Three-Dimensional Analysis of the Effect of Osteosarcoma on Sensory Nerves Innervating the Femur in a Murine Model of Osteosarcoma-Induced Bone Pain
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tumor Cells
2.3. Surgery
2.4. Experimental Endpoint
2.5. Behavioral Tests
2.6. X-Ray Imaging
2.7. Perfusion Fixation and Tissue Collection
2.8. Tissue Clearing
2.9. Antibody Specificity and Characterization
2.10. Imaging
2.11. Statistics
3. Results
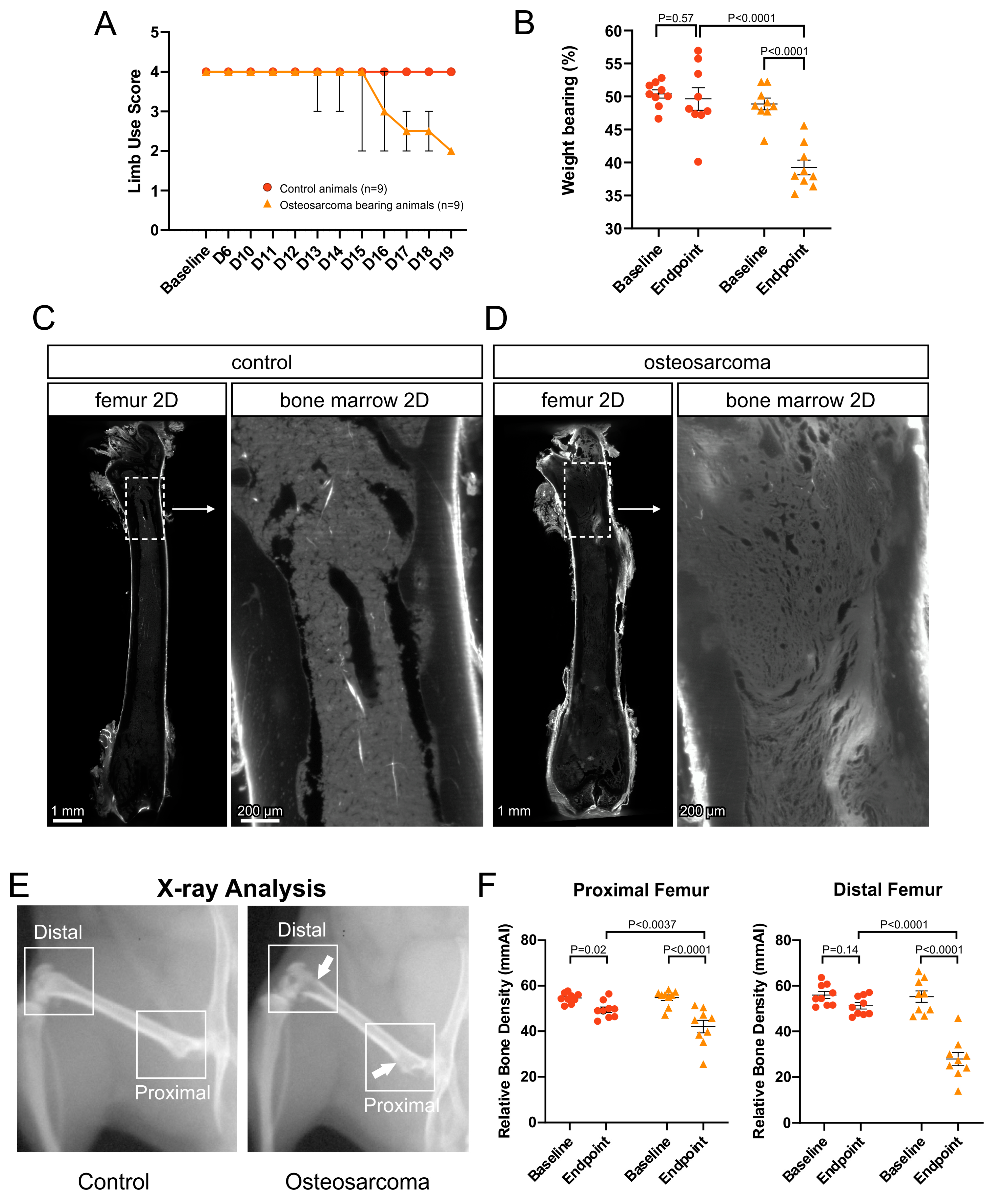
3.1. Osteosarcoma-Induced Pain Behavior and Tumor Growth
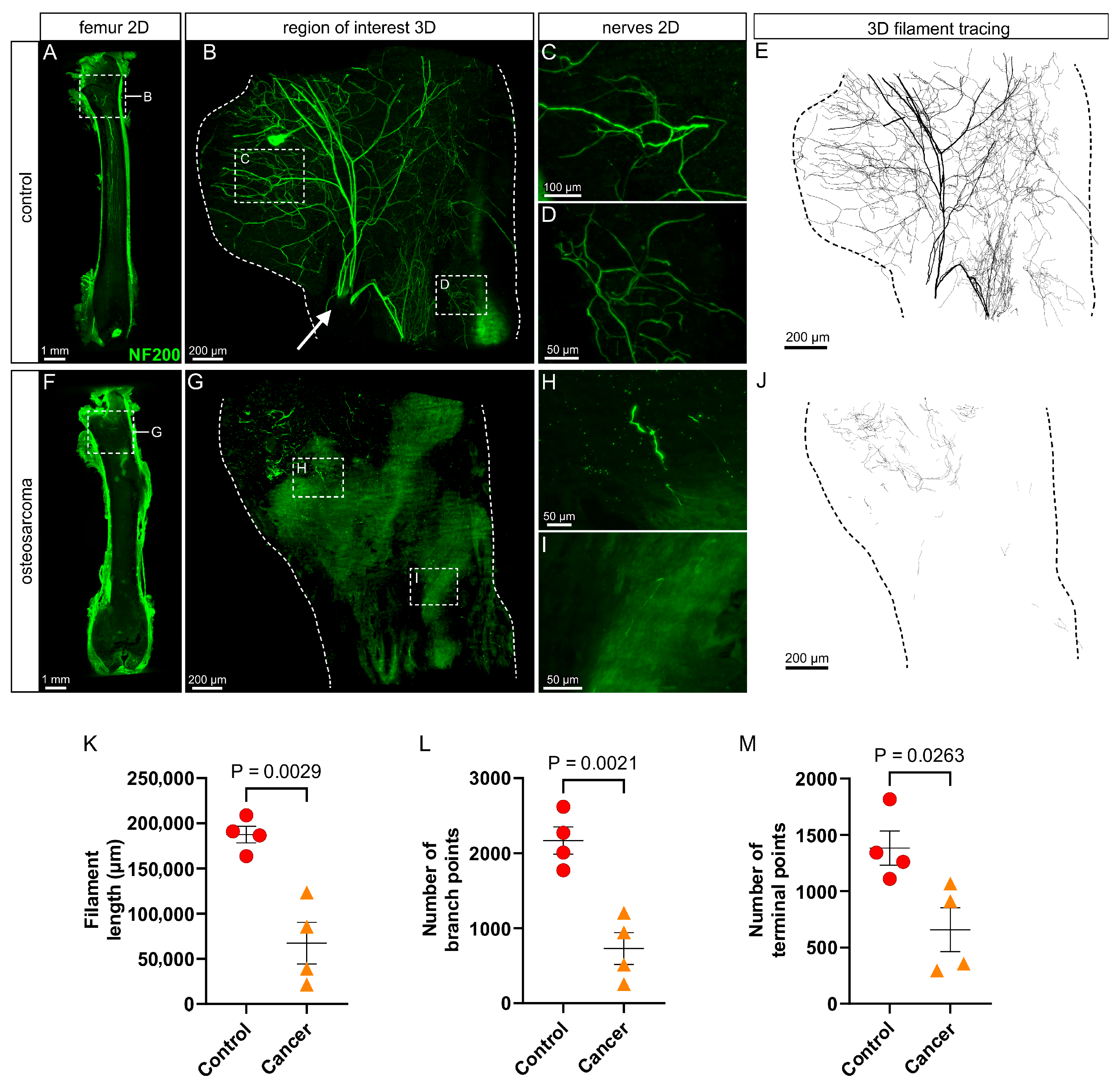
3.2. There Is a Marked Loss of Myelinated (NF200+) Nerve Profiles in the Marrow Cavity of Osteosarcoma Bearing Relative to Control Animals
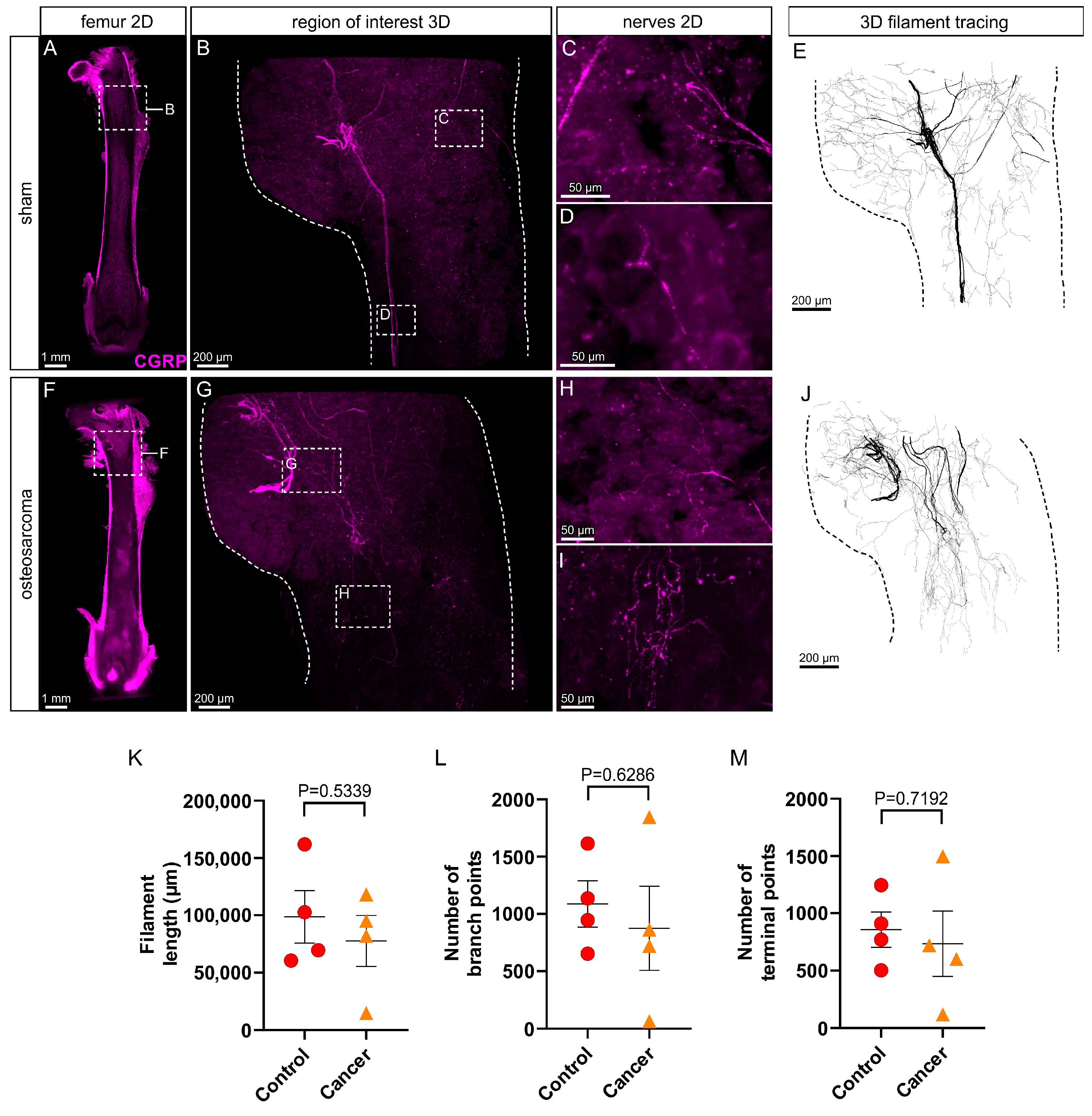
3.3. There Is No Change in Peptidergic (CGRP+) Innervation in the Marrow Cavity of Osteosarcoma Bearing Relative to Control Animals
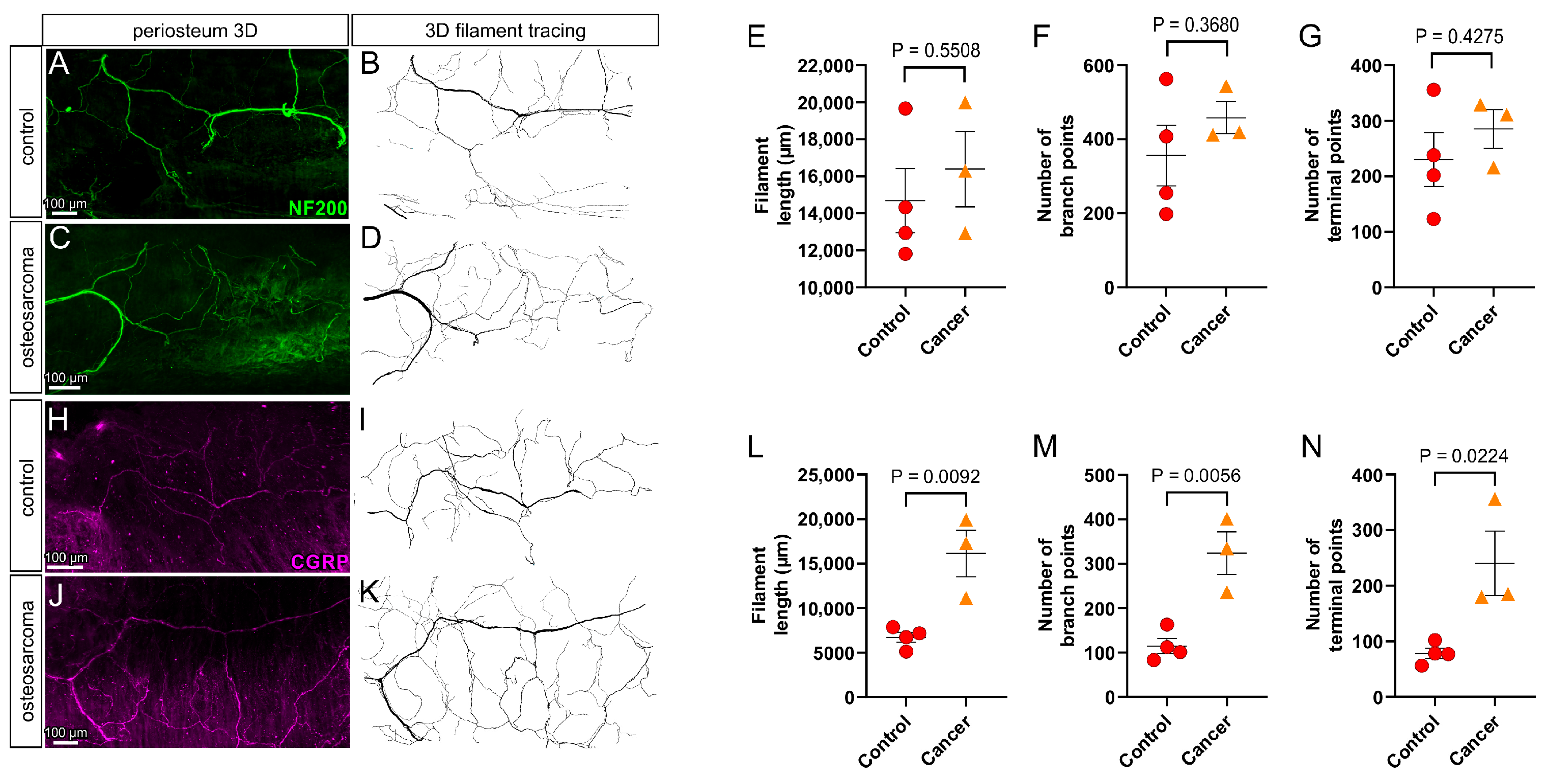
3.4. There Is an Increase in Peptidergic (CGRP+) but Not Myelinated (NF200+) Nerve Profiles in the Periosteum Overlying Bone in Osteosarcoma Bearing Relative to Control Animals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NF200 | Neurofilament 200 kDa |
| CGRP | Calcitonin gene-related peptide |
| PBS | Phosphate buffered saline |
| mmAl | Millimeter aluminum equivalents |
| DCM | Dichloromethane |
| DBE | Dibenzyl ether |
| ECi | Ethyl cinnamate |
References
- Taran, R.; Taran, S.; Malipatil, N. Pediatric osteosarcoma: An updated review. Indian J. Med. Paediatr. Oncol. 2017, 38, 33–43. [Google Scholar] [CrossRef]
- Dahlin, D.C.; Unni, K.K. Bone Tumors: General Aspects and Data on 8542 Cases, 4th ed.; Thomas: Springfield, IL, USA, 1986. [Google Scholar]
