A Systematic Review of the Cost-Effectiveness of Screening Modalities for Breast Cancer in European Countries
Simple Summary
Abstract
1. Introduction
- Evaluate the cost-effectiveness of screening modalities for breast cancer diagnosis across European countries;
- Analyze different screening strategies, including age-specific population screening approaches;
- Assess regional variations in cost-effectiveness evidence;
- Examine temporal trends in cost-effectiveness over the study period.
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Economic Standardization
2.5. Quality Assessment
2.6. Recommendation Strength Classification
2.7. Statistical Analysis
3. Results
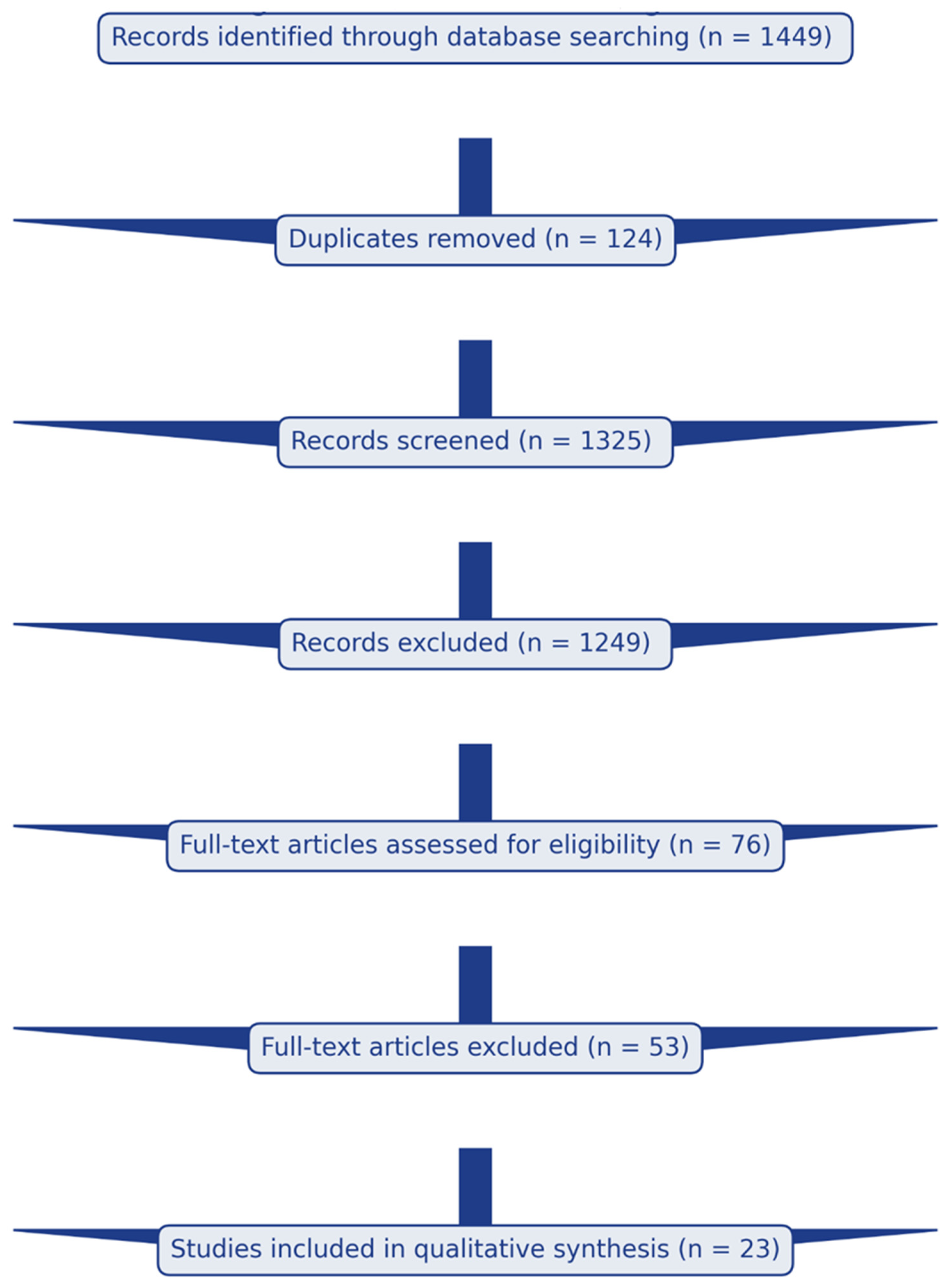
3.1. Study Selection
Temporal Distribution of Included Studies
- From 2010 to 2020: Nine studies (39%)—Introduction of digital technologies and risk-stratified approaches.
- From 2000 to 2009: Eight studies (35%)—Expansion of screening programs across Western Europe;
- From 1990 to 1999: Six studies (26%)—Early health economic evaluations primarily from the UK, Netherlands, and Sweden.
