Double-Barrel Uro-Colostomy Versus Ileal Conduit for Urinary Diversion After Pelvic Exenteration: A Systematic Review and Meta-Analysis of Comparative Outcomes
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. PICO
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Study Characteristics
3.3. Baseline Characteristics
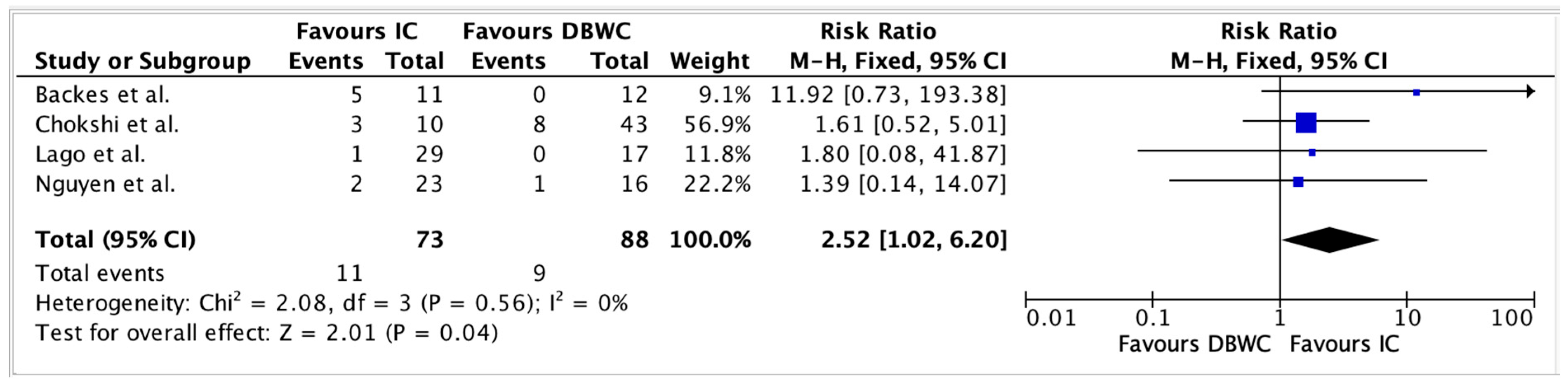
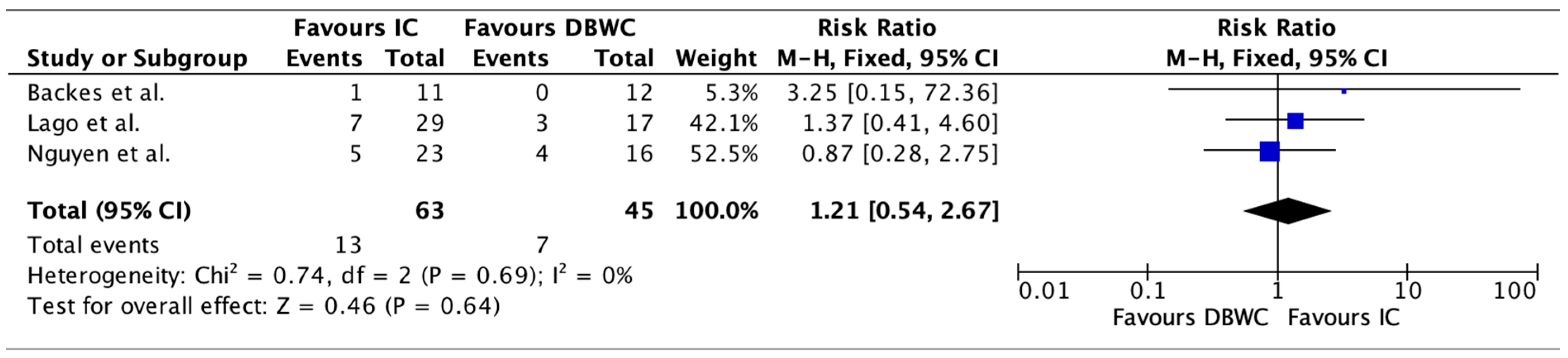
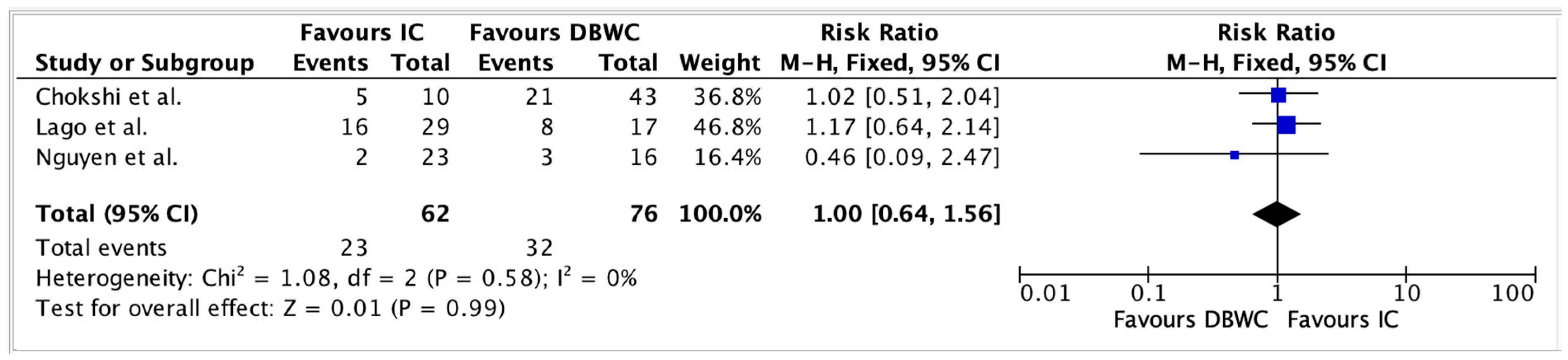
3.4. Perioperative Morbidity
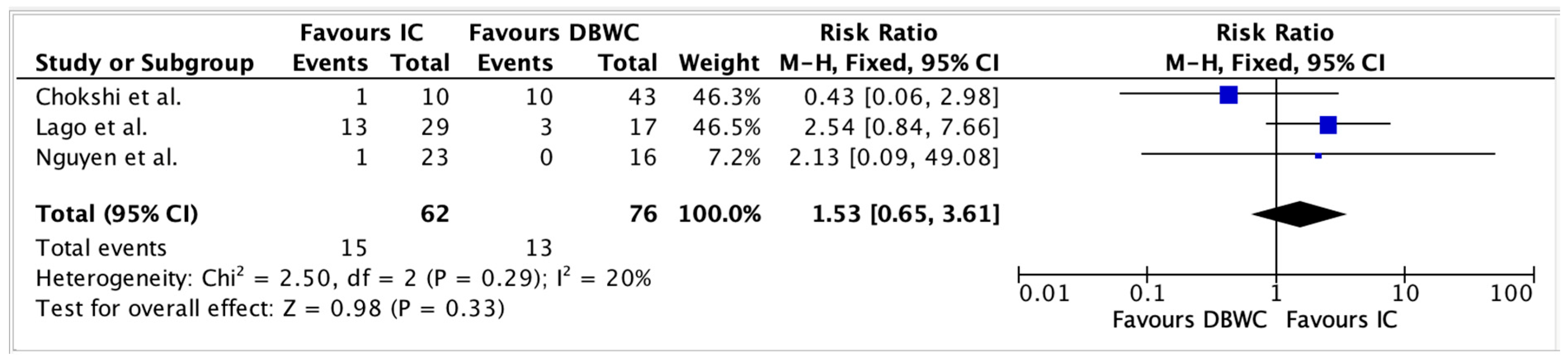
3.5. Clavien–Dindo Postoperative Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DBUC | Double-Barrel Uro-Colostomy |
| IC | Ileal Conduit |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RR | Risk Ratio |
| CI | Confidence Interval |
| LOS | Length of Stay |
| QoL | Quality of Life |
| TPE | Total Pelvic Exenteration |
| VRAM | Vertical Rectus Abdominis Myocutaneous (flap) |
References
- Srinivasaiah, N.; Shekleton, F.; Kelly, M.E.; Harji, D.; Malietzis, G.; Askari, A.; Aalbers, A.G.J.; Alberda, W.; Antoniou, A.; Austin, K.K.; et al. Minimally invasive surgery techniques in pelvic exenteration: A systematic and meta-analysis review. Surg. Endosc. 2018, 32, 4707–4715. [Google Scholar] [CrossRef]
- Sharma, S.; Odunsi, K.; Driscoll, D.; Lele, S. Pelvic exenterations for gynecological malignancies: Twenty-year experience at Roswell Park Cancer Institute. Int. J. Gynecol. Cancer 2005, 15, 475–482. [Google Scholar] [CrossRef]
