Effects of Metformin on Cancer Survival Among Men Diagnosed with Advanced Prostate Cancer Treated with Androgen-Deprivation Therapy: Emulating a Target Trial
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specification of the Target Trial
2.1.1. Study Design
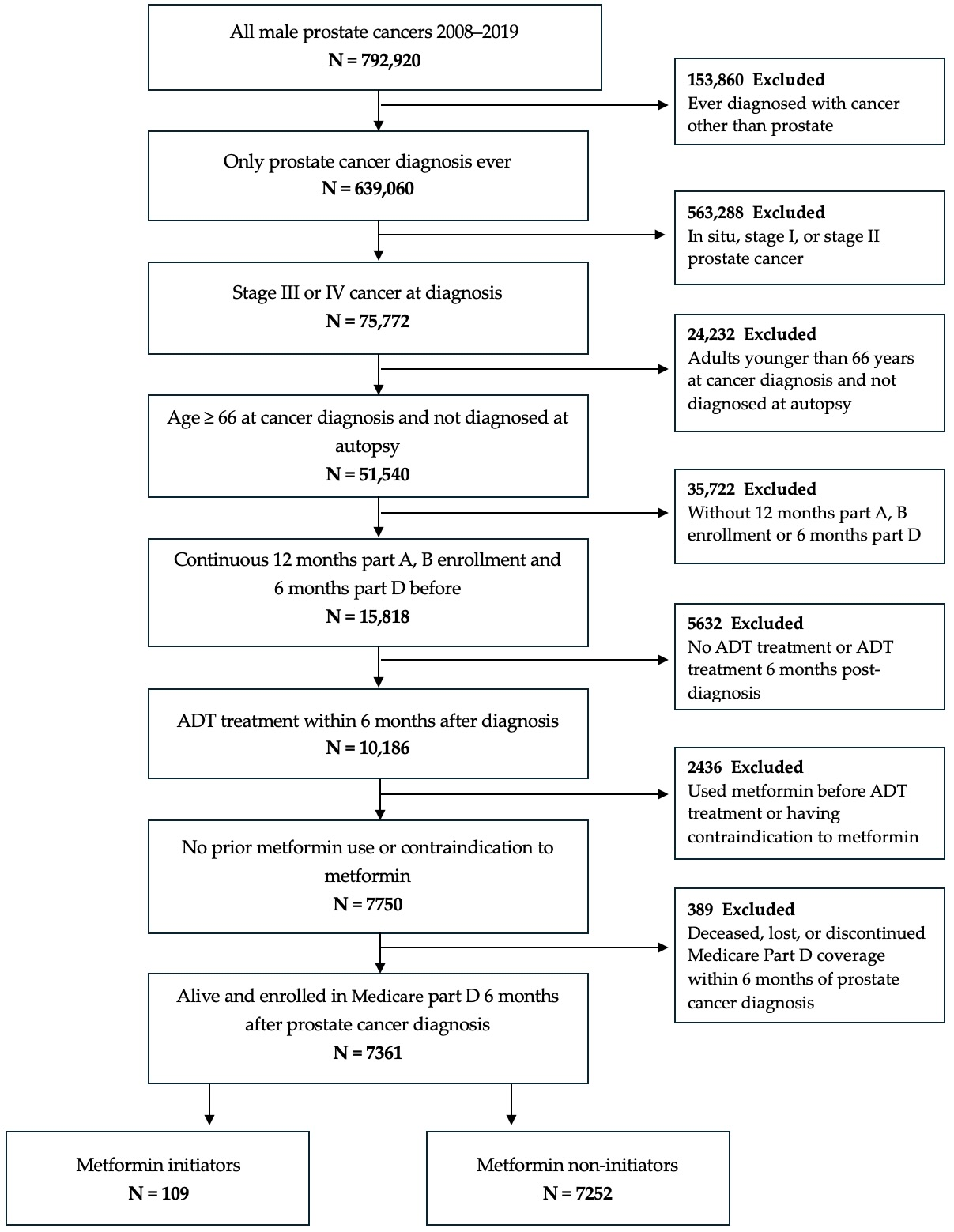
2.1.2. Participants
2.1.3. Procedures
2.2. Emulation of the Target Trial
2.2.1. Data Source
2.2.2. Variables, Endpoints, and Follow-Up
2.2.3. Statistical Analysis
2.2.4. Sensitivity Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADT | Androgen-deprivation therapy |
| AMPK | AMP-activated protein kinase |
| CHF | Congestive heart failure |
| CI | Confidence Interval |
| CRPC | Castrate-resistant prostate cancer |
| CPT | Current Procedural Terminology codes |
| FDA | Federal Drug Administration |
| HR | Hazard Ratio |
| HSPC | Hormone-sensitive prostate cancer |
| HTN | Hypertension |
| ICD | International Classification for Diseases |
| IGF-1 | Insulin/insulin-like growth factor 1 |
| IPW | Inverse probability weights |
| IRB | Institutional Review Board |
| mTOR | Mechanistic target of rapamycin |
| NDC | National Drug Codes |
| NH | Non-Hispanic |
| PCa | Prostate cancer |
| PCP | Primary care physician |
| RCT | Randomized controlled clinical trials |
| SD | Standard deviation |
| SEER | Surveillance, Epidemiology, and End Results |
| SES | Socioeconomic status |
References
- Stanford, J.L.; Stephenson, R.A.; Coyle, L.M.; Cerhan, J.; Correa, R.; Eley, J.W.; Gilliland, F.; Hankey, B.; Kolonel, L.; Kosary, C.; et al. Prostate Cancer Trends 1973–1995, SEER Program, National Cancer Institute; NIH Pub. No. 99-4543; NIH: Bethesda, MD, USA, 1999. Available online: https://seer.cancer.gov/archive/publications/prostate/index.html (accessed on 28 May 2024).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute. Available online: https://seer.cancer.gov/ (accessed on 28 May 2024).
- United States Food and Drug Administration. Hematology/Oncology (Cancer) Approvals and Safety Notifications. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications (accessed on 28 May 2024).
- Dellis, A.; Zagouri, F.; Liontos, M.; Mitropoulos, D.; Bamias, A.; Papatsoris, A.G. Management of advanced prostate cancer: A systematic review of existing guidelines and recommendations. Cancer Treat. Rev. 2019, 73, 54–61. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Schlender, L.; Martinez, Y.V.; Adeniji, C.; Reeves, D.; Faller, B.; Sommerauer, C.; Al Qur’an, T.; Woodham, A.; Kunnamo, I.; Sönnichsen, A.; et al. Efficacy and safety of metformin in the management of type 2 diabetes mellitus in older adults: A systematic review. BMC Geriatr. 2017, 17 (Suppl. S1), 227. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.H.Y.; Suissa, S. Metformin and cancer: Solutions to a real-world evidence failure. Diabetes Care 2023, 46, 904–912. [Google Scholar] [CrossRef]