- Ottaviani, G.; Jaffe, N. The Epidemiology of Osteosarcoma. In Pediatric and Adolescent Osteosarcoma; Jaffe, N., Bruland, O.S., Bielack, S., Eds.; Springer: Boston, MA, USA, 2010; pp. 3–13. [Google Scholar]
- Liu, J.-F.; Shanmugavadivel, D.; Ball-Gamble, A.; Walker, D. Clinical presentation of bone tumours in children and young people: A systematic review and meta-analysis. Arch. Dis. Child. 2025, 110, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Skeletal complications of malignancy. Cancer 1997, 80 (Suppl. S8), 1588–1594. [Google Scholar] [CrossRef]
- Mercadante, S. Malignant bone pain: Pathophysiology and treatment. Pain 1997, 69, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Schwei, M.J.; Honore, P.; Rogers, S.D.; Salak-Johnson, J.L.; Finke, M.P.; Ramnaraine, M.L.; Clohisy, D.R.; Mantyh, P.W. Neurochemical and Cellular Reorganization of the Spinal Cord in a Murine Model of Bone Cancer Pain. J. Neurosci. 1999, 19, 10886–10897. [Google Scholar] [CrossRef]
- Luger, N.M.; Mach, D.B.; Sevcik, M.A.; Mantyh, P.W. Bone Cancer Pain: From Model to Mechanism to Therapy. J. Pain Symptom Manag. 2005, 29, 32–46. [Google Scholar] [CrossRef]
- Peters, C.M.; Ghilardi, J.R.; Keyser, C.P.; Kubota, K.; Lindsay, T.H.; Luger, N.M.; Mach, D.B.; Schwei, M.J.; Sevcik, M.A.; Mantyh, P.W. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp. Neurol. 2005, 193, 85–100. [Google Scholar] [CrossRef]
- Sevcik, M.A.; Ghilardi, J.R.; Peters, C.M.; Lindsay, T.H.; Halvorson, K.G.; Jonas, B.M.; Kubota, K.; Kuskowski, M.A.; Boustany, L.; Shelton, D.L.; et al. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain 2005, 115, 128–141. [Google Scholar] [CrossRef]
- Wacnik, P.W.; Baker, C.M.; Herron, M.J.; Kren, B.T.; Blazar, B.R.; Wilcox, G.L.; Hordinsky, M.K.; Beitz, A.J.; Ericson, M.E. Tumor-induced mechanical hyperalgesia involves CGRP receptors and altered innervation and vascularization of DsRed2 fluorescent hindpaw tumors. Pain 2005, 115, 95–106. [Google Scholar] [CrossRef]
