, Y.; Hollert, H.; Shi, H.; et al. An Emerging Role of Microplastics in the Etiology of Lung Ground Glass Nodules. Environ. Sci. Eur. 2022, 34, 25. [Google Scholar] [CrossRef]
- Non-Small Cell Lung Cancer. Available online: https://www.yalemedicine.org/conditions/non-small-cell-lung-cancer (accessed on 1 September 2025).[Green Version]
- Clark, S.B.; Alsubait, S. Non–Small Cell Lung Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar][Green Version]
- Wang, Y.; Dong, A.; Jin, M.; Li, S.; Duan, Y. TEP RNA: A New Frontier for Early Diagnosis of NSCLC. J. Cancer Res. Clin. Oncol. 2024, 150, 97. [Google Scholar] [CrossRef]
- Abdipourbozorgbaghi, M.; Vancura, A.; Radpour, R.; Haefliger, S. Circulating MiRNA Panels as a Novel Non-Invasive Diagnostic, Prognostic, and Potential Predictive Biomarkers in Non-Small Cell Lung Cancer (NSCLC). Br. J. Cancer 2024, 131, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Duan, L.; Dong, Y. Diagnostic Accuracy of Exosomal Long Noncoding RNAs in Diagnosis of NSCLC: A Meta-Analysis. Mol. Diagn. Ther. 2024, 28, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Sun, H.; Yan, C.; Zhao, C.; Wang, D.; Li, M.; Ma, J. A Prediction Model Based on DNA Methylation Biomarkers and Radiological Characteristics for Identifying Malignant from Benign Pulmonary Nodules. BMC Cancer 2021, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Shariff, V.; Paritala, C.; Ankala, K.M. Optimizing Non Small Cell Lung Cancer Detection with Convolutional Neural Networks and Differential Augmentation. Sci. Rep. 2025, 15, 15640. [Google Scholar] [CrossRef]
- Kumar, A.; Salama, J.K. Role of Radiation in Oligometastases and Oligoprogression in Metastatic Non-Small Cell Lung Cancer: Consensus and Controversy. Expert Rev. Respir. Med. 2023, 17, 1033–1040. [Google Scholar] [CrossRef]
- Su, P.-L.; Furuya, N.; Asrar, A.; Rolfo, C.; Li, Z.; Carbone, D.P.; He, K. Recent Advances in Therapeutic Strategies for Non-Small Cell Lung Cancer. J. Hematol. Oncol. 2025, 18, 35. [Google Scholar] [CrossRef]
- Berger, J.M.; Tomasich, E.; Sunder-Plassmann, V.; Kleinberger, M.; Hettegger, P.; Gottmann, L.; Henao, I.S.; Korpan, M.; Setinek, U.; Valipour, A.; et al. 23P Non-Small Cell Lung Cancer DNA Methylation Profiles Correlate with Immune Checkpoint Inhibitor Response. Immuno-Oncol. Technol. 2024, 24, 100905. [Google Scholar] [CrossRef]
- Belluomini, L.; Dodi, A.; Caldart, A.; Kadrija, D.; Sposito, M.; Casali, M.; Sartori, G.; Ferrara, M.G.; Avancini, A.; Bria, E.; et al. A Narrative Review on Tumor Microenvironment in Oligometastatic and Oligoprogressive Non-Small Cell Lung Cancer: A Lot Remains to Be Done. Transl. Lung Cancer Res. 2021, 10, 3369–3384. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, Y.; Chen, H.; Li, T.; Fu, F.; Wang, J.; Li, B.; Hu, H. Identification and Validation of a DNA Methylation-Block Prognostic Model in Non-Small Cell Lung Cancer Patients. BMC Cancer 2025, 25, 999. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wu, X.; Zhang, S.; Tan, J.; Huo, Y.; Zhang, X.; Ning, B.; Ye, Y.; Wang, F. Cuproptosis: A Novel Therapeutic Mechanism in Lung Cancer. Cancer Cell Int. 2025, 25, 231. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lin, Q.; Liu, H.; Chen, Z.; Zhang, X.; Jin, L.; Peng, R.; Jin, H. Polystyrene Nanoplastics Carrying Copper Ion Induce FDX1-Mediated Cuproptosis. Ecotoxicol. Environ. Saf. 2025, 303, 118923. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, L.; Li, M.; Zhang, L.; Cheng, J.; El-Far, A.H.; Xu, Y.; Fu, J. ADAM10 Is a Key Player in the Diagnosis, Prognosis and Metastasis of Non-Small Cell Lung Cancer (NSCLC). J. Cancer 2025, 16, 1736–1746. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, L.; Ying, C.; Yu, L.; Guo, X.; Pan, K.; Zhu, D.; Chen, H. S-Adenosylmethionine Inhibits Non-Small Cell Lung Cancer and Enhances Chemosensitivity by Targeting the P62/NF-ΚB Axis and Regulating Autophagy and Oxidative Stress. Bioorganic Chem. 2025, 160, 108509. [Google Scholar] [CrossRef]
- Amadei, A.M.; Sanyé-Mengual, E.; Sala, S. Modeling the EU Plastic Footprint: Exploring Data Sources and Littering Potential. Resour. Conserv. Recycl. 2022, 178, 106086. [Google Scholar] [CrossRef]
- EU Parlament Plastic Waste and Recycling in the Eu Facts and Figures. Available online: https://www.europarl.europa.eu/topics/en/article/20181212STO21610/plastic-waste-and-recycling-in-the-eu-facts-and-figures (accessed on 30 August 2025).