- Joshua, A.M.; Armstrong, A.; Crumbaker, M.; Scher, H.I.; de Bono, J.; Tombal, B.; Hussain, M.; Sternberg, C.N.; Gillessen, S.; Carles, J.; et al. Statin and metformin use and outcomes in patients with castration-resistant prostate cancer treated with enzalutamide: A meta-analysis of AFFIRM, PREVAIL and PROSPER. Eur. J. Cancer 2022, 170, 285–295. [Google Scholar] [CrossRef]
- Ioakeim-Skoufa, I.; Tobajas-Ramos, N.; Menditto, E.; Aza-Pascual-Salcedo, M.; Gimeno-Miguel, A.; Orlando, V.; González-Rubio, F.; Fanlo-Villacampa, A.; Lasala-Aza, C.; Ostasz, E.; et al. Drug repurposing in oncology: A systematic review of randomized controlled clinical trials. Cancers 2023, 15, 2972. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xie, S.H.; Santoni, G.; Lagergren, J. Metformin use and risk of gastric adenocarcinoma in a Swedish population-based cohort study. Br. J. Cancer 2019, 121, 877–882. [Google Scholar] [CrossRef]
- Dowling, R.J.; Niraula, S.; Stambolic, V.; Goodwin, P.J. Metformin in cancer: Translational challenges. J. Mol. Endocrinol. 2012, 48, R31–R43. [Google Scholar] [CrossRef]
- Ben Sahra, I.; Laurent, K.; Loubat, A.; Giorgetti-Peraldi, S.; Colosetti, P.; Auberger, P.; Tanti, J.F.; Le Marchand-Brustel, Y.; Bost, F. The antidiabetic drug metformin exerts an antitumoral effect through a decrease of cyclin D1 level. Oncogene 2008, 27, 3576–3586. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.A.; Liou, J.I.; Cryns, V.L.; Downs, T.M.; Abel, E.J.; Jarrard, D.F. Metformin use is associated with improved survival for patients with advanced prostate cancer on androgen deprivation therapy. J. Urol. 2018, 200, 1256–1263. [Google Scholar] [CrossRef]
- Coyle, C.; Cafferty, F.H.; Vale, C.; Langley, R.E. Metformin as an adjuvant treatment for cancer: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 2184–2195. [Google Scholar] [CrossRef]
- He, X.X.; Tu, S.M.; Lee, M.H.; Yeung, S.J. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann. Oncol. 2011, 22, 2640–2645. [Google Scholar] [CrossRef]
