Radiation Necrosis in Neuro-Oncology: Diagnostic Complexity and Precision Radiotherapy Strategies
Simple Summary
Abstract
1. Introduction
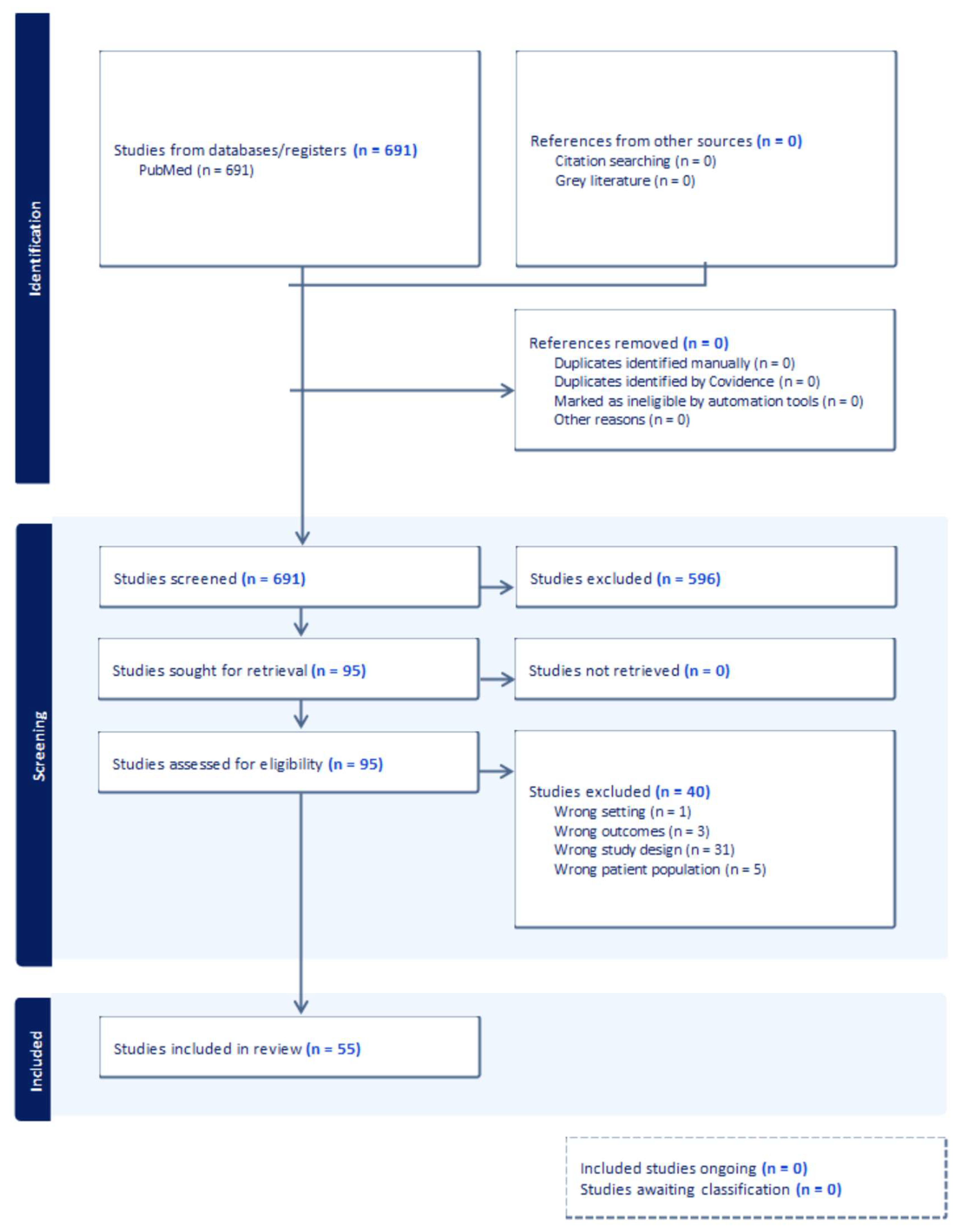
2. Materials and Methods
3. Results
3.1. Pathophysiology of RN
3.2. Risk Factors for RN
3.3. Radiation Therapy Approaches and RN Risk
3.3.1. Whole Brain Radiation Therapy (WBRT)
3.3.2. Intensity Modulated Radiation Therapy (IMRT)
3.4. Focal Techniques of Radiation Delivery
3.4.1. Stereotactic Radiosurgery (SRS) and Hypofractionated Stereotactic Radiotherapy (HFSRT)
3.4.2. Brachytherapy
3.4.3. Intra-Operative Radiotherapy (IORT)
3.4.4. Proton and Carbon Ion Therapy
3.5. Comparative Analysis of RN Risk
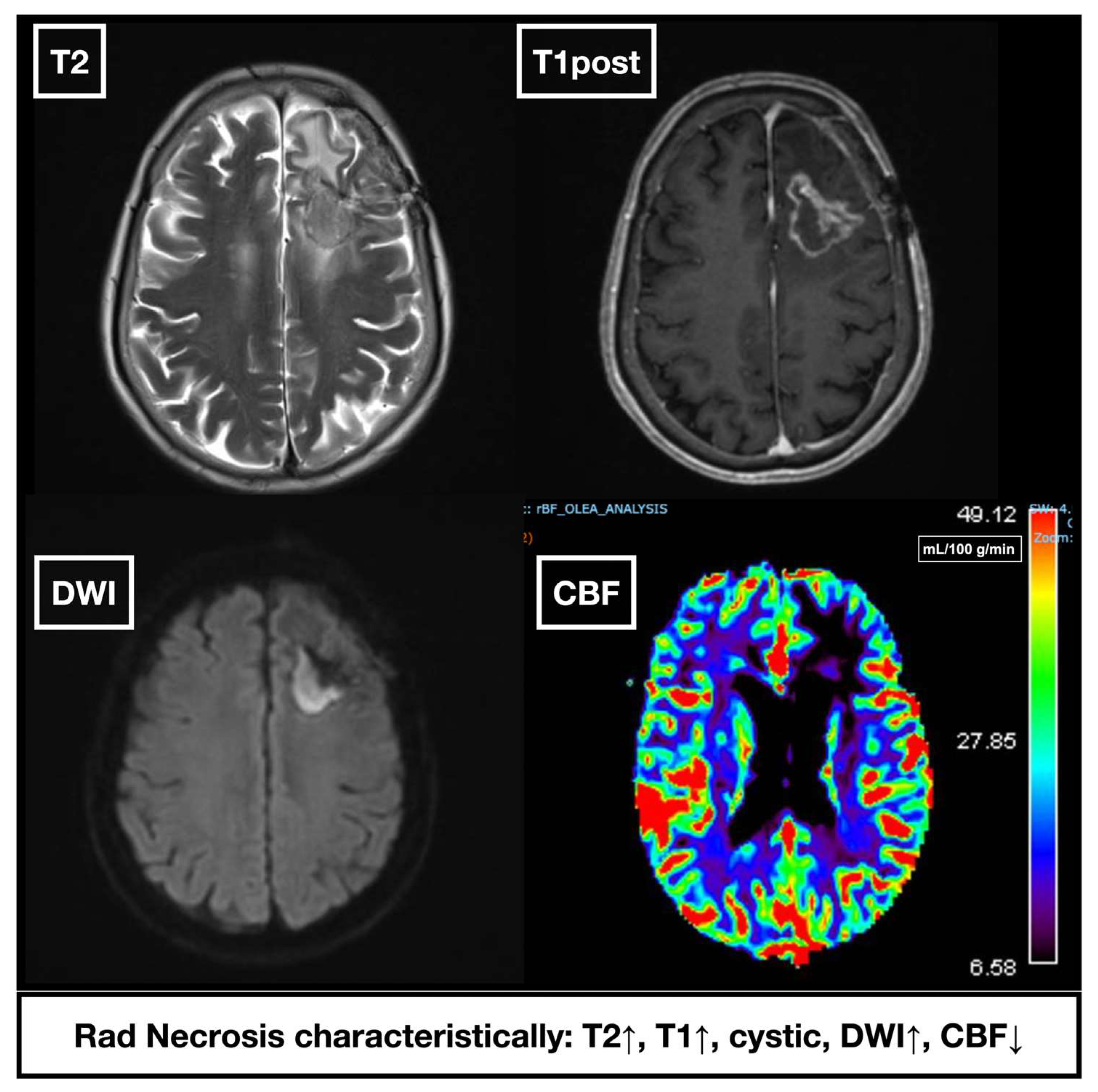
3.6. Imaging Modalities
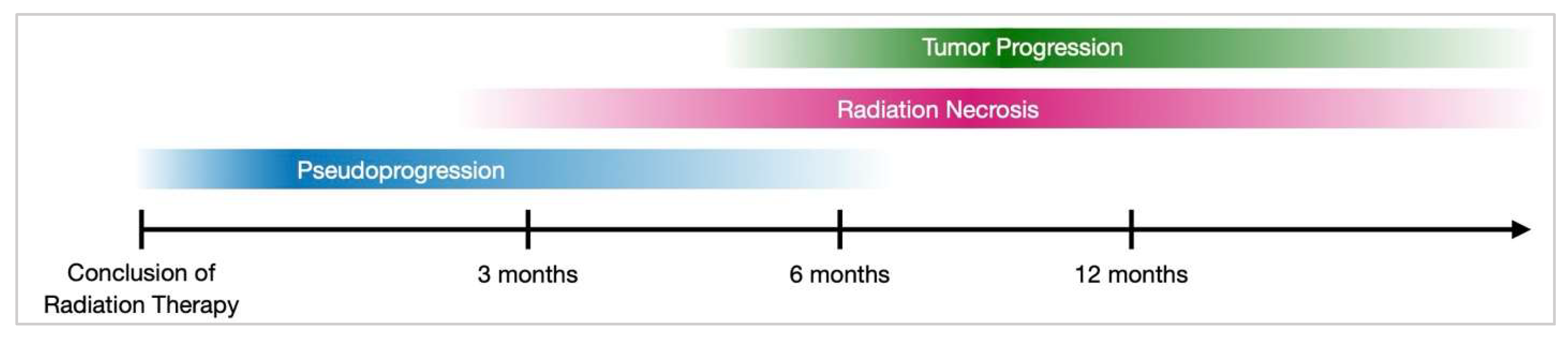
3.7. Differentiating RN from Pseudoprogression and Tumor Progression
3.8. Diagnostic Considerations
3.8.1. RN in the Context of Immunotherapy
3.8.2. Biomarkers to Differentiate RN from Recurrence
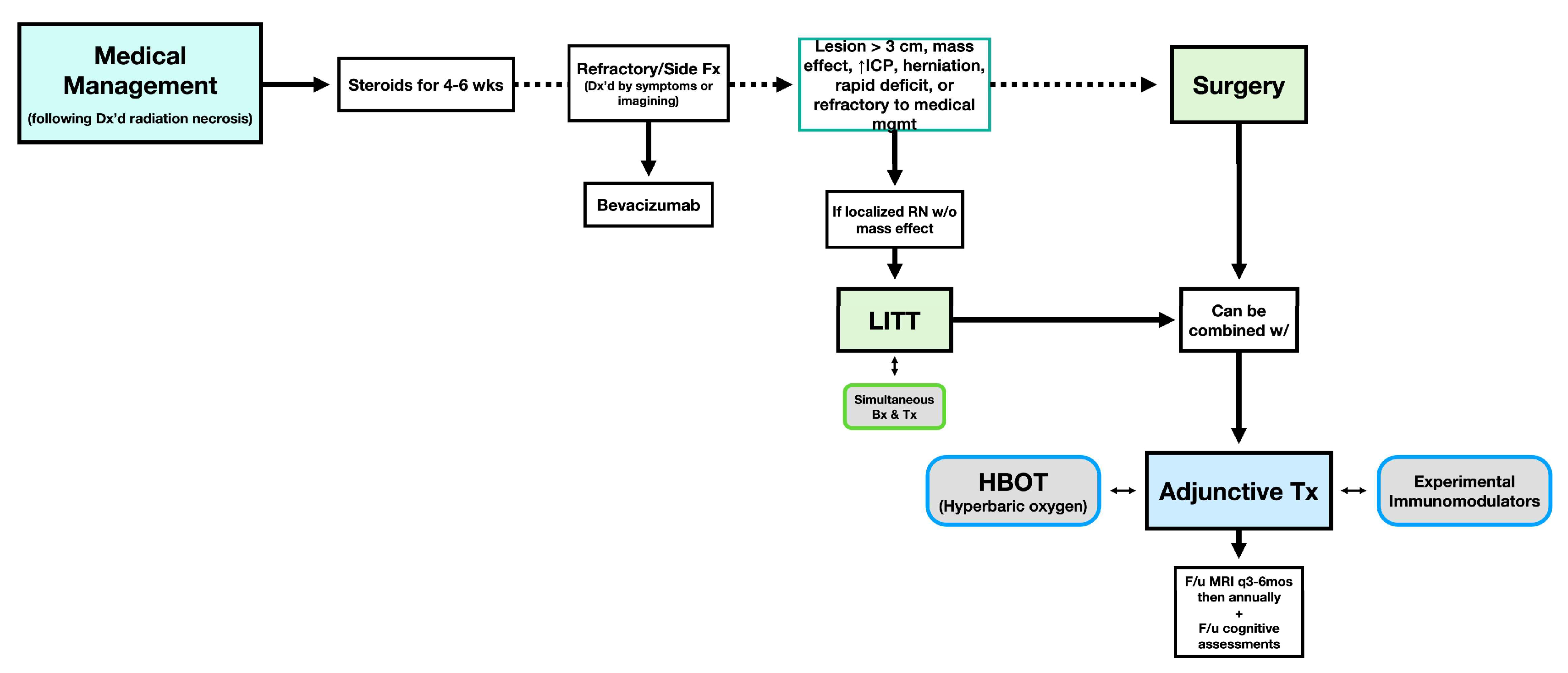
3.9. Management Strategies
3.9.1. Medical Therapies
3.9.2. Non-Pharmacological Interventions
3.9.3. Experimental and Adjunct Therapies
3.10. Connectomics in Tailoring Radiation Therapy: Tailoring Treatment to Minimize RN
3.11. Future Directions
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Suarez-Meade, P.; Marenco-Hillembrand, L.; Sherman, W.J. Neuro-oncologic Emergencies. Curr. Oncol. Rep. 2022, 24, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Mayo, Z.S.; Billena, C.; Suh, J.H.; Lo, S.S.; Chao, S.T. The dilemma of radiation necrosis from diagnosis to treatment in the management of brain metastases. Neuro-Oncology 2024, 26 (Suppl. 1), S56–S65. [Google Scholar] [CrossRef] [PubMed]
- Vaios, E.J.; Winter, S.F.; Shih, H.A.; Dietrich, J.; Peters, K.B.; Floyd, S.R.; Kirkpatrick, J.P.; Reitman, Z.J. Novel Mechanisms and Future Opportunities for the Management of Radiation Necrosis in Patients Treated for Brain Metastases in the Era of Immunotherapy. Cancers 2023, 15, 2432. [Google Scholar] [CrossRef]
- Bhandari, A.; Marwah, R.; Smith, J.; Nguyen, D.; Bhatti, A.; Lim, C.P.; Lasocki, A. Machine learning imaging applications in the differentiation of true tumour progression from treatment-related effects in brain tumours: A systematic review and meta-analysis. J. Med. Imaging Radiat. Oncol. 2022, 66, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Gecici, N.N.; Gurses, M.E.; Kaye, B.; Jimenez, N.L.F.; Berke, C.; Gökalp, E.; Lu, V.M.; Ivan, M.E.; Komotar, R.J.; Shah, A.H. Comparative analysis of bevacizumab and LITT for treating radiation necrosis in previously radiated CNS neoplasms: A systematic review and meta-analysis. J. Neuro-Oncol. 2024, 168, 1–11. [Google Scholar] [CrossRef]
- Miyatake, S.; Nonoguchi, N.; Furuse, M.; Yoritsune, E.; Miyata, T.; Kawabata, S.; Kuroiwa, T. Pathophysiology, Diagnosis, and Treatment of Radiation Necrosis in the Brain. Neurol. Med. Chir. 2015, 55, 50–59. [Google Scholar] [CrossRef]
- Trevino, C.R.; Paulino, A.C.; Kumar, V.A.; Majd, N.; Penas-Prado, M. Radiation-induced central demyelination, report of a rare subacute complication and review of the literature. Neuroimmunol. Neuroinflamm. 2021, 8, 146. [Google Scholar] [CrossRef]
- Blonigen, B.J.; Steinmetz, R.D.; Levin, L.; Lamba, M.A.; Warnick, R.E.; Breneman, J.C. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 996–1001. [Google Scholar] [CrossRef]
- Miller, J.A.; Bennett, E.E.; Xiao, R.; Kotecha, R.; Chao, S.T.; Vogelbaum, M.A.; Barnett, G.H.; Angelov, L.; Murphy, E.S.; Yu, J.S.; et al. Association Between Radiation Necrosis and Tumor Biology After Stereotactic Radiosurgery for Brain Metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 1060–1069. [Google Scholar] [CrossRef]
- Demetz, M.; Mangesius, J.; Krigers, A.; Nevinny-Stickel, M.; Thome, C.; Freyschlag, C.F.; Kerschbaumer, J. Tumor Location Impacts the Development of Radiation Necrosis in Benign Intracranial Tumors. Cancers 2023, 15, 4760. [Google Scholar] [CrossRef]
- Popp, I.; Hartong, N.E.; Nieder, C.; Grosu, A.L. PRO: Do We Still Need Whole-Brain Irradiation for Brain Metastases? Cancers 2023, 15, 3193. [Google Scholar] [CrossRef]
- Minniti, G.; Scaringi, C.; Paolini, S.; Lanzetta, G.; Romano, A.; Cicone, F.; Osti, M.; Enrici, R.M.; Esposito, V. Single-Fraction Versus Multifraction (3 × 9 Gy) Stereotactic Radiosurgery for Large (>2 cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1142–1148. [Google Scholar] [CrossRef]