- Sabino, M.A.C.; Luger, N.M.; Mach, D.B.; Rogers, S.D.; Schwei, M.J.; Mantyh, P.W. Different tumors in bone each give rise to a distinct pattern of skeletal destruction, bone cancer-related pain behaviors and neurochemical changes in the central nervous system. Int. J. Cancer 2003, 104, 550–558. [Google Scholar] [CrossRef]
- Cain, D.M.; Wacnik, P.W.; Turner, M.; Wendelschafer-Crabb, G.; Kennedy, W.R.; Wilcox, G.L.; Simone, D.A. Functional Interactions between Tumor and Peripheral Nerve: Changes in Excitability and Morphology of Primary Afferent Fibers in a Murine Model of Cancer Pain. J. Neurosci. 2001, 21, 9367–9376. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.; Rogers, S.; Schwei, M.; Salak-Johnson, J.; Luger, N.; Sabino, M.; Clohisy, D.; Mantyh, P. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 2000, 98, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Hald, A.; Nedergaard, S.; Hansen, R.R.; Ding, M.; Heegaard, A. Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur. J. Pain 2009, 13, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, J.R.; Freeman, K.T.; Jimenez-Andrade, J.M.; Mantyh, W.G.; Bloom, A.P.; A Kuskowski, M.; Mantyh, P.W. Administration of a Tropomyosin Receptor Kinase Inhibitor Attenuates Sarcoma-Induced Nerve Sprouting, Neuroma Formation and Bone Cancer Pain. Mol. Pain 2010, 6, 87. [Google Scholar] [CrossRef]
- Mantyh, W.; Jimenez-Andrade, J.; Stake, J.; Bloom, A.; Kaczmarska, M.; Taylor, R.N.; Freeman, K.; Ghilardi, J.; Kuskowski, M.; Mantyh, P. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience 2010, 171, 588–598. [Google Scholar] [CrossRef]