- Our World in Data—Plastic Pollution. Available online: https://ourworldindata.org/plastic-pollution (accessed on 31 August 2025).
- Sturm, M.T.; Horn, H.; Schuhen, K. Removal of Microplastics from Waters Through Agglomeration-Fixation Using Organosilanes—Effects of Polymer Types, Water Composition and Temperature. Water 2021, 13, 675. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Ding, Y.; Li, N.; Su, M.; Zhang, C.; Li, Y.; Wang, Q.; Sha, C.; Xia, B.; et al. Microplastics and Human Health: Exposure Pathways, Toxicity Mechanisms, and Future Research Challenges. J. Environ. Chem. Eng. 2025, 13, 118807. [Google Scholar] [CrossRef]
- Pletz, M. Ingested Microplastics: Do Humans Eat One Credit Card per Week? J. Hazard. Mater. Lett. 2022, 3, 100071. [Google Scholar] [CrossRef]
- Boccia, P.; Mondellini, S.; Mauro, S.; Zanellato, M.; Parolini, M.; Sturchio, E. Potential Effects of Environmental and Occupational Exposure to Microplastics: An Overview of Air Contamination. Toxics 2024, 12, 320. [Google Scholar] [CrossRef]
- Eberhard, T.; Casillas, G.; Zarus, G.M.; Barr, D.B. Systematic Review of Microplastics and Nanoplastics in Indoor and Outdoor Air: Identifying a Framework and Data Needs for Quantifying Human Inhalation Exposures. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 185–196. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Habibi, N.; Sajid, S.; Dupont, S.; Behbehani, M. A Preliminary Assessment of Size-Fractionated Microplastics in Indoor Aerosol—Kuwait’s Baseline. Toxics 2022, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, R.; Xia, P.; Tan, H.; Deng, Y. Unraveling Micro/Nanoplastics and Phthalates in Infusion Solutions: A Novel Integrated Approach for Quantification and Cardiovascular Cytotoxicity Evaluation. J. Hazard. Mater. 2025, 497, 139614. [Google Scholar] [CrossRef] [PubMed]
- Kwabena Danso, I.; Woo, J.-H.; Hoon Baek, S.; Kim, K.; Lee, K. Pulmonary Toxicity Assessment of Polypropylene, Polystyrene, and Polyethylene Microplastic Fragments in Mice. Toxicol. Res. 2024, 40, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using ΜFTIR Spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, D.; Pei, J.; Fei, Y.; Ouyang, D.; Zhang, H.; Luo, Y. Identification and Quantification of Microplastics Using Fourier-Transform Infrared Spectroscopy: Current Status and Future Prospects. Curr. Opin. Environ. Sci. Health 2020, 18, 14–19. [Google Scholar] [CrossRef]
- Kissel, A.; Nogowski, A.; Kienle, A.; Foschum, F. Flow Raman Spectroscopy for the Detection and Identification of Small Microplastics. Sensors 2025, 25, 1390. [Google Scholar] [CrossRef]
- Lim, J.; Shin, G.; Shin, D. Fast Detection and Classification of Microplastics Below 10 Μm Using CNN with Raman Spectroscopy. Anal. Chem. 2024, 96, 6819–6825. [Google Scholar] [CrossRef]
- Dong, C.; Xu, H.; Lin, Y.; Zhang, B.; Yu, Z.; Xie, Y.; Yu, J.; Ma, D. Microplastics Detected in Three Types of Female Reproductive Organs Using Micro-Raman Spectroscopy. Ecotoxicol. Environ. Saf. 2024, 285, 117099. [Google Scholar] [CrossRef]
- Ao, J.; Xu, G.; Wu, H.; Xie, L.; Liu, J.; Gong, K.; Ruan, X.; Han, J.; Li, K.; Wang, W.; et al. Fast Detection and 3D Imaging of Nanoplastics and Microplastics by Stimulated Raman Scattering Microscopy. Cell Rep. Phys. Sci. 2023, 4, 101623. [Google Scholar] [CrossRef]
- Isaac Chandran, P.J.; Veerasingam, S. Laser Direct Infrared Spectroscopy: A Cutting-Edge Approach to Microplastic Detection in Environmental Samples. Talanta 2025, 284, 127284. [Google Scholar] [CrossRef] [PubMed]