- Kerschbaumer, J.; Demetz, M.; Krigers, A.; Nevinny-Stickel, M.; Thomé, C.; Freyschlag, C.F. Risk Factors for Radiation Necrosis in Patients Undergoing Cranial Stereotactic Radiosurgery. Cancers 2021, 13, 4736. [Google Scholar] [CrossRef]
- Ruben, J.D.; Dally, M.; Bailey, M.; Smith, R.; McLean, C.A.; Fedele, P. Cerebral radiation necrosis: Incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 499–508. [Google Scholar] [CrossRef]
- Korytko, T.; Radivoyevitch, T.; Colussi, V.; Wessels, B.W.; Pillai, K.; Maciunas, R.J.; Einstein, D.B. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int. J. Radiat. Oncol. 2006, 64, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Doré, M.; Antoni, D.; Menoux, I.; Thillays, F.; Clavier, J.; Delpon, G.; Jarnet, D.; Bourrier, C.; Lefebvre, F.; et al. Risque de radionécrose après radiothérapie hypofractionnée en conditions stéréotaxiques du lit opératoire de métastases cérébrales. Cancer Radiother. 2017, 21, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.R.; Ryu, S.; Kalkanis, S.; Cogan, C.; Rock, J.; Movsas, B.; Kim, J.H.; Rosenblum, M. Radiosurgery to the Surgical Cavity as Adjuvant Therapy for Resected Brain Metastasis. Neurosurgery 2012, 71, 937–943. [Google Scholar] [CrossRef]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.F.; Trasimeni, G.; Bozzao, A.; Romano, A.; Enrici, R.M. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.S.; Akinyelu, T.; Vaslow, Z.K.; Matsui, J.K.; Haghighi, N.; Dan, T.; Mishra, M.V.; Murphy, E.S.; Boyles, S.; Perlow, H.K.; et al. Single-Fraction Versus Fractionated Preoperative Radiosurgery for Resected Brain Metastases: A PROPS-BM International Multicenter Cohort Study. Int. J. Radiat. Oncol. Biol. Phys. 2023, 118, 650–661. [Google Scholar] [CrossRef]
- Imber, B.S.; Kanungo, I.; Braunstein, S.; Barani, I.J.; Fogh, S.E.; Nakamura, J.L.; Berger, M.S.; Chang, E.F.; Molinaro, A.M.; Cabrera, J.R.; et al. Indications and Efficacy of Gamma Knife Stereotactic Radiosurgery for Recurrent Glioblastoma: 2 Decades of Institutional Experience. Neurosurgery 2016, 80, 129–139. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Popp, I.; Rau, S.; Hintz, M.; Schneider, J.; Bilger, A.; Fennell, J.T.; Heiland, D.H.; Rothe, T.; Egger, K.; Nieder, C.; et al. Hippocampus-avoidance whole-brain radiation therapy with a simultaneous integrated boost for multiple brain metastases. Cancer 2020, 126, 2694–2703. [Google Scholar] [CrossRef]
- Matsui, J.K.; Perlow, H.K.; Upadhyay, R.; McCalla, A.; Raval, R.R.; Thomas, E.M.; Blakaj, D.M.; Beyer, S.J.; Palmer, J.D. Advances in Radiotherapy for Brain Metastases. Surg. Oncol. Clin. N. Am. 2023, 32, 569–586. [Google Scholar] [CrossRef]
- Nieder, C.; Andratschke, N.H.; Grosu, A.L. Brain Metastases: Is There Still a Role for Whole-Brain Radiation Therapy? Semin. Radiat. Oncol. 2023, 33, 129–138. [Google Scholar] [CrossRef]
- Wiegreffe, S.; Sarria, G.R.; Layer, J.P.; Dejonckheere, E.; Nour, Y.; Schmeel, F.C.; Anton Giordano, F.; Schmeel, L.C.; Popp, I.; Grosu, A.L.; et al. Incidence of hippocampal and perihippocampal brain metastases and impact on hippocampal-avoiding radiotherapy: A systematic review and meta-analysis. Radiother. Oncol. 2024, 197, 110331. [Google Scholar] [CrossRef]
- Leskinen, S.; Shah, H.A.; Yaffe, B.; Schneider, S.J.; Ben-Shalom, N.; Boockvar, J.A.; D’Amico, R.S.; Wernicke, A.G. Hippocampal avoidance in whole brain radiotherapy and prophylactic cranial irradiation: A systematic review and meta-analysis. J. Neurooncol. 2023, 163, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Scott, C.; Souhami, L.; Dinapoli, R.; Kline, R.; Loeffler, J.; Farnan, N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 291–298. [Google Scholar] [CrossRef] [PubMed]
- McKay, W.H.; McTyre, E.R.; Okoukoni, C.; Alphonse-Sullivan, N.K.; Ruiz, J.; Munley, M.T.; Qasem, S.; Lo, H.W.; Xing, F.; Laxton, A.W.; et al. Repeat stereotactic radiosurgery as salvage therapy for locally recurrent brain metastases previously treated with radiosurgery. J. Neurosurg. 2017, 127, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Kohutek, Z.A.; Yamada, Y.; Chan, T.A.; Brennan, C.W.; Tabar, V.; Gutin, P.H.; Yang, T.J.; Rosenblum, M.K.; Ballangrud, Å.; Young, R.J.; et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J. Neurooncol. 2015, 125, 149–156. [Google Scholar] [CrossRef]
