Pharmacokinetics and Pharmacodynamics of Perfluorooctane Sulfonate (PFOS) and Its Role in the Development and Progression of Prostate, Ovarian and Breast Cancers
Simple Summary
Abstract
1. Introduction
2. Emission and Environmental Distribution of PFOS
3. Mechanisms of PFOS Toxicokinetics and Dynamics
3.1. Absorption and Distribution of PFOS in Human Tissues and Organs
3.2. PFOS–DNA Interaction Through Non-Covalent Binding
3.3. Disruption of Lipid Metabolism and Mitochondrial Function
3.4. Oxidative Stress and Reactive Oxygen Species (ROS) Generation
3.5. DNA Damage and Epigenetic Modification
3.6. Endocrine Disruption
3.7. Immunosuppressive Effects and Inflammatory Responses of PFOS
4. PFOS and Carcinogenesis
4.1. PFOS and the Hallmarks of Carcinogenesis
4.2. Prostate Cancer: Mechanism and Carcinogenesis
Evidence of PFOS in Prostate Cancer Development
4.3. Breast Cancer: Mechanism and Carcinogenesis
Evidence of PFOS in Breast Cancer Development
4.4. Ovarian Cancer: Mechanism and Carcinogenesis
Evidence of PFOS in Ovarian Cancer Development
| S/N | Study/Model | Findings | References |
|---|---|---|---|
| 1. | Human prostate stem/progenitor cell (SPC) population | PFOS increased carcinogenic risk, tumor progression and spheroid formations in the prostate gland as well as increased the expression of PPARα and RXRα. | Wen et al. [211] |
| 2. | Epidermal pre-malignant stem cells | PFOS upregulated serine and glycine metabolism and increased the growth of PCa. | Imir et al. [226]. |
| 3. | Case–control study | Significant association between elevated serum PFOS levels and an increased risk of BCa | Bonefeld-Jorgensen et al. [26]. |
| 4. | Juvenile rainbow trout (Oncorhynchus mykiss) | PFOS could interact with ERα and ERβ, potentially enhancing ER-dependent transcriptional activity | Benninghoff et al. [250] |
| 5. | H295R cells | PFOS serves as an ER agonist and Thyroid hormone receptor (THR) antagonist, as well as increased estradiol (E2) levels in H295R cells. | Du et al. [31] |
| 6. | MVLN cells | PFOS significantly induced the ER transactivity while antagonizing the activity of AR. | Kjeldsen et al. [251] |
| 7. | MCF-10A | PFOS elevated cyclin D1 and D2 levels, increased the global DNA methylation, as well as reduced the levels of the CDK inhibitor p21, occluding, E-cadherin and β-integrins. | Pierozan et al. [254] |
| 8. | Case–control study | Women aged 50 or younger were particularly susceptible to PFOS, with a stronger association observed in estrogen receptor (ER)-positive tumors within this age group. | Tsai et al. [275] |
| 9. | Case–control study | There is a strong link between PFOS exposure and elevated BCa risk. | Bonefeld-Jorgensen et al. [26]. |
| 10. | Case–control study | PFOS triggered hormonal fluctuations in adolescents aged 12–17 years. | Tsai et al. [275]. |
| 11. | Case–control study | Significant increase in PFOS levels in cases compared to the controls, indicating the correlation of PFOS with BCa. | Wielsøe et al. [281] |
| 12. | T47D human BCa cells | PFOS promoted the estrogenic effects of 17β-estradiol in T47D human BCa cells. | Sonthithai et al. [289] |
| 13. | human endometrial stromal cells (hESCs) | PFOS may drive OC by decreasing the expression levels of endometrial tolerance-related proteins Homeobox A10 (HOXA10) and integrin beta 3 (ITGB3), while increasing the expression level of Forkhead box 01 (FOXO1) protein. | Ren et al. [307]. |
| 14. | Age-Related Associations | PFOS revealed a positive association with PCa in men aged ≥ 70 years. | Alyssa et al. [216] |
| 15. | Chinese women study | PFOS exposure decreased serum E2 and prolactin levels and increased FSH levels, disrupted ovarian steriodogenesis, and caused premature ovarian insufficiency | Zhang et al. [311] |
| 16. | OC risk | PFOS exposure is associated with OC incidence. | Jones et al. [312] |
| 17. | Sex Hormones | PFOS significantly impacted the serum E2 levels among women aged 42–65 | Knox et al. [310] |
5. Regulatory Actions Against PFOS
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AVPV | Anteroventral Periventricular Nucleus |
| AFFF | Aqueous Film Forming Foam |
| BAX | BCL2 Associated X Protein |
| BCL-2 | B-Cell Lymphoma 2 |
| BRCA1 | Breast Cancer 1 Gene |
| BRCA2 | Breast Cancer 2 Gene |
| cAMP | Cyclic Adenosine Monophosphate |
| CDK | Cyclin-Dependent Kinase |
| CREB | cAMP Response Element-Binding Protein |
| CYP | Cytochrome P450 (CYP450) |
| DNA | Deoxyribonucleic Acid |
| E2 | Estradiol |
| ER | Estrogen Receptor |
| ERK | Extracellular Signal-Regulated Kinase |
| FOXO1 | Forkhead Box Protein O1 |
| FSH | Follicle-Stimulating Hormone |
| FSH. | Same as above (Follicle-Stimulating Hormone) |
| GnRH | Gonadotropin-Releasing Hormone |
| GPR30 | G Protein-Coupled Estrogen Receptor 30 (also known as GPER1) |
| GSH | Glutathione |
| GSK-3β | Glycogen Synthase Kinase 3 Beta |
| GST | Glutathione S-Transferase |
| H3K14 | Histone H3 Lysine 14 (site of acetylation or methylation) |
| HDACs | Histone Deacetylases |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HO | Heme Oxygenase |
| HO-1 | Heme Oxygenase-1 |
| IARC | International Agency for Research on Cancer |
| IGF1R | Insulin-Like Growth Factor 1 Receptor |
| ITGB3 | Integrin Beta 3 |
| JNK | c-Jun N-terminal Kinase |
| LBD | Ligand-Binding Domain |
| LH | Luteinizing Hormone |
| MAPKs | Mitogen-Activated Protein Kinases |
| MCF-10A | Human Non-Tumorigenic Mammary Epithelial Cell Line |
| MMP | Matrix Metalloproteinase |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NF-κB, | Same as above |
| p38 | p38 Mitogen-Activated Protein Kinase (MAPK) |
| P4 | Progesterone (also known as Pregn-4-ene-3,20-dione) |
| PARP | Poly (ADP-Ribose) Polymerase |
| PFAS | Per- and Polyfluoroalkyl Substances |
| PFHpA | Perfluoroheptanoic Acid |
| PFOA | Perfluorooctanoic Acid |
| PFOS | Perfluorooctane Sulfonate |
| PFPA | Perfluoropentanoic Acid |
| PPARα | Peroxisome Proliferator-Activated Receptor Alpha |
| PIN | Prostatic Intraepithelial Neoplasia |
| ATP | Adenosine Triphosphate |
| PKA | Protein Kinase A |
| POI | Primary Ovarian Insufficiency |
| PR | Progesterone Receptor |
| PSA | Prostate-Specific Antigen |
| PTEN | Phosphatase and Tensin Homolog |
| ROS | Reactive Oxygen Species |
| StAR | Steroidogenic Acute Regulatory Protein |
| TNBC | Triple-Negative Breast Cancer |
| VEGF | Vascular Endothelial Growth Factor |
| WNT1 | Wingless-Type MMTV Integration Site Family Member 1 |
| XA10 | Xanthine Dehydrogenase/Aldehyde Oxidase 1 (also known as xanthine oxidase homolog, depending on context) |
References
- Giesy, J.P.; Kannan, K. Perfluorochemical surfactants in the environment. Environ. Sci. Technol. 2002, 36, 146a–152a. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Y.; Liao, C.; Cai, Y.; Jiang, G. Perspectives on the Inclusion of Perfluorooctane Sulfonate into the Stockholm Convention on Persistent Organic Pollutants. Environ. Sci. Technol. 2009, 43, 5171–5175. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Huang, L.; Li, Y.; Zhang, Z.; Mu, R.; Liu, C.; Hu, S.; Xiao, Y.; Xu, M. A Review of Treatment Technologies for Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in Water. Processes 2023, 11, 2260. [Google Scholar] [CrossRef]
- Li, M.; Jin, Y.-T.; Yan, J.-F.; Liu, Z.; Feng, N.-X.; Han, W.; Huang, L.-W.; Li, Q.-K.; Yeung, K.-L.; Zhou, S.-Q.; et al. Exploration of perfluorooctane sulfonate degradation properties and mechanism via electron-transfer dominated radical process. Water Res. 2022, 215, 118259. [Google Scholar] [CrossRef]
- Li, M.; Mo, C.-H.; Luo, X.; He, K.-Y.; Yan, J.-F.; Wu, Q.; Yu, P.-F.; Han, W.; Feng, N.-X.; Yeung, K.L.; et al. Exploring key reaction sites and deep degradation mechanism of perfluorooctane sulfonate via peroxymonosulfate activation under electrocoagulation process. Water Res. 2021, 207, 117849. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, Y.; Wang, L.; Wang, P.; Wang, T.; Ravindran, B.; Mishra, S.; Chen, S.; Cui, X.; Yang, Y.; et al. Elucidating the degradation mechanisms of perfluorooctanoic acid and perfluorooctane sulfonate in various environmental matrices: A review of green degradation pathways. Environ. Geochem. Health 2024, 46, 349. [Google Scholar] [CrossRef]
- Bruton, T.A.; Sedlak, D.L. Treatment of perfluoroalkyl acids by heat-activated persulfate under conditions representative of in situ chemical oxidation. Chemosphere 2018, 206, 457–464. [Google Scholar] [CrossRef]
- Wang, L.; Lu, J.; Li, L.; Wang, Y.; Huang, Q. Effects of chloride on electrochemical degradation of perfluorooctanesulfonate by Magnéli phase Ti4O7 and boron doped diamond anodes. Water Res. 2020, 170, 115254. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Andaya, C.; Burant, A.; Condee, C.W.; Urtiaga, A.; Strathmann, T.J.; Higgins, C.P. Electrochemical treatment of perfluorooctanoic acid and perfluorooctane sulfonate: Insights into mechanisms and application to groundwater treatment. Chem. Eng. J. 2017, 317, 424–432. [Google Scholar] [CrossRef]
- Huang, S.; Jaffé, P.R. Defluorination of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) by Acidimicrobium sp. Strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419. [Google Scholar] [CrossRef]
- Liang, Y.; Ma, A. Investigating the degradation potential of microbial consortia for perfluorooctane sulfonate through a functional “top-down” screening approach. PLoS ONE 2024, 19, e0303904. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Zhou, T.; Wang, B.H.; Liang, T.K.; Liu, L.F. Isolation, Identification, and Biodegradation Behaviors of a Perfluorooctane Sulfonic Acid Precursor (PreFOSs) Degrading Bacterium from Contaminated Soil. Huan Jing Ke Xue 2018, 39, 3321–3328. [Google Scholar]
- Yamada, T.; Taylor, P.H.; Buck, R.C.; Kaiser, M.A.; Giraud, R.J. Thermal degradation of fluorotelomer treated articles and related materials. Chemosphere 2005, 61, 974–984. [Google Scholar] [CrossRef]
