Evaluation of the Ability to Predict Subsequent Metastasis of Early Oral Squamous Cell Carcinoma Using PET Radiomics Machine Learning Models
Simple Summary
Abstract
1. Introduction
1.1. Background
1.2. Literature Survey
1.3. The Purpose of This Study
1.4. Sections of This Article
2. Materials and Methods
2.1. Ethical Approval
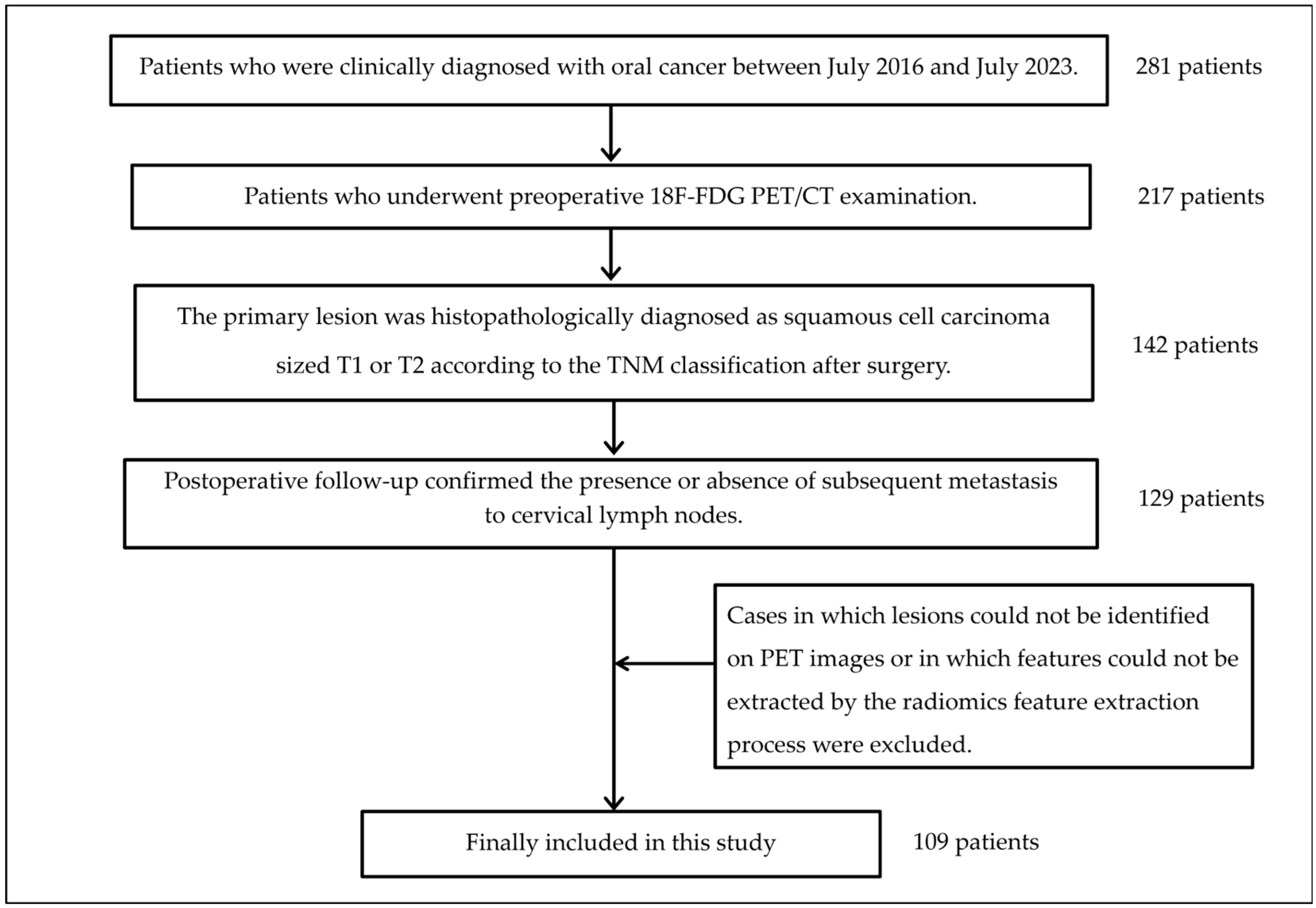
2.2. Subjects
2.3. Image Acquisition
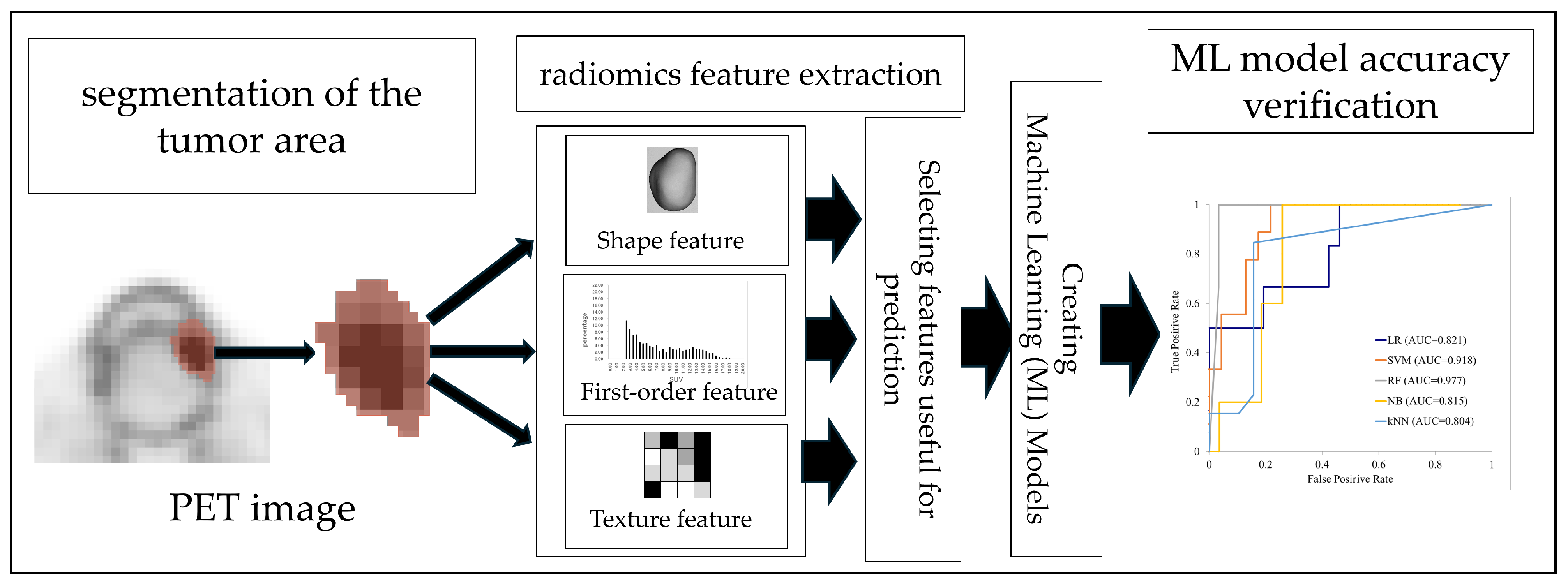
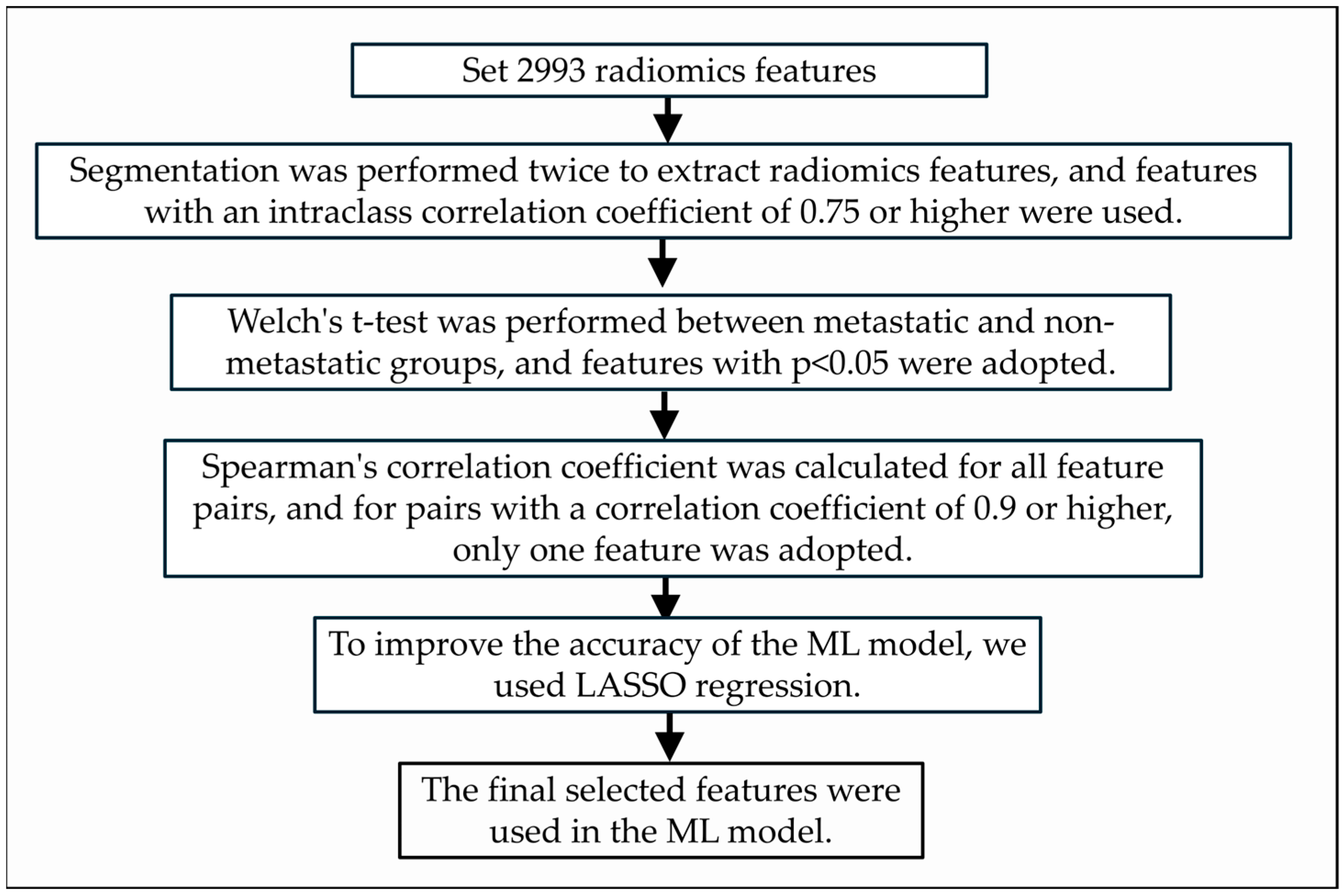
2.4. Radiomics Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OSCC | Oral squamous cell carcinoma |
| PET | Positron emission tomography |
| ML | Machine learning |
| GLCM | Gray level co-occurrence matrix |
| GLDM | Gray level dependence matrix |
| GLRLM | Gray level run length matrix |
| GLSZM | Gray level size zone matrix |
| NGTDM | Neighboring gray tone difference matrix |
| LR | Logistic regression |
| SVM | Support vector machine |
| RF | Random forest |
| NB | Naive Bayes |
| KNN | K-nearest neighbor |
| AUC | Linear dichroism |
References
- World Health Organization. International Agency for Research on Cancer. Cancer Factsheets. Available online: https://gco.iarc.who.int/today/en/fact-sheets-cancers (accessed on 29 July 2025).
- Blatt, S.; Krüger, M.; Sagheb, K.; Barth, M.; Kämmerer, P.W.; Al-Nawas, B.; Sagheb, K. Tumor Recurrence and Follow-Up Intervals in Oral Squamous Cell Carcinoma. J. Clin. Med. 2022, 23, 7061. [Google Scholar] [CrossRef]
- Al-Moraissi, E.A.; Alkhutari, A.S.; de Bree, R.; Kaur, A.; Al-Tairi, N.H.; Pérez-Sayáns, M. Management of clinically node-negative early-stage oral cancer: Network meta-analysis of randomized clinical trials. Int. J. Oral. Maxillofac. Surg. 2024, 53, 179–190. [Google Scholar] [CrossRef]
- Pasha, M.A.; Marcus, C.; Fakhry, C.; Kang, H.; Kiess, A.P.; Subramaniam, R.M. FDG PET/CT for Management and Assessing Outcomes of Squamous Cell Cancer of the Oral Cavity. AJR Am. J. Roentgenol. 2015, 205, W150–W161. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.W.L.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The process and the challenges. Magn. Reson. Imaging 2012, 30, 1234–1248. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Catalano, G.; Alaimo, L.; Chatzipanagiotou, O.P.; Ruzzenente, A.; Aucejo, F.; Marques, H.P.; Lam, V.; Hugh, T.; Bhimani, N.; Maithel, S.K.; et al. Machine learning prediction of early recurrence after surgery for gallbladder cancer. Br. J. Surg. 2024, 111, znae297. [Google Scholar] [CrossRef]