- Rae, A.; Gorovets, D.; Rava, P.; Ebner, D.; Cielo, D.; Kinsella, T.J.; DiPetrillo, T.A.; Hepel, J.T. Management approach for recurrent brain metastases following upfront radiosurgery may affect risk of subsequent radiation necrosis. Adv. Radiat. Oncol. 2016, 1, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.A.; Keggin, E.; Plowman, P.N. The developing role for intensity-modulated radiation therapy (IMRT) in the non-surgical treatment of brain metastases. Br. J. Radiol. 2010, 83, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Citrin, D.E. Recent Developments in Radiotherapy. N. Engl. J. Med. 2017, 377, 1065–1075. [Google Scholar] [CrossRef]
- Reese, A.S.; Das, S.K.; Kirkpatrick, J.P.; Marks, L.B. Quantifying the dosimetric trade-offs when using intensity-modulated radiotherapy to treat concave targets containing normal tissues. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 585–593. [Google Scholar] [CrossRef]
- Mukwada, G.; Chamunyonga, C.; Rowshanfarzad, P.; Gill, S.; Ebert, M.A. Insights into the dosimetric and geometric characteristics of stereotactic radiosurgery for multiple brain metastases: A systematic review. PLoS ONE 2024, 19, e0307088. [Google Scholar] [CrossRef]
- El Shafie, R.A.; Tonndorf-Martini, E.; Schmitt, D.; Celik, A.; Weber, D.; Lang, K.; Konig, L.; Hone, S.; Forster, T.; von Nettelbladt, B.; et al. Single-Isocenter Volumetric Modulated Arc Therapy vs. CyberKnife M6 for the Stereotactic Radiosurgery of Multiple Brain Metastases. Front. Oncol. 2020, 10, 568. [Google Scholar] [CrossRef]
- Carlson, M.L.; Link, M.J. Vestibular Schwannomas. N. Engl. J. Med. 2021, 384, 1335–1348. [Google Scholar] [CrossRef]
- Alongi, F.; Fiorentino, A.; Mancosu, P.; Navarria, P.; Giaj Levra, N.; Mazzola, R.; Scorsetti, M. Stereotactic radiosurgery for intracranial metastases: Linac-based and gamma-dedicated unit approach. Expert. Rev. Anticancer. Ther. 2016, 16, 731–740. [Google Scholar] [CrossRef]
- Han, E.Y.; Wang, H.; Luo, D.; Li, J.; Wang, X. Dosimetric comparison of fractionated radiosurgery plans using frameless Gamma Knife ICON and CyberKnife systems with linear accelerator-based radiosurgery plans for multiple large brain metastases. J. Neurosurg. 2020, 132, 1473–1479. [Google Scholar] [CrossRef]
- Seung, S.K.; Larson, D.A.; Galvin, J.M.; Mehta, M.P.; Potters, L.; Schultz, C.J.; Yajnik, S.V.; Hartford, A.C.; Rosenthal, S.A. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) Practice Guideline for the Performance of Stereotactic Radiosurgery (SRS). Am. J. Clin. Oncol. 2013, 36, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Treuer, H.; Hoevels, M.; Luyken, K.; Visser-Vandewalle, V.; Wirths, J.; Kocher, M.; Ruge, M. Intracranial stereotactic radiosurgery with an adapted linear accelerator vs. robotic radiosurgery: Comparison of dosimetric treatment plan quality. Strahlenther. Onkol. 2015, 191, 470–476. [Google Scholar] [CrossRef]
- Hendricks, B.K.; DiDomenico, J.D.; Barani, I.J.; Barranco, F.D. ZAP-X Gyroscopic Radiosurgery System: A Preliminary Analysis of Clinical Applications within a Retrospective Case Series. Stereotact. Funct. Neurosurg. 2022, 100, 99–107. [Google Scholar] [CrossRef]
- Garsa, A.; Jang, J.K.; Baxi, S.; Chen, C.; Akinniranye, O.; Hall, O.; Larkin, J.; Motala, A.; Hempel, S. Radiation Therapy for Brain Metastases: A Systematic Review. Pract. Radiat. Oncol. 2021, 11, 354–365. [Google Scholar] [CrossRef]
- Milano, M.T.; Grimm, J.; Niemierko, A.; Soltys, S.G.; Moiseenko, V.; Redmond, K.J.; Yorke, E.; Sahgal, A.; Xue, J.; Mahadevan, A.; et al. Single- and Multifraction Stereotactic Radiosurgery Dose/Volume Tolerances of the Brain. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 68–86. [Google Scholar] [CrossRef]
- Lehrer, E.J.; Peterson, J.L.; Zaorsky, N.G.; Brown, P.D.; Sahgal, A.; Chiang, V.L.; Chao, S.T.; Sheehan, J.P.; Trifiletti, D.M. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Ruge, M.I.; Kickingereder, P.; Grau, S.; Treuer, H.; Sturm, V.; Voges, J. Stereotactic iodine-125 brachytherapy for brain tumors: Temporary versus permanent implantation. Radiat. Oncol. 2012, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Gessler, D.J.; Neil, E.C.; Shah, R.; Levine, J.; Shanks, J.; Wilke, C.; Reynolds, M.; Zhang, S.; Özütemiz, C.; Gencturk, M.; et al. GammaTile® brachytherapy in the treatment of recurrent glioblastomas. Neuro-Oncol. Adv. 2021, 4, vdab185. [Google Scholar] [CrossRef]
- Mouli, S.; Goyal, P.; Tate, M.; Dixit, K.; Primdahl, D.; Boockvar, J.; Quinones-Hinojosa, A.; Weiss, C.; Butowski, N.; Dreher, M.; et al. TIPS-17 A First-In-Human Feasibility Study to Evaluate the Safety of Selective Intra-Arterial Yttrium-90 Microsphere Treatment in Patients with Recurrent Glioblastoma (the Frontier Trial). Neuro-Oncol. Adv. 2023; 5, (Suppl. 3), iii37–iii38. [Google Scholar]