- Taylor, P.H.; Yamada, T.; Striebich, R.C.; Graham, J.L.; Giraud, R.J. Investigation of waste incineration of fluorotelomer-based polymers as a potential source of PFOA in the environment. Chemosphere 2014, 110, 17–22, Erratum in Chemosphere 2022, 298, 134601. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- Xiaopeng, C.; Jin, T. Perfluorooctane sulfonate (PFOS) causes aging damage in the liver through the mt-DNA-mediated NLRP3 signaling pathway. Ecotoxicol. Environ. Saf. 2023, 262, 115121. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhu, J.; Zhuge, S.; Yu, J.; Jiang, G. Perfluorooctane sulfonate induces hepatotoxicity through promoting inflammation, cell death and autophagy in a rat model. J. Toxicol. Sci. 2025, 50, 45–55. [Google Scholar] [CrossRef]
- Ahmad, M.; Liu, M.; Yang, Z.; Zhang, H.; Nabi, G.; Hao, Y.; Chen, L. Perfluorooctane sulfonate causes DNA damage and apoptosis via oxidative stress in umbilical cord fibroblast cells of Yangtze finless porpoise. Sci. Total Environ. 2025, 958, 178030. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ren, T.; Yu, G.; Meng, X.; Feng, L.; Li, F.; Zhang, J.; Wang, C. Unraveling the long-term gastrointestinal impact of perinatal perfluorobutane sulfonate exposure on rat offspring: Intestinal barrier dysfunction and Th17/Treg imbalance. Sci. Total Environ. 2024, 955, 176858. [Google Scholar] [CrossRef]
- Wen, Y.; Juhasz, A.; Cui, X. Regulating the absorption and excretion of perfluorooctane sulfonate and its alternatives through influencing enterohepatic circulation. Sci. Total Environ. 2024, 933, 173161. [Google Scholar] [CrossRef]
- Ling, J.; Hua, L.; Qin, Y.; Gu, T.; Jiang, S.; Zhao, J. Perfluorooctane sulfonate promotes hepatic lipid accumulation and steatosis in high-fat diet mice through AMP-activated protein kinase/acetyl-CoA carboxylase (AMPK/ACC) pathway. J. Appl. Toxicol. 2023, 43, 312–322. [Google Scholar] [CrossRef]
- Gallo, V.; Leonardi, G.; Genser, B.; Lopez-Espinosa, M.J.; Frisbee, S.J.; Karlsson, L.; Ducatman, A.M.; Fletcher, T. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ. Health Perspect. 2012, 120, 655–660. [Google Scholar] [CrossRef]
- Wu, T.; Li, Y.; Gong, L.; Lu, J.G.; Du, X.L.; Zhang, W.D.; He, X.L.; Wang, J.Q. Multi-step process of human breast carcinogenesis: A role for BRCA1, BECN1, CCND1, PTEN and UVRAG. Mol. Med. Rep. 2012, 5, 305–312. [Google Scholar] [PubMed]
- Narsinh, K.H.; Cui, J.; Papadatos, D.; Sirlin, C.B.; Santillan, C.S. Hepatocarcinogenesis and LI-RADS. Abdom. Radiol. 2018, 43, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Masutani, M.; Fujimori, H. Poly(ADP-ribosyl)ation in carcinogenesis. Mol. Asp. Med. 2013, 34, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Bonefeld-Jorgensen, E.C.; Long, M.; Bossi, R.; Ayotte, P.; Asmund, G.; Krüger, T.; Ghisari, M.; Mulvad, G.; Kern, P.; Nzulumiki, P.; et al. Perfluorinated compounds are related to breast cancer risk in Greenlandic Inuit: A case control study. Environ. Health 2011, 10, 88. [Google Scholar] [CrossRef]
- Pierozan, P.; Höglund, A.; Theodoropoulou, E.; Karlsson, O. Perfluorooctanesulfonic acid (PFOS) induced cancer related DNA methylation alterations in human breast cells: A whole genome methylome study. Sci. Total Environ. 2024, 949, 174864. [Google Scholar] [CrossRef]
- Pierozan, P.; Karlsson, O. PFOS induces proliferation, cell-cycle progression, and malignant phenotype in human breast epithelial cells. Arch. Toxicol. 2018, 92, 705–716. [Google Scholar] [CrossRef]
- Cunat, S.; Hoffmann, P.; Pujol, P. Estrogens and epithelial ovarian cancer. Gynecol. Oncol. 2004, 94, 25–32. [Google Scholar] [CrossRef]
- Tachachartvanich, P.; Singam, E.R.A.; Durkin, K.A.; Furlow, J.D.; Smith, M.T.; La Merrill, M.A. In Vitro characterization of the endocrine disrupting effects of per- and poly-fluoroalkyl substances (PFASs) on the human androgen receptor. J. Hazard. Mater. 2022, 429, 128243. [Google Scholar] [CrossRef]
- Du, G.; Hu, J.; Huang, H.; Qin, Y.; Han, X.; Wu, D.; Song, L.; Xia, Y.; Wang, X. Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo. Environ. Toxicol. Chem. 2013, 32, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.C.; Rhee, J.; Berndt, S.I.; Moore, S.C.; Freedman, N.D.; Jones, R.R.; Silverman, D.T.; Gierach, G.L.; Hofmann, J.N.; Purdue, M.P. Serum perfluorooctane sulfonate and perfluorooctanoate and risk of postmenopausal breast cancer according to hormone receptor status: An analysis in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int. J. Cancer 2023, 153, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Ducatman, A.; Ward, R.; Leonard, S.; Bukowski, V.; Lan Guo, N.; Shi, X.; Vallyathan, V.; Castranova, V. Perfluorooctane sulfonate (PFOS) induces reactive oxygen species (ROS) production in human microvascular endothelial cells: Role in endothelial permeability. J. Toxicol. Environ. Health A 2010, 73, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Jiang, X.; Liu, Y.; Junaid, M.; Ahmad, M.; Bi, C.; Guo, W.; Jiang Ca Liu, S. Chronic environmental level exposure to perfluorooctane sulfonate overshadows graphene oxide to induce apoptosis through activation of the ROS-p53-caspase pathway in marine medaka Oryzias melastigma. Chemosphere 2024, 365, 143374. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef]
- Owumi, S.; Agbarogi, H.; Oluwawibe, B.J.; Otunla, M.T.; Anifowose, M.M.; Arunsi, U.O. Modulation of the Nrf-2 and HO-1 signalling axis is associated with Betaine’s abatement of fluoride-induced hepatorenal toxicities in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 7725–7745. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Chen, A.; Huang, H.; Fang, S.; Hang, Q. ROS. A “booster” for chronic inflammation and tumor metastasis. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189175. [Google Scholar] [CrossRef]
- Porter, C.M.; Shrestha, E.; Peiffer, L.B.; Sfanos, K.S. The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 345–354. [Google Scholar] [CrossRef]
- Cole, S.W. Chronic inflammation and breast cancer recurrence. J. Clin. Oncol. 2009, 27, 3418–3419. [Google Scholar] [CrossRef] [PubMed]
- Jiajing, C.; Shuqi, Y.; Haoyan, M.; Pingwei, W.; Dongge, L.; Yanping, L.; Qianqian, C.; Saleh, F.; Shuping, R. Perfluorooctane sulfonate causes damage to L-02 cells via Wnt/β-catenin signal path and endoplasmic reticulum stress pathway. Toxicol. Ind. Health 2024, 40, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, L.; Peng, B.-X.; Lei, Y.; Li, M.; Guo, L.-H. Perfluorooctane sulfonate promotes the migration of colorectal cancer cells by inducing epithelial-mesenchymal transition. J. Environ. Sci. 2024, 145, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef]
- Maxwell, D.L.; Oluwayiose, O.A.; Houle, E.; Roth, K.; Nowak, K.; Sawant, S.; Paskavitz, A.L.; Liu, W.; Gurdziel, K.; Petriello, M.C.; et al. Mixtures of per- and polyfluoroalkyl substances (PFAS) alter sperm methylation and long-term reprogramming of offspring liver and fat transcriptome. Environ. Int. 2024, 186, 108577. [Google Scholar] [CrossRef]
- Wan, Y.J.; Li, Y.Y.; Xia, W.; Chen, J.; Lv, Z.Q.; Zeng, H.C.; Zhang, L.; Yang, W.J.; Chen, T.; Lin, Y.; et al. Alterations in tumor biomarker GSTP gene methylation patterns induced by prenatal exposure to PFOS. Toxicology 2010, 274, 57–64. [Google Scholar] [CrossRef]
- van den Dungen, M.W.; Murk, A.J.; Kok, D.E.; Steegenga, W.T. Persistent organic pollutants alter DNA methylation during human adipocyte differentiation. Toxicol. Vitr. 2017, 40, 79–87. [Google Scholar] [CrossRef]
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer development, progression, and therapy: An epigenetic overview. Int. J. Mol. Sci. 2013, 14, 21087–21113. [Google Scholar] [CrossRef]
- Darwiche, N. Epigenetic mechanisms and the hallmarks of cancer: An intimate affair. Am. J. Cancer Res. 2020, 10, 1954–1978. [Google Scholar]
- Mancini, F.R.; Cano-Sancho, G.; Gambaretti, J.; Marchand, P.; Boutron-Ruault, M.C.; Severi, G.; Arveux, P.; Antignac, J.P.; Kvaskoff, M. Perfluorinated alkylated substances serum concentration and breast cancer risk: Evidence from a nested case-control study in the French E3N cohort. Int. J. Cancer 2020, 146, 917–928. [Google Scholar] [CrossRef]
- Rhee, J.; Barry, K.H.; Huang, W.Y.; Sampson, J.N.; Hofmann, J.N.; Silverman, D.T.; Calafat, A.M.; Botelho, J.C.; Kato, K.; Purdue, M.P.; et al. A prospective nested case-control study of serum concentrations of per- and polyfluoroalkyl substances and aggressive prostate cancer risk. Environ. Res. 2023, 228, 115718. [Google Scholar] [CrossRef]
- Zushi, Y.; Masunaga, S. GIS-based source identification and apportionment of diffuse water pollution: Perfluorinated compound pollution in the Tokyo Bay basin. Chemosphere 2011, 85, 1340–1346. [Google Scholar] [CrossRef]
- Chen, H.; Reinhard, M.; Nguyen, T.V.; You, L.; He, Y.; Gin, K.Y. Characterization of occurrence, sources and sinks of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in a tropical urban catchment. Environ. Pollut. 2017, 227, 397–405. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, B.; Wang, W.; Li, W.C.; Huang, J.; Deng, S.B.; Wang, Y.J.; Yu, G. Occurrence and source apportionment of Per- and poly-fluorinated compounds (PFCs) in North Canal Basin, Beijing. Sci. Rep. 2016, 6, 36683. [Google Scholar] [CrossRef]
- D’Ambro, E.L.; Murphy, B.N.; Bash, J.O.; Gilliam, R.C.; Pye, H.O.T. Predictions of PFAS regional-scale atmospheric deposition and ambient air exposure. Sci. Total Environ. 2023, 902, 166256. [Google Scholar] [CrossRef]
- Koch, A.; Kärrman, A.; Yeung, L.W.Y.; Jonsson, M.; Ahrens, L.; Wang, T. Point source characterization of per- and polyfluoroalkyl substances (PFASs) and extractable organofluorine (EOF) in freshwater and aquatic invertebrates. Environ. Sci. Process Impacts 2019, 21, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Yukioka, S.; Tanaka, S.; Yeung, L.W.Y.; Kärrman, A.; Wang, T. Characterization of an AFFF impacted freshwater environment using total fluorine, extractable organofluorine and suspect per- and polyfluoroalkyl substance screening analysis. Chemosphere 2021, 276, 130179. [Google Scholar] [CrossRef]