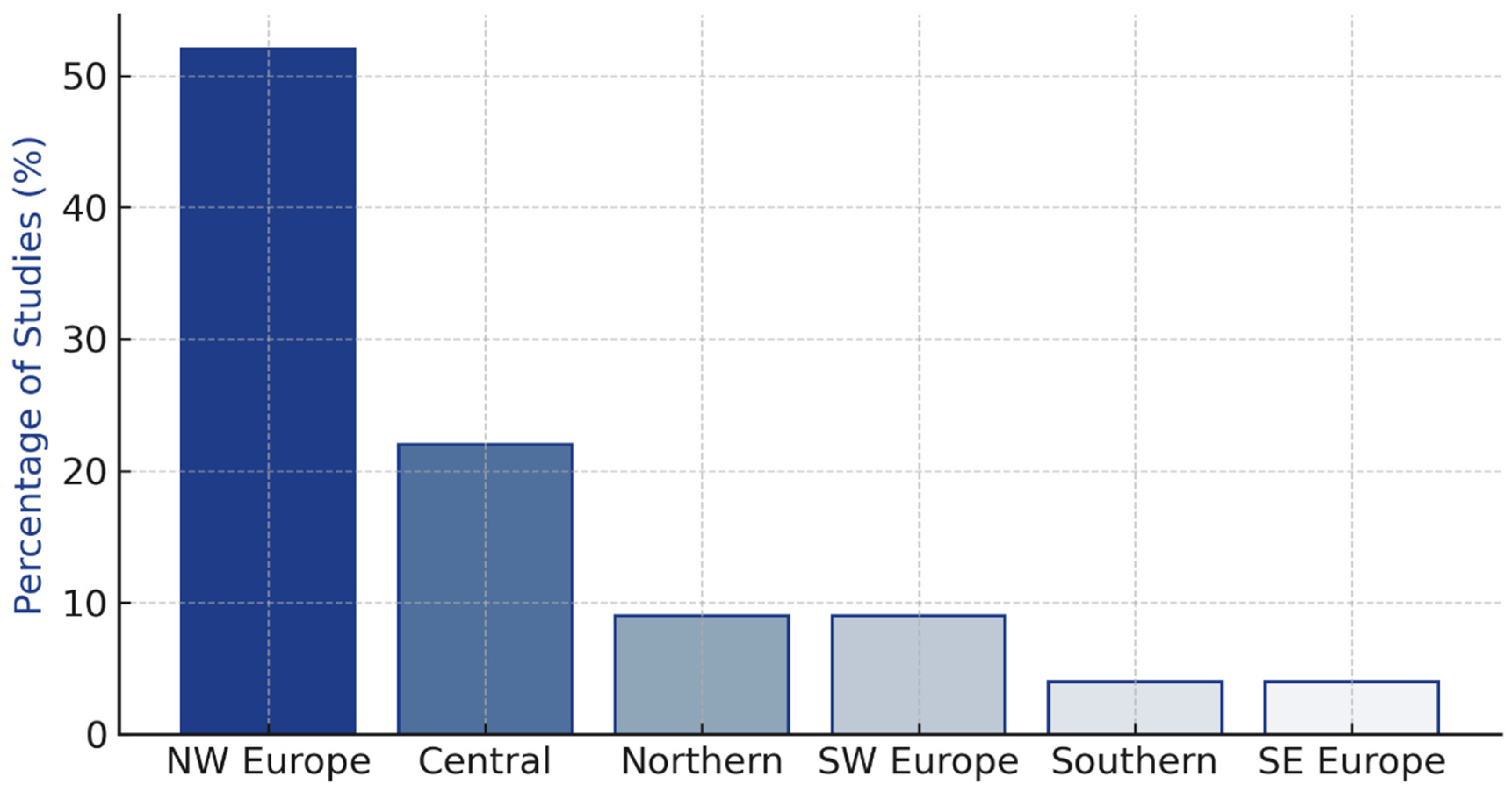
3.2. Geographic Distribution and Study Quality
3.3. Cost-Effectiveness by Screening Modality
3.4. Mammography Screening (Ages 50–69 Years)
3.5. MRI Screening (High-Risk Populations)
3.6. Digital Breast Tomosynthesis
3.7. Special Populations: Dense Breasts and Women Under 50
- Austria: 94.87% adherence; 19% survival increase.
- Germany: 47% adherence; 11% annual mortality reduction.
- Netherlands: 70% adherence; 10–16% mortality reduction.
- Norway: 74.5–77% adherence; 30% mortality reduction.
- Switzerland: 80% adherence; 39.1/100;000 survival rate.
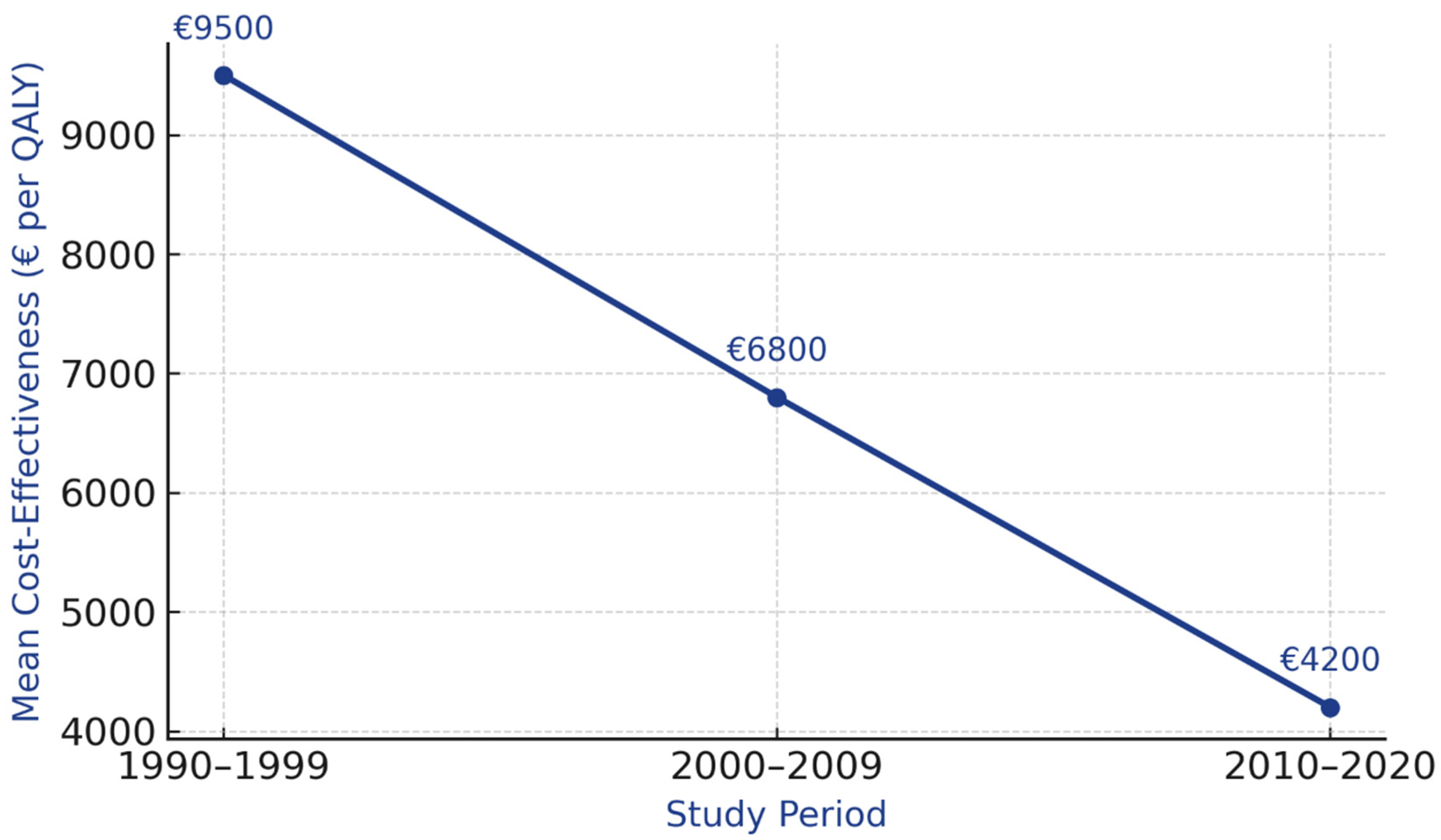
3.8. Temporal Evolution
4. Discussion
4.1. Principal Findings
4.2. Contextualization Within European Healthcare Policy Frameworks
Alignment with European Commission and National Guidelines
4.3. National HTA Recommendations and Implementation
4.4. Correlation with Breast Cancer Survival Outcomes
4.4.1. Mortality Trends and Screening Impact
4.4.2. Geographic Variations in Survival
4.5. Evidence Quality and Recommendation Strength: GRADE Framework Application
4.5.1. Methodological Justification for Recommendation Categories
- High quality (≥8 studies): Consistent results across multiple populations and healthcare systems; low heterogeneity in cost-effectiveness ratios; robust methodology (CHEERS quality score ≥20/28); and coverage across multiple European regions.
- Moderate quality (4–7 studies): Mostly consistent results; moderate heterogeneity; geographic coverage limited to 2–3 European regions; and some methodological limitations.
- Low quality (≤3 studies): Variable results; high heterogeneity; limited geographic coverage; and emerging technology with limited long-term data.
4.5.2. Recommendation Strength Determination:
- Cost-effectiveness ratio consistently <EUR 10,000/QALY across diverse healthcare contexts;
- Large, consistent benefit across populations;
- High certainty that benefits outweigh harms;
- Alignment with established willingness-to-pay thresholds in European countries;
- Supported by ≥8 high-quality economic evaluations;
- Example: Biennial mammography ages 50–69 (EUR 3000–8000/QALY; 12 studies; all regions).
- Cost-effectiveness ratio EUR 10,000-EUR 35,000/QALY (within acceptable range but higher uncertainty);
- Benefits likely outweigh harms, but closer balance;
- Moderate certainty of evidence;
- Supported by 4–7 studies with moderate geographic coverage;
- Resource constraints may influence implementation decisions;
- Example: MRI screening for high-risk populations (EUR 18,201–33,534/QALY; 4 studies).
- Limited evidence (≤3 studies) or substantial uncertainty;
- Emerging technologies requiring further evaluation;
- High geographic limitation (single region only);
- Insufficient data on long-term outcomes or implementation feasibility;
- Example: Digital breast tomosynthesis in general population screening (3 studies; limited data).
4.6. Implementation Considerations and Healthcare System Context