- Pop, L.A.; van der Plas, M.; Ruifrok, A.C.; Schalkwijk, L.J.; Hanssen, A.E.; van der Kogel, A.J. Tolerance of rat spinal cord to continuous interstitial irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Brenner, D.J. The dose-rate effect revisited: Radiobiological considerations of importance in radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 1403–1414. [Google Scholar] [CrossRef]
- Huang, K.; Sneed, P.K.; Kunwar, S.; Kragten, A.; Larson, D.A.; Berger, M.S.; Chan, A.; Pouliot, J.; Mcdermott, M.W. Surgical resection and permanent iodine-125 brachytherapy for brain metastases. J. Neurooncol. 2008, 91, 83–93. [Google Scholar] [CrossRef]
- Wernicke, A.G.; Hirschfeld, C.B.; Smith, A.W.; Taube, S.; Yondorf, M.Z.; Parashar, B.; Nedialkova, L.; Kulidzhanov, F.; Trichter, S.; Sabbas, A.; et al. Clinical Outcomes of Large Brain Metastases Treated With Neurosurgical Resection and Intraoperative Cesium-131 Brachytherapy: Results of a Prospective Trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, G.; Zadeh, G.; Gingras-Hill, G.; Millar, B.A.; Laperriere, N.J.; Bernstein, M.; Jiang, H.; Ménard, C.; Chung, C. Salvage radiosurgery for brain metastases: Prognostic factors to consider in patient selection. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 137–142. [Google Scholar] [CrossRef]
- Layer, J.P.; Hamed, M.; Potthoff, A.L.; Dejonckheere, C.S.; Layer, K.; Sarria, G.R.; Scafa, D.; Koch, D.; Köksal, M.; Kugel, F.; et al. Outcome assessment of intraoperative radiotherapy for brain metastases: Results of a prospective observational study with comparative matched-pair analysis. J. Neurooncol. 2023, 164, 107–116. [Google Scholar] [CrossRef]
- Cifarelli, C.P.; Jacobson, G.M. Intraoperative Radiotherapy in Brain Malignancies: Indications and Outcomes in Primary and Metastatic Brain Tumors. Front. Oncol. 2021, 11, 768168. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, D.; Bert, J.; Dupre, P.F.; Benhalouche, S.; Pradier, O.; Boussion, N.; Visvikis, D. Monte-Carlo dosimetry for intraoperative radiotherapy using a low energy x-ray source. Acta Oncol. 2015, 54, 1788–1795. [Google Scholar] [CrossRef]
- Diehl, C.D.; Pigorsch, S.U.; Gempt, J.; Krieg, S.M.; Reitz, S.; Waltenberger, M.; Barz, M.; Meyer, H.S.; Wagner, A.; Wilkens, J.; et al. Low-Energy X-Ray Intraoperative Radiation Therapy (Lex-IORT) for Resected Brain Metastases: A Single-Institution Experience. Cancers 2022, 15, 14. [Google Scholar] [CrossRef]
- Liu, H.; Chang, J.Y. Proton therapy in clinical practice. Chin. J. Cancer 2011, 30, 315–326. [Google Scholar] [CrossRef]
- Semenova, J. Proton beam radiation therapy in the treatment of pediatric central nervous system malignancies: A review of the literature. J. Pediatr. Oncol. Nurs. 2009, 26, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Grosshans, D. Proton therapy—Present and future. Adv. Drug Deliv. Rev. 2017, 109, 26–44. [Google Scholar] [CrossRef]
- Oden, J.; Toma-Dasu, I.; Witt Nystrom, P.; Traneus, E.; Dasu, A. Spatial correlation of linear energy transfer and relative biological effectiveness with suspected treatment-related toxicities following proton therapy for intracranial tumors. Med. Phys. 2020, 47, 342–351. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Eulitz, J.; Peters, N.; Wohlfahrt, P.; Enghardt, W.; Richter, C.; Luhr, A. Impact of range uncertainty on clinical distributions of linear energy transfer and biological effectiveness in proton therapy. Med. Phys. 2020, 47, 6151–6162. [Google Scholar] [CrossRef]
- Barbarite, E.; Sick, J.T.; Berchmans, E.; Bregy, A.; Shah, A.H.; Elsayyad, N.; Komotar, R.J. The role of brachytherapy in the treatment of glioblastoma multiforme. Neurosurg. Rev. 2016, 40, 195–211. [Google Scholar] [CrossRef]
- Valk, P.E.; Dillon, W.P. Radiation injury of the brain. AJNR Am. J. Neuroradiol. 1991, 12, 45–62. [Google Scholar]
- Fatterpekar, G.M.; Galheigo, D.; Narayana, A.; Johnson, G.; Knopp, E. Treatment-Related Change Versus Tumor Recurrence in High-Grade Gliomas: A Diagnostic Conundrum—Use of Dynamic Susceptibility Contrast-Enhanced (DSC) Perfusion MRI. Am. J. Roentgenol. 2012, 198, 19–26. [Google Scholar] [CrossRef]
- Shah, R.; Vattoth, S.; Jacob, R.; Manzil, F.F.P.; O’Malley, J.P.; Borghei, P.; Patel, B.N.; Curé, J.K. Radiation Necrosis in the Brain: Imaging Features and Differentiation from Tumor Recurrence. RadioGraphics 2012, 32, 1343–1359. [Google Scholar] [CrossRef]