- Alabi, R.O.; Youssef, O.; Pirinen, M.; Elmusrati, M.; Mäkitie, A.A.; Leivo, I.; Almangush, A. Machine learning in oral squamous cell carcinoma: Current status, clinical concerns and prospects for future-A systematic review. Artif. Intell. Med. 2021, 115, 102060. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Lee, N.P.; Adeoye, J.; Thomson, P.; Choi, S.W. Machine learning and treatment outcome prediction for oral cancer. J. Oral. Pathol. Med. 2020, 49, 977–985. [Google Scholar] [CrossRef]
- Adeoye, J.; Hui, L.; Koohi-Moghadam, M.; Tan, J.Y.; Choi, S.W.; Thomson, P. Comparison of time-to-event machine learning models in predicting oral cavity cancer prognosis. Int. J. Med. Inform. 2022, 157, 104635. [Google Scholar] [CrossRef]
- Konishi, M.; Kakimoto, N. Radiomics analysis of intraoral ultrasound images for prediction of late cervical lymph node metastasis in patients with tongue cancer. Head Neck 2023, 45, 2619–2626. [Google Scholar] [CrossRef]
- Yuan, Y.; Ren, J.; Tao, X. Machine learning-based MRI texture analysis to predict occult lymph node metastasis in early-stage oral tongue squamous cell carcinoma. Eur. Radiol. 2021, 31, 6429–6437. [Google Scholar] [CrossRef]
- Kubo, K.; Kawahara, D.; Murakami, Y.; Takeuchi, Y.; Katsuta, T.; Imano, N.; Nishibuchi, I.; Saito, A.; Konishi, M.; Kakimoto, N.; et al. Development of a radiomics and machine learning model for predicting occult cervical lymph node metastasis in patients with tongue cancer. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2022, 134, 93–101. [Google Scholar] [CrossRef]
- Wang, F.; Tan, R.; Feng, K.; Hu, J.; Zhuang, Z.; Wang, C.; Hou, J.; Liu, X. Magnetic Resonance Imaging-Based Radiomics Features Associated with Depth of Invasion Predicted Lymph Node Metastasis and Prognosis in Tongue Cancer. J. Magn. Reson. Imaging 2022, 56, 196–209. [Google Scholar] [CrossRef]