- Belontz, S.L.; Brahney, J.; Caplan, C.E.; Dillon, E.; Yan, T.; Dominguez, G. Combining Submicron Spectroscopy Techniques (AFM-IR and O-PTIR) To Detect and Quantify Microplastics and Nanoplastics in Snow from a Utah Ski Resort. Environ. Sci. Technol. 2025, 59, 13362–13373. [Google Scholar] [CrossRef] [PubMed]
- Chalannavar, R.K.; Kamble, A.A.; Malabadi, R.B.; Divakar, M.S.; Swathi, K.K.; Kolkar, K.P.; Moramazi, S.; Munhoz, A.N.R.; Castaño Coronado, K.V. Microplastics: Detection Methods—An Update. World J. Adv. Res. Rev. 2025, 26, 2809–2824. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Mielańczuk, M.; Syczewski, M. The Raman Spectroscopy and SEM/EDS Investigation of the Primary Sources of Microplastics from Cosmetics Available in Poland. Chemosphere 2022, 308, 136407. [Google Scholar] [CrossRef]
- Shi, B.; Patel, M.; Yu, D.; Yan, J.; Li, Z.; Petriw, D.; Pruyn, T.; Smyth, K.; Passeport, E.; Miller, R.J.D.; et al. Automatic Quantification and Classification of Microplastics in Scanning Electron Micrographs via Deep Learning. Sci. Total Environ. 2022, 825, 153903. [Google Scholar] [CrossRef]
- Xie, D.; Fang, H.; Zhao, X.; Lin, Y.; Su, Z. Identification of Microplastics and Nanoplastics in Environmental Water by AFM-IR. Anal. Chim. Acta. 2025, 1354, 343992. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, B.; Wang, H. Analytical Methods for Microplastics in the Environment: A Review. Environ. Chem. Lett. 2023, 21, 383–401. [Google Scholar] [CrossRef]
- Liu, R.; Guo, X.; Yang, G.; Lu, S.; Chen, F.; Jia, W.; Li, J.; Niu, J.; Guo, H.; Zhu, H. Formation of Metal-Microplastic Complexes in Lung Adenocarcinoma Is Associated with Increased Risk of Cancer Progression. J. Hazard. Mater. 2025, 494, 138517. [Google Scholar] [CrossRef]
- Picó, Y.; Barceló, D. Pyrolysis Gas Chromatography-Mass Spectrometry in Environmental Analysis: Focus on Organic Matter and Microplastics. TrAC Trends Anal. Chem. 2020, 130, 115964. [Google Scholar] [CrossRef]
- Mansa, R.; Zou, S. Thermogravimetric Analysis of Microplastics: A Mini Review. Environ. Adv. 2021, 5, 100117. [Google Scholar] [CrossRef]
- Liang, M.; Huang, X.; Luo, S.; Zeng, Y.; Chen, K.; Wang, X.; Li, Y.; Liu, C.; Cui, L.; Huang, W.; et al. Aggregation and Deposition Kinetics of Polystyrene Nanoplastics in Lung Fluids: Influence of Particle Property, Fluid Condition, and Surfactant Protein. J. Hazard. Mater. 2025, 495, 138978. [Google Scholar] [CrossRef]
- Dzierżyński, E.; Blicharz-Grabias, E.; Komaniecka, I.; Panek, R.; Forma, A.; Gawlik, P.J.; Puźniak, D.; Flieger, W.; Choma, A.; Suśniak, K.; et al. Post-Mortem Evidence of Microplastic Bioaccumulation in Human Organs: Insights from Advanced Imaging and Spectroscopic Analysis. Arch. Toxicol. 2025, 99, 4051–4066. [Google Scholar] [CrossRef]
- Zhu, L.; Kang, Y.; Ma, M.; Wu, Z.; Zhang, L.; Hu, R.; Xu, Q.; Zhu, J.; Gu, X.; An, L. Tissue Accumulation of Microplastics and Potential Health Risks in Human. Sci. Total Environ. 2024, 915, 170004. [Google Scholar] [CrossRef]
- Paget, V.; Dekali, S.; Kortulewski, T.; Grall, R.; Gamez, C.; Blazy, K.; Aguerre-Chariol, O.; Chevillard, S.; Braun, A.; Rat, P.; et al. Specific Uptake and Genotoxicity Induced by Polystyrene Nanobeads with Distinct Surface Chemistry on Human Lung Epithelial Cells and Macrophages. PLoS ONE 2015, 10, e0123297. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Singh, A.; Kumar Gupta, V.; Mishra, Y.K. Nano/Micro-Plastic, an Invisible Threat Getting into the Brain. Chemosphere 2024, 361, 142380. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Guchhait, R.; De, S.; Pramanick, K. High Doses of Nano-Polystyrene Aggravate the Oxidative Stress, DNA Damage, and the Cell Death in Onions. Environ. Pollut. 2023, 316, 120611. [Google Scholar] [CrossRef]