4.6.1. Financing and Organizational Models
4.6.2. Resource-Stratified Implementation Framework
- Biennial mammography screening ages 50–69;
- Organized population-based call–recall system;
- Double reading protocols;
- Centralized quality assurance;
- Target participation ≥70%;
- Expected cost-effectiveness: EUR 3000–8000/QALY.
- Extend screening to ages 45–74;
- Implement systematic screening coverage monitoring;
- Develop strategies to address socioeconomic and geographic disparities;
- Improve participation rates through targeted interventions;
- Expected cost-effectiveness: EUR 4000–10,000/QALY.
- Integrate MRI screening for high-risk populations (BRCA carriers, strong family history);
- Consider DBT implementation in high-volume centers;
- Implement risk-stratified screening protocols incorporating breast density;
- Develop personalized screening intervals based on risk assessment;
- Expected cost-effectiveness: EUR 18,000–35,000/QALY for additional interventions.
4.6.3. Addressing Health Inequities
4.6.4. Temporal Evolution and Technological Advances
- Technological advances: Transition from film to digital mammography, introduction of DBT, and improved image quality reducing false positives;
- Treatment improvements: More effective therapies for screen-detected cancers, improving survival gains;
- Program maturation: Learning curves in program organization, quality assurance systems, and efficient diagnostic pathways;
- Healthcare system optimization: Better integration of screening with diagnostic and treatment services.
4.7. Emerging Technologies and Future Directions
4.7.1. Artificial Intelligence in Screening
4.7.2. Risk-Stratified Screening
4.7.3. Study Strengths and Limitations
- Comprehensive systematic search across five databases following PRISMA guidelines;
- Rigorous quality assessment using CHEERS 2022 checklist;
- Novel three-step economic standardization methodology accounting for healthcare-specific inflation, exchange rate variations, and purchasing power parity;
- Inclusion of diverse European healthcare contexts;
- Transparent application of evidence quality criteria using the modified GRADE framework;
- Integration of clinical effectiveness, economic evidence, and survival outcomes.
- Exclusion of non-English studies may have biased results toward Northern and Western European contexts where English-language publication is more common.
- Gray literature from national HTA agencies in non-English languages was not systematically searched.
- This may have contributed to the geographic imbalance in included studies.
- Included studies varied in which costs were incorporated: some included only direct medical costs, while others included indirect costs (productivity losses, transportation).
- Treatment costs used in models often predate recent expensive targeted therapies approved since 2016, potentially underestimating true costs in contemporary settings.
- Variation in cost perspectives (healthcare system, societal, payer) limits direct comparability.