- Houvenaeghel, G.; Moutardier, V.; Karsenty, G.; Bladou, F.; Lelong, B.; Buttarelli, M.; Delpero, J.R. Major complications of urinary diversion after pelvic exenteration for gynecologic malignancies: A 23-year mono-institutional experience in 124 patients. Gynecol. Oncol. 2004, 92, 680–683. [Google Scholar] [CrossRef]
- Kelly, M.E.; Ryan, E.J.; Aalbers, A.G.J.; Abdul, A.N.; Abraham-Nordling, M.; Alberda, W.; Antoniou, A.; Austin, K.K.; Baker, R.; Bali, M.; et al. Pelvic Exenteration for Advanced Nonrectal Pelvic Malignancy. Ann. Surg. 2019, 270, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.E.; Glynn, R.; Aalbers, A.; Alberda, W.; Antoniou, A.; Austin, K.; de Wilt, J.; Versteegen, M.; Yip, J.; Winter, D. Surgical and survival outcomes following pelvic exenteration for locally advanced primary rectal cancer results from an international collaboration. Ann. Surg. 2019, 269, 315–321. [Google Scholar]
- Khan, O.; Patsouras, D.; Ravindraanandan, M.; Abrar, M.M.; Schizas, A.; George, M.; Malde, S.; Thurairaja, R.; Khan, M.S.; Sahai, A. Total Pelvic Exenteration for Locally Advanced and Recurrent Rectal Cancer: Urological Outcomes and Adverse Events. Eur. Urol. Focus 2021, 7, 638–643. [Google Scholar] [CrossRef]