- Boctor, J.; Hoyle, F.C.; Farag, M.A.; Ebaid, M.; Walsh, T.; Whiteley, A.S.; Murphy, D.V. Microplastics and Nanoplastics: Fate, Transport, and Governance from Agricultural Soil to Food Webs and Humans. Environ. Sci. Eur. 2025, 37, 68. [Google Scholar] [CrossRef]
- Woo, J.-H.; Seo, H.J.; Lee, J.-Y.; Lee, I.; Jeon, K.; Kim, B.; Lee, K. Polypropylene Nanoplastic Exposure Leads to Lung Inflammation Through P38-Mediated NF-ΚB Pathway Due to Mitochondrial Damage. Part. Fibre Toxicol. 2023, 20, 2. [Google Scholar] [CrossRef]
- Nihart, A.J.; Garcia, M.A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J.D.; Castillo, E.F.; Gullapalli, R.R.; Howard, T.; Bleske, B.; et al. Bioaccumulation of Microplastics in Decedent Human Brains. Nat. Med. 2025, 31, 1114–1119. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of Airborne Microplastics in Human Lung Tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, L.; Weng, J.; Jin, Z.; Cao, Y.; Jiang, H.; Zhang, Z. Detection and Characterization of Microplastics in the Human Testis and Semen. Sci. Total Environ. 2023, 877, 162713. [Google Scholar] [CrossRef]
- Liu, S.; Guo, J.; Liu, X.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. Detection of Various Microplastics in Placentas, Meconium, Infant Feces, Breastmilk and Infant Formula: A Pilot Prospective Study. Sci. Total Environ. 2023, 854, 158699. [Google Scholar] [CrossRef] [PubMed]
- How Microplastics and Asbestos Are Similar. Available online: https://www.kelleyferraro.com/blog/2024/01/how-microplastics-and-asbestos-are-similar/ (accessed on 30 August 2025).
- Prata, J.C. Microplastics and Human Health: Integrating Pharmacokinetics. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1489–1511. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, Y.; Shi, P.; Ni, Y.; Zeng, H.; Zhang, Z.; Zhao, C.; Sun, W.; Yi, Q. Mitigating Microplastic-Induced Organ Damage: Mechanistic Insights from the Microplastic-Macrophage Axes. Redox Biol. 2025, 84, 103688. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C. Energetic Stress-Induced Metabolic Regulation by Extracellular Vesicles. Compr. Physiol. 2023, 13, 5051–5068. [Google Scholar] [CrossRef]
- Duan, J.; Huang, Z.; Qin, S.; Li, B.; Zhang, Z.; Liu, R.; Wang, K.; Nice, E.C.; Jiang, J.; Huang, C. Oxidative Stress Induces Extracellular Vesicle Release by Upregulation of HEXB to Facilitate Tumour Growth in Experimental Hepatocellular Carcinoma. J. Extracell. Vesicles 2024, 13, e12468. [Google Scholar] [CrossRef]
- Wang, X.; Koffi, P.F.; English, O.F.; Lee, J.C. Staphylococcus Aureus Extracellular Vesicles: A Story of Toxicity and the Stress of 2020. Toxins 2021, 13, 75. [Google Scholar] [CrossRef]
- Dellar, E.R.; Hill, C.; Carter, D.R.F.; Baena-Lopez, L.A. Oxidative Stress-induced Changes in the Transcriptomic Profile of Extracellular Vesicles. J. Extracell. Biol. 2024, 3, e150. [Google Scholar] [CrossRef]
- Chiaradia, E.; Tancini, B.; Emiliani, C.; Delo, F.; Pellegrino, R.M.; Tognoloni, A.; Urbanelli, L.; Buratta, S. Extracellular Vesicles Under Oxidative Stress Conditions: Biological Properties and Physiological Roles. Cells 2021, 10, 1763. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Wang, Y.; Qu, C.; Yi, W.; Yang, J.; Pan, H.; Zhang, J.; Chen, C.; Bai, C.; Zhou, P.-K.; et al. Exposure to Polystyrene Nanoplastics Induces Abnormal Activation of Innate Immunity via the CGAS-STING Pathway. Ecotoxicol. Environ. Saf. 2024, 275, 116255. [Google Scholar] [CrossRef] [PubMed]
