Peritoneal Cancer Index Dominates Prognosis After CRS–HIPEC for Colorectal Peritoneal Metastases: A Consecutive Single-Centre Cohort with 3-Year Follow-Up
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Data Collection and Variable Definitions
- Pre-operative qualification for CRS–HIPEC was based on contrast-enhanced computed tomography (CT) performed as part of staging work-up, with the Peritoneal Cancer Index (PCI) estimated to assess disease extent and operability. The definitive PCI was recorded intra-operatively during laparotomy using the standard 13-region Sugarbaker method and used for all analyses.
- Completeness of cytoreduction (CCR) was assigned at the end of CRS:
- ○
- CCR-0—no macroscopic residual disease
- ○
- CCR-1—residual nodules ≤ 2.5 mm
- ○
- CCR-2—residual nodules > 2.5 mm
- The HIPEC procedure was performed immediately after CRS using a closed-abdomen technique in all cases. The most commonly used agent was oxaliplatin (460 mg/m2), administered over 30 min at 42–43 °C, and used in 70.7% of patients. Other agents included doxorubicin (14.7%), mitomycin C (10.7%), and cisplatin (4.0%), chosen according to institutional protocols based on prior systemic therapy, tumour biology, and known drug intolerances. No intravenous chemotherapy was co-administered. The perfusion parameters were continuously monitored during the procedure. The extent of resection was coded as peritoneum-only versus multivisceral, according to the operative report.
2.3. Survival End Points
- Survivor—no recorded death within 36 months of surgery (blank follow-up dates were considered alive under this rule);
- Non-survivor—death recorded ≤ 36 months;
- Censored—not applicable, as the study window ensured ≥ 36 months of potential follow-up for every case.
- PCI: 0–10, 11–20, >20
- CCR: CCR-0 vs. CCR-1/2
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Patient Characteristics
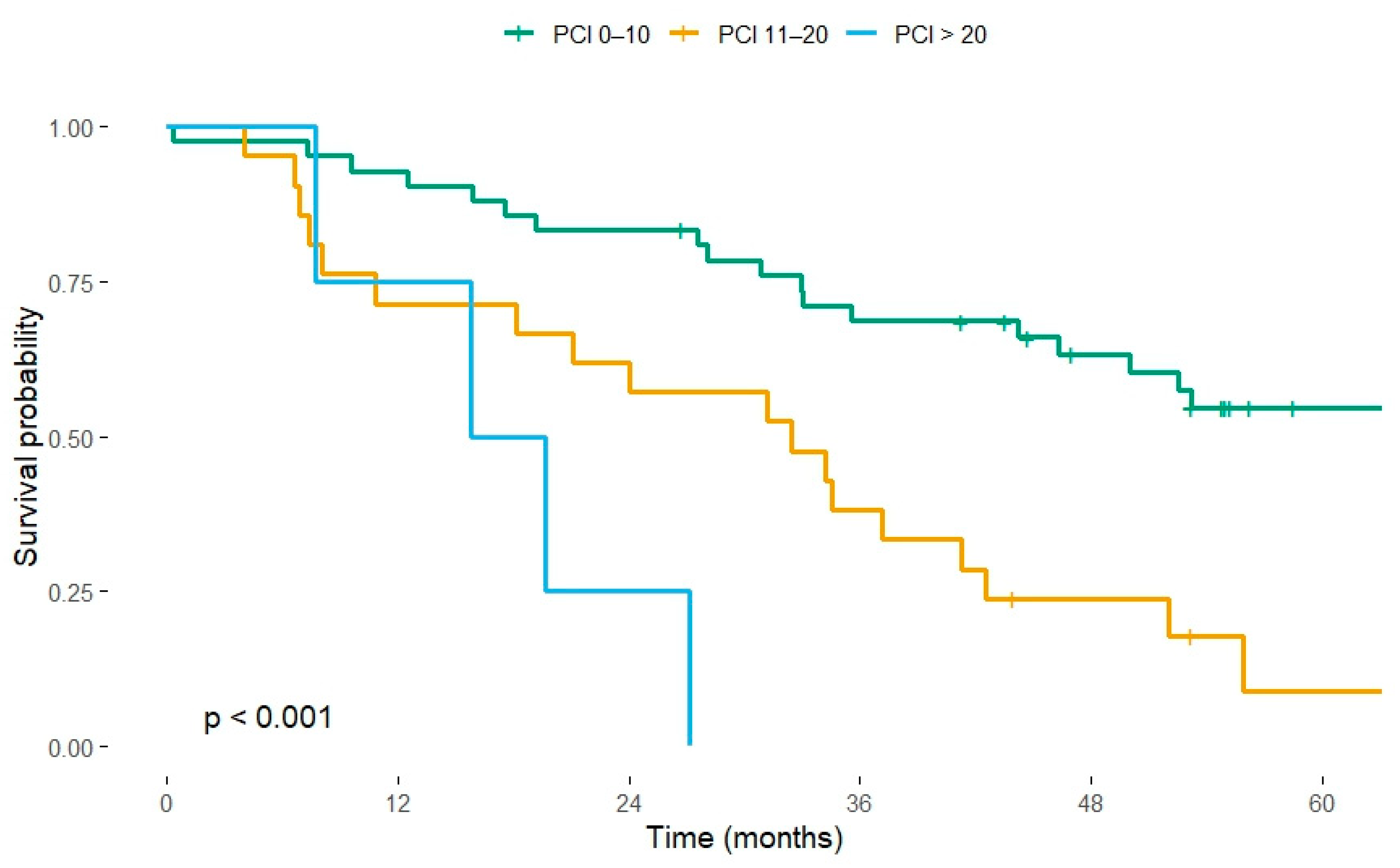
3.2. Survival by Peritoneal Cancer Index
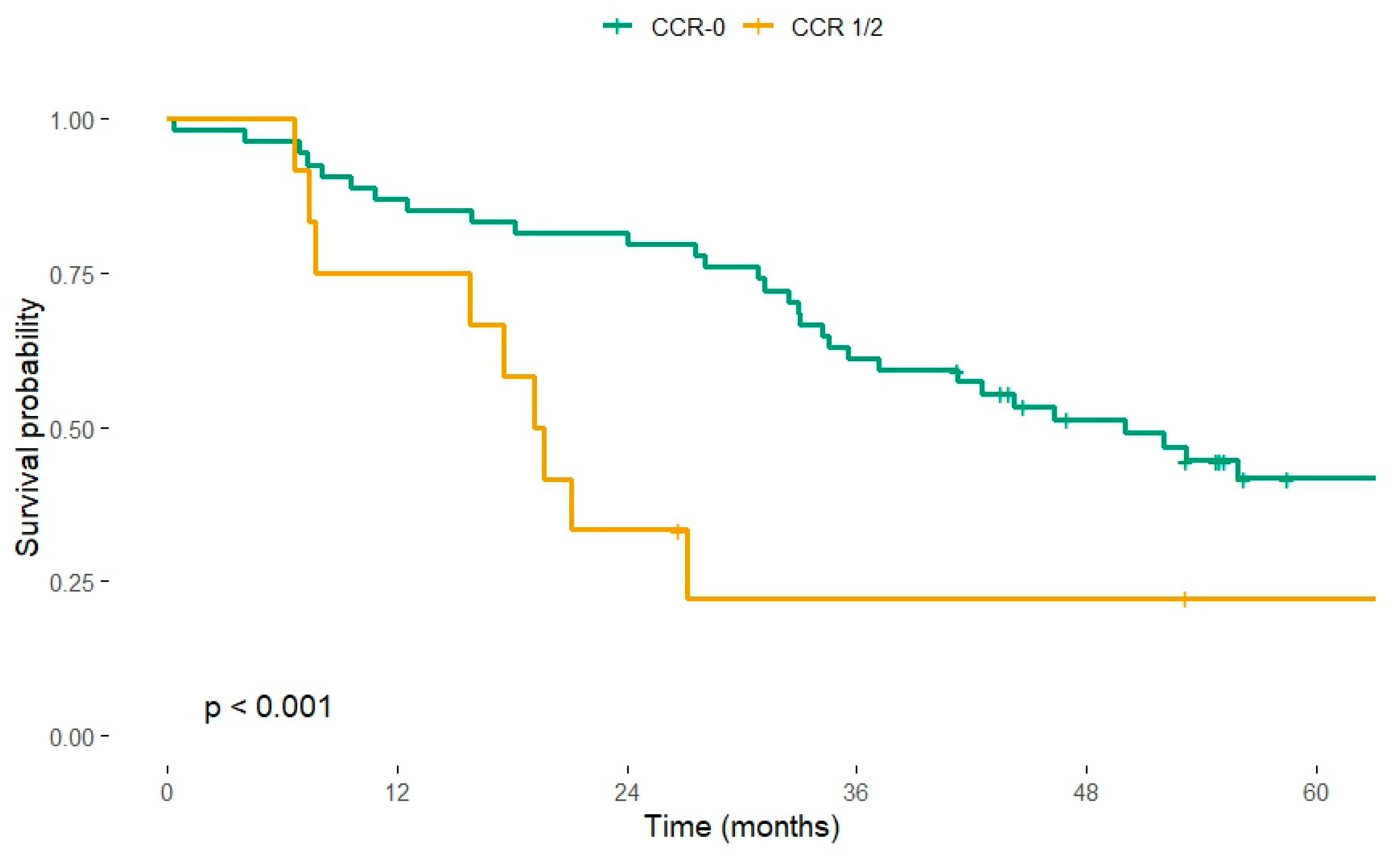
3.3. Completeness of Cytoreduction
3.4. Extent of Resection
3.5. Relationship Between PCI and Completeness of Cytoreduction
3.6. Influence of the HIPEC Agent
3.7. Multivariable Cox Model
- PCI 11–20 carried an adjusted HR of 3.02 (95% CI 1.52–6.03, p = 0.002).