- Gilchrist, L.S.; Cain, D.M.; Harding-Rose, C.; Kov, A.N.; Wendelschafer-Crabb, G.; Kennedy, W.R.; Simone, D.A. Re-organization of P2X3 receptor localization on epidermal nerve fibers in a murine model of cancer pain. Brain Res. 2005, 1044, 197–205. [Google Scholar] [CrossRef]
- McCaffrey, G.; Thompson, M.L.; Majuta, L.; Fealk, M.N.; Chartier, S.; Longo, G.; Mantyh, P.W. NGF blockade at early times during bone cancer development attenuates bone destruction and increases limb use. Cancer Res. 2014, 74, 7014–7023. [Google Scholar] [CrossRef]
- Thai, J.; Fuller-Jackson, J.; Ivanusic, J.J. Using tissue clearing and light sheet fluorescence microscopy for the three-dimensional analysis of sensory and sympathetic nerve endings that innervate bone and dental tissue of mice. J. Comp. Neurol. 2024, 532, e25582. [Google Scholar] [CrossRef]
- Mach, D.; Rogers, S.; Sabino, M.; Luger, N.; Schwei, M.; Pomonis, J.; Keyser, C.; Clohisy, D.; Adams, D.; O’leary, P.; et al. Origins of skeletal pain: Sensory and sympathetic innervation of the mouse femur. Neuroscience 2002, 113, 155–166. [Google Scholar] [CrossRef]
- Thai, J.; Kyloh, M.; Travis, L.; Spencer, N.J.; Ivanusic, J.J. Identifying spinal afferent (sensory) nerve endings that innervate the marrow cavity and periosteum using anterograde tracing. J. Comp. Neurol. 2020, 528, 1903–1916. [Google Scholar] [CrossRef]
- Brazill, J.M.; Beeve, A.T.; Craft, C.S.; Ivanusic, J.J.; Scheller, E.L. Nerves in Bone: Evolving Concepts in Pain and Anabolism. J. Bone Miner. Res. 2019, 34, 1393–1406. [Google Scholar] [CrossRef]
- Ivanusic, J.J. Size, neurochemistry, and segmental distribution of sensory neurons innervating the rat tibia. J. Comp. Neurol. 