- Xie, S.; Wang, T.; Liu, S.; Jones, K.C.; Sweetman, A.J.; Lu, Y. Industrial source identification and emission estimation of perfluorooctane sulfonate in China. Environ. Int. 2013, 52, 1–8. [Google Scholar] [CrossRef]
- Gebbink, W.A.; van Leeuwen, S.P.J. Environmental contamination and human exposure to PFASs near a fluorochemical production plant: Review of historic and current PFOA and GenX contamination in the Netherlands. Environ. Int. 2020, 137, 105583. [Google Scholar] [CrossRef] [PubMed]
- Dalmijn, J.; Glüge, J.; Scheringer, M.; Cousins, I.T. Emission inventory of PFASs and other fluorinated organic substances for the fluoropolymer production industry in Europe. Environ. Sci. Process. Impacts 2024, 26, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Lian, Y.; Sun, X.; Wu, Y.; Qiao, L.; Wang, M. Source, transportation, bioaccumulation, distribution and food risk assessment of perfluorinated alkyl substances in vegetables: A review. Food Chem. 2021, 349, 129137. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.; Choi, S.; Choo, G.; Yang, M.; Park, J.H.; Oh, J.E. Organophosphate Flame Retardants and Perfluoroalkyl Substances in Drinking Water Treatment Plants from Korea: Occurrence and Human Exposure. Int. J. Environ. Res. Public Health 2021, 18, 2645. [Google Scholar] [CrossRef] [PubMed]
- Pico, Y.; Blasco, C.; Farré, M.; Barceló, D. Occurrence of perfluorinated compounds in water and sediment of L’Albufera Natural Park (València, Spain). Environ. Sci. Pollut. Res. Int. 2012, 19, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Shoeib, M.; Harner, T.; Lee, S.C.; Guo, R.; Reiner, E.J. Wastewater treatment plant and landfills as sources of polyfluoroalkyl compounds to the atmosphere. Environ. Sci. Technol. 2011, 45, 8098–8105. [Google Scholar] [CrossRef]
- Paul, A.G.; Jones, K.C.; Sweetman, A.J. A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ. Sci. Technol. 2009, 43, 386–392. [Google Scholar] [CrossRef]
- Scheringer, M.; Trier, X.; Cousins, I.T.; de Voogt, P.; Fletcher, T.; Wang, Z.; Webster, T.F. Helsingør statement on poly- and perfluorinated alkyl substances (PFASs). Chemosphere 2014, 114, 337–339. [Google Scholar] [CrossRef]
- D’Hollander, W.; de Voogt, P.; De Coen, W.; Bervoets, L. Perfluorinated substances in human food and other sources of human exposure. Rev. Environ. Contam. Toxicol. 2010, 208, 179–215. [Google Scholar]
- Mohamed, B.A.; Nicomel, N.R.; Hamid, H.; Li, L.Y. Using circular economy principles in the optimisation of sludge-based activated carbon production for the removal of perfluoroalkyl substances. Sci. Total Environ. 2023, 874, 162392. [Google Scholar] [CrossRef]
- Sepulvado, J.G.; Blaine, A.C.; Hundal, L.S.; Higgins, C.P. Occurrence and fate of perfluorochemicals in soil following the land application of municipal biosolids. Environ. Sci. Technol. 2011, 45, 8106–8112. [Google Scholar] [CrossRef]
- Houtz, E.F.; Higgins, C.P.; Field, J.A.; Sedlak, D.L. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environ. Sci. Technol. 2013, 47, 8187–8195. [Google Scholar] [CrossRef]
- Anderson, R.H.; Long, G.C.; Porter, R.C.; Anderson, J.K. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 2016, 150, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Houtz, E.F.; Sutton, R.; Park, J.S.; Sedlak, M. Poly- and perfluoroalkyl substances in wastewater: Significance of unknown precursors, manufacturing shifts, and likely AFFF impacts. Water Res. 2016, 95, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Houde, M.; Martin, J.W.; Letcher, R.J.; Solomon, K.R.; Muir, D.C. Biological monitoring of polyfluoroalkyl substances: A review. Environ. Sci. Technol. 2006, 40, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Nadal, M. Human exposure to per-and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef]
- Solan, M.E.; Lavado, R. Effects of short-chain per- and polyfluoroalkyl substances (PFAS) on human cytochrome P450 (CYP450) enzymes and human hepatocytes: An in vitro study. Curr. Res. Toxicol. 2023, 5, 100116. [Google Scholar] [CrossRef]
- Deepika, D.; Rovira, J.; Sabuz, Ó.; Balaguer, J.; Schuhmacher, M.; Domingo, J.L.; Kumar, V. Framework for risk assessment of PFAS utilizing experimental studies and in-silico models. Environ. Res. 2022, 208, 112722. [Google Scholar] [CrossRef]
- Starnes, H.M.; Jackson, T.W.; Rock, K.D.; Belcher, S.M. Quantitative cross-species comparison of serum albumin binding of per-and polyfluoroalkyl substances from five structural classes. Toxicol. Sci. 2024, 199, 132–149. [Google Scholar] [CrossRef]
- Olsen, G.W.; Hansen, K.J.; Stevenson, L.A.; Burris, J.M.; Mandel, J.H. Human donor liver and serum concentrations of perfluorooctanesulfonate and other perfluorochemicals. Environ. Sci. Technol. 2003, 37, 888–891. [Google Scholar] [CrossRef]
- Maestri, L.; Negri, S.; Ferrari, M.; Ghittori, S.; Fabris, F.; Danesino, P.; Imbriani, M. Determination of perfluorooctanoic acid and perfluorooctanesulfonate in human tissues by liquid chromatography/single quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Up Minute Res. Mass Spectrom. 2006, 20, 2728–2734. [Google Scholar] [CrossRef]
- Völkel, W.; Eisenmenger, W.; Fromme, H. Perfluorinated substances in liver tissue of humans. In Report of a Pilot Study; Department of Environmental Medicine, Bavarian Health and Food Safety Authority: Oberschleissheim, Germany, 2007. [Google Scholar]
- Liu, Y.; Lin, N.; Dai, C.; Xu, J.; Zhang, Y.; Xu, M.; Wang, F.; Li, Y.; Chen, D. Occurrence and distribution of per-and polyfluoroalkyl substances (PFASs) in human livers with liver cancer. Environ. Res. 2021, 202, 111775. [Google Scholar] [CrossRef] [PubMed]
- Pirali, B.; Negri, S.; Chytiris, S.; Perissi, A.; Villani, L.; La Manna, L.; Cottica, D.; Ferrari, M.; Imbriani, M.; Rotondi, M. Perfluorooctane sulfonate and perfluorooctanoic acid in surgical thyroid specimens of patients with thyroid diseases. Thyroid 2009, 19, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Mamsen, L.S.; Björvang, R.D.; Mucs, D.; Vinnars, M.-T.; Papadogiannakis, N.; Lindh, C.H.; Andersen, C.Y.; Damdimopoulou, P. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ. Int. 2019, 124, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, B.; Yuan, Y.; Chen, N.; Guo, H.; Zhang, H.; Zhang, Z. Integrated computational analysis of molecular mechanisms underlying perfluorooctane sulfonic acid induced thyroid toxicity. Sci. Rep. 2025, 15, 7920. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Fei, X.-C.; Ma, Y.-S.; Gao, H.-W. Binding of PFOS to serum albumin and DNA. insight into the molecular toxicity of perfluorochemicals. BMC Mol. Biol. 2009, 10, 16. [Google Scholar] [CrossRef]
- Arshad, N.; Abbas, N.; Perveen, F.; Mirza, B.; Almuhaini, A.M.; Alkahtani, S. Molecular docking analysis and spectroscopic investigations of zinc(II), nickel(II) N-phthaloyl-β-alanine complexes for DNA binding: Evaluation of antibacterial and antitumor activities. J. Saudi Chem. Soc. 2021, 25, 101323. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Thirunavukarasu, K.; Tamizhmani, P.; Michael, A.A.; Velusamy, T. Toxicological Impact of Chronic Chlorpyrifos Exposure: DNA Damage and Epigenetic Alterations Induces Neoplastic Transformation of Liver Cells. Biochem. Biophys. Res. Commun. 2025, 746, 151287. [Google Scholar] [CrossRef]
- Behr, A.C.; Plinsch, C.; Braeuning, A.; Buhrke, T. Activation of human nuclear receptors by perfluoroalkylated substances (PFAS). Toxicol. Vitr. 2020, 62, 104700. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D.; Yan, S.; Cui, J.; Liang, Y.; Ren, S. Adverse Effects of Perfluorooctane Sulfonate on the Liver and Relevant Mechanisms. Toxics 2022, 10, 265. [Google Scholar] [CrossRef]
- Chang, E.T.; Adami, H.O.; Boffetta, P.; Cole, P.; Starr, T.B.; Mandel, J.S. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and cancer risk in humans. Crit. Rev. Toxicol. 2014, 44 (Suppl. 1), 1–81. [Google Scholar] [CrossRef]
- Sadrabadi, F.; Alarcan, J.; Sprenger, H.; Braeuning, A.; Buhrke, T. Impact of perfluoroalkyl substances (PFAS) and PFAS mixtures on lipid metabolism in differentiated HepaRG cells as a model for human hepatocytes. Arch. Toxicol. 2024, 98, 507–524. [Google Scholar] [CrossRef]
- Kersten, S.; Stienstra, R. The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie 2017, 136, 75–84. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Qiu, T.; Wu, J.; Sun, X.; Jiang, L.; Liu, X.; Yang, G.; Cao, J.; Yao, X. Mitochondrial iron overload mediated by cooperative transfer of plasma membrane ATP5B and TFR2 to mitochondria triggers hepatic insulin resistance under PFOS exposure. Ecotoxicol. Environ. Saf. 2023, 253, 114662. [Google Scholar] [CrossRef]
- Wei, K.N.; Wang, X.J.; Zeng, Z.C.; Gu, R.T.; Deng, S.Z.; Jiang, J.; Xu, C.L.; Li, W.; Wang, H.L. Perfluorooctane sulfonate affects mouse oocyte maturation in vitro by promoting oxidative stress and apoptosis induced bymitochondrial dysfunction. Ecotoxicol. Environ. Saf. 2021, 225, 112807. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Liu, N.; Xu, Y.; Qiao, H. Perfluorononanoic acid impedes mouse oocyte maturation by inducing mitochondrial dysfunction and oxidative stress. Reprod. Toxicol. 2021, 104, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Sun, W.; Sun, H.; Zhang, L. Perfluorooctane sulfonate continual exposure impairs glucose-stimulated insulin secretion via SIRT1-induced upregulation of UCP2 expression. Environ. Pollut. 2021, 278, 116840. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Mishra, J.S.; Yadav, P.; Dangudubiyyam, S.V.; Blesson, C.S.; Kumar, S. PFOS Impairs Mitochondrial Biogenesis and Dynamics and Reduces Oxygen Consumption in Human Trophoblasts. J. Environ. Sci. Public Health 2023, 7, 164–175. [Google Scholar] [CrossRef]
- Liu, Y.; Eliot, M.N.; Papandonatos, G.D.; Kelsey, K.T.; Fore, R.; Langevin, S.; Buckley, J.; Chen, A.; Lanphear, B.P.; Cecil, K.M.; et al. Gestational Perfluoroalkyl Substance Exposure and DNA Methylation at Birth and 12 Years of Age: A Longitudinal Epigenome-Wide Association Study. Environ. Health Perspect. 2022, 130, 37005. [Google Scholar] [CrossRef]