- Wide variation in model types (Markov cohort models, microsimulation, decision trees), time horizons (10 years to lifetime), and discount rates (0–5%);
- Different assumptions about overdiagnosis rates (ranging from 1% to 30% across studies) and quality-of-life decrements from false positives;
- Limited ability to synthesize across diverse methodologies precluded traditional meta-analysis;
- Sensitivity to model assumptions not always adequately explored in primary studies.
- Limited data on cost-effectiveness stratified by socioeconomic status, ethnicity, or rural/urban location;
- Insufficient evidence on optimal strategies for reaching underserved populations;
- No studies specifically evaluated screening programs in migrant populations despite this being a substantial and growing demographic in Europe;
- Inability to assess whether cost-effectiveness varies for disadvantaged groups.
- Limited data on quality-of-life impacts beyond clinical endpoints;
- Insufficient evidence on psychological harms from false-positive results and overdiagnosis;
- Few studies incorporated patient values and preferences into decision models;
- Overdiagnosis rates remain controversial and poorly quantified in many contexts.
- No included studies explicitly compared screening programs with alternative uses of healthcare resources (e.g., treatment innovations, other cancer screening programs).
- Limited evidence on optimal resource allocation across the full cancer control continuum (prevention, screening, treatment, survivorship care).
- Threshold values for cost-effectiveness vary across countries and may not reflect true opportunity costs.
- Most studies assumed full or optimal participation rates; few modeled actual observed participation.
- Limited evidence on costs and effectiveness of interventions to improve screening uptake.
- Insufficient data on implementation costs and learning curves for new technologies.
- Gap between ideal program performance assumed in models and real-world program effectiveness.
- Rapidly evolving field with limited long-term cost-effectiveness data for AI-assisted screening, abbreviated MRI protocols, and molecular imaging;
- Uncertainty about optimal integration of new technologies into existing pathways;
- Technology costs declining over time may make current estimates outdated quickly.
- Severe underrepresentation of Southern and Eastern European studies (8% combined) despite these regions comprising 40% of the European population;
- Limited generalizability of findings to healthcare systems with different resource constraints and organizational structures;
- Evidence gaps reflect broader patterns in health economics research investment rather than lack of screening program need.
5. Conclusions
5.1. Evidence-Based Recommendations
5.1.1. Strong Recommendations (High Confidence)
- Biennial mammography screening for women aged 50–69 years demonstrates consistent cost-effectiveness (EUR 3000–8000/QALY), falls well below established European HTA thresholds, and should be prioritized in organized population-based screening programs with systematic quality assurance.
5.1.2. Conditional Recommendations (Moderate Confidence)
- 2.
- MRI screening for high-risk populations (BRCA1/2 carriers, strong family history) shows acceptable cost-effectiveness (EUR 18,201–33,534/QALY) and warrants implementation where healthcare resources and infrastructure permit.
- 3.
- Organized screening programs demonstrate superior cost-effectiveness compared to opportunistic approaches across all European regions studied, supporting systematic call–recall systems over physician- or patient-initiated screening [54].
5.1.3. Research Recommendations (Low Confidence)
- 4.
- Digital breast tomosynthesis shows promise but requires further cost-effectiveness evaluation across diverse healthcare contexts before widespread implementation as a replacement for standard digital mammography.
- 5.
- Risk-stratified screening approaches incorporating breast density, genetic risk, and family history require validation across diverse European populations and healthcare systems.
5.2. Applicability and Generalizability
5.3. Research Priorities
Critical Evidence Gaps Requiring Urgent Research
- Geographic representation: Conduct cost-effectiveness analyses in underrepresented European regions, particularly Southern and Eastern Europe, to assess generalizability and inform context-specific implementation strategies.
- Equity and access: Evaluate cost-effectiveness of interventions to improve screening participation among underserved populations, including low-income women, migrants, and rural residents.
- Emerging technologies: Conduct rigorous economic evaluations of AI-assisted screening, abbreviated MRI protocols, and risk-stratified approaches in real-world implementation settings.
- Optimal protocols for special populations: Determine cost-effective screening intervals and modalities for women with dense breasts across diverse European healthcare contexts.
- Methodological standardization: Develop consensus guidelines for conducting and reporting breast cancer screening economic evaluations, including standardized approaches to cost estimation, quality-of-life assessment, and modeling overdiagnosis.
- Implementation science: Evaluate barriers and facilitators to screening program implementation, participation, and quality across different healthcare financing models.
- Whole-pathway optimization: Assess optimal resource allocation across the entire breast cancer control continuum from prevention through survivorship.
- Patient-centered outcomes: Incorporate patient values, preferences, and experiences into economic evaluations and decision-making frameworks.
6. Key Points
- Biennial mammography screening for women aged 50–69 years demonstrates consistent cost-effectiveness (EUR 3000–8000 per quality-adjusted life year (QALY)) across European healthcare systems and should be prioritized in organized screening programs.
- MRI screening for high-risk populations (BRCA carriers, strong family history) shows acceptable cost-effectiveness (EUR 18,201–33,534 per QALY) and warrants implementation where healthcare resources permit.
- Screening women under 50 years shows substantially higher costs (EUR 105,000 per year of life saved) compared to older populations, supporting risk-stratified approaches incorporating breast density assessment.
- Organized population-based screening programs demonstrate superior cost-effectiveness compared to opportunistic screening approaches across all European regions studied.
Future Research Directions
- Eastern and Southern European Evidence: Expansion of cost-effectiveness research in under-represented regions to support evidence-based implementation across all European healthcare contexts.
- Emerging Technologies: Rigorous health economic evaluation of artificial intelligence-assisted screening, contrast-enhanced mammography, and molecular breast imaging as these technologies approach clinical maturity.
- Risk-Stratified Screening Implementation: Large-scale evaluation of personalized screening intervals based on genetic risk, breast density, and family history across diverse European populations.
- Long-term Outcomes: Extended follow-up studies (>20 years) assessing cost-effectiveness under contemporary overdiagnosis estimates [23] and changing treatment paradigms.