2009, 517, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Aso, K.; Ikeuchi, M.; Izumi, M.; Sugimura, N.; Kato, T.; Ushida, T.; Tani, T. Nociceptive phenotype of dorsal root ganglia neurons innervating the subchondral bone in rat knee joints. Eur. J. Pain 2013, 18, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Corral, G.; Jimenez-Andrade, J.; Bloom, A.; Taylor, R.N.; Mantyh, W.; Kaczmarska, M.; Ghilardi, J.; Mantyh, P. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience 2011, 178, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Andrade, J.M.; Bloom, A.P.; Stake, J.I.; Mantyh, W.G.; Taylor, R.N.; Freeman, K.T.; Ghilardi, J.R.; Kuskowski, M.A.; Mantyh, P.W. Pathological Sprouting of Adult Nociceptors in Chronic Prostate Cancer-Induced Bone Pain. J. Neurosci. 2010, 30, 14649–14656. [Google Scholar] [CrossRef]
- Sliepen, S.H.J.; Diaz-Delcastillo, M.; Korioth, J.; Olsen, R.B.; Appel, C.K.; Christoph, T.; Heegaard, A.-M.; Rutten, K. Cancer-induced Bone Pain Impairs Burrowing Behaviour in Mouse and Rat. In Vivo 2019, 33, 1125–1132. [Google Scholar] [CrossRef]
- Sabino, M.A.C.; Ghilardi, J.R.; Jongen, J.L.M.; Keyser, C.P.; Luger, N.M.; Mach, D.B.; Peters, C.M.; Rogers, S.D.; Schwei, M.J.; De Felipe, C.; et al. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2. Cancer Res. 2002, 62, 7343–7349. [Google Scholar]
- Irie, K.; Hara-Irie, F.; Ozawa, H.; Yajima, T. Calcitonin gene-related peptide (CGRP)-containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc. Res. Tech. 2002, 58, 85–90. [Google Scholar] [CrossRef]
- Imai, S.; Rauvala, H.; Konttinen, Y.T.; Tokunaga, T.; Maeda, T.; Hukuda, S.; Santavirta, S. Efferent Targets of Osseous CGRP-Immunoreactive Nerve Fiber Before and After Bone Destruction in Adjuvant Arthritic Rat: An Ultramorphological Study on Their Terminal-Target Relations. J. Bone Miner. Res. 1997, 12, 1018–1027. [Google Scholar] [CrossRef]
- Hill, E.L.; Elde, R. Distribution of CGRP-, VIP-, D beta H-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991, 264, 469–480. [Google Scholar] [CrossRef]