- Xia, W.; Wan, Y.; Li, Y.Y.; Zeng, H.; Lv, Z.; Li, G.; Wei, Z.; Xu, S.Q. PFOS prenatal exposure induce mitochondrial injury and gene expression change in hearts of weaned SD rats. Toxicology 2011, 282, 23–29. [Google Scholar] [CrossRef]
- Owumi, S.; Otunla, M.; Arunsi, U.; Asogwa, E.; Chimezie, J.; Babalola, J.O.; Altayyar, A.; Owoeye, O. Sub-chronic Berberine supplementation in prepubertal male rats relieved pro-inflammatory stressors and enhanced reproductive functional parameters. Discov. Mol. 2024, 1, 7. [Google Scholar] [CrossRef]
- Alsharif, I.A.; Fayed, H.M.; Abdel-Rahman, R.F.; Abd-Elsalam, R.M.; Ogaly, H.A. Miconazole Mitigates Acetic Acid-Induced Experimental Colitis in Rats: Insight into Inflammation, Oxidative Stress and Keap1/Nrf-2 Signaling Crosstalk. Biology 2022, 11, 303. [Google Scholar] [CrossRef]
- Ijaz, M.U.; Shahid, H.; Hayat, M.F.; Khan, H.A.; Al-Ghanim, K.A.; Riaz, M.N. The therapeutic potential of isosakuranetin against perfluorooctane sulfonate instigated cardiac toxicity via modulating Nrf-2/Keap-1 pathway, inflammatory, apoptotic, and histological profile. Cell Biochem. Funct. 2024, 42, e4060. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, G.; Li, M.; Huo, M.; Zong, W.; Liu, R. Probing the Cell Apoptosis Pathway Induced by Perfluorooctanoic Acid and Perfluorooctane Sulfonate at the Subcellular and Molecular Levels. J. Agric. Food Chem. 2020, 68, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yu, J.; Zhuge, S.; Chen, H.; Zhang, L.; Jiang, G. Oxidative stress and Cx43-mediated apoptosis are involved in PFOS-induced nephrotoxicity. Toxicology 2022, 478, 153283. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Jiang, Y.; Chen, P.; Xiao, F.; Zhang, J.; Ma, Y.; Chen, T. PFOS and PFOSA induce oxidative stress-mediated cardiac defects in zebrafish via PPARγ and AHR pathways, respectively. Sci. Total Environ. 2024, 951, 175716. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Tang, Y.; Zhao, Y.; Fang, H.; Zhang, Y.; Hou, X.; Tan, H.; Yu, S.; Zhang, H.; et al. PFOS Exposure Promotes Hepatotoxicity in Quails by Exacerbating Oxidative Stress and Inflammation-Induced Apoptosis through Activating TLR4/MyD88/NF-κb Signaling. ACS Omega 2024, 9, 25370–25380. [Google Scholar] [CrossRef]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef]
- Mao, Z.; Xia, W.; Wang, J.; Chen, T.; Zeng, Q.; Xu, B.; Li, W.; Chen, X.; Xu, S. Perfluorooctane sulfonate induces apoptosis in lung cancer A549 cells through reactive oxygen species-mediated mitochondrion-dependent pathway. J. Appl. Toxicol. 2013, 33, 1268–1276. [Google Scholar] [CrossRef]
- Lee, Y.G.; Chou, H.C.; Chen, Y.T.; Tung, S.Y.; Ko, T.L.; Buyandelger, B.; Wen, L.L.; Juan, S.H. L-Carnitine reduces reactive oxygen species/endoplasmic reticulum stress and maintains mitochondrial function during autophagy-mediated cell apoptosis in perfluorooctanesulfonate-treated renal tubular cells. Sci. Rep. 2022, 12, 4673. [Google Scholar] [CrossRef]
- Wen, L.L.; Chen, Y.T.; Lee, Y.G.; Ko, T.L.; Chou, H.C.; Juan, S.H. Perfluorooctane sulfonate induces autophagy-associated apoptosis through oxidative stress and the activation of extracellular signal-regulated kinases in renal tubular cells. PLoS ONE 2021, 16, e0245442. [Google Scholar] [CrossRef]
- Xing, J.; Wang, G.; Zhao, J.; Wang, E.; Yin, B.; Fang, D.; Zhao, J.; Zhang, H.; Chen, Y.Q.; Chen, W. Toxicity assessment of perfluorooctane sulfonate using acute and subchronic male C57BL/6J mouse models. Environ. Pollut. 2016, 210, 388–396. [Google Scholar] [CrossRef]
- Cichoz-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Khansari, M.R.; Yousefsani, B.S.; Kobarfard, F.; Faizi, M.; Pourahmad, J. In vitro toxicity of perfluorooctane sulfonate on rat liver hepatocytes: Probability of distructive binding to CYP 2E1 and involvement of cellular proteolysis. Environ. Sci. Pollut. Res. Int. 2017, 24, 23382–23388. [Google Scholar] [CrossRef]
- Renu, K.; Chakraborty, R.; Myakala, H.; Koti, R.; Famurewa, A.C.; Madhyastha, H.; Vellingiri, B.; George, A.; Valsala Gopalakrishnan, A. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium)—Induced hepatotoxicity—A review. Chemosphere 2021, 271, 129735. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Wu, W.; Ge, S.; Jia, R.; Lin, T.; Yuan, Y.; Kuang, H.; Yang, B.; Wu, L.; Wei, J.; et al. Naringin protects against perfluorooctane sulfonate-induced liver injury by modulating NRF2 and NF-kappaB in mice. Int. Immunopharmacol. 2018, 65, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.S.; Rychkov, G.Y.; Barritt, G.J. TRPM2 Non-Selective Cation Channels in Liver Injury Mediated by Reactive Oxygen Species. Antioxidants 2021, 10, 1243. [Google Scholar] [CrossRef]
- Liu, C.; Chang, V.W.; Gin, K.Y.; Nguyen, V.T. Genotoxicity of perfluorinated chemicals (PFCs) to the green mussel (Perna viridis). Sci. Total Environ. 2014, 487, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Wang, J.; Dong, G.H.; Liu, M.M.; Wang, D.; Zheng, L.; Jin, Y.H. Mechanism of perfluorooctanesulfonate (PFOS)-induced apoptosis in the immunocyte. J. Immunotoxicol. 2013, 10, 49–58. [Google Scholar] [CrossRef]
- Wielsøe, M.; Long, M.; Ghisari, M.; Bonefeld-Jørgensen, E.C. Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere 2015, 129, 239–245. [Google Scholar] [CrossRef]
- Eke, D.; Çelik, A. Curcumin prevents perfluorooctane sulfonate-induced genotoxicity and oxidative DNA damage in rat peripheral blood. Drug Chem. Toxicol. 2016, 39, 97–103. [Google Scholar] [CrossRef]
- Guo, X.; Li, Q.; Shi, J.; Shi, L.; Li, B.; Xu, A.; Zhao, G.; Wu, L. Perfluorooctane sulfonate exposure causes gonadal developmental toxicity in Caenorhabditis elegans through ROS-induced DNA damage. Chemosphere 2016, 155, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L.; Kouzarides, T.; Shiekhattar, R.; Shilatifard, A. An operational definition of epigenetics. Genes. Dev. 2009, 23, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Perng, W.; Nakiwala, D.; Goodrich, J.M. What Happens In Utero Does Not Stay In Utero: A Review of Evidence for Prenatal Epigenetic Programming by Per- and Polyfluoroalkyl Substances (PFAS) in Infants, Children, and Adolescents. Curr. Environ. Health Rep. 2023, 10, 35–44. [Google Scholar] [CrossRef]
- Robinson, S.L.; Zeng, X.; Guan, W.; Sundaram, R.; Mendola, P.; Putnick, D.L.; Waterland, R.A.; Gunasekara, C.J.; Kannan, K.; Gao, C.; et al. Perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS) and DNA methylation in newborn dried blood spots in the Upstate KIDS cohort. Environ. Res. 2021, 194, 110668. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thapar, I.; Brooks, B.W. Epigenetic changes by per- and polyfluoroalkyl substances (PFAS). Environ. Pollut. 2021, 279, 116929. [Google Scholar] [CrossRef]
- Lu, Y.; Chan, Y.T.; Tan, H.Y.; Li, S.; Wang, N.; Feng, Y. Epigenetic regulation in human cancer: The potential role of epi-drug in cancer therapy. Mol. Cancer 2020, 19, 79. [Google Scholar] [CrossRef]
- Durham, J.; Tessmann, J.W.; Deng, P.; Hennig, B.; Zaytseva, Y.Y. The role of perfluorooctane sulfonic acid (PFOS) exposure in inflammation of intestinal tissues and intestinal carcinogenesis. Front. Toxicol. 2023, 5, 1244457. [Google Scholar] [CrossRef]
- Wen, Y.; Rashid, F.; Fazal, Z.; Singh, R.; Spinella, M.J.; Irudayaraj, J. Nephrotoxicity of perfluorooctane sulfonate (PFOS)-effect on transcription and epigenetic factors. Environ. Epigenetics 2022, 8, dvac010. [Google Scholar] [CrossRef]
- Nuñez, J.K.; Chen, J.; Pommier, G.C.; Cogan, J.Z.; Replogle, J.M.; Adriaens, C.; Ramadoss, G.N.; Shi, Q.; Hung, K.L.; Samelson, A.J.; et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell 2021, 184, 2503–2519.e2517. [Google Scholar] [CrossRef]
- Kulis, M.; Esteller, M. DNA methylation and cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar]
- Hervouet, E.; Peixoto, P.; Delage-Mourroux, R.; Boyer-Guittaut, M.; Cartron, P.-F. Specific or not specific recruitment of DNMTs for DNA methylation, an epigenetic dilemma. Clin. Epigenetics 2018, 10, 17. [Google Scholar] [CrossRef]
- Tian, J.; Xu, H.; Zhang, Y.; Shi, X.; Wang, W.; Gao, H.; Bi, Y. SAM targeting methylation by the methyl donor, a novel therapeutic strategy for antagonize PFOS transgenerational fertilitty toxicity. Ecotoxicol. Environ. Saf. 2019, 184, 109579. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, T.; Walker, D.I.; Thomas, D.C.; Qiu, C.; Chatzi, L.; Alderete, T.L.; Kim, J.S.; Conti, D.V.; Breton, C.V.; et al. Dysregulated lipid and fatty acid metabolism link perfluoroalkyl substances exposure and impaired glucose metabolism in young adults. Environ. Int. 2020, 145, 106091. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Huang, L.; Jiang, Y.; Xu, Y.; Zhu, M.; Chen, M. Metabolic characterizations of PFOS-induced disruptions in early embryonic development. Ecotoxicol. Environ. Saf. 2025, 293, 118024. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wei, Y.; Zhang, Z.; Wang, F.; He, J.; Wang, R.; Xu, Y.; Keerman, M.; Zhang, S.; Zhang, Y.; et al. Plasma PFOA and PFOS Levels, DNA Methylation, and Blood Lipid Levels: A Pilot Study. Environ. Sci. Technol. 2022, 56, 17039–17051. [Google Scholar] [CrossRef]
- Watkins, D.J.; Wellenius, G.A.; Butler, R.A.; Bartell, S.M.; Fletcher, T.; Kelsey, K.T. Associations between serum perfluoroalkyl acids and LINE-1 DNA methylation. Environ. Int. 2014, 63, 71–76. [Google Scholar] [CrossRef]
- McGrath, J.; Trojer, P. Targeting histone lysine methylation in cancer. Pharmacol. Ther. 2015, 150, 1–22. [Google Scholar] [CrossRef]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef]
- Arifuzzaman, S.; Khatun, M.R.; Khatun, R. Emerging of lysine demethylases (KDMs): From pathophysiological insights to novel therapeutic opportunities. Biomed. Pharmacother. 2020, 129, 110392. [Google Scholar] [CrossRef]
- Wu, X.; Deng, Y.; Zu, Y.; Yin, J. Histone demethylase KDM4C activates HIF1α/VEGFA signaling through the costimulatory factor STAT3 in NSCLC. Am. J. Cancer Res. 2020, 10, 491–506. [Google Scholar]