- Matsusue, E.; Fink, J.R.; Rockhill, J.K.; Ogawa, T.; Maravilla, K.R. Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology 2010, 52, 297–306. [Google Scholar] [CrossRef]
- Park, Y.W.; Choi, D.; Park, J.E.; Ahn, S.S.; Kim, H.; Chang, J.H.; Kim, S.H.; Kim, H.S.; Lee, S.-K. Differentiation of recurrent glioblastoma from radiation necrosis using diffusion radiomics with machine learning model development and external validation. Sci. Rep. 2021, 11, 2913. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.; Steffensen, E.; Larsson, E.M. Perfusion MRI (dynamic susceptibility contrast imaging) with different measurement approaches for the evaluation of blood flow and blood volume in human gliomas. Acta Radiol. 2012, 53, 95–101. [Google Scholar] [CrossRef]
- Clement, P.; Petr, J.; Dijsselhof, M.B.J.; Padrela, B.; Pasternak, M.; Dolui, S.; Jarutyte, L.; Pinter, N.; Hernandez-Garcia, L.; Jahn, A.; et al. A Beginner’s Guide to Arterial Spin Labeling (ASL) Image Processing. Front. Radiol. 2022, 2, 929533. [Google Scholar] [CrossRef] [PubMed]
- Yunqi, Y.; Aihua, N.; Zhiming, Z.; Yingchao, L.; Qiang, W.; Yang, M.; Yi, Z. Quantitative MR Perfusion for the Differentiation of Recurrence and Radionecrosis in Hypoperfusion and Hyperperfusion Brain Metastases After Gamma Knife Radiosurgery. Front. Neurol. 2022, 13, 823731. [Google Scholar] [CrossRef]
- Chae, W.H.; Niesel, K.; Schulz, M.; Klemm, F.; Joyce, J.A.; Prummer, M.; Brill, B.; Bergs, J.; Rodel, F.; Pilatus, U.; et al. Evaluating Magnetic Resonance Spectroscopy as a Tool for Monitoring Therapeutic Response of Whole Brain Radiotherapy in a Mouse Model for Breast-to-Brain Metastasis. Front. Oncol. 2019, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Kwock, L.; Mukherji, S.K. Clinical applications of proton MR spectroscopy. Am. J. Neuroradiol. 1996, 17, 1–15. [Google Scholar] [PubMed]
- Galijasevic, M.; Steiger, R.; Mangesius, S.; Mangesius, J.; Kerschbaumer, J.; Freyschlag, C.F.; Gruber, N.; Janjic, T.; Gizewski, E.R.; Grams, A.E. Magnetic Resonance Spectroscopy in Diagnosis and Follow-Up of Gliomas: State-of-the-Art. Cancers 2022, 14, 3197. [Google Scholar] [CrossRef]
- Hangel, G.; Niess, E.; Lazen, P.; Bednarik, P.; Bogner, W.; Strasser, B. Emerging methods and applications of ultra-high field MR spectroscopic imaging in the human brain. Anal. Biochem. 2022, 638, 114479. [Google Scholar] [CrossRef]
- Bernstock, J.D.; Gary, S.E.; Klinger, N.; Valdes, P.A.; Ibn Essayed, W.; Olsen, H.E.; Chagoya, G.; Elsayed, G.; Yamashita, D.; Schuss, P.; et al. Standard clinical approaches and emerging modalities for glioblastoma imaging. Neurooncol. Adv. 2022, 4, vdac080. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Cowperthwaite, M.C.; Burnett, M.G.; Markey, M.K. Differentiating tumor recurrence from treatment necrosis: A review of neuro-oncologic imaging strategies. Neuro-Oncology 2013, 15, 515–534. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Dong, Q.; Zhan, L.; Zhang, J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am. J. Cancer Res. 2019, 9, 1546–1553. [Google Scholar]
- Ellingson, B.M.; Chung, C.; Pope, W.B.; Boxerman, J.L.; Kaufmann, T.J. Pseudoprogression, radionecrosis, inflammation or true tumor progression? challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J. Neuro-Oncol. 2017, 134, 495–504. [Google Scholar] [CrossRef]
- Cruz Lc, H.; Rodriguez, I.; Domingues, R.C.; Gasparetto, E.L.; Sorensen, A.G. Pseudoprogression and Pseudoresponse: Imaging Challenges in the Assessment of Posttreatment Glioma. Am. J. Neuroradiol. 2011, 32, 1978–1985. [Google Scholar] [CrossRef]
- Lee, D.; Riestenberg, R.A.; Haskell-Mendoza, A.; Bloch, O. Brain Metastasis Recurrence Versus Radiation Necrosis. Neurosurg. Clin. N. Am. 2020, 31, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, D.; El Shafie, R.; Thomas, M.; Bozorgmehr, F.; Schiele, A.; Schmitt, D.; Welzel, T.; Thalmann, P.; Paul, A.; König, L.; et al. Stereotactic Radiotherapy vs. Whole Brain Radiation Therapy for Patients with 1–10 Brain Metastases from Small Cell Lung Cancer: Results of the Randomized ENCEPHALON (ARO 2018–9) Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, e5. [Google Scholar]
- Meixner, E.; Horner-Rieber, J.; Lischalk, J.W.; Eichkorn, T.; Kramer, A.; Sandrini, E.; Paul, A.; Hoegen, P.; Deng, M.; Welzel, T.; et al. Management of initial and recurrent radiation-induced contrast enhancements following radiotherapy for brain metastases: Clinical and radiological impact of bevacizumab and corticosteroids. Clin. Transl. Radiat. Oncol. 2023, 39, 100600. [Google Scholar] [CrossRef]