- Hill, E.L.; Elde, R. Calcitonin gene-related peptide-immunoreactive nerve fibers in mandibular periosteum of rat: Evidence for primary afferent origin. Neurosci. Lett. 1988, 85, 172–178. [Google Scholar] [CrossRef]
- Bjurholm, A.; Kreicbergs, A.; Brodin, E.; Schultzberg, M. Substance P- and CGRP-immunoreactive nerves in bone. Peptides 1988, 9, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Sapio, M.R.; Vazquez, F.A.; Loydpierson, A.J.; Maric, D.; Kim, J.J.; LaPaglia, D.M.; Puhl, H.L.; Lu, V.B.; Ikeda, S.R.; Mannes, A.J.; et al. Comparative Analysis of Dorsal Root, Nodose and Sympathetic Ganglia for the Development of New Analgesics. Front. Neurosci. 2020, 14, 615362. [Google Scholar] [CrossRef] [PubMed]
- Mantyh, P.W. Bone cancer pain: From mechanism to therapy. Curr. Opin. Support Palliat. Care 2014, 8, 83–90. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.X.; Nanthakumar, S.S.; Nielsen, G.P.; E Rosenberg, A. Osteoid osteoma: The uniquely innervated bone tumor. Mod. Pathol. 1998, 11, 175–180. [Google Scholar]
- Hasegawa, T.; Hirose, T.; Sakamoto, R.; Seki, K.; Ikata, T.; Hizawa, K. Mechanism of pain in osteoid osteomas: An immunohistochemical study. Histopathology 1993, 22, 487–491. [Google Scholar] [CrossRef]
- Ortiz, Y.T.; Shamir, L.G.; McMahon, L.R.; Wilkerson, J.L. Characterization of commercially available murine fibrosarcoma NCTC-2472 cells both in vitro and as a model of bone cancer pain in vivo. PLoS ONE 2024, 19, e0309398. [Google Scholar] [CrossRef]
- Sen, H.S.; Uzunhan, T.A. Evaluation of neuropathic pain with diverse pathophysiologies in childhood cancers. North. Clin. Istanb. 2022, 9, 241–247. [Google Scholar] [CrossRef]