- Das, A. The Emerging Role of Microplastics in Systemic Toxicity: Involvement of Reactive Oxygen Species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef] [PubMed]
- Chirichigno, J.W.; Manfredi, G.; Beal, M.F.; Albers, D.S. Stress-Induced Mitochondrial Depolarization and Oxidative Damage in PSP Cybrids. Brain Res. 2002, 951, 31–35. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Zhang, Q.; Su, Z.; Yan, T.; Zhou, S.; Wang, T.; Wei, X.; Chen, Z.; Hu, G.; et al. DNA Damage, Serum Metabolomic Alteration and Carcinogenic Risk Associated with Low-Level Air Pollution. Environ. Pollut. 2022, 297, 118763. [Google Scholar] [CrossRef]
- Ong, G.; Logue, S.E. Unfolding the Interactions Between Endoplasmic Reticulum Stress and Oxidative Stress. Antioxidants 2023, 12, 981. [Google Scholar] [CrossRef]
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre d’Hellencourt, C.; Ravanan, P. A Molecular Web: Endoplasmic Reticulum Stress, Inflammation, and Oxidative Stress. Front. Cell. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Chen, T.T.; Zhang, D.W.; Zhang, Y.; Li, F.; Ding, Y.C.; Wang, M.Y.; Zhang, L.; Chen, K.G.; Fei, G.H. Microplastics Exacerbate Ferroptosis via Mitochondrial Reactive Oxygen Species-Mediated Autophagy in Chronic Obstructive Pulmonary Disease. Autophagy 2025, 21, 1717–1743. [Google Scholar] [CrossRef]
- Mu, Y.; Sun, J.; Li, Z.; Zhang, W.; Liu, Z.; Li, C.; Peng, C.; Cui, G.; Shao, H.; Du, Z. Activation of Pyroptosis and Ferroptosis Is Involved in the Hepatotoxicity Induced by Polystyrene Microplastics in Mice. Chemosphere 2022, 291, 132944. [Google Scholar] [CrossRef]
- Jung, W.; Yang, M.-J.; Kang, M.-S.; Kim, J.-B.; Yoon, K.-S.; Yu, T.; Yoon, C.; Yang, H.W.; Choi, S.-J.; Park, E.-J. Chronic Lung Tissue Deposition of Inhaled Polyethylene Microplastics May Lead to Fibrotic Lesions. Toxicol. Rep. 2025, 15, 102111. [Google Scholar] [CrossRef]
- González-Ruíz, J.; Baccarelli, A.A.; Cantu-de-Leon, D.; Prada, D. Air Pollution and Lung Cancer: Contributions of Extracellular Vesicles as Pathogenic Mechanisms and Clinical Utility. Curr. Environ. Health Rep. 2023, 10, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative Stress—Current Problems and Prospects. Antioxidants 2024, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, L.; Lei, R.; Zhang, P.; Yang, Y.; Liu, H.; Zhang, Y. DEHP and DBP, Common Phthalates, Induce Glucose Metabolism Disorders in Rats via Oxidative Damage of PI3K/Akt/GLUT4 Signaling. Environ. Pollut. 2024, 341, 122948. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, G.; Yu, H.; Liu, H.; An, T. The Respiratory Cytotoxicity of Typical Organophosphorus Flame Retardants on Five Different Respiratory Tract Cells: Which Are the Most Sensitive One? Environ. Pollut. 2022, 307, 119564. [Google Scholar] [CrossRef]
- Deng, X.; Gui, Y.; Zhao, L. The Micro(Nano)Plastics Perspective: Exploring Cancer Development and Therapy. Mol. Cancer 2025, 24, 30. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Wieland, S.; Wilde, M.V.; Fröhlich, T.; Kress, H.; Laforsch, C. Cellular Internalization Pathways of Environmentally Exposed Microplastic Particles: Phagocytosis or Macropinocytosis? J. Hazard. Mater. 2025, 489, 137647. [Google Scholar] [CrossRef]
- Liu, L.; Xu, K.; Zhang, B.; Ye, Y.; Zhang, Q.; Jiang, W. Cellular Internalization and Release of Polystyrene Microplastics and Nanoplastics. Sci. Total Environ. 2021, 779, 146523. [Google Scholar] [CrossRef]