- Health Equity Analysis: Investigation of cost-effectiveness across socioeconomic groups, ethnic minorities, and vulnerable populations to inform equitable screening access policies.
- Evidence gaps in Southern and Eastern Europe limit generalizability, requiring contextual adaptation of screening policies rather than uniform implementation across European healthcare systems.
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today (Version 1.1); International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.fr/today (accessed on 20 January 2025).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dyba, T.; Randi, G.; Bray, F.; Martos, C.; Giusti, F.; Nicholson, N.; Gavin, A.; Flego, M.; Neamtiu, L.; Dimitrova, N.; et al. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur. J. Cancer 2021, 157, 308–347. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, F.; Aase, H.S.; Álvarez, M.; Azavedo, E.; Baarslag, H.J.; Balleyguier, C.; Baltzer, P.A.; Beslagic, V.; Bick, U.; Bogdanovic-Stojanovic, D.; et al. Position paper on screening for breast cancer by the European Society of Breast Imaging (EUSOBI) and 30 national breast radiology bodies from Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Israel, Lithuania, Moldova, The Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Spain, Sweden, Switzerland and Turkey. Eur. Radiol. 2017, 27, 2737–2743. [Google Scholar] [CrossRef]
- Marcon, M.; Fuchsjäger, M.H.; Clauser, P.; Mann, R.M. ESR Essentials: Screening for breast cancer—General recommendations by EUSOBI. Eur. Radiol. 2024, 34, 6348–6357. [Google Scholar] [CrossRef]
- Mann, R.M.; Athanasiou, A.; Baltzer, P.A.T.; Camps-Herrero, J.; Clauser, P.; Fallenberg, E.M.; Forrai, G.; Fuchsjäger, M.H.; Helbich, T.H.; Killburn-Toppin, F.; et al. Breast cancer screening in women with extremely dense breasts recommendations of the European Society of Breast Imaging (EUSOBI). Eur. Radiol. 2022, 32, 4036–4045. [Google Scholar] [CrossRef]
- Sardanelli, F.; Fallenberg, E.M.; Clauser, P.; Trimboli, R.M.; Camps-Herrero, J.; Helbich, T.H.; Forrai, G.; European Society of Breast Imaging (EUSOBI), with language review by Europa Donna–The European Breast Cancer Coalition. Mammography: An update of the EUSOBI recommendations on information for women. Insights Imaging 2017, 8, 11–18. [Google Scholar] [CrossRef]
- Canelo-Aybar, C.; Posso, M.; Montero, N.; Solà, I.; Saz-Parkinson, Z.; Duffy, S.W.; Follmann, M.; Gräwingholt, A.; Giorgi Rossi, P.; Alonso-Coello, P. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: A systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br. J. Cancer 2022, 126, 673–688. [Google Scholar] [CrossRef]
- Ponti, A.; Anttila, A.; Ronco, G.; Senore, C.; Basu, P.; Segnan, N.; Tomatis, M.; Žakelj, M.P.; Dillner, J.; Fernan, M.; et al. Cancer Screening in the European Union: Report on the Implementation of the Council Recommendation on Cancer Screening. European Commission 2017. Available online: https://health.ec.europa.eu/document/download/911ecf9b-0ae2-4879-93e6-b750420e9dc0_en (accessed on 20 January 2025).
- Marmot, M.G.; Altman, D.G.; Cameron, D.A.; Dewar, J.A.; Thompson, S.G.; Wilcox, M. The benefits and harms of breast cancer screening: An independent review. Br. J. Cancer 2013, 108, 2205–2240. [Google Scholar] [CrossRef]
- Independent UK Panel on Breast Cancer Screening. The benefits and harms of breast cancer screening: An independent review. Lancet 2012, 380, 1778–1786. [Google Scholar] [CrossRef]
- Duffy, S.W.; Vulkan, D.; Cuckle, H.; Parmar, D.; Sheikh, S.; Smith, R.A.; Evans, A.; Blyuss, O.; Johns, L.; Ellis, I.O.; et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): Final results of a randomised, controlled trial. Lancet Oncol. 2020, 21, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.M.; Wale, C.; Smith, R.; Evans, A.; Cuckle, H.; Duffy, S.W. Effect of mammographic screening from age 40 years on breast cancer mortality in the UK Age trial at 17 years’ follow-up: A randomised controlled trial. Lancet Oncol. 2015, 16, 1123–1132. [Google Scholar] [CrossRef]
- Sankatsing, V.D.V.; Heijnsdijk, E.A.M.; van Luijt, P.A.; van Ravesteyn, N.T.; Fracheboud, J.; de Koning, H.J. Cost-effectiveness of digital mammography screening before the age of 50 in The Netherlands. Int. J. Cancer 2015, 137, 1990–1999. [Google Scholar] [CrossRef]
- van den Broek, J.J.; Schechter, C.B.; van Ravesteyn, N.T.; Janssens, A.C.J.W.; Wolfson, M.C.; Trentham-Dietz, A.; Simard, J.; Easton, D.F.; Mandelblatt, J.S.; Kraft, P.; et al. Personalizing breast cancer screening based on polygenic risk (25) and family history. J. Natl. Cancer Inst. 2021, 113, 434–442. [Google Scholar] [CrossRef]
- Alagoz, O.; Berry, D.A.; de Koning, H.J.; Feuer, E.J.; Lee, S.J.; Plevritis, S.K.; Schechter, C.B.; Stout, N.K.; Trentham-Dietz, A.; Mandelblatt, J.S.; et al. Introduction to the Cancer Intervention and Surveillance Model (27)ing Network (CISNET) Breast Cancer Model (27)s. Med. Decis. Making 2018, 38, 3S–8S. [Google Scholar] [CrossRef]