- Caraceni, A.; Zecca, E.; Bonezzi, C.; Arcuri, E.; Tur, R.Y.; Maltoni, M.; Visentin, M.; Gorni, G.; Martini, C.; Tirelli, W.; et al. Gabapentin for Neuropathic Cancer Pain: A Randomized Controlled Trial From the Gabapentin Cancer Pain Study Group. J. Clin. Oncol. 2004, 22, 2909–2917. [Google Scholar] [CrossRef]
- Anghelescu, D.L.; Steen, B.D.; Wu, H.; Wu, J.; Daw, N.C.; Rao, B.N.; Neel, M.D.; Navid, F. Prospective study of neuropathic pain after definitive surgery for extremity osteosarcoma in a pediatric population. Pediatr. Blood Cancer 2016, 64, e26162. [Google Scholar] [CrossRef]
- Knoerl, R.; Sohn, M.B.; Foust, M.; Francar, L.; O’Rourke, M.A.; Morrow, G.R.; Mustian, K.M.; Gauthier, L.; Gewandter, J.S. Exploring Analgesic Use Patterns Among Cancer Survivors with Chronic Chemotherapy-Induced Peripheral Neuropathy. Oncol. Nurs. Forum 2024, 51, 445–450. [Google Scholar] [CrossRef]
- Jimenez-Andrade, J.M.; Mantyh, W.G.; Bloom, A.P.; Ferng, A.S.; Geffre, C.P.; Mantyh, P.W. Bone cancer pain. Ann. New York Acad. Sci. 2010, 1198, 173–181. [Google Scholar] [CrossRef]
- Nencini, S.; Ivanusic, J. Mechanically sensitive Aδ nociceptors that innervate bone marrow respond to changes in intra-osseous pressure. J. Physiol. 2017, 595, 4399–4415. [Google Scholar] [CrossRef] [PubMed]
- Nencini, S.; Ivanusic, J.J. The Physiology of Bone Pain. How Much Do We Really Know? Front. Physiol. 2016, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Nencini, S.; Thai, J.; Ivanusic, J.J. TRPV1 activation alters the function of Aδ and C fiber sensory neurons that innervate bone. Bone 2019, 123, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Thai, J.; Trinh, P.; Habib, M.; Effendi, K.N.; Ivanusic, J.J. ASIC3 inhibition modulates inflammation-induced changes in the activity and sensitivity of Aδ and C fiber sensory neurons that innervate bone. Mol. Pain 2020, 16. [Google Scholar] [CrossRef]