- Lin, N.C.; Hsu, J.T.; Chen, M.Y.C.; Tsai, K.Y. Maximum standardised uptake value is prognostic in patients with early-stage squamous cell carcinoma of the tongue. Br. J. Oral. Maxillofac. Surg. 2022, 60, 1209–1215. [Google Scholar] [CrossRef]
- Tellman, R.S.; Donders, D.N.V.; Arens, A.I.J.; Boeve, K.; Brouwers, A.H.; Eerenstein, S.E.J.; van Egmond, S.L.; Nulent, T.J.W.K.; Klop, W.M.C.; Lacko, M.; et al. [18F]FDG PET/CT to reduce the need for sentinel lymph node biopsy in early-stage oral cancer: PETN0-study protocol. PLoS ONE 2025, 20, e0325032. [Google Scholar] [CrossRef]
- Minamitake, A.; Murakami, R.; Shiraishi, S.; Yoshida, R.; Sakata, J.; Hirosue, A.; Kawahara, K.; Yamana, K.; Nakayama, H.; Kitajima, M.; et al. Laterality on FDG-PET/CT in clinically node-negative early-stage oral squamous cell carcinoma: A retrospective analysis of patients with late neck metastasis. Oral Radiol. 2022, 38, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, T.; Haga, A.; Kudoh, K.; Takahashi, A.; Sasaki, M.; Kudo, Y.; Ikushima, H.; Miyamoto, Y. Radiomics analysis of [18F]-fluoro-2-deoxyglucose positron emission tomography for the prediction of cervical lymph node metastasis in tongue squamous cell carcinoma. Oral Radiol. 2023, 39, 41–50. [Google Scholar] [CrossRef]
- Pfaehler, E.; Schindele, A.; Dierks, A.; Busse, C.; Brumberg, J.; Kübler, A.C.; Buck, A.K.; Linz, C.; Lapa, C.; Brands, R.C.; et al. Value of PET radiomic features for diagnosis and reccurence prediction of newly diagnosed oral squamous cell carcinoma. Sci. Rep. 2025, 20, 17475. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; He, X.; Huang, C.; Li, W.; Li, H.; Huang, C.; Hu, C. Magnetic resonance imaging-based radiomics and deep learning models for predicting lymph node metastasis of squamous cell carcinoma of the tongue. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2024, 138, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, S.; Li, J.; Feng, C.; Zhu, L.; Li, J.; Lin, L.; Lv, X.; Su, K.; Lao, X.; et al. Diagnosis of lymph node metastasis in oral squamous cell carcinoma by an MRI-based deep learning model. Oral Oncol. 2025, 161, 107165. [Google Scholar] [CrossRef]