- Mandelblatt, J.S.; Stout, N.K.; Schechter, C.B.; van den Broek, J.J.; Miglioretti, D.L.; Krapcho, M.; Trentham-Dietz, A.; Munoz, D.; Lee, S.J.; Berry, D.A.; et al. Collaborative Modeling (28,29) of the Benefits and Harms Associated With Different U.S. Breast Cancer Screening Strategies. Ann. Intern. Med. 2016, 164, 215–225. [Google Scholar] [CrossRef]
- van Ravesteyn, N.T.; Miglioretti, D.L.; Stout, N.K.; Lee, S.J.; Schechter, C.B.; Buist, D.S.; Huang, H.; Tosteson, A.N.; Trentham-Dietz, A.; Mandelblatt, J.S.; et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: A comparative modeling (28,29) study of risk. Ann. Intern. Med. 2012, 156, 609–617. [Google Scholar] [CrossRef]
- Trentham-Dietz, A.; Kerlikowske, K.; Stout, N.K.; Miglioretti, D.L.; Schechter, C.B.; Ergun, M.A.; van den Broek, J.J.; Alagoz, O.; Sprague, B.L.; van Ravesteyn, N.T.; et al. Tailoring Breast Cancer Screening Intervals by Breast Density (30) and Risk for Women Aged 50 Years or Older: Collaborative Modeling (28,29) of Screening Outcomes. Ann. Intern. Med. 2016, 165, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Heijnsdijk, E.A.M.; Wever, E.M.; Auvinen, A.; Hugosson, J.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Villers, A.; Páez, A.; Moss, S.M.; et al. Quality-of-life effects of prostate-specific antigen screening. N. Engl. J. Med. 2012, 367, 595–605. [Google Scholar] [CrossRef] [PubMed]
- de Gelder, R.; Heijnsdijk, E.A.M.; van Ravesteyn, N.T.; Fracheboud, J.; Draisma, G.; de Koning, H.J. Interpreting overdiagnosis estimates (32) in population-based mammography screening. Epidemiol. Rev. 2011, 33, 111–121. [Google Scholar] [CrossRef]
- Broeders, M.; Moss, S.; Nyström, L.; Njor, S.; Jonsson, H.; Paap, E.; Massat, N.; Duffy, S.; Lynge, E.; Paci, E.; et al. The impact of mammographic screening on breast cancer mortality in Europe: A review of observational studies. J. Med. Screen. 2012, 19, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Njor, S.H.; Olsen, A.H.; Blichert-Toft, M.; Schwartz, W.; Vejborg, I.; Lynge, E. Overdiagnosis in screening mammography in Denmark: Population based cohort study. BMJ 2013, 346, f1064. [Google Scholar] [CrossRef][Green Version]
- Mühlberger, N.; Sroczynski, G.; Gogollari, A.; Jahn, B.; Pashayan, N.; Sutcliffe, S.; Widschwendter, M.; Stojkov, I.; Siebert, U. Cost effectiveness of breast cancer screening and prevention: A systematic review with a focus on risk-adapted strategies. Eur. J. Health Econ. 2021, 22, 1291–1313. [Google Scholar] [CrossRef] [PubMed]
- Sroczynski, G.; Gogollari, A.; Kuehne, F.; Hallsson, L.R.; Widschwendter, M.; Pashayan, N.; Siebert, U. A Systematic Review of the Cost Effectiveness of Risk-Reducing Salpingo-Oophorectomy and Bilateral Mastectomy in BRCA Mutation Carriers. Cancer Prev. Res. 2020, 13, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Corley, D.A.; Sedki, M.; Ritzwoller, D.P.; Greenlee, R.T.; Karliner, L.; Williams, A.; Hunter, W.G.; Honda, S.A.; Joseph, D.; Zheng, Y.; et al. Systematic Review of the Cost Effectiveness of Breast Cancer Prevention, Screening, and Treatment Interventions. J. Clin. Oncol. 2020, 38, 332–350. [Google Scholar] [CrossRef]
- Gordon, L.G.; Elliott, T.M.; Olsen, C.M.; Pandeya, N.; Whiteman, D.C. Out-of-pocket medical expenses for Queenslanders with a major cancer. Med. J. Aust. 2018, 208, 497. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Middleton, R.; Doré, J.F.; Héry, C.; Zheng, T.; Gavin, A. Advanced breast cancer incidence following population-based mammographic screening. Ann. Oncol. 2011, 22, 1726–1735. [Google Scholar] [CrossRef]
- Zelle, S.G.; Vidaurre, T.; Abugattas, J.E.; Manrique, J.E.; Sarria, G.; Jeronimo, J.; Ganoza, A.; Vallejos, C.; Napo, N.; Venegas, D.; et al. Cost-Effectiveness Analysis of Breast Cancer Control Interventions in Peru. PLoS ONE 2013, 8, e82575. [Google Scholar] [CrossRef][Green Version]
- Hofvind, S.; Ursin, G.; Tretli, S.; Sebuødegård, S.; Møller, B. Breast cancer mortality in participants of the Norwegian Breast Cancer Screening Program. Cancer 2013, 119, 3106–3112. [Google Scholar] [CrossRef]
- Weedon-Fekjær, H.; Romundstad, P.R.; Vatten, L.J. Modern mammography screening and breast cancer mortality: Population study. BMJ 2014, 348, g3701. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Calabrese, M.; Mariscotti, G.; Durando, M.; Tosto, S.; Monetti, F.; Airaldi, S.; Bignotti, B.; Nori, J.; Bagni, A.; et al. Adjunct Screening with Tomosynthesis or Ultrasound in Women with Mammography-Negative Dense Breasts: Interim Report of a Prospective Comparative Trial. J. Clin. Oncol. 2016, 34, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Zhang, Z.; Lehrer, D.; Jong, R.A.; Pisano, E.D.; Barr, R.G.; Böhm-Vélez, M.; Mahoney, M.C.; Evans, W.P., 3rd; Larsen, L.H.; et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012, 307, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Kalager, M.; Zelen, M.; Langmark, F.; Adami, H.O. Effect of screening mammography on breast-cancer mortality in Norway. N. Engl. J. Med. 2010, 363, 1203–1210. [Google Scholar] [CrossRef]