- Nencini, S.; Ringuet, M.; Kim, D.-H.; Chen, Y.-J.; Greenhill, C.; Ivanusic, J.J. Mechanisms of nerve growth factor signaling in bone nociceptors and in an animal model of inflammatory bone pain. Mol. Pain 2017, 13. [Google Scholar] [CrossRef]
- Nencini, S.; Ringuet, M.; Kim, D.-H.; Greenhill, C.; Ivanusic, J.J. GDNF, Neurturin, and Artemin Activate and Sensitize Bone Afferent Neurons and Contribute to Inflammatory Bone Pain. J. Neurosci. 2018, 38, 4899–4911. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuller-Jackson, J.-P.; Hopkins, C.; Thai, J.; Lassen, M.B.; Heegaard, A.-M.; Ivanusic, J. Three-Dimensional Analysis of the Effect of Osteosarcoma on Sensory Nerves Innervating the Femur in a Murine Model of Osteosarcoma-Induced Bone Pain. Cancers 2025, 17, 3533. https://doi.org/10.3390/cancers17213533
Fuller-Jackson J-P, Hopkins C, Thai J, Lassen MB, Heegaard A-M, Ivanusic J. Three-Dimensional Analysis of the Effect of Osteosarcoma on Sensory Nerves Innervating the Femur in a Murine Model of Osteosarcoma-Induced Bone Pain. Cancers. 2025; 17(21):3533. https://doi.org/10.3390/cancers17213533
Chicago/Turabian StyleFuller-Jackson, John-Paul, Chelsea Hopkins, Jenny Thai, Mie Brandt Lassen, Anne-Marie Heegaard, and Jason Ivanusic. 2025. "Three-Dimensional Analysis of the Effect of Osteosarcoma on Sensory Nerves Innervating the Femur in a Murine Model of Osteosarcoma-Induced Bone Pain" Cancers 17, no. 21: 3533. https://doi.org/10.3390/cancers17213533
APA StyleFuller-Jackson, J.-P., Hopkins, C., Thai, J., Lassen, M. B., Heegaard, A.-M., & Ivanusic, J. (2025). Three-Dimensional Analysis of the Effect of Osteosarcoma on Sensory Nerves Innervating the Femur in a Murine Model of Osteosarcoma-Induced Bone Pain. Cancers, 17(21), 3533. https://doi.org/10.3390/cancers17213533