- Zhuang, H.; Shi, S.; Yuan, Z.; Chang, J.Y. Bevacizumab treatment for radiation brain necrosis: Mechanism, efficacy and issues. Mol. Cancer 2019, 18, 21. [Google Scholar] [CrossRef]
- Xu, Y.; Rong, X.; Hu, W.; Huang, X.; Li, Y.; Zheng, D.; Cai, Z.; Zuo, Z.; Tang, Y. Bevacizumab Monotherapy Reduces Radiation-induced Brain Necrosis in Nasopharyngeal Carcinoma Patients: A Randomized Controlled Trial. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1087–1095. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, J.W.; Kong, D.S.; Seol, H.J.; Nam, D.H.; Lee, J.I. Effect of Bevacizumab Treatment in Cerebral Radiation Necrosis: Investigation of Response Predictors in a Single-Center Experience. J. Korean Neurosurg. Soc. 2023, 66, 562–572. [Google Scholar] [CrossRef]
- Furuse, M.; Nonoguchi, N.; Kuroiwa, T.; Miyamoto, S.; Arakawa, Y.; Shinoda, J.; Miwa, K.; Iuchi, T.; Tsuboi, K.; Houkin, K.; et al. A prospective, multicentre, single-arm clinical trial of bevacizumab for patients with surgically untreatable, symptomatic brain radiation necrosis. Neurooncol. Pract. 2016, 3, 272–280. [Google Scholar] [CrossRef]
- Li, H.; Rong, X.; Hu, W.; Yang, Y.; Lei, M.; Wen, W.; Yue, Z.; Huang, X.; Chua, M.L.K.; Li, Y.; et al. Bevacizumab Combined with Corticosteroids Does Not Improve the Clinical Outcome of Nasopharyngeal Carcinoma Patients With Radiation-Induced Brain Necrosis. Front. Oncol. 2021, 11, 746941. [Google Scholar] [CrossRef] [PubMed]
- Newman, W.C.; Goldberg, J.; Guadix, S.W.; Brown, S.; Reiner, A.S.; Panageas, K.; Beal, K.; Brennan, C.W.; Tabar, V.; Young, R.J.; et al. The effect of surgery on radiation necrosis in irradiated brain metastases: Extent of resection and long-term clinical and radiographic outcomes. J. Neuro-Oncol. 2021, 153, 507–518. [Google Scholar] [CrossRef]
- Telera, S.; Fabi, A.; Pace, A.; Vidiri, A.; Anelli, V.; Carapella, C.M.; Marucci, L.; Crispo, F.; Sperduti, I.; Pompili, A. Radionecrosis induced by stereotactic radiosurgery of brain metastases: Results of surgery and outcome of disease. J. Neurooncol. 2013, 113, 313–325. [Google Scholar] [CrossRef]
- Chung, C.; Bryant, A.; Brown, P.D. Interventions for the treatment of brain radionecrosis after radiotherapy or radiosurgery. Cochrane Database Syst. Rev. 2018, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, M.; Tatter, S.; Chiang, V.; Fecci, P.; Strowd, R.; Prabhu, S.; Hadjipanayis, C.; Kirkpatrick, J.; Sun, D.; Sinicrope, K.; et al. Efficacy of laser interstitial thermal therapy for biopsy-proven radiation necrosis in radiographically recurrent brain metastases. Neuro-Oncol. Adv. 2023, 5, vdad031. [Google Scholar] [CrossRef] [PubMed]
- Co, J.; Moraes, M.V.; Katznelson, R.; Evans, A.W.; Shultz, D.; Laperriere, N.; Millar, B.-A.; Berlin, A.; Kongkham, P.; Tsang, D.S. Hyperbaric Oxygen for Radiation Necrosis of the Brain. Can. J. Neurol. Sci./J. Can. Des Sci. Neurol. 2019, 47, 92–99. [Google Scholar] [CrossRef]
- Patel, J.S.; Salari, E.; Chen, X.; Switchenko, J.; Eaton, B.R.; Zhong, J.; Yang, X.; Shu, H.G.; Sudmeier, L.J. Radiomic Analysis of Treatment Effect for Patients with Radiation Necrosis Treated with Pentoxifylline and Vitamin E. Tomography 2024, 10, 1501–1512. [Google Scholar] [CrossRef]
- Wang, M.; Ma, H.; Wang, X.; Guo, Y.; Xia, X.; Xia, H.; Guo, Y.; Huang, X.; He, H.; Jia, X.; et al. Integration of BOLD-fMRI and DTI into radiation treatment planning for high-grade gliomas located near the primary motor cortexes and corticospinal tracts. Radiat. Oncol. 2015, 10, 64. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Robbins, M.E. Radiation-induced cognitive impairment—From bench to bedside. Neuro-Oncology 2012, 14 (Suppl. 4), iv37–iv44. [Google Scholar]
- Diehl, C.D.; Rosenkranz, E.; Schwendner, M.; Misslbeck, M.; Sollmann, N.; Ille, S.; Meyer, B.; Combs, S.E.; Krieg, S.M. Dose Reduction to Motor Structures in Adjuvant Fractionated Stereotactic Radiotherapy of Brain Metastases: nTMS-Derived DTI-Based Motor Fiber Tracking in Treatment Planning. Cancers 2022, 15, 282. [Google Scholar] [CrossRef]
- Liang, M.Z.; Tang, Y.; Chen, P.; Tang, X.N.; Knobf, M.T.; Hu, G.Y.; Sun, Z.; Liu, M.L.; Yu, Y.L.; Ye, Z.J. Brain connectomics improve prediction of 1-year decreased quality of life in breast cancer: A multi-voxel pattern analysis. Eur. J. Oncol. Nurs. 2024, 68, 102499. [Google Scholar] [CrossRef] [PubMed]