- PCI > 20 carried an adjusted HR of 7.29 (95% CI 1.72–30.81, p = 0.007).
3.8. Post-Operative Morbidity and Early Outcomes
3.9. Predictors of Overall Survival
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sugarbaker, P.H. Management of peritoneal-surface malignancy: The surgeon’s role. Langenbeck’s Arch. Surg. 1999, 384, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.A.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D.J. Treatment of Colorectal Peritoneal Carcinomatosis with Systemic Chemotherapy: A Pooled Analysis of North Central Cancer Treatment Group Phase III Trials N9741 and N9841. J. Clin. Oncol. 2012, 30, 263–267. [Google Scholar] [CrossRef]
- Riediger, C.; Schuster, T.; Bork, U.; Schweipert, J.; Sigg, M.; Weiss, J.; Weitz, J. Do certain surgical steps increase postoperative morbidity after cytoreductive surgery and HIPEC- a retrospective analysis. Surg. Oncol. 2022, 45, 101884. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Slooten, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized Trial of Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy Versus Systemic Chemotherapy and Palliative Surgery in Patients with Peritoneal Carcinomatosis of Colorectal Cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.-M.; Ferron, G.; Guilloit, J.-M.; Meeus, P.; Goéré, D.; et al. Complete Cytoreductive Surgery Plus Intraperitoneal Chemohyperthermia with Oxaliplatin for Peritoneal Carcinomatosis of Colorectal Origin. J. Clin. Oncol. 2009, 27, 681–685. [Google Scholar] [CrossRef]
- Mirnezami, R.; Mehta, A.M.; Chandrakumaran, K.; Cecil, T.; Moran, B.J.; Carr, N.; Verwaal, V.J.; Mohamed, F.; Mirnezami, A.H. Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. Br. J. Cancer 2014, 111, 1500–1508. [Google Scholar] [CrossRef]
- Baratti, D.; Kusamura, S.; Pietrantonio, F.; Guaglio, M.; Niger, M.; Deraco, M. Progress in treatments for colorectal cancer peritoneal metastases during the years 2010–2015. A systematic review. Crit. Rev. Oncol. 2016, 100, 209–222. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Passot, G.; Glehen, O. New Insights on the Treatment of Colorectal Peritoneal Metastases from the CAIRO6 Trial. JAMA Surg. 2021, 157, 171. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Hida, K.; Ishibashi, H.; Sako, S.; Mizumoto, A.; Ichinose, M.; Padmanabhan, N.; Yoshida, S.; Yonemura, Y. Thirty-three long-term survivors after cytoreductive surgery in patients with peritoneal metastases from colorectal cancer: A retrospective descriptive study. World J. Surg. Oncol. 2021, 19, 31. [Google Scholar] [CrossRef]
- Narasimhan, V.; Tan, S.; Kong, J.; Pham, T.; Michael, M.; Ramsay, R.; Warrier, S.; Heriot, A. Prognostic factors influencing survival in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for isolated colorectal peritoneal metastases: A systematic review and meta-analysis. Color. Dis. 2020, 22, 1482–1495. [Google Scholar] [CrossRef]
- Glehen, O.; Kwiatkowski, F.; Sugarbaker, P.H.; Elias, D.; Levine, E.A.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive Surgery Combined with Perioperative Intraperitoneal Chemotherapy for the Management of Peritoneal Carcinomatosis from Colorectal Cancer: A Multi-Institutional Study. J. Clin. Oncol. 2004, 22, 3284–3292. [Google Scholar] [CrossRef]
- Gervais, M.-K.; Dubé, P.; McConnell, Y.; Drolet, P.; Mitchell, A.; Sideris, L. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with oxaliplatin for peritoneal carcinomatosis arising from colorectal cancer. J. Surg. Oncol. 2013, 108, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Birgisson, H.; Enblad, M.; Artursson, S.; Ghanipour, L.; Cashin, P.; Graf, W. Patients with colorectal peritoneal metastases and high peritoneal cancer index may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Segelman, J.; Granath, F.; Holm, T.; Machado, M.; Mahteme, H.; Martling, A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2012, 99, 699–705. [Google Scholar] [CrossRef]
- Cashin, P.H.; Graf, W.; Nygren, P.; Mahteme, H. Comparison of Prognostic Scores for Patients with Colorectal Cancer Peritoneal Metastases Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2013, 20, 4183–4189. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Geng, Y.; Hu, W. Peritoneal Metastasis: A Dilemma and Challenge in the Treatment of Metastatic Colorectal Cancer. Cancers 2023, 15, 5641. [Google Scholar] [CrossRef]