- Liu, W.; Zhang, X.; Wen, Y.; Anastasio, M.A.; Irudayaraj, J. A machine learning approach to elucidating PFOS-induced alterations of repressive epigenetic marks in kidney cancer cells with single-cell imaging. Environ. Adv. 2023, 11, 100344. [Google Scholar] [CrossRef]
- Wilson, C.; Krieg, A.J. KDM4B. A Nail for Every Hammer? Genes 2019, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, L.; Lin, K.; Zhang, Y.; Deng, A.; Liang, Y.; Li, C.; Wen, T. The KMT1A-GATA3-STAT3 Circuit Is a Novel Self-Renewal Signaling of Human Bladder Cancer Stem Cells. Clin. Cancer Res. 2017, 23, 6673–6685. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Fan, L.; Song, Z.; Fang, S.; Huang, M.; Chen, P. The KMT1A/TIMP3/PI3K/AKT circuit regulates tumor growth in cervical cancer. Reprod. Biol. 2022, 22, 100644. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.W.; Lee, M.H.; Jothi, M.; Mal, M.; Li, F.; Mal, A.K. Camptothecin exhibits topoisomerase1-independent KMT1A suppression and myogenic differentiation in alveolar rhabdomyosarcoma cells. Oncotarget 2018, 9, 25796–25807. [Google Scholar] [CrossRef][Green Version]
- Fuks, F.; Hurd, P.J.; Deplus, R.; Kouzarides, T. The DNA methyltransferases associate with HP1 and the SUV39H1 histone methyltransferase. Nucleic Acids Res. 2003, 31, 2305–2312. [Google Scholar] [CrossRef]
- Ghassabian, A.; Bongers-Schokking, J.J.; de Rijke, Y.B.; van Mil, N.; Jaddoe, V.W.; de Muinck Keizer-Schrama, S.M.; Hooijkaas, H.; Hofman, A.; Visser, W.; Roman, G.C.; et al. Maternal thyroid autoimmunity during pregnancy and the risk of attention deficit/hyperactivity problems in children: The Generation R Study. Thyroid 2012, 22, 178–186. [Google Scholar] [CrossRef]
- Coperchini, F.; Pignatti, P.; Lacerenza, S.; Negri, S.; Sideri, R.; Testoni, C.; de Martinis, L.; Cottica, D.; Magri, F.; Imbriani, M.; et al. Exposure to perfluorinated compounds: In vitro study on thyroid cells. Environ. Sci. Pollut. Res. 2015, 22, 2287–2294. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Leonard, J.L.; Davis, P.J. Molecular Aspects of Thyroid Hormone Actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef]
- Oetting, A.; Yen, P.M. New insights into thyroid hormone action. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 193–208. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Brent, G.A. Graves’ Disease. N. Engl. J. Med. 2008, 358, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Bali, S.K.; Martin, R.; Almeida, N.M.S.; Saunders, C.; Wilson, A.K. Per- and Polyfluoroalkyl (PFAS) Disruption of Thyroid Hormone Synthesis. ACS Omega 2024, 9, 39554–39563. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Strazzeri, C.; Rhoden, K.J. Perfluorooctane sulfonic acid, a persistent organic pollutant, inhibits iodide accumulation by thyroid follicular cells in vitro. Mol. Cell Endocrinol. 2020, 515, 110922. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xu, P.; Chen, Y.; Wang, X. Possible mechanisms of toxic effects of per-and polyfluoroalkyl substances exposure on thyroid. J. Environ. Occup. Med. 2023, 40, 1327–1333. [Google Scholar]
- Shi, X.; Liu, C.; Wu, G.; Zhou, B. Waterborne exposure to PFOS causes disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae. Chemosphere 2009, 77, 1010–1018. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Song, M.; Kim, Y.-J.; Park, Y.-K.; Ryu, J.-C. Changes in thyroid peroxidase activity in response to various chemicals. J. Environ. Monit. 2012, 14, 2121–2126. [Google Scholar] [CrossRef]
- Xin, Y.; Ren, X.-M.; Ruan, T.; Li, C.-H.; Guo, L.-H.; Jiang, G. Chlorinated Polyfluoroalkylether Sulfonates Exhibit Similar Binding Potency and Activity to Thyroid Hormone Transport Proteins and Nuclear Receptors as Perfluorooctanesulfonate. Environ. Sci. Technol. 2018, 52, 9412–9418. [Google Scholar] [CrossRef]
- Croce, L.; Coperchini, F.; Tonacchera, M.; Imbriani, M.; Rotondi, M.; Chiovato, L. Effect of long- and short-chain perfluorinated compounds on cultured thyroid cells viability and response to TSH. J. Endocrinol. Investig. 2019, 42, 1329–1335. [Google Scholar] [CrossRef]
- DeWitt, J.C.; Germolec, D.R.; Luebke, R.W.; Johnson, V.J. Associating Changes in the Immune System with Clinical Diseases for Interpretation in Risk Assessment. Curr. Protoc. Toxicol. 2016, 67, 1811–18122. [Google Scholar] [CrossRef]
- vonderEmbse, A.N.; DeWitt, J.C. Developmental Immunotoxicity (DIT) Testing: Current Recommendations and the Future of DIT Testing. Methods Mol. Biol. 2018, 1803, 47–56. [Google Scholar]
- Fang, C.; Huang, Q.; Ye, T.; Chen, Y.; Liu, L.; Kang, M.; Lin, Y.; Shen, H.; Dong, S. Embryonic exposure to PFOS induces immunosuppression in the fish larvae of marine medaka. Ecotoxicol. Environ. Saf. 2013, 92, 104–111. [Google Scholar] [CrossRef]
- Pachkowski, B.; Post, G.B.; Stern, A.H. The derivation of a Reference Dose (RfD) for perfluorooctane sulfonate (PFOS) based on immune suppression. Environ. Res. 2019, 171, 452–469. [Google Scholar] [CrossRef]
- Grandjean, P.; Andersen, E.W.; Budtz-Jørgensen, E.; Nielsen, F.; Mølbak, K.; Weihe, P.; Heilmann, C. Serum Vaccine Antibody Concentrations in Children Exposed to Perfluorinated Compounds. JAMA 2012, 307, 391–397. [Google Scholar] [CrossRef]
- Grandjean, P.; Heilmann, C.; Weihe, P.; Nielsen, F.; Mogensen, U.B.; Budtz-Jørgensen, E. Serum Vaccine Antibody Concentrations in Adolescents Exposed to Perfluorinated Compounds. Environ. Health Perspect. 2017, 125, 077018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xue, L.; Deji, Z.; Wang, X.; Liu, P.; Lu, J.; Zhou, R.; Huang, Z. Effects of exposure to per- and polyfluoroalkyl substances on vaccine antibodies: A systematic review and meta-analysis based on epidemiological studies. Environ. Pollut. 2022, 306, 119442. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, V.; Bil, W.; Vandebriel, R.; Granum, B.; Luijten, M.; Lindeman, B.; Grandjean, P.; Kaiser, A.M.; Hauzenberger, I.; Hartmann, C.; et al. Consideration of pathways for immunotoxicity of per- and polyfluoroalkyl substances (PFAS). Environ. Health 2023, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Pan, Y.; Bin, L.; Liu, Y.; Huang, W.; Li, R.; Lai, K.P. Immunotoxicity mechanisms of perfluorinated compounds PFOA and PFOS. Chemosphere 2022, 291, 132892. [Google Scholar] [CrossRef]
- Qazi, M.R.; Xia, Z.; Bogdanska, J.; Chang, S.C.; Ehresman, D.J.; Butenhoff, J.L.; Nelson, B.D.; DePierre, J.W.; Abedi-Valugerdi, M. The atrophy and changes in the cellular compositions of the thymus and spleen observed in mice subjected to short-term exposure to perfluorooctanesulfonate are high-dose phenomena mediated in part by peroxisome proliferator-activated receptor-alpha (PPARalpha). Toxicology 2009, 260, 68–76. [Google Scholar]
- Dong, G.H.; Liu, M.M.; Wang, D.; Zheng, L.; Liang, Z.F.; Jin, Y.H. Sub-chronic effect of perfluorooctanesulfonate (PFOS) on the balance of type 1 and type 2 cytokine in adult C57BL6 mice. Arch. Toxicol. 2011, 85, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.H.; Zhang, Y.H.; Zheng, L.; Liu, W.; Jin, Y.H.; He, Q.C. Chronic effects of perfluorooctanesulfonate exposure on immunotoxicity in adult male C57BL/6 mice. Arch. Toxicol. 2009, 83, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Peden-Adams, M.M.; Keller, J.M.; Eudaly, J.G.; Berger, J.; Gilkeson, G.S.; Keil, D.E. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol. Sci. 2008, 104, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Dong, G.H.; Jin, Y.H.; He, Q.C. Immunotoxic changes associated with a 7-day oral exposure to perfluorooctanesulfonate (PFOS) in adult male C57BL/6 mice. Arch. Toxicol. 2009, 83, 679–689. [Google Scholar] [CrossRef]
- Szilagyi, J.T.; Freedman, A.N.; Kepper, S.L.; Keshava, A.M.; Bangma, J.T.; Fry, R.C. Per- and Polyfluoroalkyl Substances Differentially Inhibit Placental Trophoblast Migration and Invasion In Vitro. Toxicol. Sci. 2020, 175, 210–219. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef]
- Lipinski, M.M.; Jacks, T. The retinoblastoma gene family in differentiation and development. Oncogene 1999, 18, 7873–7882. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef]
- Blasco, M.A. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet. 2005, 6, 611–622. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, U.; Christofori, G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer 2004, 4, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.H. The Metabolism of Tumours: Investigations from the Kaiser Wilhelm Institute for Biology, Berlin-Dahlem. Translated from the German Edition, with Accounts of Additional Recent Researches, by Frank Dickens; Constable & Company Limited: London, UK, 1931. [Google Scholar]
- Vajdic, C.M.; Van Leeuwen, M.T. Cancer incidence and risk factors after solid organ transplantation. Int. J. Cancer 2009, 125, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Lobo, N.A.; Shimono, Y.; Qian, D.; Clarke, M.F. The Biology of Cancer Stem Cells. Annu. Rev. Cell Dev. Biol. 2007, 23, 675–699. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Q.; Fan, S.; He, Y.; Duan, J.; Zhang, S.; Xiong, H.; Zeng, D. PFOS promotes lung adenocarcinoma cell proliferation through PI3K/AKT/NF-κB mediated EMT. Food Chem. Toxicol. 2025, 205, 115714. [Google Scholar] [CrossRef]
- Zhang, L.; Louie, A.; Rigutto, G.; Guo, H.; Zhao, Y.; Ahn, S.; Dahlberg, S.; Sholinbeck, M.; Smith, M.T. A systematic evidence map of chronic inflammation and immunosuppression related to per- and polyfluoroalkyl substance (PFAS) exposure. Environ. Res. 2023, 220, 115188. [Google Scholar] [CrossRef]
- Smith, M.T.; Guyton, K.Z.; Gibbons, C.F.; Fritz, J.M.; Portier, C.J.; Rusyn, I.; DeMarini, D.M.; Caldwell, J.C.; Kavlock, R.J.; Lambert, P.F.; et al. Key Characteristics of Carcinogens as a Basis for Organizing Data on Mechanisms of Carcinogenesis. Environ. Health Perspect. 2016, 124, 713–721. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Rosen, M.B.; Das, K.P.; Rooney, J.; Abbott, B.; Lau, C.; Corton, J.C. PPARα-independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling. Toxicology 2017, 387, 95–107. [Google Scholar] [CrossRef]