- Farmer, R.E.; Ford, D.; Mathur, R.; Chaturvedi, N.; Kaplan, R.; Smeeth, L.; Bhaskaran, K. Metformin use and risk of cancer in patients with type 2 diabetes: A cohort study using inverse probability weighting. Int. J. Epidemiol. 2019, 48, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.S.; Agay, N.; Farmer, R.; Murad, H.; Olmer, L.; Dankner, R. Metformin treatment among men with diabetes and the risk of prostate cancer: A population-based historical cohort study. Am. J. Epidemiol. 2022, 191, 626–635. [Google Scholar] [CrossRef]
- Alghandour, R.; Ebrahim, M.A.; Elshal, A.M.; Ghobrial, F.; Elzaafarany, M.; Elbaiomy, M.A. Repurposing metformin as an anticancer drug: Randomized controlled trial in advanced prostate cancer (MANSMED). Urol. Oncol. 2021, 39, 831.e1–831.e10. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, J.H.; Jang, H.J.; Lee, J. The addition of metformin to systemic anticancer therapy in advanced or metastatic cancers: A meta-analysis of randomized controlled trials. Int. J. Med. Sci. 2020, 17, 2551–2560. [Google Scholar] [CrossRef]
- Franciosi, M.; Lucisano, G.; Lapice, E.; Strippoli, G.F.; Pellegrini, F.; Nicolucci, A. Metformin therapy and risk of cancer in patients with type 2 diabetes: Systematic review. PLoS ONE 2013, 8, e71583. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Liu, Y.; Platz, E.A.; Stampfer, M.J.; Willett, W.C. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int. J. Cancer 2007, 121, 1571–1578. [Google Scholar] [CrossRef]
- Dickerman, B.A.; García-Albéniz, X.; Logan, R.W.; Denaxas, S.; Hernán, M.A. Evaluating metformin strategies for cancer prevention: A target trial emulation using electronic health records. Epidemiology 2023, 34, 690–699. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.J.; Hellberg, K.; Turner, M.; Talbott, G.; Kolar, M.J.; Ross, D.S.; Hoxhaj, G.; Saghatelian, A.; Shaw, R.J.; Manning, B.D. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017, 25, 463–471. [Google Scholar] [CrossRef]
- Rozengurt, E.; Sinnett-Smith, J.; Kisfalvi, K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling: A novel target for metformin in pancreatic cancer. Clin. Cancer Res. 2010, 16, 2505–2511. [Google Scholar] [CrossRef]
- Cashin, A.G.; Hansford, H.J.; Hernán, M.A.; Swanson, S.A.; Lee, H.; Jones, M.D.; Dahabreh, I.J.; Dickerman, B.A.; Egger, M.; Garcia-Albeniz, X.; et al. Transparent Reporting of Observational Studies Emulating a Target Trial—The TARGET Statement. JAMA 2025, 334, 1084–1093. [Google Scholar] [CrossRef]
- SEER Data & Software. U.S. Datasets. U.S. Mortality Data, 1969–2023. Available online: https://seer.cancer.gov/mortality (accessed on 5 October 2025).