- Finzel, B. Current methods in explainable artificial intelligence and future prospects for integrative physiology. Pflugers Arch. 2025, 477, 513–529. [Google Scholar] [CrossRef]
- Cheng, N.M.; Fang, Y.H.D.; Lee, L.Y.; Chang, J.T.C.; Tsan, D.L.; Ng, S.H.; Wang, H.M.; Liao, C.T.; Yang, L.Y.; Hsu, C.H.; et al. Zone-size nonuniformity of 18F-FDG PET regional textural features predicts survival in patients with oropharyngeal cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 419–428. [Google Scholar] [CrossRef]
- Chung, M.K.; Jeong, H.S.; Son, Y.I.; So, Y.K.; Park, G.Y.; Choi, J.Y.; Hyun, S.H.; Kim, H.J.; Ko, Y.H.; Baek, D.H. Metabolic tumor volumes by [18F]-fluorodeoxyglucose PET/CT correlate with occult metastasis in oral squamous cell carcinoma of the tongue. Ann. Surg. Oncol. 2009, 16, 3111–3117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Shi, A.; Ding, X.; Wang, J. The value of a nomogram based on 18F-FDG PET/CT metabolic parameters and metabolic heterogeneity in predicting distant metastasis in gastric cancer. Jpn. J. Clin. Oncol. 2025, 55, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Pahk, K.; Chung, J.H.; Yi, E.; Kim, S.; Lee, S.H. Metabolic tumor heterogeneity analysis by F-18 FDG PET/CT predicts mediastinal lymph node metastasis in non-small cell lung cancer patients with clinically suspected N2. Eur. J. Radiol. 2018, 106, 145–149. [Google Scholar] [CrossRef]
- Cheng, N.M.; Fang, Y.H.D.; Tsan, D.L.; Lee, L.Y.; Chang, J.T.C.; Wang, H.W.; Ng, S.H.; Liao, C.T.; Yang, L.Y.; Yen, T.C. Heterogeneity and irregularity of pretreatment 18F-fluorodeoxyglucose positron emission tomography improved prognostic stratification of p16-negative high-risk squamous cell carcinoma of the oropharynx. Oral Oncol. 2018, 78, 156–162. [Google Scholar] [CrossRef]
- Cheng, N.M.; Hsieh, C.E.; Liao, C.T.; Ng, S.H.; Wang, H.W.; Fang, Y.H.D.; Chou, W.C.; Lin, C.Y.; Yen, T.C. Prognostic Value of Tumor Heterogeneity and SUVmax of Pretreatment 18F-FDG PET/CT for Salivary Gland Carcinoma With High-Risk Histology. Clin. Nucl. Med. 2019, 44, 351–358. [Google Scholar] [CrossRef]