- Ernhofer, B.; Spittler, A.; Ferk, F.; Mišík, M.; Zylka, M.M.; Glatt, L.; Boettiger, K.; Solta, A.; Kirchhofer, D.; Timelthaler, G.; et al. Small Particles, Big Problems: Polystyrene Nanoparticles Induce DNA Damage, Oxidative Stress, Migration, and Mitogenic Pathways Predominantly in Non-Malignant Lung Cells. J. Hazard. Mater. 2025, 495, 139129. [Google Scholar] [CrossRef]
- Delaney, S.; Rodriguez, C.; Sarrett, S.M.; Dayts, E.J.; Zeglis, B.M.; Keinänen, O. Unraveling the In Vivo Fate of Inhaled Micro- and Nanoplastics with PET Imaging. Sci. Total Environ. 2023, 904, 166320. [Google Scholar] [CrossRef]
- Ge, Y.; Yang, S.; Zhang, T.; Li, J.; Gong, S.; Fang, Y.; Liang, Y.; Yin, L.; Pu, Y.; Chen, Z.; et al. Integrative Lipidomic and Transcriptomic Analysis Unraveled Polystyrene Nanoplastics-Induced Liver Injury via Oral and Inhalation Exposure: All Roads Lead to Rome? Environ. Int. 2025, 202, 109717. [Google Scholar] [CrossRef]
- Donkers, J.M.; Höppener, E.M.; Grigoriev, I.; Will, L.; Melgert, B.N.; van der Zaan, B.; van de Steeg, E.; Kooter, I.M. Advanced Epithelial Lung and Gut Barrier Models Demonstrate Passage of Microplastic Particles. Microplast. Nanoplast. 2022, 2, 6. [Google Scholar] [CrossRef]
- Sjöholm, I.; Edman, P. Acrylic Microspheres In Vivo. I. Distribution and Elimination of Polyacrylamide Microparticles After Intravenous and Intraperitoneal Injection in Mouse and Rat. J. Pharmacol. Exp. Ther. 1979, 211, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.E.; Hare, J.T.; Khamis, Z.I.; Hua, T.; Sang, Q.-X.A. Exposure of Human Lung Cells to Polystyrene Microplastics Significantly Retards Cell Proliferation and Triggers Morphological Changes. Chem. Res. Toxicol. 2021, 34, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Oh, J.; Hevia-Ramos, G.; Ha, E.; Hong, Y.-C.; Kim, H.; Lim, Y.-H. A Systematic Review and Meta-Analysis on Long-Term Exposure to Particulate Matter and All-Cause and Cause-Specific Mortality in the Asia-Pacific States. J. Korean Med. Sci. 2025, 40, e156. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Wang, Z.; Wu, H.; Yu, P. Meta-Analysis of the Relationship Between Internal Microplastic and Health Outcomes. Eur. J. Intern. Med. 2025, 106552. [Google Scholar] [CrossRef]
- Saha, S.C.; Saha, G. Effect of Microplastics Deposition on Human Lung Airways: A Review with Computational Benefits and Challenges. Heliyon 2024, 10, e24355. [Google Scholar] [CrossRef]
- Han, M.; Liang, J.; Wang, K.; Si, Q.; Zhu, C.; Zhao, Y.; Khan, N.A.K.; Abdullah, A.L.B.; Shau-Hwai, A.T.; Li, Y.M.; et al. Integrin A5B1-Mediated Endocytosis of Polystyrene Nanoplastics: Implications for Human Lung Disease and Therapeutic Targets. Sci. Total Environ. 2024, 953, 176017. [Google Scholar] [CrossRef]
- Shi, J.; Yu, X.; Zhao, J.; Wang, T.; Li, N.; Yu, J.; Yao, L. Integrated Transcriptomics and Metabolomics Reveal the Mechanism of Polystyrene Nanoplastics Toxicity to Mice. Ecotoxicol. Environ. Saf. 2024, 284, 116925. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, H.; Wang, C.; Su, X.-L.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.-H.; Cai, Z.; Zheng, C. Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells. Environ. Sci. Technol. 2022, 56, 12483–12493. [Google Scholar] [CrossRef]
- Gutiérrez-García, J.; Egea, R.; Barguilla, I.; Nymark, P.; García-Rodríguez, A.; Guyot, B.; Maguer-Satta, V.; Marcos, R.; Rubio, L.; Hernández, A. Long-Term Exposure to Real-Life Polyethylene Terephthalate Nanoplastics Induces Carcinogenesis In Vitro. Environ. Sci. Technol. 2025, 59, 10891–10904. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, S.; Chu, J.; Yang, Y.; Yuan, B.; Zhang, H. Multi-Omics Analysis Reveals the Toxicity of Polyvinyl Chloride Microplastics Toward BEAS-2B Cells. Toxics 2024, 12, 399. [Google Scholar] [CrossRef] [PubMed]