- Warren, J.L.; Klabunde, C.N.; Schrag, D.; Bach, P.B.; Riley, G.F. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med. Care 2002, 40 (Suppl. S8), IV-3–IV-18. [Google Scholar] [CrossRef]
- SEER Program. About the SEER Program. Surveillance, Epidemiology, and End Results (SEER) Program 2023. Available online: https://seer.cancer.gov/about/overview.html (accessed on 1 July 2024).
- Halabi, S.; Roy, A.; Rydzewska, L.; Guo, S.; Godolphin, P.; Hussain, M.; Tangen, C.; Thompson, I.; Xie, W.; Carducci, M.A.; et al. Radiographic progression-free survival and clinical progression-free survival as potential surrogates for overall survival in men with metastatic hormone-sensitive prostate cancer. J. Clin. Oncol. 2024, 42, 1044–1054. [Google Scholar] [CrossRef]
- National Cancer Institute. Hormone Therapy for Prostate Cancer. Available online: https://www.cancer.gov/types/prostate/prostate-hormone-therapy-fact-sheet (accessed on 1 August 2024).
- Hernán, M.A.; Sauer, B.C.; Hernández-Díaz, S.; Platt, R.; Shrier, I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J. Clin. Epidemiol. 2016, 79, 70–75. [Google Scholar] [CrossRef]
- Lapini, A.; Caffo, O.; Pappagallo, G.; Iacovelli, R.; D’Angelillo, R.M.; Vavassori, V.; Ceccarelli, R.; Bracarda, S.; Jereczek-Fossa, B.A.; Da Pozzo, L.; et al. Monitoring patients with metastatic hormone-sensitive and metastatic castration-resistant prostate cancer: A multidisciplinary consensus document. Cancers 2019, 11, 1908. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.A.; Hernández-Díaz, S.; Werler, M.M.; Mitchell, A.A. Causal knowledge as a prerequisite for confounding evaluation: An application to birth defects epidemiology. Am. J. Epidemiol. 2002, 155, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.S.; Kim, H.; Polychronopoulou, E.; Torres-Sanchez, L.E.; Villasante-Tezanos, A.; Baillargeon, J.; Canfield, S.; Kuo, Y.F. Use of weight loss medications in relation with prostate, colorectal and male breast cancers among older men: SEER-Medicare 2007–2015. J. Cancer Res. Clin. Oncol. 2023, 149, 8255–8265. [Google Scholar] [CrossRef] [PubMed]
| Target Trial Specification | Emulating Target Trial | |
|---|---|---|
| Causal question | Does metformin initiation, compared to no initiation, affect the risk of all-cause and prostate cancer (PCa)-specific mortality in patients with an advanced PCa diagnosis who have been treated with androgen-deprivation therapy (ADT)? | Same as for the target trial. |
| Eligibility criteria |
| Same as for the target trial. 1-year prior health information availability was determined by having continuous Medicare parts A and B enrollment in the year before. No prior metformin used required at least 6 months of Medicare part D enrollment prior to diagnosis. All variables were identified using National Drug Codes (NDC), Current Procedural Terminology (CPT) codes., and International Classification for Diseases (ICD) versions 9 and 10 codes. |
| Treatment strategies |
| Same as for the target trial. Metformin was identified by NDC. |
| Treatment assignment | Individuals are randomly assigned to a treatment strategy at baseline and are aware of the strategy to which they have been assigned. | Because randomization is not feasible in observational settings, individuals are classified to a strategy based on their metformin prescription data in the 6 months after diagnosis. |