| Author Year Study Type Sample Size | Primary Brain Tumor or Metastasis | Histology (Tissue of Origin for Metastasis) | Type of Radiation Delivery | Radiation Dose | Additional Therapies | Radian Necrosis Incidence | % Radiographic RN Only | % Symptomatic RN | Follow-Up Duration | Time to SRS After Surgery (Days) | Time from SRS to RN | RN Management Strategies | Factors Assessed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demetz et al., 2023 [10] Retrospective N = 205 | Primary Brain Tumor | All benign neoplasms (vestibular schwannoma, meningioma, glomus jugulare tumors, ependymoma, schwannoma of other cranial nerves, others) | LINAC based SRS (unspecificed number of fractions) | 12–18 Gy (median 13 Gy) | Steroid taper (routine) | 15.6% (total) | 53% | 47% | Mean 42 months, standard deviation 16.3 months, range 0–192 months | N/A | Median 10 months | Dexamethasone in 12 cases, bevacizumab in 2 cases, surgical resection in 2 cases | Tumor location, applied radiation dose |
| Kerschbaumer et al., 2021 [13] Retrospective N = 388 | Mixed | Mixed (NSCLC, melanoma, breast, renal, unknown met, vestibular schwannoma, meningiomas, ependymoma, glomus tumors, gliomas) | Single staged LINAC based SRS | 14–25 Gy (median 16 Gy) | Steroid taper (routine) | 15.7% (total) | 53% | 47% | Mean 24 months, range 0–192 months) | N/A | Median 8 months (range 1–41 months) | Medical management for 23, surgery for 2, 2 palliative | Tumor diameter, radiation dose |
| Ruben et al., 2006 [14] Retrospective N = 426 | Primary Brain Tumor | Glioma | EBRT | 16–60 Gy (median 50 Gy) | SRS (12%), Conformal EBRT (0.5%), interstital brachytherapy (0.7%), chemotherapy | 4.90% | Did not specify | Did not specify | Did not specify | N/A | Mean 11.6 months, range 2–32 months | Valproate and chemotherapy drugs | Dose, fractionation, and time |
| Korytko et al., 2006 [15] Retrospective N = 129 | Both | Mixed (excluding AVMs) | SF GK SRS | 12 Gy | Did not specify | 23% | Did not specify | Did not specify | Every 3–6 months | N/A | Did not specify | Did not specify | Brain volume, location, previous WBRT, sex |
| Keller et al., 2017 [16] Retrospective N = 181 | Metastasis | NSCLC, breast, and other mets | 3 fraction GK SRS | 23.1 Gy | Did not specify | 18.50% | Did not specify | Did not specify | Median 15 months (range: 3–38 months | Did not specify | Did not specify | Did not specify | Location and volume |
| Robbins et al., 2012 [17] Retrospective N = 85 | Metastasis | Lung (59%), Breast (11%), Melanoma/Renal (13%), Gynecologic (6%), Colon (4%), and Other (7%) | SRS to the surgical cavity (with WBRT as salvage) | 12–18 Gy (median 16 Gy) | Surgery, Salvage SRS, Salvage WBRT (used in 35% of cases) | 8% (7 patients) | Did not specify | Did not specify | Median 11.2 months (range: 1–93 months) | Median: 18 days (95% received SRS <2 months post-surgery) | Median: 8.4 months (range: 5.8–16.5 months) | Steroids, surgical excision, CT perfusion studies, MRI monitoring | Tumor location, radiation dose, target volume, extent of surgical resection, active systemic disease |
| Blonigen et al., 2010 [8] Retrospective N = 63 patients, 173 lesions | Metastasis | Most common: Breast and Lung | Linear accelerator-based SRS (Single-fraction) | Mean: 18 Gy (range: 12–22 Gy) | Steroid therapy (all symptomatic cases), pentoxifylline/vitamin E, hyperbaric oxygen (11%), surgical resection (33%) | 14% | 4% | 10% | Median 13.7 months (range: 3.5–51 months) | Did not specify | Median: 11.5 months | Steroids, surgical resection, pentoxifylline, vitamin E, hyperbaric oxygen | Brain volume receiving V8 Gy–V18 Gy, conformality index, lesion size, tumor location |
| Minniti et al., 2011 [18] Retrospective N = 206 patients, 310 lesions | Metastasis | Lung (51%), Breast (18%), Melanoma (17%), Others (14%) | LINAC-based SRS (Single-fraction) | Mean: 18 Gy (range 15–20 Gy) | Steroid therapy (all symptomatic cases), high-dose dexamethasone (>4 months in 7.8% of patients), salvage WBRT (22.8%), salvage SRS (10.2%) | 24% (total) | 14% | 10% | Median: 9.4 months (range: 2–42 months) | Did not specify | Median: 11 months (symptomatic), 10 months (asymptomatic) | Steroids, high-dose dexamethasone, hypofractionated radiotherapy, salvage SRS, WBRT | V10 Gy–V16 Gy, lesion volume, conformality index, tumor location, KPS, extracranial disease |
| Prabhu (2023) [19] Retrospective N = 404 | Metastasis | NSCLC (47.3%), Breast (16.4%), Melanoma (12.3%), Renal cell (8.5%), GI (6.3%), Other (9.1%) | Single-fraction (SF) or multifraction (MF) preoperative SRS | SF-SRS: 14–17 Gy (median 15 Gy); MF-SRS: 24–30 Gy (median 24 Gy) | Surgical resection | 6.7% (SF-SRS), 10.7% (MF-SRS) | Not reported | 58% (SF-SRS), 75% (MF-SRS) | Steroids, surgical resection, and palliative care | SF-SRS: Median 1 day; MF-SRS: Median 2 days | Not reported | Steroids, surgical excision, and advanced imaging for differentiation | Radiation dose, lesion size, fractionation, tumor location |