- Śniadach, J.; Kicman, A.; Szymkowiak, S.; Waszkiewicz, N. The Hidden Threat of Microplastics in Traditional Cigarettes: A Narrative Review of Health and Environmental Risks. J. Clin. Med. 2025, 14, 3721. [Google Scholar] [CrossRef] [PubMed]
- Morataya-Reyes, M.; Villacorta, A.; Gutiérrez-García, J.; Egea, R.; Martín-Pérez, J.; Barguilla, I.; Marcos, R.; Hernández, A. The Long-Term In Vitro Co-Exposure of Polyethylene Terephthalate (PET) Nanoplastics and Cigarette Smoke Condensate Exacerbates the Induction of Carcinogenic Traits. J. Hazard. Mater. 2025, 493, 138359. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, M.; Wu, Y.; Zheng, P.; Gao, X.; Wang, J. Impact of Air Pollution on the Progress-Free Survival of Non-Small Cell Lung Cancer Patients with Anti-PD-1/PD-L1 Immunotherapy: A Cohort Study. Environ. Pollut. 2025, 368, 125683. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and Toxicity: A Preliminary Study of Effects of Nanoplastic Particles on Human Lung Epithelial Cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef]
- Shahzadi, C.; Di Serafino, A.; Aruffo, E.; Mascitelli, A.; Di Carlo, P. A549 as an In Vitro Model to Evaluate the Impact of Microplastics in the Air. Biology 2023, 12, 1243. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Huang, R.; Tang, C.; Hu, C.; Ning, P.; Wang, F. Cytotoxicity and Genotoxicity of Polystyrene Micro- and Nanoplastics with Different Size and Surface Modification in A549 Cells. Int. J. Nanomed. 2022, 17, 4509–4523. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Tang, H.; Wang, P.; Zhang, Y.; Liu, S.; Qiu, J.; Chen, H.; Wang, L.; Wang, R.; et al. Microplastics Exposure Causes the Senescence of Human Lung Epithelial Cells and Mouse Lungs by Inducing ROS Signaling. Environ. Int. 2024, 185, 108489. [Google Scholar] [CrossRef]
- 9 Common Uses of Polylactic Acid. Available online: https://plamfg.com/blog/uses-of-polylactic-acid/ (accessed on 9 September 2025).
- García-Rodríguez, A.; Gutiérrez, J.; Villacorta, A.; Arribas Arranz, J.; Romero-Andrada, I.; Lacoma, A.; Marcos, R.; Hernández, A.; Rubio, L. Polylactic Acid Nanoplastics (PLA-NPLs) Induce Adverse Effects on an In Vitro Model of the Human Lung Epithelium: The Calu-3 Air-Liquid Interface (ALI) Barrier. J. Hazard. Mater. 2024, 475, 134900. [Google Scholar] [CrossRef]
- da Luz, C.M.; Boyles, M.S.P.; Falagan-Lotsch, P.; Pereira, M.R.; Tutumi, H.R.; de Oliveira Santos, E.; Martins, N.B.; Himly, M.; Sommer, A.; Foissner, I.; et al. Poly-Lactic Acid Nanoparticles (PLA-NP) Promote Physiological Modifications in Lung Epithelial Cells and Are Internalized by Clathrin-Coated Pits and Lipid Rafts. J. Nanobiotechnol. 2017, 15, 11. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, G.; Sun, S.; Xu, P. Medical Applications and Prospects of Polylactic Acid Materials. iScience 2024, 27, 111512. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, Biodegradation and Excretion of Polylactic Acid (PLA) in Medical Implants and Theranostic Systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Zhang, X.; Chang, S.; Xue, Y.; Shang, P.; Liu, Y.; Chen, H.; Zhou, X.; Wang, W.; Wang, P.; et al. Co-Exposure of Polystyrene Nanoplastics and Ozone Synergistically Induced Airway Inflammation: Evidence and Biomarkers Screening. Ecotoxicol. Environ. Saf. 2025, 302, 118643. [Google Scholar] [CrossRef] [PubMed]
- Kaluç, N.; Bertorello, S.; Tombul, O.K.; Baldi, S.; Nannini, G.; Bartolucci, G.; Niccolai, E.; Amedei, A. Gut-Lung Microbiota Dynamics in Mice Exposed to Nanoplastics. NanoImpact 2024, 36, 100531. [Google Scholar] [CrossRef]