| Outcomes | All-cause and PCa-specific mortality, occurring within 3 years from diagnosis. | Same as for the target trial. |
| Follow-up initiation | Starts on the day of treatment assignment (baseline). | Follow-up begins at the baseline date (time zero), defined as the date in which all eligibility criteria are met (both ADT and metformin have initiated for treatment strategy a, or just ADT for treatment strategy b). |
| Follow-up endpoint | The earliest occurrence of death, loss of follow-up, 3 years after diagnosis, or at the administrative end date of follow-up. | The end of follow-up is defined similarly as the earliest occurrence of death, loss of follow up, 3 years after diagnosis, or at the administrative end date of follow-up, which is 31 December 2020 |
| Causal contrasts | Intention-to-treat and per-protocol effects. | The emulation estimates the observational analog of the intention-to-treat and per-protocol effects. The resulting association reflects the hazard ratio for all-cause and PCa-specific death. |
| Statistical analysis | Descriptive statistics. Hazard ratios (HRs) and their 95% confidence intervals (CIs) for the risk of death are estimated using Cox proportional hazards models for intention-to-treat and per-protocol effects. | Descriptive statistics. HRs with 95% CIs for death in the intention-to-treat analysis were estimated using Cox proportional hazards models, applying inverse probability weighting (IPW) to adjust for baseline factors associated with treatment probability. For the per-protocol analysis, HRs with 95% CIs for death were estimated using Cox proportional hazards models, censoring individuals at the time they deviated from their assigned treatment strategy, and applying IPW to adjust for both baseline and time-varying factors related to treatment adherence. |
| Characteristics | Category | Non-Initiators n = 7252 (%) | Metformin Initiators n = 109 (%) | p-Value |
|---|---|---|---|---|
| Age, mean (SD) | 75.4 (6.8) | 74.3 (6.4) | 0.0964 | |
| Race/ethnicity | Hispanic | 512 (7.06) | 13 (11.93) | 0.0074 * |
| Non-Hispanic Black | 608 (8.38) | 17 (15.6) | ||
| Non-Hispanic White | 5655 (77.98) | >68 (>66.97) | ||
| Other | 477 (6.58) | <11 (<5.5) ** | ||
| Marital status | Divorced/Widowed | 1149 (15.84) | 11 (10.09) | 0.4403 |
| Married | 4749 (65.49) | >73 (>69.72) | ||
| Never Married | 843 (11.62) | 14 (12.84) | ||
| Unknown | 511 (7.05) | <11 (<7.34) ** | ||
| Percent of adults with less than high school education | 1st quartile | 1933 (26.65) | 29 (26.61) | 0.0657 |
| 2nd quartile | 1748 (24.1) | 25 (22.94) | ||
| 3rd quartile | 1627 (22.44) | 24 (22.02) | ||
| 4th quartile | 1359 (18.74) | >21 (>26.61) | ||
| Unknown | 585 (8.07) | <11 (<1.83) ** | ||
| Percent of households below poverty | 1st quartile | 1854 (25.57) | 25 (22.94) | 0.0744 |
| 2nd quartile | 1727 (23.81) | >23 (>29.36) | ||
| 3rd quartile | 1603 (22.1) | 22 (20.18) | ||
| 4th quartile | 1483 (20.45) | 28 (25.69) | ||
| Unknown | 585 (8.07) | <11 (<1.83) ** | ||
| Rural/urban residence | Metro | >5957 (>82.28) | >78 (>79.82) | 0.8628 |
| Rural | 162 (2.23) | <11 (<1.83) ** | ||
| Urban | 1122 (15.47) | 20 (18.35) | ||