- Arunsi, U.O.; Olugbami, J.O.; Oyelere, A.K. Anticancer Effects of Ascorbic Acid: Not All Sides Fit All. Cancers 2025, 17, 2877. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; Babb, P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: International comparisons. BJU Int. 2002, 90, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Verze, P.; Cai, T.; Lorenzetti, S. The role of the prostate in male fertility, health and disease. Nat. Rev. Urol. 2016, 13, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, S.; Narciso, L.; Marcoccia, D.; Altieri, I. A novel in vitro toxicological approach to identify chemicals with a prostate-mediated effect on male reproduction. J. Biol. Res.-Boll. Della Soc. Ital. Di Biol. Sper. 2011, 84, 36–41. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Cellular and Molecular Mechanisms Underlying Prostate Cancer Development: Therapeutic Implications. Medicines 2019, 6, 82. [Google Scholar] [CrossRef]
- Evans, A.J. Treatment effects in prostate cancer. Mod. Pathol. 2018, 31 (Suppl. S1), 110–121. [Google Scholar] [CrossRef]
- van de Wijngaart, D.J.; Dubbink, H.J.; van Royen, M.E.; Trapman, J.; Jenster, G. Androgen receptor coregulators: Recruitment via the coactivator binding groove. Mol. Cell. Endocrinol. 2012, 352, 57–69. [Google Scholar] [CrossRef]
- Aurilio, G.; Cimadamore, A.; Mazzucchelli, R.; Lopez-Beltran, A.; Verri, E.; Scarpelli, M.; Massari, F.; Cheng, L.; Santoni, M.; Montironi, R. Androgen Receptor Signaling Pathway in Prostate Cancer: From Genetics to Clinical Applications. Cells 2020, 9, 2653. [Google Scholar] [CrossRef]
- Michmerhuizen, A.R.; Spratt, D.E.; Pierce, L.J.; Speers, C.W. ARe we there yet? Understanding androgen receptor signaling in breast cancer. NPJ Breast Cancer 2020, 6, 47. [Google Scholar] [CrossRef]
- He, Y.; Xu, W.; Xiao, Y.T.; Huang, H.; Gu, D.; Ren, S. Targeting signaling pathways in prostate cancer: Mechanisms and clinical trials. Signal Transduct. Target. Ther. 2022, 7, 198. [Google Scholar] [CrossRef]
- Bhumireddy, A.; Bandaru, N.; Raghurami Reddy, B.; Gore, S.T.; Mukherjee, S.; Balasubramanian, W.R.; Sumanth Kumar, V.; Alapati, K.S.; Venkata Gowri Chandra Sekhar, K.; Nellore, K.; et al. Design, synthesis, and biological evaluation of phenyl thiazole-based AR-V7 degraders. Bioorganic Med. Chem. Lett. 2022, 55, 128448. [Google Scholar] [CrossRef]
- Xiao, M.; Ha, S.; Zhu, J.; Tao, W.; Fu, Z.; Wei, H.; Hou, Q.; Luo, G.; Xiang, H. Structure-Activity Relationship (SAR) Studies of Novel Monovalent AR/AR-V7 Dual Degraders with Potent Efficacy against Advanced Prostate Cancer. J. Med. Chem. 2024, 67, 5567–5590. [Google Scholar] [CrossRef]
- Ha, S.; Luo, G.; Xiang, H. A Comprehensive Overview of Small-Molecule Androgen Receptor Degraders: Recent Progress and Future Perspectives. J. Med. Chem. 2022, 65, 16128–16154. [Google Scholar] [CrossRef]
- Jia, X.; Han, X. Targeting androgen receptor degradation with PROTACs from bench to bedside. Biomed. Pharmacother. 2023, 158, 114112. [Google Scholar] [CrossRef]
- Zhao, L.; Han, X.; Lu, J.; McEachern, D.; Wang, S. A highly potent PROTAC androgen receptor (AR) degrader ARD-61 effectively inhibits AR-positive breast cancer cell growth in vitro and tumor growth in vivo. Neoplasia 2020, 22, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-generation antiandrogens: From discovery to standard of care in castration resistant prostate cancer. Front. Oncol. 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Wadosky, K.M.; Koochekpour, S. Molecular mechanisms underlying resistance to androgen deprivation therapy in prostate cancer. Oncotarget 2016, 7, 64447. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Hu, M.C.; Makino, K.; Spohn, B.; Bartholomeusz, G.; Yan, D.-H.; Hung, M.-C. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000, 60, 6841–6845. [Google Scholar]
- Hu, W.Y.; Hu, D.P.; Xie, L.; Li, Y.; Majumdar, S.; Nonn, L.; Hu, H.; Shioda, T.; Prins, G.S. Isolation and functional interrogation of adult human prostate epithelial stem cells at single cell resolution. Stem Cell Res. 2017, 23, 1–12. [Google Scholar] [CrossRef]
- Tomasetti, C.; Vogelstein, B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347, 78–81. [Google Scholar] [CrossRef]
- Tomasetti, C.; Li, L.; Vogelstein, B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 2017, 355, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.Y.; Shi, G.B.; Hu, D.P.; Nelles, J.L.; Prins, G.S. Actions of estrogens and endocrine disrupting chemicals on human prostate stem/progenitor cells and prostate cancer risk. Mol. Cell. Endocrinol. 2012, 354, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Troeschel, A.N.; Teras, L.R.; Hodge, J.M.; Rodriguez, J.; Wang, Y.; Daniel, J.; Diver, W.R.; Winquist, A. A case-cohort study of per- and polyfluoroalkyl substance concentrations and incident prostate cancer in the cancer prevention Study-II LifeLink cohort study. Environ. Res. 2024, 259, 119560. [Google Scholar] [CrossRef] [PubMed]
- Takacs, M.L.; Abbott, B.D. Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol. Sci. 2007, 95, 108–117. [Google Scholar] [CrossRef]
- Buhrke, T.; Kibellus, A.; Lampen, A. In vitro toxicological characterization of perfluorinated carboxylic acids with different carbon chain lengths. Toxicol. Lett. 2013, 218, 97–104. [Google Scholar] [CrossRef]
- Hu, W.Y.; Hu, D.P.; Xie, L.; Nonn, L.; Lu, R.; Abern, M.; Shioda, T.; Prins, G.S. Keratin Profiling by Single-Cell RNA-Sequencing Identifies Human Prostate Stem Cell Lineage Hierarchy and Cancer Stem-Like Cells. Int. J. Mol. Sci. 2021, 22, 8109. [Google Scholar] [CrossRef]
- Germann, M.; Wetterwald, A.; Guzmán-Ramirez, N.; van der Pluijm, G.; Culig, Z.; Cecchini, M.G.; Williams, E.D.; Thalmann, G.N. Stem-like cells with luminal progenitor phenotype survive castration in human prostate cancer. Stem Cells 2012, 30, 1076–1086. [Google Scholar] [CrossRef]
- Alumkal, J.J.; Sun, D.; Lu, E.; Beer, T.M.; Thomas, G.V.; Latour, E.; Aggarwal, R.; Cetnar, J.; Ryan, C.J.; Tabatabaei, S.; et al. Transcriptional profiling identifies an androgen receptor activity-low, stemness program associated with enzalutamide resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 12315–12323. [Google Scholar] [CrossRef]
- Carey, B.W.; Finley, L.W.; Cross, J.R.; Allis, C.D.; Thompson, C.B. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 2015, 518, 413–416. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, A.M.; Finley, L.W.S. Metabolic signatures of cancer cells and stem cells. Nat. Metab. 2019, 1, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.; Marin de Mas, I.; Zodda, E.; Marin, S.; Morrish, F.; Selivanov, V.; Meca-Cortés, Ó.; Delowar, H.; Pons, M.; Izquierdo, I.; et al. Metabolic Reprogramming and Dependencies Associated with Epithelial Cancer Stem Cells Independent of the Epithelial-Mesenchymal Transition Program. Stem Cells 2016, 34, 1163–1176. [Google Scholar] [CrossRef]
- Imir, O.B.; Kaminsky, A.Z.; Zuo, Q.Y.; Liu, Y.J.; Singh, R.; Spinella, M.J.; Irudayaraj, J.; Hu, W.Y.; Prins, G.S.; Madak Erdogan, Z. Per- and Polyfluoroalkyl Substance Exposure Combined with High-Fat Diet Supports Prostate Cancer Progression. Nutrients 2021, 13, 3902. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020, GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424, Erratum in CA A Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef]
- Cao, S.S.; Lu, C.T. Recent perspectives of breast cancer prognosis and predictive factors (Review). Oncol. Lett. 2016, 12, 3674–3678. [Google Scholar] [CrossRef]
- Dias, K.; Dvorkin-Gheva, A.; Hallett, R.M.; Wu, Y.; Hassell, J.; Pond, G.R.; Levine, M.; Whelan, T.; Bane, A.L. Claudin-Low Breast Cancer; Clinical & Pathological Characteristics. PLoS ONE 2017, 12, e0168669. [Google Scholar]
- Khan, M.Z.I.; Uzair, M.; Nazli, A.; Chen, J.Z. An overview on Estrogen receptors signaling and its ligands in breast cancer. Eur. J. Med. Chem. 2022, 241, 114658. [Google Scholar] [CrossRef]
- Acconcia, F.; Fiocchetti, M.; Marino, M. Xenoestrogen regulation of ERα/ERβ balance in hormone-associated cancers. Mol. Cell Endocrinol. 2017, 457, 3–12. [Google Scholar] [CrossRef]
- Malainou, C.P.; Stachika, N.; Damianou, A.K.; Anastopoulos, A.; Ploumaki, I.; Triantafyllou, E.; Drougkas, K.; Gomatou, G.; Kotteas, E. Estrogen-Receptor-Low-Positive Breast Cancer: Pathological and Clinical Perspectives. Curr. Oncol. 2023, 30, 9734–9745. [Google Scholar] [CrossRef] [PubMed]
- Gallez, A.; Dias Da Silva, I.; Wuidar, V.; Foidart, J.-M.; Péqueux, C. Estetrol and Mammary Gland: Friends or Foes? J. Mammary Gland. Biol. Neoplasia 2021, 26, 297–308. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Chapter Three—Estrogen receptor signaling mechanisms. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 116, pp. 135–170. [Google Scholar]
- Patel, S.; Homaei, A.; Raju, A.B.; Meher, B.R. Estrogen: The necessary evil for human health, and ways to tame it. Biomed. Pharmacother. 2018, 102, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Kim, K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J. Mol. Endocrinol. 2008, 41, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Björnström, L.; Sjöberg, M. Estrogen receptor-dependent activation of AP-1 via non-genomic signalling. Nucl. Recept. 2004, 2, 3. [Google Scholar] [CrossRef][Green Version]
- Powell, E.; Wang, Y.; Shapiro, D.J.; Xu, W. Differential Requirements of Hsp90 and DNA for the Formation of Estrogen Receptor Homodimers and Heterodimers. J. Biol. Chem. 2010, 285, 16125–16134. [Google Scholar] [CrossRef]
- West, D.C.; Pan, D.; Tonsing-Carter, E.Y.; Hernandez, K.M.; Pierce, C.F.; Styke, S.C.; Bowie, K.R.; Garcia, T.I.; Kocherginsky, M.; Conzen, S.D. GR and ER Coactivation Alters the Expression of Differentiation Genes and Associates with Improved ER+ Breast Cancer Outcome. Mol. Cancer Res. 2016, 14, 707–719. [Google Scholar] [CrossRef]
- Jeong, K.W.; Lee, Y.-H.; Stallcup, M.R. Recruitment of the SWI/SNF chromatin remodeling complex to steroid hormone-regulated promoters by nuclear receptor coactivator flightless-I. J. Biol. Chem. 2009, 284, 29298–29309. [Google Scholar] [CrossRef]