- Otto, S.J.; Fracheboud, J.; Verbeek, A.L.; Boer, R.; Reijerink-Verheij, J.C.; Otten, J.D.; Broeders, M.J.; de Koning, H.J.; National Evaluation Team for Breast Cancer Screening (NETB). Mammography screening and risk of breast cancer death: A population-based case-control study. Cancer Epidemiol. Biomark. Prev. 2012, 21, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. Value Health 2022, 25, 3–9. [Google Scholar] [CrossRef]
- Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes, 4th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Morton, R.; Sayma, M.; Sura, S.M. Economic analysis of the breast cancer screening program used by the UK NHS: Should the program be maintained? Breast Cancer 2017, 9, 217–225. [Google Scholar]
- Schünemann, H.J.; Wiercioch, W.; Brozek, J.; Etxeandia-Ikobaltzeta, I.; Mustafa, R.A.; Manja, V.; Brignardello-Petersen, R.; Neumann, I.; Falavigna, M.; Alhazzani, W.; et al. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J. Clin. Epidemiol. 2017, 81, 101–110. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Sanders, G.D.; Neumann, P.J.; Basu, A.; Brock, D.W.; Feeny, D.; Krahn, M.; Kuntz, K.M.; Meltzer, D.O.; Owens, D.K.; Prosser, L.A.; et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016, 316, 1093–1103. [Google Scholar] [CrossRef]
- Neumann, P.J.; Cohen, J.T.; Weinstein, M.C. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N. Engl. J. Med. 2014, 371, 796–797. [Google Scholar] [CrossRef] [PubMed]
- Marseille, E.; Larson, B.; Kazi, D.S.; Kahn, J.G.; Rosen, S. Thresholds for the cost-effectiveness of interventions: Alternative approaches. Bull. World Health Organ. 2015, 93, 118–124. [Google Scholar] [CrossRef]
- Sardanelli, F.; Magni, V.; Rossini, G.; Kilburn-Toppin, F.; Healy, N.A.; Gilbert, F.J. Breast MRI screening: A comprehensive review of the evidence and practical recommendations from the European Society of Breast Imaging. Insights Imaging 2024, 15, 96. [Google Scholar] [CrossRef]
- Gøtzsche, P.C.; Jørgensen, K.J. Screening for breast cancer with mammography. Cochrane Database Syst. Rev. 2013, 6, CD001877. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.R.; Moorman, P.; Gierisch, J.M.; Havrilesky, L.J.; Grimm, L.J.; Ghate, S.; Davidson, B.; Mongtomery, R.C.; Crowley, M.J.; McCrory, D.C.; et al. Benefits and Harms of Breast Cancer Screening: A Systematic Review. JAMA 2015, 314, 1615–1634, Erratum in JAMA 2016, 315, 1406. https://doi.org/10.1001/jama.2016.3295. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Fu, R.; Cantor, A.; Pappas, M.; Daeges, M.; Humphrey, L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann. Intern. Med. 2016, 164, 244–255. [Google Scholar] [CrossRef]
- Hofvind, S.; Tsuruda, K.; Mangerud, G.; Ertzaas, A.K.; Holen, Å.; Pedersen, K.; Haldorsen, T. The Norwegian Breast Cancer Screening Program, 1996–2016: Celebrating 20 Years of Organised Mammographic Screening. Available online: https://www.fhi.no/contentassets/bd9f68efbc534972a3dd4a31c63190d8/celebrating-20-years-of-organised-mammographic-screening.pdf (accessed on 10 October 2025).
- Puliti, D.; Miccinesi, G.; Manneschi, G.; De Lisi, V.; Federico, M.; Ferretti, S.; Grazzini, G.; Paci, E.; Zappa, M.; IMPACT COHORT Working Group. Does an organised screening programme reduce the inequalities in breast cancer survival? Ann. Oncol. 2012, 23, 319–323. [Google Scholar] [CrossRef]
- Feig, S.A. Cost-effectiveness of mammography, MRI, and ultrasonography for breast cancer screening. Radiol. Clin. N. Am. 2010, 48, 879–891. [Google Scholar] [CrossRef]
- Niell, B.L.; Freer, P.E.; Weinfurtner, R.J.; Arleo, E.K.; Drukteinis, J.S. Screening for Breast Cancer. Radiol. Clin. N. Am. 2017, 55, 1145–1162. [Google Scholar] [CrossRef]
- Perry, N.; Broeders, M.; de Wolf, C.; Törnberg, S.; Holland, R.; von Karsa, L. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition--summary document. Ann. Oncol. 2008, 19, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Benbrahim-Tallaa, L.; Bouvard, V.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Breast-cancer screening--viewpoint of the IARC Working Group. N. Engl. J. Med. 2015, 372, 2353–2358. [Google Scholar] [CrossRef]
- Løberg, M.; Lousdal, M.L.; Bretthauer, M.; Kalager, M. Benefits and harms of mammography screening. Breast Cancer Res. 2015, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Yankaskas, B.C.; Taplin, S.H.; Ichikawa, L.; Geller, B.M.; Rosenberg, R.D.; Carney, P.A.; Kerlikowske, K.; Buist, D.S.; Sickles, E.A.; Ernster, V.L. Association between mammography timing and measures of screening performance in the United States. Radiology 2005, 234, 363–373. [Google Scholar] [CrossRef]
- Plevritis, S.K.; Munoz, D.; Kurian, A.W.; Stout, N.K.; Alagoz, O.; Near, A.M.; Lee, S.J.; van den Broek, J.J.; Huang, X.; Schechter, C.B.; et al. Association of Screening and Treatment with Breast Cancer Mortality by Molecular Subtype in US Women, 2000–2012. JAMA 2018, 319, 154–164. [Google Scholar] [CrossRef]