- Yamakawa, N.; Nakayama, Y.; Ueda, N.; Yagyuu, T.; Tamaki, S.; Kirita, T. Volume-based 18F-fluorodeoxyglucose positron emission tomography/computed tomography parameters correlate with delayed neck metastasis in clinical early-stage oral squamous cell carcinoma. Oral Radiol. 2023, 39, 668–682. [Google Scholar] [CrossRef]
- Lim, R.S.M.; Ramdave, S.; Beech, P.; Billah, B.; Karim, N.; Smith, J.A.; Safdar, A.; Sigston, E. Utility of SUVmax on 18 F-FDG PET in detecting cervical nodal metastases. Cancer Imaging 2016, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Alqutub, A.; Alqutub, A.; Bakhshwin, A.; Mofti, Z.; Alqutub, S.; Alkhamesi, A.A.; Nujoom, M.A.; Rammal, A.; Merdad, M.; Marzouki, H.Z. Histopathological predictors of lymph node metastasis in oral cavity squamous cell carcinoma: A systematic review and meta-analysis. Front. Oncol. 2024, 14, 1401211. [Google Scholar] [CrossRef]
- Bjerkli, I.H.; Laurvik, H.; Nginamau, E.S.; Søland, T.M.; Costea, D.; Uhlin-Hansen, H.H.L.; Hadler-Olsen, E.; Steigen, S.E. Tumor budding score predicts lymph node status in oral tongue squamous cell carcinoma and should be included in the pathology report. PLoS ONE 2020, 15, e0239783. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Bansal, V.; Malik, V.; Bhagat, R.; Punia, R.S.; Handa, U.; Gupta, A.; Dass, A. Tumor Budding and Worse Pattern of Invasion Can Predict Nodal Metastasis in Oral Cancers and Associated With Poor Survival in Early-Stage Tumors. Ear Nose Throat J. 2019, 98, E112–E119. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Ishida, M.; Ueno, Y.; Fujisawa, T.; Iwai, H.; Tsuta, K. Novel pathological predictive factors for extranodal extension in oral squamous cell carcinoma: A retrospective cohort study based on tumor budding, desmoplastic reaction, tumor-infiltrating lymphocytes, and depth of invasion. BMC Cancer 2022, 22, 402. [Google Scholar] [CrossRef]
- Mittlmeier, L.M.; Brendel, M.; Beyer, L.; Albert, N.L.; Todica, A.; Zacherl, M.J.; Wenter, V.; Herlemann, A.; Kretschmer, A.; Ledderose, S.T.; et al. Feasibility of Different Tumor Delineation Approaches for 18F-PSMA-1007 PET/CT Imaging in Prostate Cancer Patients. Front. Oncol. 2021, 11, 663631. [Google Scholar] [CrossRef]