- Yang, F.; Ma, Q.; Liu, Z.; Li, W.; Tan, Y.; Jin, C.; Ma, W.; Hu, Y.; Shen, J.; Ohgi, K.A. Glucocorticoid receptor: MegaTrans switching mediates the repression of an ERα-regulated transcriptional program. Mol. Cell 2017, 66, 321–331.e6. [Google Scholar] [CrossRef]
- Watters, J.J.; Campbell, J.S.; Cunningham, M.J.; Krebs, E.G.; Dorsa, D.M. Rapid Membrane Effects of Steroids in Neuroblastoma Cells: Effects of Estrogen on Mitogen Activated Protein Kinase Signalling Cascade and c-fos Immediate Early Gene Transcription. Endocrinology 1997, 138, 4030–4033. [Google Scholar] [CrossRef]
- Rocca, A.; Braga, L.; Volpe, M.C.; Maiocchi, S.; Generali, D. The Predictive and Prognostic Role of RAS–RAF–MEK–ERK Pathway Alterations in Breast Cancer: Revision of the Literature and Comparison with the Analysis of Cancer Genomic Datasets. Cancers 2022, 14, 5306. [Google Scholar] [CrossRef]
- Ciruelos Gil, E.M. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat. Rev. 2014, 40, 862–871. [Google Scholar] [CrossRef]
- Heery, D.M.; Kalkhoven, E.; Hoare, S.; Parker, M.G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 1997, 387, 733–736. [Google Scholar] [CrossRef]
- Zhang, P.; Torres, K.; Liu, X.; Liu, C.G.; Pollock, R.E. An Overview of Chromatin-Regulating Proteins in Cells. Curr. Protein Pept. Sci. 2016, 17, 401–410. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Mandel, J.H.; Zobel, L.R. Serum perfluorooctane sulfonate and hepatic and lipid clinical chemistry tests in fluorochemical production employees. J. Occup. Environ. Med. 1999, 41, 799–806. [Google Scholar] [CrossRef]
- Olsen Geary, W.; Burris Jean, M.; Ehresman David, J.; Froehlich John, W.; Seacat Andrew, M.; Butenhoff John, L.; Zobel Larry, R. Half-Life of Serum Elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- Benninghoff, A.D.; Bisson, W.H.; Koch, D.C.; Ehresman, D.J.; Kolluri, S.K.; Williams, D.E. Estrogen-Like Activity of Perfluoroalkyl Acids In Vivo and Interaction with Human and Rainbow Trout Estrogen Receptors In Vitro. Toxicol. Sci. 2011, 120, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.S.; Bonefeld-Jørgensen, E.C. Perfluorinated compounds affect the function of sex hormone receptors. Environ. Sci. Pollut. Res. 2013, 20, 8031–8044. [Google Scholar] [CrossRef] [PubMed]
- Maras, M.; Vanparys, C.; Muylle, F.; Robbens, J.; Berger, U.; Barber, J.L.; Blust, R.; De Coen, W. Estrogen-like properties of fluorotelomer alcohols as revealed by mcf-7 breast cancer cell proliferation. Environ. Health Perspect. 2006, 114, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, H.; Ishida, H.; Matsuoka, M.; Tominaga, N.; Arizono, K. Estrogenic effects of fluorotelomer alcohols for human estrogen receptor isoforms alpha and beta in vitro. Biol. Pharm. Bull. 2007, 30, 1358–1359. [Google Scholar] [CrossRef]
- Pierozan, P.; Cattani, D.; Karlsson, O. Correction to: Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) induce epigenetic alterations and promote human breast cell carcinogenesis in vitro. Arch. Toxicol. 2020, 94, 3907–3909, Erratum in Arch. Toxicol. 2020, 94, 3907–3909. [Google Scholar] [CrossRef]
- Samatar, A.A.; Poulikakos, P.I. Targeting RAS-ERK signalling in cancer: Promises and challenges. Nat. Rev. Drug Discov. 2014, 13, 928–942. [Google Scholar] [CrossRef]
- Karin, M. The regulation of AP-1 activity by mitogen-activated protein kinases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 127–134. [Google Scholar] [CrossRef]
- Burton, P.B.; Anderson, C.J.; Corbishly, C.M. Caspase 3 and p27 as predictors of invasive bladder cancer. N. Engl. J. Med. 2000, 343, 1418–1420. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Alkarain, A.; Jordan, R.; Slingerland, J. p27 deregulation in breast cancer: Prognostic significance and implications for therapy. J. Mammary Gland. Biol. Neoplasia 2004, 9, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Belletti, B.; Baldassarre, G. New light on p27(kip1) in breast cancer. Cell Cycle 2012, 11, 3701–3702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weigelt, B.; Peterse, J.L.; van’t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Scully, O.J.; Bay, B.H.; Yip, G.; Yu, Y. Breast cancer metastasis. Cancer Genom. Proteom. 2012, 9, 311–320. [Google Scholar]
- Zutter, M.M.; Mazoujian, G.; Santoro, S.A. Decreased expression of integrin adhesive protein receptors in adenocarcinoma of the breast. Am. J. Pathol. 1990, 137, 863–870. [Google Scholar]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Osanai, M.; Murata, M.; Nishikiori, N.; Chiba, H.; Kojima, T.; Sawada, N. Epigenetic silencing of occludin promotes tumorigenic and metastatic properties of cancer cells via modulations of unique sets of apoptosis-associated genes. Cancer Res. 2006, 66, 9125–9133. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Mansel, R.E.; Jiang, W.G. Loss of occludin leads to the progression of human breast cancer. Int. J. Mol. Med. 2010, 26, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Hosoda, K.; Nishizawa, N.; Katoh, H.; Watanabe, M. Epigenetic biomarkers of promoter DNA methylation in the new era of cancer treatment. Cancer Sci. 2018, 109, 3695–3706. [Google Scholar] [CrossRef]
- Jovanovic, J.; Rønneberg, J.A.; Tost, J.; Kristensen, V. The epigenetics of breast cancer. Mol. Oncol. 2010, 4, 242–254. [Google Scholar] [CrossRef]
- Lo, P.K.; Sukumar, S. Epigenomics and breast cancer. Pharmacogenomics 2008, 9, 1879–1902. [Google Scholar] [CrossRef]
- Esteller, M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet. 2007, 8, 286–298. [Google Scholar] [CrossRef]
- Radpour, R.; Barekati, Z.; Kohler, C.; Schumacher, M.M.; Grussenmeyer, T.; Jenoe, P.; Hartmann, N.; Moes, S.; Letzkus, M.; Bitzer, J.; et al. Integrated epigenetics of human breast cancer: Synoptic investigation of targeted genes, microRNAs and proteins upon demethylation treatment. PLoS ONE 2011, 6, e27355. [Google Scholar] [CrossRef]
- Kondo, Y. Epigenetic cross-talk between DNA methylation and histone modifications in human cancers. Yonsei Med. J. 2009, 50, 455–463. [Google Scholar] [CrossRef]
- Baylin, S.B.; Ohm, J.E. Epigenetic gene silencing in cancer—A mechanism for early oncogenic pathway addiction? Nat. Rev. Cancer 2006, 6, 107–116. [Google Scholar] [CrossRef]
- Fang, J.Y.; Lu, Y.Y. Effects of histone acetylation and DNA methylation on p21 (WAF1) regulation. World J. Gastroenterol. 2002, 8, 400–405. [Google Scholar] [CrossRef]
- Tsai, M.S.; Chang, S.H.; Kuo, W.H.; Kuo, C.H.; Li, S.Y.; Wang, M.Y.; Chang, D.Y.; Lu, Y.S.; Huang, C.S.; Cheng, A.L.; et al. A case-control study of perfluoroalkyl substances and the risk of breast cancer in Taiwanese women. Environ. Int. 2020, 142, 105850. [Google Scholar] [CrossRef]
- Bonefeld-Jørgensen, E.C.; Long, M.; Fredslund, S.O.; Bossi, R.; Olsen, J. Breast cancer risk after exposure to perfluorinated compounds in Danish women: A case–control study nested in the Danish National Birth Cohort. Cancer Causes Control 2014, 25, 1439–1448. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, P.C.; Lo, S.C.; Torng, P.L.; Sung, F.C.; Su, T.C. The association of carotid intima-media thickness with serum Level of perfluorinated chemicals and endothelium-platelet microparticles in adolescents and young adults. Environ. Int. 2016, 94, 292–299. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Tsai, M.S.; Lin, C.Y.; Lin, C.C.; Chen, M.H.; Hsu, S.H.; Chien, K.L.; Sung, F.C.; Chen, P.C.; Su, T.C. Association between perfluoroalkyl substances and reproductive hormones in adolescents and young adults. Int. J. Hyg. Environ. Health 2015, 218, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Folkerd, E.; Dowsett, M. Sex hormones and breast cancer risk and prognosis. Breast 2013, 22, S38–S43. [Google Scholar] [CrossRef] [PubMed]
- Wielsøe, M.; Kern, P.; Bonefeld-Jørgensen, E.C. Serum levels of environmental pollutants is a risk factor for breast cancer in Inuit: A case control study. Environ. Health 2017, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Azim, H.A.; Partridge, A.H., Jr. Biology of breast cancer in young women. Breast Cancer Res. 2014, 16, 427. [Google Scholar] [CrossRef]
- Kortenkamp, A. Breast cancer, oestrogens and environmental pollutants: A re-evaluation from a mixture perspective. Int. J. Androl. 2006, 29, 193–198. [Google Scholar] [CrossRef]
- Barry, V.; Winquist, A.; Steenland, K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ. Health Perspect. 2013, 121, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, J.Y.; Lee, H.J. Human Evidence of Perfluorooctanoic Acid (PFOA) Exposure on Hepatic Disease: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 11318. [Google Scholar] [CrossRef] [PubMed]
- Hardisty, J.F.; Willson, G.A.; Brown, W.R.; McConnell, E.E.; Frame, S.R.; Gaylor, D.W.; Kennedy, G.L.; Butenhoff, J.L. Pathology Working Group review and evaluation of proliferative lesions of mammary gland tissues in female rats fed ammonium perfluorooctanoate (APFO) in the diet for 2 years. Drug Chem. Toxicol. 2010, 33, 131–137. [Google Scholar] [CrossRef] [PubMed]
- White, S.S.; Calafat, A.M.; Kuklenyik, Z.; Villanueva, L.; Zehr, R.D.; Helfant, L.; Strynar, M.J.; Lindstrom, A.B.; Thibodeaux, J.R.; Wood, C.; et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol. Sci. 2007, 96, 133–144. [Google Scholar] [CrossRef]
- Yang, Q.; Kurotani, R.; Yamada, A.; Kimura, S.; Gonzalez, F.J. Peroxisome proliferator-activated receptor alpha activation during pregnancy severely impairs mammary lobuloalveolar development in mice. Endocrinology 2006, 147, 4772–4780. [Google Scholar] [CrossRef]
- Sonthithai, P.; Suriyo, T.; Thiantanawat, A.; Watcharasit, P.; Ruchirawat, M.; Satayavivad, J. Perfluorinated chemicals, PFOS and PFOA, enhance the estrogenic effects of 17β-estradiol in T47D human breast cancer cells. J. Appl. Toxicol. 2016, 36, 790–801. [Google Scholar] [CrossRef]