- Zha, H.; Xia, J.; Li, S.; Lv, J.; Zhuge, A.; Tang, R.; Wang, S.; Wang, K.; Chang, K.; Li, L. Airborne Polystyrene Microplastics and Nanoplastics Induce Nasal and Lung Microbial Dysbiosis in Mice. Chemosphere 2023, 310, 136764. [Google Scholar] [CrossRef]
- Zha, H.; Xia, J.; Wang, K.; Xu, L.; Chang, K.; Li, L. Foodborne and Airborne Polyethersulfone Nanoplastics Respectively Induce Liver and Lung Injury in Mice: Comparison with Microplastics. Environ. Int. 2024, 183, 108350. [Google Scholar] [CrossRef]
- Gurczynski, S.J.; Lipinski, J.H.; Strauss, J.; Alam, S.; Huffnagle, G.B.; Ranjan, P.; Kennedy, L.H.; Moore, B.B.; O’Dwyer, D.N. Horizontal Transmission of Gut Microbiota Attenuates Mortality in Lung Fibrosis. JCI Insight 2023, 9, e164572. [Google Scholar] [CrossRef]
- Yu, Z.; Kress, S.; Blay, N.; Gregor, P.; Kukk, H.-M.; Leskien, M.; Majewska, R.; Oosterwegel, M.J.; Szabó, D.; ten Have, M.; et al. External Exposome and Incident Asthma across the Life Course in 14 European Cohorts: A Prospective Analysis Within the EXPANSE Project. Lancet Reg. Health-Eur. 2025, 54, 101314. [Google Scholar] [CrossRef]
- Paplińska-Goryca, M.; Misiukiewicz-Stępień, P.; Wróbel, M.; Mycroft-Rzeszotarska, K.; Adamska, D.; Rachowka, J.; Królikowska, M.; Goryca, K.; Krenke, R. The Impaired Response of Nasal Epithelial Cells to Microplastic Stimulation in Asthma and COPD. Sci. Rep. 2025, 15, 4242. [Google Scholar] [CrossRef]
- Yang, S.; Cheng, Y.; Chen, Z.; Liu, T.; Yin, L.; Pu, Y.; Liang, G. In Vitro Evaluation of Nanoplastics Using Human Lung Epithelial Cells, Microarray Analysis and Co-Culture Model. Ecotoxicol. Environ. Saf. 2021, 226, 112837. [Google Scholar] [CrossRef]
| Material | Symbol | Application | Localisation of Aggregation in the Human Organism | Source |
|---|---|---|---|---|
| Polypropylene | PP | Containers, fibres, protective masks, car parts | Liver, lungs, testicles, breast milk | [67,68,69,70] |
| Low- and high-density polyethylene | LDPE HDPE | Plastic bags, bottles, disposable cutlery (LDPE), industrial containers, bottle caps (HDPE) | Lungs, brain, liver, kidneys, testicles | [42,68,69,70] |
| Polystyrene | PS | Building insulation, containers, and vessels | Brain, lungs, kidneys, heart, thyroid | [60,69] |
| Polylactic acid | PLA | 3D printing, packaging, implants | Breast milk | [71] |
| Polyvinyl chloride | PCV | Construction, cables, packaging | Liver, lungs | [61,68,69] |
| Cellulose derivatives | Textiles | Respiratory tract | [60,69] | |
| Polyethylene terephthalate (polyester) | PET | Bottles, textiles, photovoltaic cells | Kidneys | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sychowski, G.; Romanowicz, H.; Cieślik-Wolski, B.; Wojciechowska-Durczyńska, K.; Smolarz, B. Microplastics Exposure Impact on Lung Cancer—Literature Review. Cancers 2025, 17, 3616. https://doi.org/10.3390/cancers17223616
Sychowski G, Romanowicz H, Cieślik-Wolski B, Wojciechowska-Durczyńska K, Smolarz B. Microplastics Exposure Impact on Lung Cancer—Literature Review. Cancers. 2025; 17(22):3616. https://doi.org/10.3390/cancers17223616
Chicago/Turabian StyleSychowski, Grzegorz, Hanna Romanowicz, Bartosz Cieślik-Wolski, Katarzyna Wojciechowska-Durczyńska, and Beata Smolarz. 2025. "Microplastics Exposure Impact on Lung Cancer—Literature Review" Cancers 17, no. 22: 3616. https://doi.org/10.3390/cancers17223616
APA StyleSychowski, G., Romanowicz, H., Cieślik-Wolski, B., Wojciechowska-Durczyńska, K., & Smolarz, B. (2025). Microplastics Exposure Impact on Lung Cancer—Literature Review. Cancers, 17(22), 3616. https://doi.org/10.3390/cancers17223616