- Yuan, Z.; Ai, S.; He, Q.; Wu, K.; Yang, M.; Zheng, K.; He, Y.; Tang, X.; Liu, Y.; Wu, Z.; et al. Intratumoral and peritumoral habitat radiomics of MRI predicts pathologic complete response to neoadjuvant chemoimmunotherapy in oral squamous cell carcinoma. Int. J. Surg. 2025, 111, 6232–6244. [Google Scholar] [CrossRef] [PubMed]

| Late Cervical Lymph Node Metastasis | Total (n = 109) | ||
|---|---|---|---|
| Absent (n = 78) | Present (n = 31) | ||
| Average age | 68.9 | 68.6 | 68.8 |
| Sex | |||
| Male | 42 | 18 | 60 |
| Female | 36 | 13 | 49 |
| Site of Primary tumor | |||
| Tongue | 44 | 18 | 62 |
| Floor of oral mouth | 5 | 2 | 7 |
| Gingiva of maxilla | 6 | 8 | 14 |
| Gingiva of mandible | 11 | 2 | 13 |
| Buccal mucosa | 11 | 1 | 12 |
| Palate | 0 | 0 | 0 |
| Lip | 1 | 0 | 1 |
| pT classification | |||
| pT1 | 42 | 16 | 58 |
| pT2 | 36 | 15 | 51 |
| Feature | N = 148 |
|---|---|
| Feature type | |
| Shape feature | 1 |
| First-order feature | 2 |
| Texture feature | 145 |
| Texture features by matrix | |
| GLCM | 48 |
| GLDM | 37 |
| GLRLM | 11 |
| GLSZM | 33 |
| NGTDM | 16 |
| Image | |
| Original image | 61 |
| Wavelet HHH image | 37 |
| Wavelet LLL image | 49 |
| Bins | |
| 0.01–0.05 | 34 |
| 0.1–0.5 | 49 |
| 1–5 | 55 |
| 10 | 9 |
| Feature | Coefficient |
|---|---|
| bins 0.03 original GLCM MCC | 0.00592462 |
| bins 1 original GLSZM Small Area Emphasis | 0.00803585 |
| bins 2 original NGTDM Strength | 0.0149423 |
| bins 10 WL_LLL NGTDM Strength | 0.0172186 |
| bins 0.02 WL_HHH GLSZM Size Zone Non Uniformity Normalized | 0.0256454 |
| bins 2 WL_LLL NGTDM Contrast | 0.0558602 |
| bins 1 original GLSZM Zone Percentage | 0.0579938 |
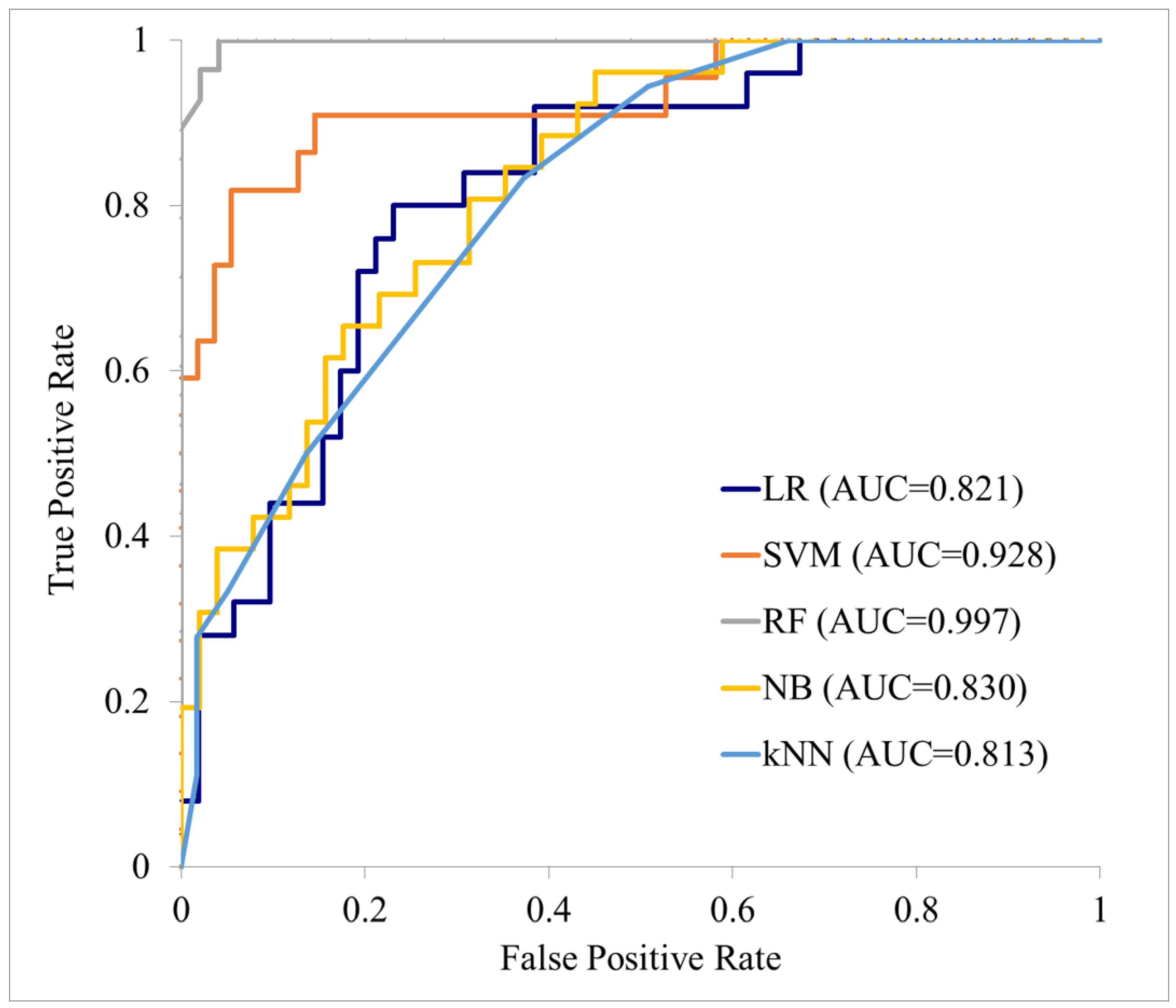
| Cohort | ML Model | AUC | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||
| Training cohort (n = 76) | LR | 0.821 (0.735–0.906) | 72.73 (62.78–82.67) | 48.00 (28.42–67.58) | 84.62 (74.81–94.42) | 60.00 (38.53–81.47) | 77.19 (66.30–88.09) |
| SVM | 0.928 (0.870–0.986) | 88.31 (81.14–95.49) | 59.09 (38.55–79.64) | 100.00 (100.00–100.00) | 100.00 (100.00–100.00) | 85.94 (77.42–94.45) | |
| RF | 0.997 (0.986–1.000) | 96.10 (91.78–100.00) | 92.86 (83.32–100.00) | 97.96 (94.00–100.00) | 96.30 (89.17–100.00) | 96.00 (90.57–100.00 | |