| Unknown | <11 (<11) ** | 0 (0) | ||
| Charlson Comorbidity index | 0 | 4433 (61.13) | 47 (43.12) | 0.0011 * |
| 1 | 1487 (20.5) | 33 (30.28) | ||
| 2 | 725 (10) | 13 (11.93) | ||
| 3 or more | 607 (8.37) | 16 (14.68) | ||
| Diabetes | No | 6483 (89.4) | 59 (54.13) | 0.0001 * |
| Yes | 769 (10.6) | 50 (45.87) | ||
| CHF | No | 6998 (96.5) | >98 (>95.41) | 0.5418 |
| Yes | 254 (3.5) | <11 (<4.59) ** | ||
| Cerebrovascular disease | No | 6770 (93.35) | >98 (>93.58) | 0.9256 |
| Yes | 482 (6.65) | <11(6.42) ** | ||
| Hypertension | No | 2370 (32.68) | 31 (28.44) | 0.3486 |
| Yes | 4882 (67.32) | 78 (71.56) | ||
| Depression | No | 6690 (92.25) | 98 (89.91) | 0.3650 |
| Yes | 562 (7.75) | 11 (10.09) | ||
| Number of PCP visits in the year before diagnosis, mean (SD) | 4.9 (5.5) | 5.0 (5.7) | 0.6166 | |
| All-cause mortality at any time | No | 4200 (57.92) | 57 (52.29) | 0.2381 |
| Yes | 3052 (42.08) | 52 (47.71) | ||
| PCa-specific mortality at any time | No | 5333 (73.54) | 73 (66.97) | 0.1234 |
| Yes | 1919 (26.46) | 36 (33.03) |
| Metformin Initiators vs. Non-Initiators | ||
|---|---|---|
| Intent-to-Treat | Hazard Ratio (HR) | 95% Confidence Interval (CI) |
| All-cause mortality | 1.389 | 0.987–1.955 |
| PCa-specific mortality | 0.997 | 0.636–1.556 |
| Per-Protocol A | Hazard Ratio (HR) | 95% Confidence Interval (CI) |
| All-cause mortality | 1.068 | 0.688–1.660 |
| PCa-specific mortality | 1.417 | 0.868–2.321 |
| Metformin Initiators vs. Non-Initiators | ||
|---|---|---|
| Per-Protocol A | Hazard Ratio (HR) | 95% Confidence Interval (CI) |
| All-cause mortality | 1.038 | 0.663–1.625 |
| PCa-specific mortality | 1.379 | 0.835–2.276 |
| Per-Protocol B | Hazard Ratio (HR) | 95% Confidence Interval (CI) |
| All-cause mortality | 1.229 | 0.852–1.773 |
| PCa-specific mortality | 1.401 | 0.923–2.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, D.S.; Polychronopoulou, E.; Abdelgadir, O.; Greenberg, R.; Cowell, L.G.; Messiah, S.E.; Kuo, Y.-F. Effects of Metformin on Cancer Survival Among Men Diagnosed with Advanced Prostate Cancer Treated with Androgen-Deprivation Therapy: Emulating a Target Trial. Cancers 2025, 17, 3579. https://doi.org/10.3390/cancers17213579
Lopez DS, Polychronopoulou E, Abdelgadir O, Greenberg R, Cowell LG, Messiah SE, Kuo Y-F. Effects of Metformin on Cancer Survival Among Men Diagnosed with Advanced Prostate Cancer Treated with Androgen-Deprivation Therapy: Emulating a Target Trial. Cancers. 2025; 17(21):3579. https://doi.org/10.3390/cancers17213579
Chicago/Turabian StyleLopez, David S., Efstathia Polychronopoulou, Omer Abdelgadir, Raymond Greenberg, Lindsay G. Cowell, Sarah E. Messiah, and Yong-Fang Kuo. 2025. "Effects of Metformin on Cancer Survival Among Men Diagnosed with Advanced Prostate Cancer Treated with Androgen-Deprivation Therapy: Emulating a Target Trial" Cancers 17, no. 21: 3579. https://doi.org/10.3390/cancers17213579
APA StyleLopez, D. S., Polychronopoulou, E., Abdelgadir, O., Greenberg, R., Cowell, L. G., Messiah, S. E., & Kuo, Y.-F. (2025). Effects of Metformin on Cancer Survival Among Men Diagnosed with Advanced Prostate Cancer Treated with Androgen-Deprivation Therapy: Emulating a Target Trial. Cancers, 17(21), 3579. https://doi.org/10.3390/cancers17213579