- Koumpa, F.S.; Xylas, D.; Konopka, M.; Galea, D.; Veselkov, K.; Antoniou, A.; Mehta, A.; Mirnezami, R. Colorectal Peritoneal Metastases: A Systematic Review of Current and Emerging Trends in Clinical and Translational Research. Gastroenterol. Res. Pr. 2019, 2019, 5180895. [Google Scholar] [CrossRef]
- D’aNnibale, G.; Aulicino, M.; Abatini, C.; Orsini, C.; Barberis, L.; Lodoli, C.; El Halabieh, M.A.; Di Giorgio, A.; Ferracci, F.; Calegari, M.A.; et al. Evaluating long-term outcomes after cytoreductive surgery for colorectal peritoneal metastasis: Do long survivors really exist? World J. Surg. Oncol. 2025, 23, 313. [Google Scholar] [CrossRef]
- Graf, W.; Cashin, P.H.; Ghanipour, L.; Enblad, M.; Botling, J.; Terman, A.; Birgisson, H. Prognostic Impact of BRAF and KRAS Mutation in Patients with Colorectal and Appendiceal Peritoneal Metastases Scheduled for CRS and HIPEC. Ann. Surg. Oncol. 2020, 27, 293–300. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Beagan, J.J.; Sluiter, N.R.; Bach, S.; Eijk, P.P.; Vlek, S.L.; Heideman, D.A.M.; Kusters, M.; Pegtel, D.M.; Kazemier, G.; van Grieken, N.C.T.; et al. Circulating Tumor DNA as a Preoperative Marker of Recurrence in Patients with Peritoneal Metastases of Colorectal Cancer: A Clinical Feasibility Study. J. Clin. Med. 2020, 9, 1738. [Google Scholar] [CrossRef]
- Simkens, G.A.; van Oudheusden, T.R.; Nieboer, D.; Steyerberg, E.W.; Rutten, H.J.; Luyer, M.D.; Nienhuijs, S.W.; de Hingh, I.H. Development of a Prognostic Nomogram for Patients with Peritoneally Metastasized Colorectal Cancer Treated with Cytoreductive Surgery and HIPEC. Ann. Surg. Oncol. 2016, 23, 4214–4221. [Google Scholar] [CrossRef]
- Qiu, B.; Shen, Z.; Wu, S.; Qin, X.; Yang, D.; Wang, Q. A machine learning-based model for predicting distant metastasis in patients with rectal cancer. Front. Oncol. 2023, 13, 1235121. [Google Scholar] [CrossRef]
- Bakkers, C.; van Erning, F.; Rovers, K.; Nienhuijs, S.; Burger, J.; Lemmens, V.; Aalbers, A.; Kok, N.; Boerma, D.; Brandt, A.; et al. Long-term survival after hyperthermic intraperitoneal chemotherapy using mitomycin C or oxaliplatin in colorectal cancer patients with synchronous peritoneal metastases: A nationwide comparative study. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Gusani, N.J.; Holtzman, M.P.; Ahrendt, S.A.; Jones, H.L.; Zeh, H.J.; Bartlett, D.L. Multivisceral Resection Does Not Affect Morbidity and Survival After Cytoreductive Surgery and Chemoperfusion for Carcinomatosis from Colorectal Cancer. Ann. Surg. Oncol. 2008, 15, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Yan, T.D.; Saxena, A.; Morris, D.L. Should the Treatment of Peritoneal Carcinomatosis by Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Still be Regarded as a Highly Morbid Procedure? Ann. Surg. 2009, 249, 900–907. [Google Scholar] [CrossRef]
- Kok, N.F.M.; de Hingh, I.H.J.T. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal metastases of colorectal origin. Br. J. Surg. 2017, 104, 313–315. [Google Scholar] [CrossRef]
- Hentzen, J.E.K.R.; Constansia, R.D.N.; Been, L.B.; Hoogwater, F.J.H.; van Ginkel, R.J.; van Dam, G.M.; Hemmer, P.H.J.; Kruijff, S. Diagnostic Laparoscopy as a Selection Tool for Patients with Colorectal Peritoneal Metastases to Prevent a Non-therapeutic Laparotomy During Cytoreductive Surgery. Ann. Surg. Oncol. 2020, 27, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Hida, K.; Yonemura, Y.; Sugarbaker, P.H.; Ghabra, S.; Ishihara, S.; Nagata, H.; Murono, K.; Goi, T.; Katayama, K.; et al. The Characteristics of 206 Long-Term Survivors with Peritoneal Metastases from Colorectal Cancer Treated with Curative Intent Surgery: A Multi-Center Cohort from PSOGI. Cancers 2021, 13, 2964. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Kameyama, M.; Kobayashi, H.; Kotake, K.; Sugihara, K. Investigation of the Japanese Classification of Peritoneal Metastasis from Colorectal Cancer Referring to the Correlation with PCI. J. Anus, Rectum Colon 2020, 4, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Kusamura, S.; Niger, M.; Perrone, F.; Milione, M.; Cattaneo, L.; Guaglio, M.; Bartolini, V.; Pietrantonio, F.; Deraco, M. Prognostic Impact of Primary Side and RAS/RAF Mutations in a Surgical Series of Colorectal Cancer with Peritoneal Metastases. Ann. Surg. Oncol. 2020, 28, 3332–3342. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value |
|---|---|
| Total patients | 75 |
| Median age (IQR) | 60 (54–67) |
| Gender (male/female) | 34/41 |
| Median PCI (IQR) | 10 (6–15) |
| CCR-0 (%) | 58 (77.3%) |
| Peritoneum only resection (%) | 23 (30.7%) |