| NB | 0.830 (0.747–0.914) | 75.32 (65.70–84.95) | 65.39 (47.10–83.67) | 80.39 (69.50–91.29) | 62.96 (44.75–81.18) | 82.00 (71.35–92.65) | |
| KNN | 0.813 (0.726–0.900) | 90.91 (84.49–97.33) | 11.11 (0.00–25.63) | 98.31 (95.01–100.00) | 66.67 (13.32–100.00) | 78.38 (69.00–87.76) | |
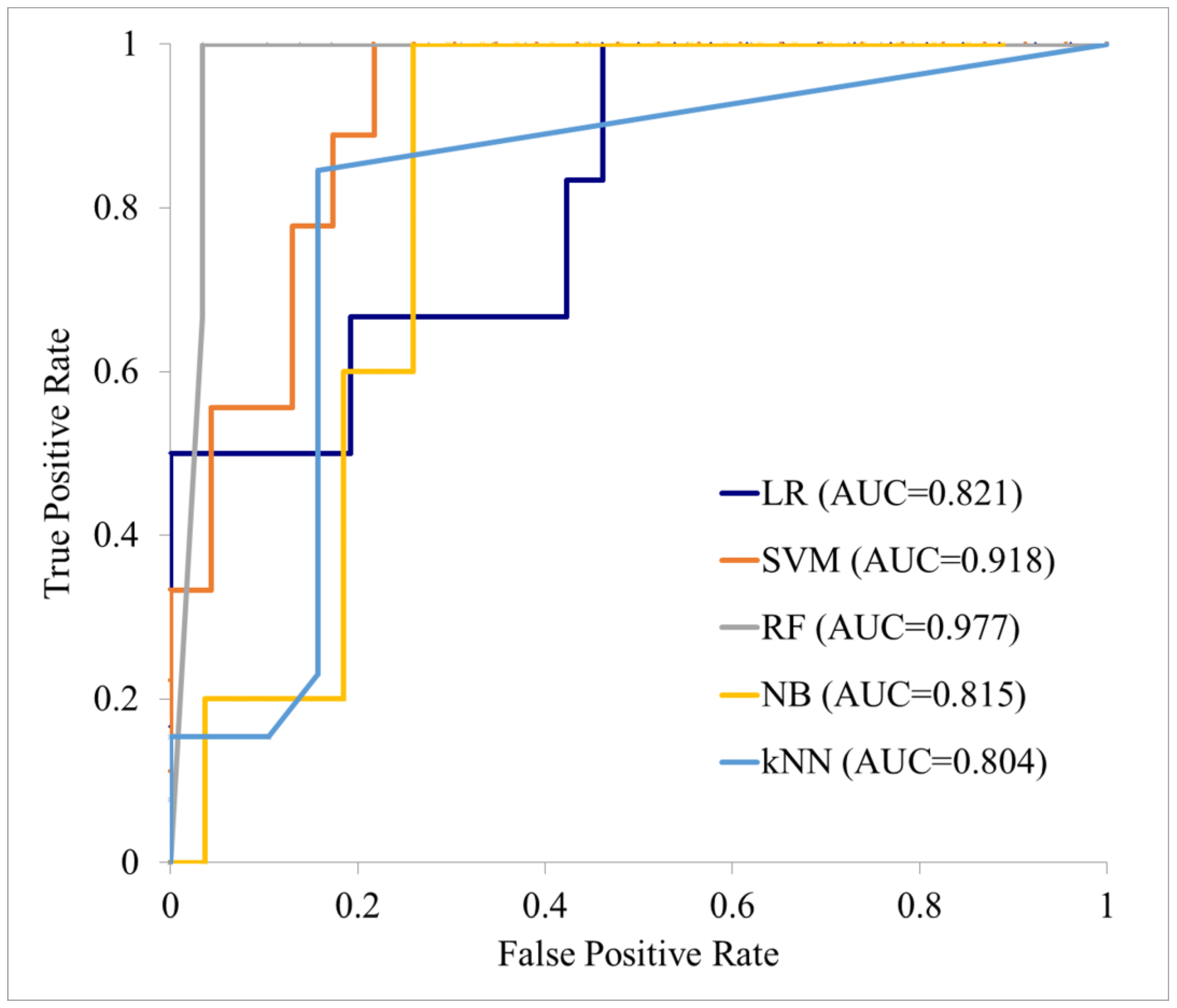
| Test cohort (n = 33) | LR | 0.821 (0.688–0.954) | 81.25 (67.73–94.77) | 50.00 (9.99–90.01) | 88.46 (76.18–100.00) | 5 0.00 (9.99–90.01) | 88.46 (76.18–100.00) |
| SVM | 0.918 (0.822–1.000) | 84.38 (71.79–96.96) | 55.566 (23.09–88.02) | 95.65 (87.32–100.00) | 83.33 (53.51–100.00) | 4.62 (70.75–98.48) | |
| RF | 0.977 (0.925–1.000) | 87.50 (76.04–98.96) | 100.00 (100.00–100.00) | 86.21 (73.66–98.76) | 42.86 (6.20–79.52) | 100.00 (100.00–100.00) | |
| NB | 0.815 (0.680–0.949) | 78.13 (63.80–92.45) | 100.00 (100.00–100.00) | 74.07 (57.54–90.60) | 41.67 (13.77–69.56) | 100.00 (100.00–100.00) | |
| KNN | 0.804 (0.666–0.941) | 62.50 (45.73–79.27) | 7.69 (0.00–22.18) | 100.00 (100.00–100.00) | 100.00 (100.00–100.00) | 61.29 (44.14–78.44) |
| Cohort | ML Model | AUC | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||
| Training cohort (n = 76) | LR | 0.969 (0.956–0.982) | 90.65 (86.97–94.33) | 70.06 (60.70–79.43) | 98.51 (96.59–100.00) | 95.45 (89.81–100.00) | 89.53 (85.82–93.23) |
| SVM | 0.726 (0.604–0.848) | 69.35 (56.02–82.68) | 67.30 (61.86–72.74) | 69.74 (51.18–88.31) | 49.99 (36.59–63.38) | 83.60 (77.39–89.81) | |
| RF | 0.985 (0.977–0.993) | 94.29 (92.84–95.73) | 84.51 (78.51–90.51) | 97.86 (95.99–99.72) | 93.80 (88.33–99.26) | 94.52 (92.98–96.07) | |
| NB | 0.835 (0.822–0.848) | 75.39 (72.90–77.88) | 74.97 (69.28–80.66) | 75.65 (71.18–80.13) | 55.54 (51.02–60.06) | 88.17 (84.92–91.42) | |
| KNN | 0.770 (0.733–0.806) | 75.58 (72.09–79.08) | 25.22 (21.51–28.93) | 97.04 (93.53–100.00) | 80.45 (61.42–99.48) | 75.27 (72.52–78.02) | |
| Test cohort (n = 33) | LR | 0.721 (0.634–0.808) | 68.13 (58.46–77.79) | 50.39 (15.34–85.43) | 77.25 (56.11–98.39) | 46.15 (30.72–61.58) | 81.56 (68.16–94.96) |
| SVM | 0.485 (0.277–0.692) | 53.75 (41.67–65.83) | 50.33 (24.47–76.20) | 55.63 (37.72–73.54) | 36.08 (16.11–56.04) | 69.71 (56.51–82.91) | |