- Ghisari, M.; Long, M.; Røge, D.M.; Olsen, J.; Bonefeld-Jørgensen, E.C. Polymorphism in xenobiotic and estrogen metabolizing genes, exposure to perfluorinated compounds and subsequent breast cancer risk: A nested case-control study in the Danish National Birth Cohort. Environ. Res. 2017, 154, 325–333. [Google Scholar] [CrossRef]
- Lacey, J.V.; Kreimer, A.R., Jr.; Buys, S.S.; Marcus, P.M.; Chang, S.C.; Leitzmann, M.F.; Hoover, R.N.; Prorok, P.C.; Berg, C.D.; Hartge, P. Breast cancer epidemiology according to recognized breast cancer risk factors in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Cohort. BMC Cancer 2009, 9, 84. [Google Scholar] [CrossRef]
- Danaei, G.; Vander Hoorn, S.; Lopez, A.D.; Murray, C.J.; Ezzati, M. Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005, 366, 1784–1793. [Google Scholar] [CrossRef]
- Johnson, S.M.; Shaw, J.A.; Walker, R.A. Sporadic breast cancer in young women: Prevalence of loss of heterozygosity at p53, BRCA1 and BRCA2. Int. J. Cancer 2002, 98, 205–209. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih Ie, M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am. J. Pathol. 2016, 186, 733–747. [Google Scholar] [CrossRef] [PubMed]
- Poole, E.M.; Merritt, M.A.; Jordan, S.J.; Yang, H.P.; Hankinson, S.E.; Park, Y.; Rosner, B.; Webb, P.M.; Cramer, D.W.; Wentzensen, N.; et al. Hormonal and reproductive risk factors for epithelial ovarian cancer by tumor aggressiveness. Cancer Epidemiol. Biomark. Prev. 2013, 22, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Bodelon, C.; Wentzensen, N.; Schonfeld, S.J.; Visvanathan, K.; Hartge, P.; Park, Y.; Pfeiffer, R.M. Hormonal risk factors and invasive epithelial ovarian cancer risk by parity. Br. J. Cancer 2013, 109, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Moorman, P.G.; Calingaert, B.; Palmieri, R.T.; Iversen, E.S.; Bentley, R.C.; Halabi, S.; Berchuck, A.; Schildkraut, J.M. Hormonal risk factors for ovarian cancer in premenopausal and postmenopausal women. Am. J. Pathol. 2008, 167, 1059–1069. [Google Scholar] [CrossRef]
- Kozieł, M.J.; Piastowska-Ciesielska, A.W. Estrogens, Estrogen Receptors and Tumor Microenvironment in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 14673. [Google Scholar] [CrossRef]
- Reade, C.J.; McVey, R.M.; Tone, A.A.; Finlayson, S.J.; McAlpine, J.N.; Fung-Kee-Fung, M.; Ferguson, S.E. The fallopian tube as the origin of high grade serous ovarian cancer: Review of a paradigm shift. J. Obstet. Gynaecol. Can. 2014, 36, 133–140. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Zhu, X.; Zhong, L.; Jiang, Q.; Wang, Y.; Tang, Q.; Li, Q.; Zhang, C.; Wang, H.; et al. Drug resistance in ovarian cancer: From mechanism to clinical trial. Mol. Cancer 2024, 23, 66. [Google Scholar] [CrossRef]
- Kulkarni, S.; Gajjar, K.; Madhusudan, S. Poly (ADP-ribose) polymerase inhibitor therapy and mechanisms of resistance in epithelial ovarian cancer. Front. Oncol. 2024, 14, 1414112. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chang, J.-Y. New Insights into Mechanisms of Cisplatin Resistance: From Tumor Cell to Microenvironment. Int. J. Mol. Sci. 2019, 20, 4136. [Google Scholar] [CrossRef]
- Feng, X.; Wang, X.; Cao, X.; Xia, Y.; Zhou, R.; Chen, L. Chronic Exposure of Female Mice to an Environmental Level of Perfluorooctane Sulfonate Suppresses Estrogen Synthesis Through Reduced Histone H3K14 Acetylation of the StAR Promoter Leading to Deficits in Follicular Development and Ovulation. Toxicol. Sci. 2015, 148, 368–379. [Google Scholar] [CrossRef]
- Suba, Z. Amplified Crosstalk Between Estrogen Binding and GFR Signaling Mediated Pathways of ER Activation Drives Responses in Tumors Treated with Endocrine Disruptors. Recent Pat. Anti-Cancer Drug Discov. 2018, 13, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Gogola-Mruk, J.; Hoffmann-Młodzianowska, M.; Kamińska, K.; Ptak, A. Mixtures of persistent organic pollutants increase ovarian granulosa tumor cell line migration and spheroid invasion by upregulating MMP2 expression and activity via IGF1R. Toxicology 2021, 452, 152715. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Zhou, X.; Jia, T.; Wang, B.; Liu, A.; Gao, M.; Song, J.; Wang, L.; Jing, Y.; Yu, L.; et al. Developmental exposure to perfluorooctane sulfonate(PFOS) impairs the endometrial receptivity. Sci. Rep. 2025, 15, 1747. [Google Scholar] [CrossRef] [PubMed]
- Rickard, B.P.; Tan, X.; Fenton, S.E.; Rizvi, I. Select Per- and Polyfluoroalkyl Substances (PFAS) Induce Resistance to Carboplatin in Ovarian Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 5176. [Google Scholar] [CrossRef]
- Ding, N.; Harlow, S.D.; Randolph, J.F.; Loch-Caruso, R., Jr.; Park, S.K. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum. Reprod. Update 2020, 26, 724–752. [Google Scholar] [CrossRef]
- Knox, S.S.; Jackson, T.; Javins, B.; Frisbee, S.J.; Shankar, A.; Ducatman, A.M. Implications of early menopause in women exposed to perfluorocarbons. J. Clin. Endocrinol. Metab. 2011, 96, 1747–1753. [Google Scholar] [CrossRef]
- Zhang, S.; Tan, R.; Pan, R.; Xiong, J.; Tian, Y.; Wu, J.; Chen, L. Association of Perfluoroalkyl and Polyfluoroalkyl Substances With Premature Ovarian Insufficiency in Chinese Women. J. Clin. Endocrinol. Metab. 2018, 103, 2543–2551. [Google Scholar] [CrossRef]
- Jones, R.R.; Madrigal, J.M.; Medgyesi, D.N.; Fisher, J.A.; Calafat, A.M.; Botelho, J.C.; Kato, K.; Albert, P.S.; Silverman, D.T.; Hofmann, J.N.; et al. Serum concentrations of per- and polyfluoroalkyl substances and risk of ovarian cancer. J. Natl. Cancer Inst. 2025, 113, 580–587. [Google Scholar] [CrossRef]
- UNEP. UNEP: Stockholm Convention on Persistent Organic Pollutants (POPs); Programme UNE, Ed.; Programme UNE: Genève, Switzerland, 2009. [Google Scholar]
- EPA. Our Current Understanding of the Human Health and Environmental Risks of PFAS; Agency UEP, Ed.; Agency UEP: Washington, DC, USA, 2024. [Google Scholar]
- ECHA ECA. Perfluorooctane Sulfonic Acid and its Derivatives (PFOS); Agency EC, Ed.; Agency EC: Helsinki, Finland, 2020. [Google Scholar]
- Shearer, J.J.; Callahan, C.L.; Calafat, A.M.; Huang, W.Y.; Jones, R.R.; Sabbisetti, V.S.; Freedman, N.D.; Sampson, J.N.; Silverman, D.T.; Purdue, M.P.; et al. Serum Concentrations of Per- and Polyfluoroalkyl Substances and Risk of Renal Cell Carcinoma. J. Natl. Cancer Inst. 2021, 113, 580–587. [Google Scholar] [CrossRef]
- Rhee, J.; Chang, V.C.; Cheng, I.; Calafat, A.M.; Botelho, J.C.; Shearer, J.J.; Sampson, J.N.; Setiawan, V.W.; Wilkens, L.R.; Silverman, D.T.; et al. Serum concentrations of per- and polyfluoroalkyl substances and risk of renal cell carcinoma in the Multiethnic Cohort Study. Environ. Int. 2023, 180, 108197. [Google Scholar] [CrossRef]
- Purdue, M.P.; Rhee, J.; Denic-Roberts, H.; McGlynn, K.A.; Byrne, C.; Sampson, J.; Botelho, J.C.; Calafat, A.M.; Rusiecki, J. A Nested Case-Control Study of Serum Per- and Polyfluoroalkyl Substances and Testicular Germ Cell Tumors among U.S. Air Force Servicemen. Environ. Health Perspect. 2023, 131, 77007. [Google Scholar] [CrossRef]
- Jones, R.R.; Madrigal, J.M.; Troisi, R.; Surcel, H.-M.; Öhman, H.; Kivelä, J.; Kiviranta, H.; Rantakokko, P.; Koponen, J.; Medgyesi, D.N.; et al. Maternal serum concentrations of per- and polyfluoroalkyl substances and childhood acute lymphoblastic leukemia. JNCI J. Natl. Cancer Inst. 2023, 116, 728–736. [Google Scholar] [CrossRef]
- Tessmann, J.W.; Deng, P.; Durham, J.; Li, C.; Banerjee, M.; Wang, Q.; Goettl, R.A.; He, D.; Wang, C.; Lee, E.Y.; et al. Perfluorooctanesulfonic acid exposure leads to downregulation of 3-hydroxy-3-methylglutaryl-CoA synthase 2 expression and upregulation of markers associated with intestinal carcinogenesis in mouse intestinal tissues. Chemosphere 2024, 359, 142332. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Environmental impacts, exposure pathways, and health effects of PFOA and PFOS. Ecotoxicol. Environ. Saf. 2023, 267, 115663. [Google Scholar] [CrossRef]
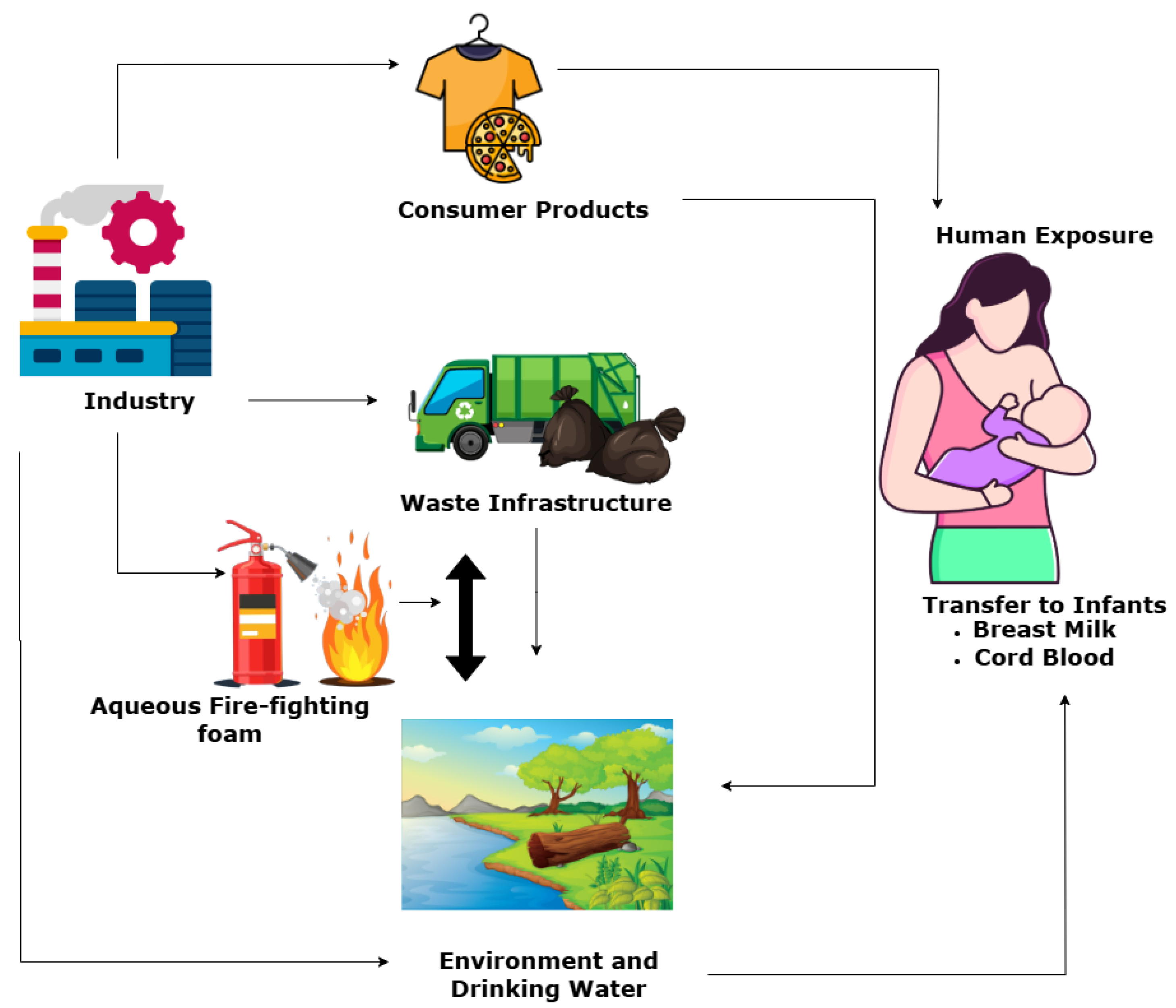

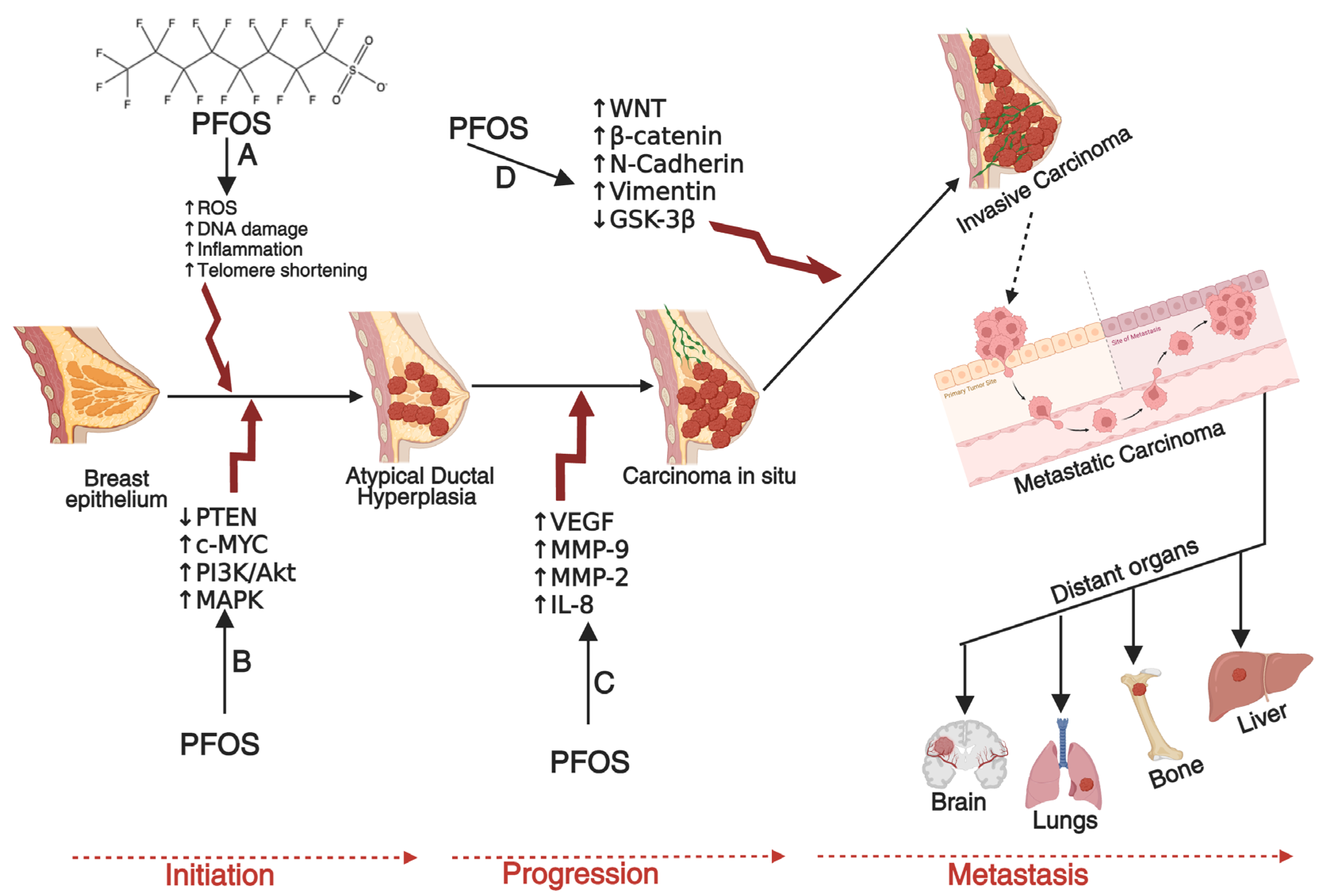
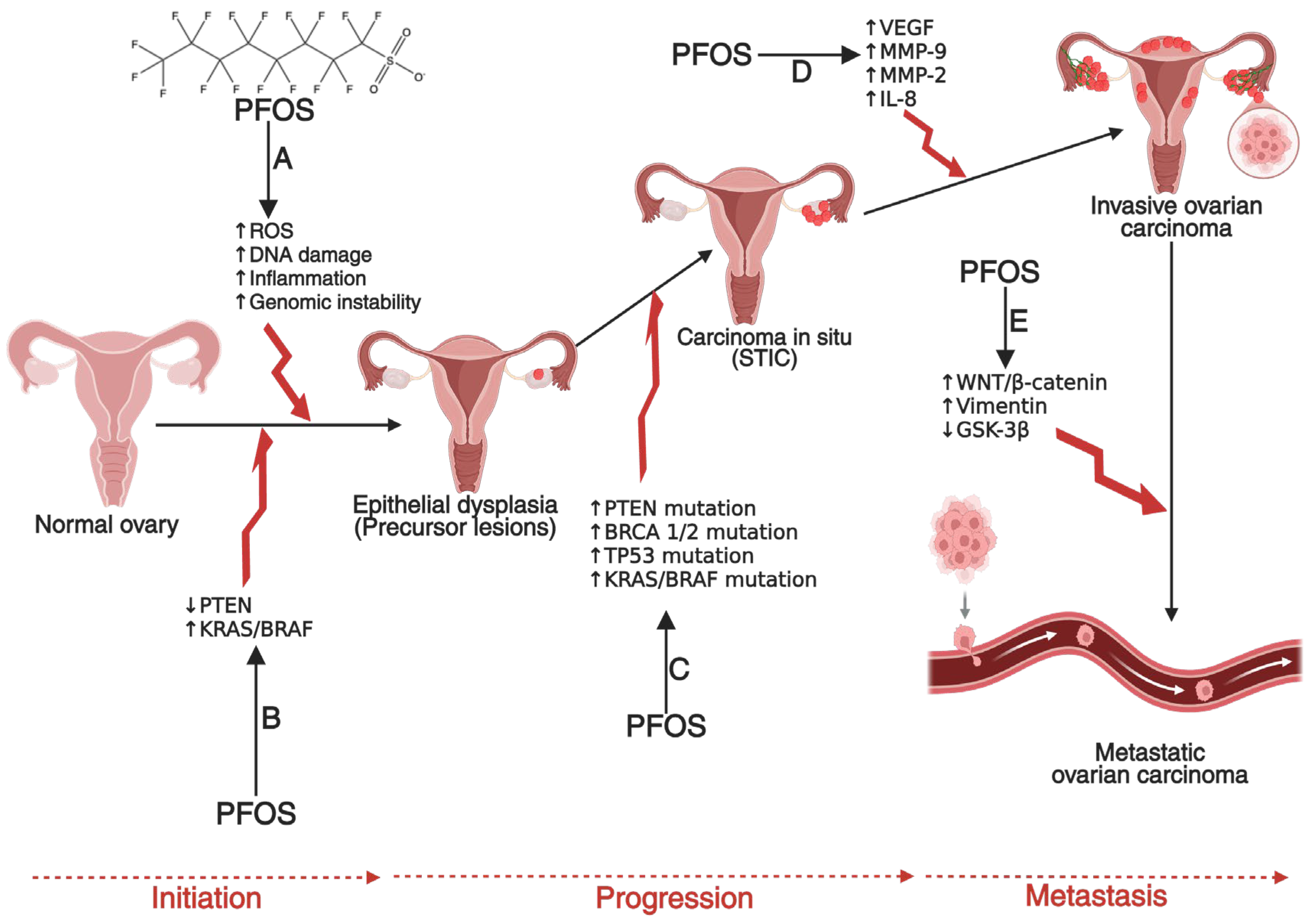
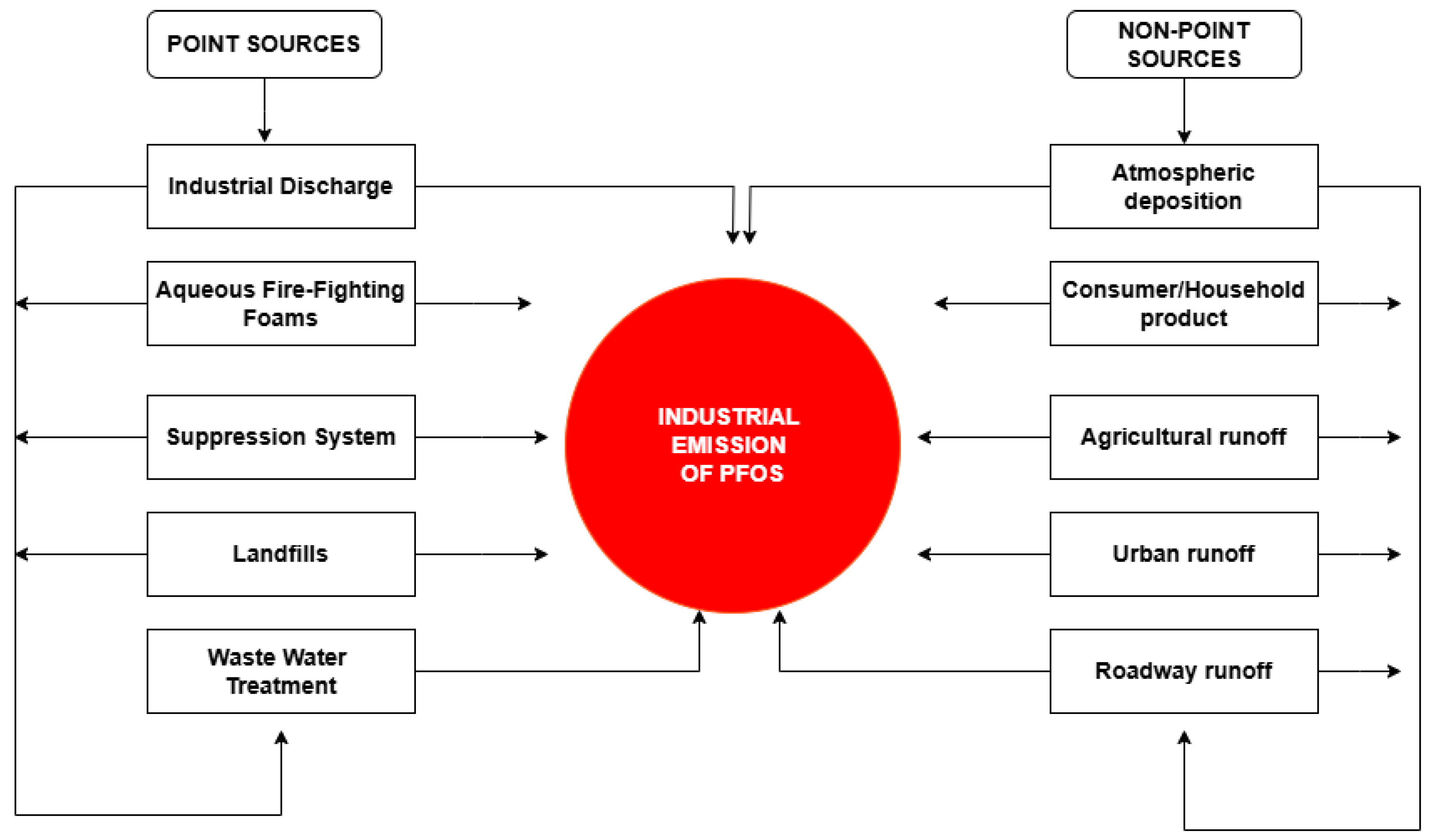

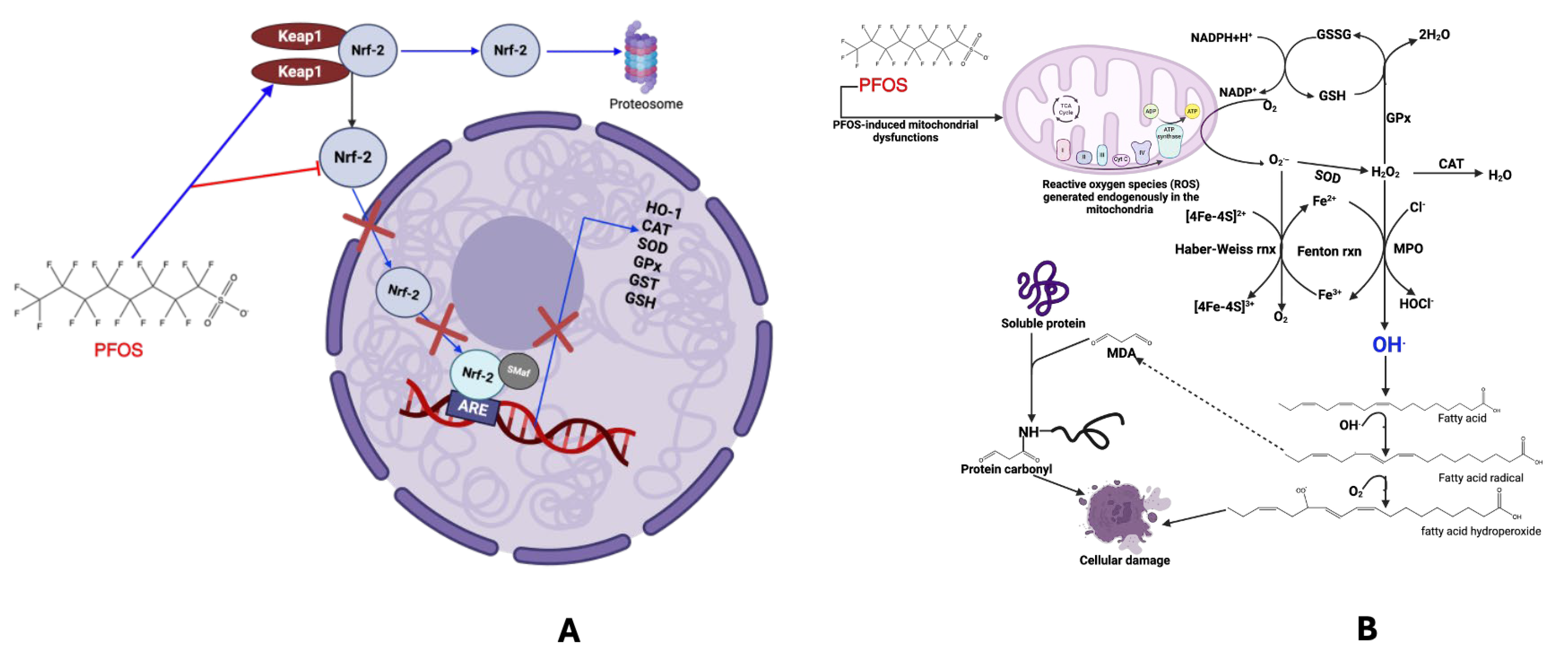
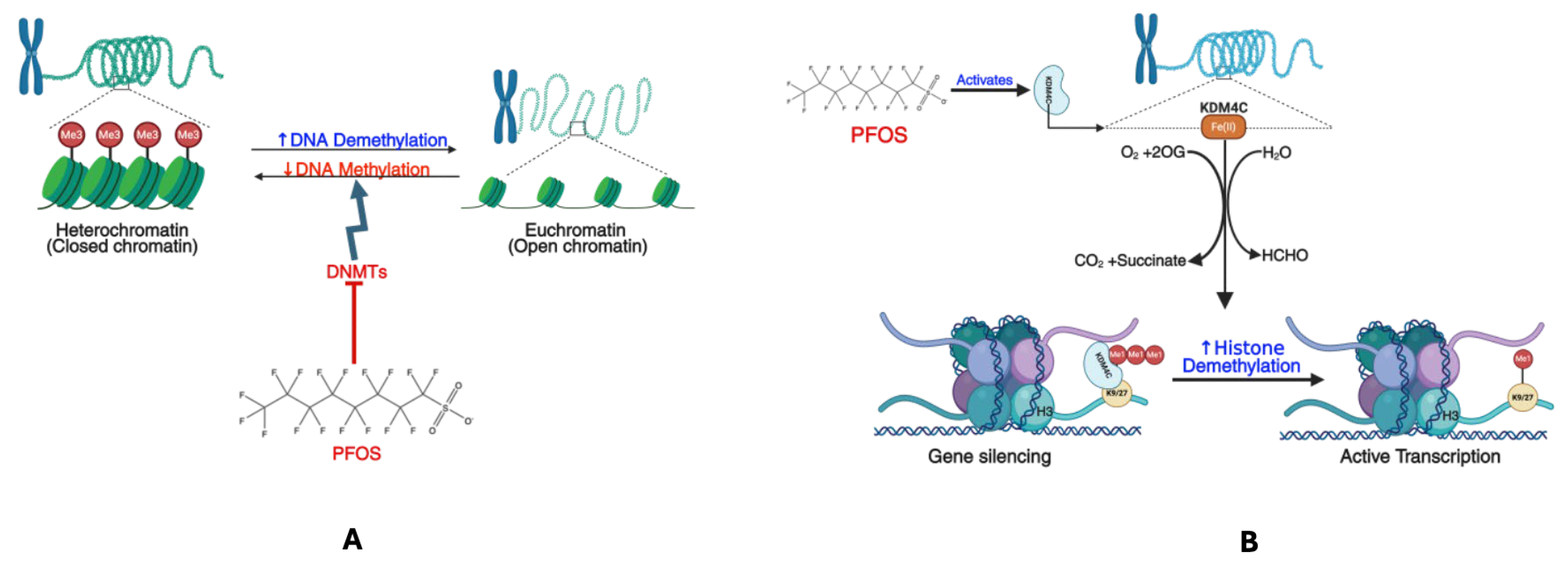
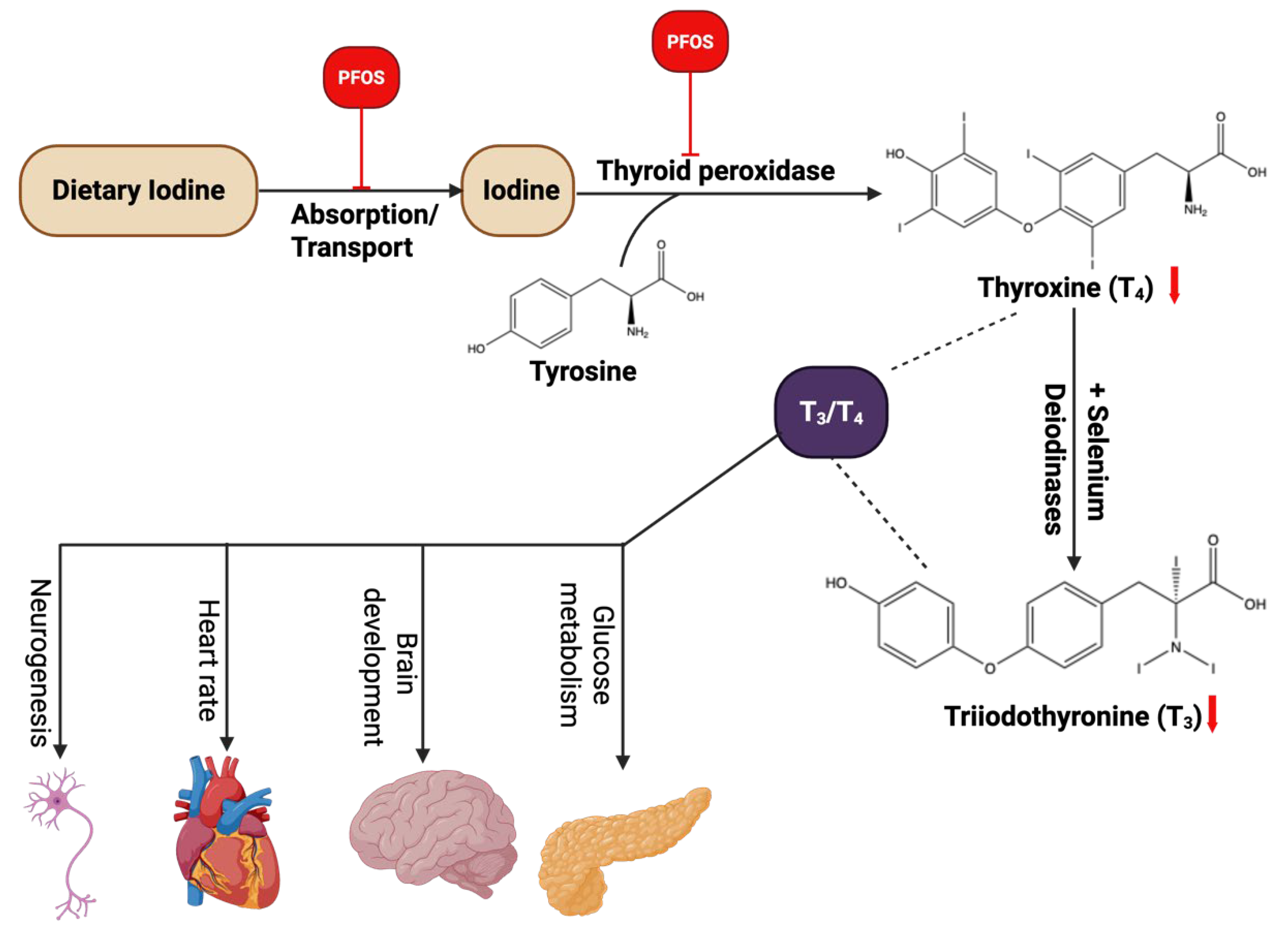
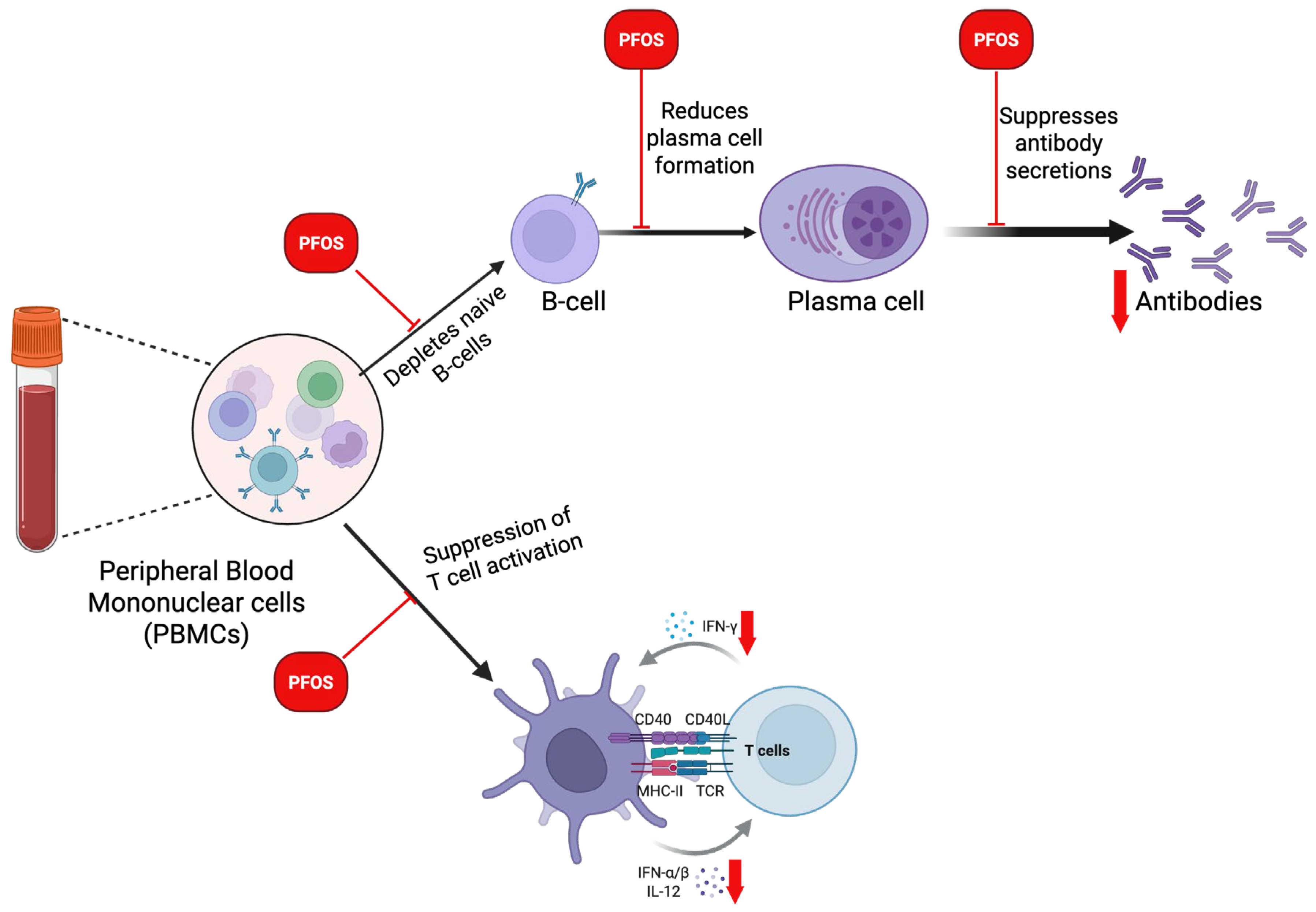
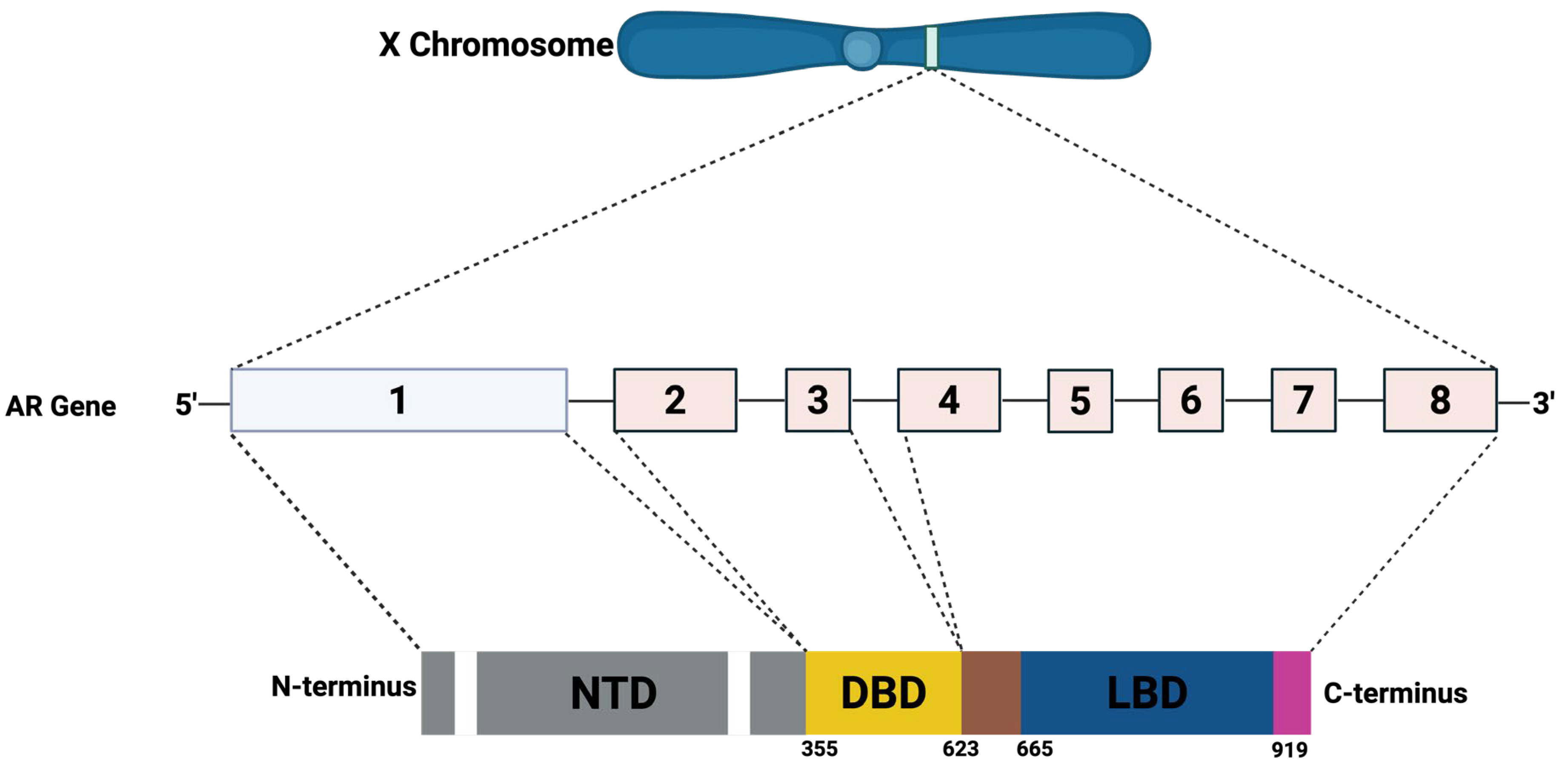
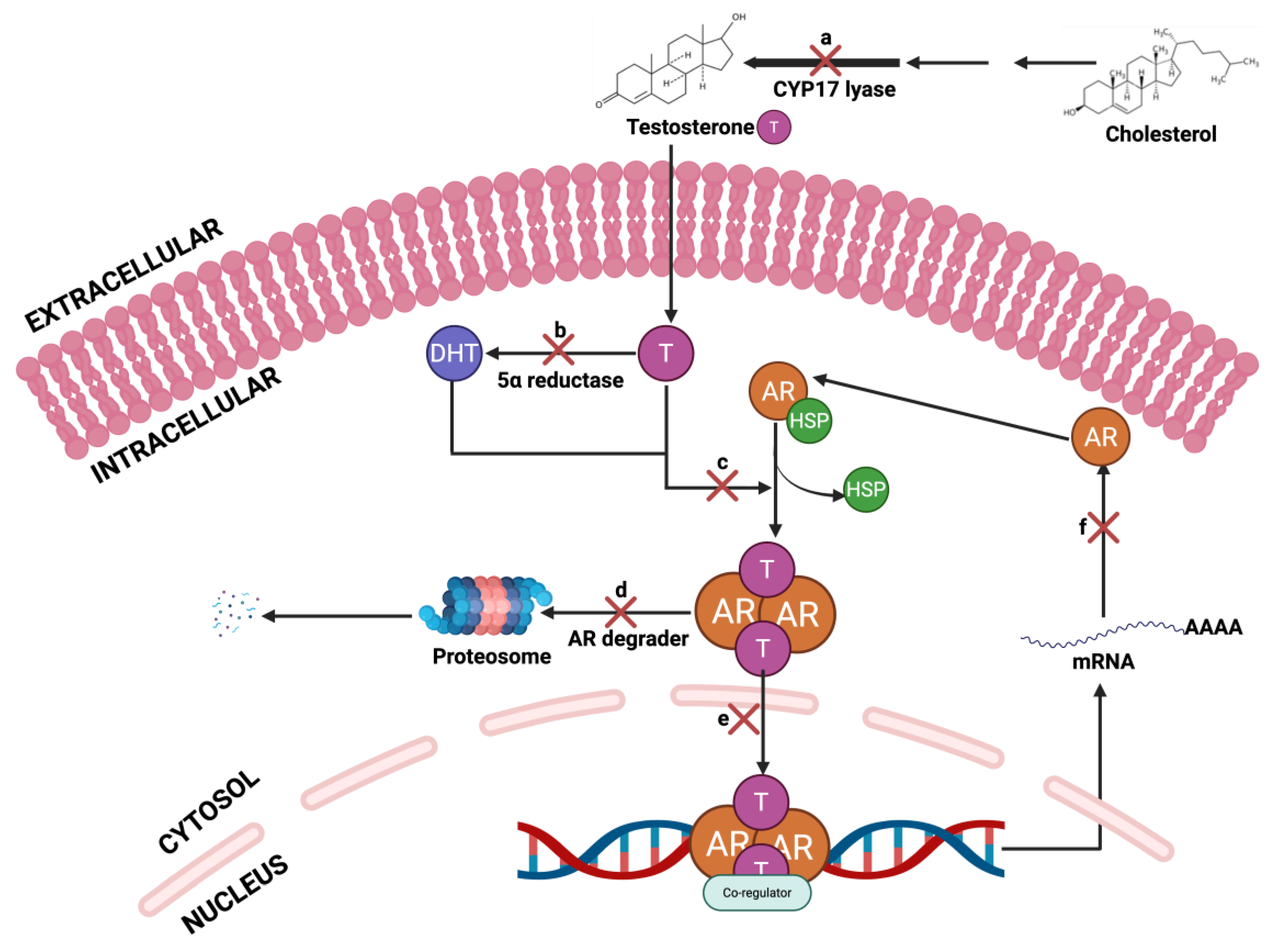
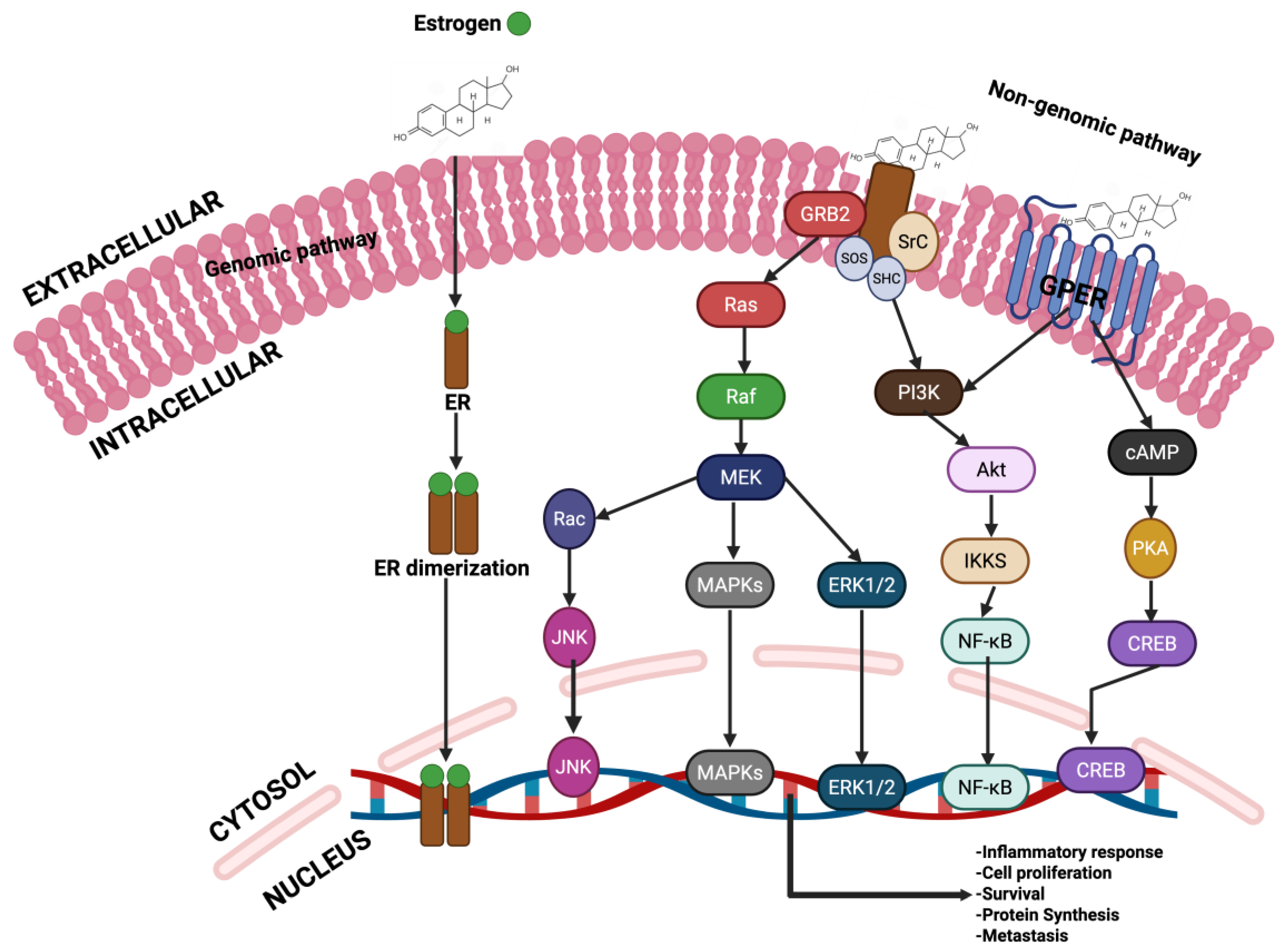
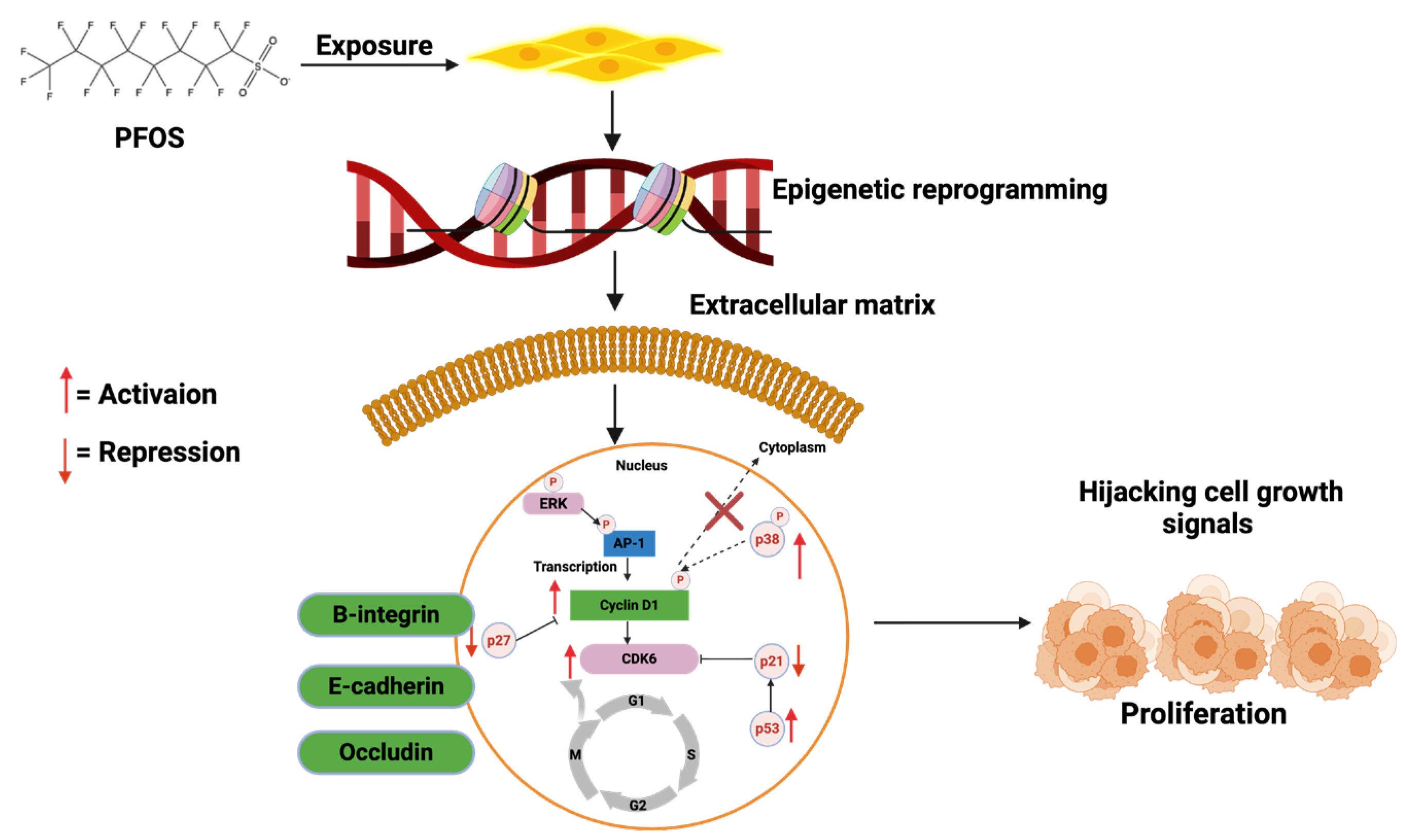
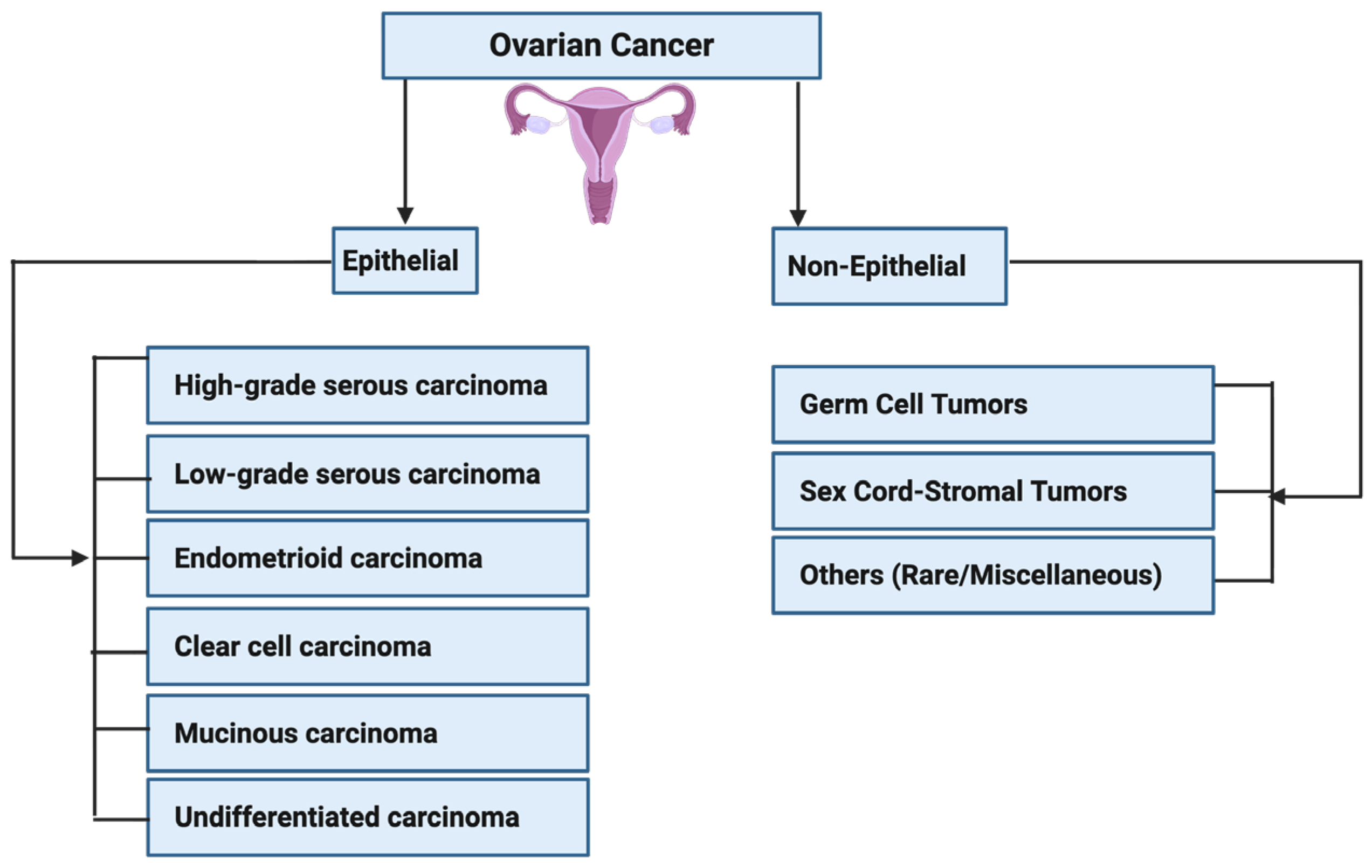
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunsi, U.O.; Ezirim, D.C.; Arunsi, C.C.; Altayyar, A.; Uche, E.G.; Jonathan, F.C.; Opieh, A.K.; Anadi, I.V.; Ofoegbu, C.O.; Nwankwo, V.C.; et al. Pharmacokinetics and Pharmacodynamics of Perfluorooctane Sulfonate (PFOS) and Its Role in the Development and Progression of Prostate, Ovarian and Breast Cancers. Cancers 2025, 17, 3507. https://doi.org/10.3390/cancers17213507
Arunsi UO, Ezirim DC, Arunsi CC, Altayyar A, Uche EG, Jonathan FC, Opieh AK, Anadi IV, Ofoegbu CO, Nwankwo VC, et al. Pharmacokinetics and Pharmacodynamics of Perfluorooctane Sulfonate (PFOS) and Its Role in the Development and Progression of Prostate, Ovarian and Breast Cancers. Cancers. 2025; 17(21):3507. https://doi.org/10.3390/cancers17213507
Chicago/Turabian StyleArunsi, Uche Okuu, Daniel Chukwuebuka Ezirim, Chinonye Courage Arunsi, Ahmad Altayyar, Eke Godswill Uche, Favour Chidera Jonathan, Aluba Kalu Opieh, Ifeoma Vivian Anadi, Clinton Ositadinma Ofoegbu, Victor Chukwubuike Nwankwo, and et al. 2025. "Pharmacokinetics and Pharmacodynamics of Perfluorooctane Sulfonate (PFOS) and Its Role in the Development and Progression of Prostate, Ovarian and Breast Cancers" Cancers 17, no. 21: 3507. https://doi.org/10.3390/cancers17213507
APA StyleArunsi, U. O., Ezirim, D. C., Arunsi, C. C., Altayyar, A., Uche, E. G., Jonathan, F. C., Opieh, A. K., Anadi, I. V., Ofoegbu, C. O., Nwankwo, V. C., Ugbogu, E. A., Etusim, P. E., & Owumi, S. (2025). Pharmacokinetics and Pharmacodynamics of Perfluorooctane Sulfonate (PFOS) and Its Role in the Development and Progression of Prostate, Ovarian and Breast Cancers. Cancers, 17(21), 3507. https://doi.org/10.3390/cancers17213507