| Imber et al., 2017 [20] Retrospective N = 174 | Primary Brain Tumor | Glioblastoma | Gamma Knife SRS | Median: 16 Gy (range: 10–22 Gy) | Salvage craniotomy in 26.4% of patients, systemic chemotherapy, subset received bevacizumab | Salvage craniotomy (26.4%), systemic chemotherapy, subset received bevacizumab | Not reported | Not reported | Median 8.7 months (range: 0–120.1 months) | Did not specify | Median: 6.6 months (range: 1.1–83.6 months) | Steroids, surgical resection, and palliative care | Age, marginal prescription dose, treatment volume, surgery-to-Gamma Knife interval |
| Modality | RN Rate (Glioma) | RN Rate (Metastasis) | Re-Irradiation Risk Impact | Evidence Level |
|---|---|---|---|---|
| WBRT | 4.9% (EBRT data) | 10–30% | High | RCTs and retrospective data |
| SRS (Single-Fraction) | 15–25% | 23% | >24% symptomatic RN in repeat SRS | Multiple retrospective cohorts |
| HFSRT (Multi-Fraction SRS) | ~7% | 7% | Moderate | Multicenter retrospective and ongoing RCT (NCT04114981) |
| IMRT | Varies, typically moderate | Variable; depends on tumor burden | Moderate | Retrospective series |
| Brachytherapy (I-125) | Up to 23% | 17.50% | High | Early trials and retrospective data |
| Brachytherapy (Cs-131) | 1.3–5% | 1.3–4.8% | Lower with Cs-131 due to shorter half-life | Prospective single-center studies |
| IORT (LEX-IORT) | ~2.9% | 2.90% | Unknown | Phase II prospective (INTRAMET) |
| Proton Therapy | Lower than photons | Lower; especially in pediatric and eloquent areas | Potentially lower with Bragg peak | Phase II and registry data |
| Carbon Ion Therapy | Unknown due to limited data | Theoretical advantage | Potentially lower (but unclear) | Experimental, limited clinical access |
| Therapy | Mechanism | Expected Duration/Response | Limitations |
|---|---|---|---|
| Corticosteroids | Reduces vasogenic edema and stabilizes vascular permeability1 | Temporary benefit; median PFS ≈ 2.9 months; ~50% recurrence after discontinuation | Long term toxicity, rebound edema |
| Bevacizumab | Anti-VEGF antibody decreasing vascular permeability | Rapid edema reduction; 65.5% response vs 31.5% with steroids; relief in ≈ 90% on repeat cycles; some recurrence (~34%) | Hypertension; transient effect; risk of ischemia with prolonged use |
| Surgical resection | Physically removes necrotic tissue, relieves mass effect | Clinical improvement in > 50%; edema resolution within 2–4 weeks | Invasive; surgical morbidity; unsuitable for deep lesions |
| LITT | Minimally invasive laser ablation of necrotic tissue | Neurologic improvement in 50–70%; functional improvement in 70–80% | Limited data; thermalinjury risk |
| HBOT | Increases oxygenation and angiogenesis | 70–80% improvement in small series | Potential to stimulate tumor growth; access/cost |
| Pentoxifylline + Vitamin E | Anti-inflammatory and antioxidant effects reduce fibrosis/necrosis | Reported radiologic improvements; adjunctive use | Limited evidence; mild side effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittelman, L.; Duehr, J.; Kazmi, J.S.; Vargas, L.O.; Donahue, N.; Chen, J.; Leskinen, S.; Syed, S.A.; Wernicke, A.G.; D’Amico, R.S. Radiation Necrosis in Neuro-Oncology: Diagnostic Complexity and Precision Radiotherapy Strategies. Cancers 2025, 17, 3542. https://doi.org/10.3390/cancers17213542
Mittelman L, Duehr J, Kazmi JS, Vargas LO, Donahue N, Chen J, Leskinen S, Syed SA, Wernicke AG, D’Amico RS. Radiation Necrosis in Neuro-Oncology: Diagnostic Complexity and Precision Radiotherapy Strategies. Cancers. 2025; 17(21):3542. https://doi.org/10.3390/cancers17213542
Chicago/Turabian StyleMittelman, Laura, James Duehr, Jacob S. Kazmi, Luis O. Vargas, Nora Donahue, John Chen, Sandra Leskinen, Shoaib A. Syed, A. Gabriella Wernicke, and Randy S. D’Amico. 2025. "Radiation Necrosis in Neuro-Oncology: Diagnostic Complexity and Precision Radiotherapy Strategies" Cancers 17, no. 21: 3542. https://doi.org/10.3390/cancers17213542
APA StyleMittelman, L., Duehr, J., Kazmi, J. S., Vargas, L. O., Donahue, N., Chen, J., Leskinen, S., Syed, S. A., Wernicke, A. G., & D’Amico, R. S. (2025). Radiation Necrosis in Neuro-Oncology: Diagnostic Complexity and Precision Radiotherapy Strategies. Cancers, 17(21), 3542. https://doi.org/10.3390/cancers17213542