| RF | 0.642 (0.585–0.698) | 70.00 (59.88–80.12) | 25.98 (17.33–54.63) | 84.35 (76.56–92.14) | 48.10 (24.41–71.78) | 76.30 (66.21–86.39) | |
| NB | 0.654 (0.567–0.742) | 63.13 (55.66–70.59) | 63.06 (46.62–79.49) | 62.97 (54.01–71.93) | 37.19 (28.25–46.14) | 83.47 (77.55–89.38) | |
| KNN | 0.694 (0.580–0.808) | 75.63 (69.87–81.38) | 23.06 (6.44–39.67) | 94.89 (90.47–99.32) | 62.67 (29.38–95.96) | 77.16 (73.06–81.26) |
| Authors | Year | Modality | AUC | Accuracy (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| Konishi et al. [11] | 2023 | Ultrasonography | 0.967 | 95.0 | 90.0 | 96.7 |
| Yuan et al. [12] | 2021 | MRI | 0.802 | 74.1 | 63.3 | 82.1 |
| Kudoh et al. [18] | 2023 | PET | 0.790 | 68.0 | 65.0 | 70.0 |
| Wang et al. [20] | 2024 | MRI | 0.872 | 87.34 | 78.78 | 93.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikkuni, Y.; Nishiyama, H.; Takamura, M.; Kobayashi, T.; Soga, M.; Ike, M.; Katsura, K.; Hayashi, T. Evaluation of the Ability to Predict Subsequent Metastasis of Early Oral Squamous Cell Carcinoma Using PET Radiomics Machine Learning Models. Cancers 2025, 17, 3573. https://doi.org/10.3390/cancers17213573
Nikkuni Y, Nishiyama H, Takamura M, Kobayashi T, Soga M, Ike M, Katsura K, Hayashi T. Evaluation of the Ability to Predict Subsequent Metastasis of Early Oral Squamous Cell Carcinoma Using PET Radiomics Machine Learning Models. Cancers. 2025; 17(21):3573. https://doi.org/10.3390/cancers17213573
Chicago/Turabian StyleNikkuni, Yutaka, Hideyoshi Nishiyama, Masaki Takamura, Taichi Kobayashi, Marie Soga, Makiko Ike, Kouji Katsura, and Takafumi Hayashi. 2025. "Evaluation of the Ability to Predict Subsequent Metastasis of Early Oral Squamous Cell Carcinoma Using PET Radiomics Machine Learning Models" Cancers 17, no. 21: 3573. https://doi.org/10.3390/cancers17213573
APA StyleNikkuni, Y., Nishiyama, H., Takamura, M., Kobayashi, T., Soga, M., Ike, M., Katsura, K., & Hayashi, T. (2025). Evaluation of the Ability to Predict Subsequent Metastasis of Early Oral Squamous Cell Carcinoma Using PET Radiomics Machine Learning Models. Cancers, 17(21), 3573. https://doi.org/10.3390/cancers17213573