- Saadatmand, S.; Tilanus-Linthorst, M.M.; Rutgers, E.J.; Hoogerbrugge, N.; Oosterwijk, J.C.; Tollenaar, R.A.; Hooning, M.; Loo, C.E.; Obdeijn, I.M.; Heijnsdijk, E.A.; et al. Cost-effectiveness of screening women with familial risk for breast cancer with magnetic resonance imaging. J. Natl. Cancer Inst. 2013, 105, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Phi, X.A.; Greuter, M.J.W.; Obdeijn, I.M.; Oosterwijk, J.C.; Feenstra, T.L.; Houssami, N.; de Bock, G.H. Should women with a BRCA1/2 mutation aged 60 and older be offered intensified breast cancer screening?—A cost-effectiveness analysis. Breast 2019, 45, 82–88. [Google Scholar] [CrossRef]
- Giordano, L.; von Karsa, L.; Tomatis, M.; Majek, O.; de Wolf, C.; Lancucki, L.; Hofvind, S.; Nyström, L.; Segnan, N.; Ponti, A.; et al. Mammographic screening programmes in Europe: Organization, coverage and participation. J. Med. Screen. 2012, 19, 72–82. [Google Scholar] [CrossRef]
- Skaane, P.; Bandos, A.I.; Gullien, R.; Eben, E.B.; Ekseth, U.; Haakenaasen, U.; Izadi, M.; Jebsen, I.N.; Jahr, G.; Krager, M.; et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013, 267, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ciatto, S.; Houssami, N.; Bernardi, D.; Caumo, F.; Pellegrini, M.; Brunelli, S.; Tuttobene, P.; Bricolo, P.; Fantò, C.; Valentini, M.; et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): A prospective comparison study. Lancet Oncol. 2013, 14, 583–589. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Schrading, S.; Leutner, C.C.; Morakkabati-Spitz, N.; Wardelmann, E.; Fimmers, R.; Kuhn, W.; Schild, H.H. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J. Clin. Oncol. 2005, 23, 8469–8476. [Google Scholar] [CrossRef]
- Saslow, D.; Boetes, C.; Burke, W.; Harms, S.; Leach, M.O.; Lehman, C.D.; Morris, E.; Pisano, E.; Schnall, M.; Sener, S.; et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J. Clin. 2007, 57, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Biller-Andorno, N.; Jüni, P. Abolishing mammography screening programs? A view from the Swiss Medical Board. N. Engl. J. Med. 2014, 370, 1965–1967. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.J.; Gøtzsche, P.C.; Kalager, M.; Zahl, P.H. Breast Cancer Screening in Denmark: A Cohort Study of Tumor Size and Overdiagnosis (36). Ann. Intern. Med. 2017, 166, 313–323. [Google Scholar] [CrossRef]
| Region | Studies (n) | Percentage | Quality Score Range | Sample Size Range |
|---|---|---|---|---|
| North-Western Europe | 12 | 52% | 18–26 | 5000–850,000 |
| Central Europe | 5 | 22% | 17–24 | 8000–450,000 |
| Northern Europe | 2 | 9% | 19–22 | 12,000–180,000 |
| South-Western Europe | 2 | 9% | 16–20 | 15,000–95,000 |
| Southern Europe | 1 | 4% | 18 | 25,000 |
| South-Eastern Europe | 1 | 4% | 19 | 18,000 |
| Total | 23 | 100% | 16–26 | 5000–850,000 |
| Screening Modality | Target Population | Cost-Effectiveness Range | Evidence Quality | Studies (n) | Geographic Coverage |
|---|---|---|---|---|---|
| Mammography | Ages 50–69 years | EUR 3000–8000/QALY | High | 12 | All regions |
| Mammography | Ages < 50 years | EUR 105,000/life-year saved | Moderate | 4 | NW/Central Europe |
| MRI | High-risk populations | EUR 18,201–33,534/QALY | Moderate | 4 | NW/Central Europe |
| MRI | Dense breasts | EUR 37,181/QALY (4-year intervals) | Low | 2 | Northern Europe |
| Digital Breast Tomosynthesis | General population | Limited data available | Low | 3 | Northern Europe |
| Risk-stratified screening | Ages 40–50 years | EUR 36,200/QALY | Low | 2 | NW Europe |
| Country | Program Type | Adherence Rate | Mortality Reduction | Survival Improvement | Cost-Effectiveness |
|---|---|---|---|---|---|
| Austria | Organized | 94.87% | Not reported | 19% increase | EUR 4200/QALY |
| Germany | Organized | 47% | 11% annual reduction | Not reported | EUR 6100/QALY |
| Netherlands | Organized | 70% | 10–16% reduction | Not reported | EUR 3800/QALY |
| Norway | Organized | 74.5–77% | 30% reduction | Not reported | EUR 5200/QALY |
| Switzerland | Mixed | 80% | Not reported | 39.1/100,000 rate | EUR 7400/QALY |
| Greece | Opportunistic | 35% | Not reported | Not reported | EUR 8900/QALY |
| Time Period | Studies (n) | Mean Cost-Effectiveness | Range | Technology Improvements |
|---|---|---|---|---|
| 1990–1999 | 6 | EUR 9500/QALY | EUR 7200–12,800 | Film mammography |
| 2000–2009 | 8 | EUR 6800/QALY | EUR 4500–9200 | Digital mammography introduction |
| 2010–2020 | 9 | EUR 4200/QALY | EUR 3000–6500 | DBT, MRI, improved protocols |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidiropoulou, Z.; Fonseca, V. A Systematic Review of the Cost-Effectiveness of Screening Modalities for Breast Cancer in European Countries. Cancers 2025, 17, 3585. https://doi.org/10.3390/cancers17213585
Sidiropoulou Z, Fonseca V. A Systematic Review of the Cost-Effectiveness of Screening Modalities for Breast Cancer in European Countries. Cancers. 2025; 17(21):3585. https://doi.org/10.3390/cancers17213585
Chicago/Turabian StyleSidiropoulou, Zacharoula, and Vasco Fonseca. 2025. "A Systematic Review of the Cost-Effectiveness of Screening Modalities for Breast Cancer in European Countries" Cancers 17, no. 21: 3585. https://doi.org/10.3390/cancers17213585
APA StyleSidiropoulou, Z., & Fonseca, V. (2025). A Systematic Review of the Cost-Effectiveness of Screening Modalities for Breast Cancer in European Countries. Cancers, 17(21), 3585. https://doi.org/10.3390/cancers17213585