| Most common HIPEC agent (oxaliplatin) (%) | 53 (70.7%) |
| PCI Group | CCR-0 | CCR-1/2 | CCR-0 (%) |
|---|---|---|---|
| 0–10 | 55 | 5 | 92% |
| 11–20 | 36 | 8 | 82% |
| >20 | 1 | 6 | 14% |
| Variable | Category (Reference) | Univariable HR (95% CI) | p | Multivariable HR (95% CI) | p |
|---|---|---|---|---|---|
| Peritoneal Cancer Index (PCI) | 0–10 (ref) | — | — | — | — |
| 11–20 | 3.10 (1.60–6.00) | 0.001 | 3.02 (1.52–6.03) | 0.002 | |
| >20 | 8.15 (2.54–26.18) | <0.001 | 7.29 (1.72–30.81) | 0.007 | |
| Completeness of cytoreduction (CCR) | CCR-0 (ref) | — | — | — | — |
| CCR 1/2 | 2.33 (1.23–4.35) † | 0.009 | 1.12 (0.45–2.86) ‡ | 0.795 | |
| Extent of resection | Multivisceral (ref) | — | — | — | — |
| Peritoneum only | 0.74 (0.38–1.43) | 0.373 | 0.99 (0.48–2.04) | 0.969 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazanowski, M.; Lesiak, P.; Wierzbicki, J.; Kapturkiewicz, B.; Maciejewski, P.; Pawłowski, M.; Jastrzębski, T.; Bębenek, M. Peritoneal Cancer Index Dominates Prognosis After CRS–HIPEC for Colorectal Peritoneal Metastases: A Consecutive Single-Centre Cohort with 3-Year Follow-Up. Cancers 2025, 17, 3614. https://doi.org/10.3390/cancers17223614
Kazanowski M, Lesiak P, Wierzbicki J, Kapturkiewicz B, Maciejewski P, Pawłowski M, Jastrzębski T, Bębenek M. Peritoneal Cancer Index Dominates Prognosis After CRS–HIPEC for Colorectal Peritoneal Metastases: A Consecutive Single-Centre Cohort with 3-Year Follow-Up. Cancers. 2025; 17(22):3614. https://doi.org/10.3390/cancers17223614
Chicago/Turabian StyleKazanowski, Michał, Paweł Lesiak, Jędrzej Wierzbicki, Bartosz Kapturkiewicz, Paweł Maciejewski, Marcin Pawłowski, Tomasz Jastrzębski, and Marek Bębenek. 2025. "Peritoneal Cancer Index Dominates Prognosis After CRS–HIPEC for Colorectal Peritoneal Metastases: A Consecutive Single-Centre Cohort with 3-Year Follow-Up" Cancers 17, no. 22: 3614. https://doi.org/10.3390/cancers17223614
APA StyleKazanowski, M., Lesiak, P., Wierzbicki, J., Kapturkiewicz, B., Maciejewski, P., Pawłowski, M., Jastrzębski, T., & Bębenek, M. (2025). Peritoneal Cancer Index Dominates Prognosis After CRS–HIPEC for Colorectal Peritoneal Metastases: A Consecutive Single-Centre Cohort with 3-Year Follow-Up. Cancers, 17(22), 3614. https://doi.org/10.3390/cancers17223614