- Kazi, M.; Rohila, J.; Kumar, N.A.; Bankar, S.; Engineer, R.; Desouza, A.; Saklani, A. Urinary reconstruction following total pelvic exenteration for locally advanced rectal cancer: Complications and factors affecting outcomes. Langenbecks Arch. Surg. 2021, 406, 329–337. [Google Scholar] [CrossRef]
- Wright, J.P.; Guerrero, W.M.; Lucking, J.R.; Bustamante-Lopez, L.; Monson, J.R.T. The double-barrel wet colostomy: An alternative for urinary diversion after pelvic exenteration. Surgeon 2023, 21, 375–380. [Google Scholar] [CrossRef]
- Brunschwig, A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer 1948, 1, 177–183. [Google Scholar] [CrossRef]
- Brunschwig, A.; Daniel, W. Pelvic exenteration operations: With summary of sixty-six cases surviving more than five years. Ann. Surg. 1960, 151, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Brunschwig, A.; Pierce, V.K. Partial and complete pelvic exenteration; a progress report based upon the first 100 operations. Cancer 1950, 3, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Bricker, E.M.; Modlin, J. The role of pelvic evisceration in surgery. Surgery 1951, 30, 76–94. [Google Scholar]
- Boey, J.; Wong, J.; Ong, G.B. Pelvic exenteration for locally advanced colorectal carcinoma. Ann. Surg. 1982, 195, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Bricker, E.M. Substitution for the urinary bladder by the use of isolated ileal segments. Surg. Clin. N. Am. 1956, 36, 1117–1130. [Google Scholar] [CrossRef]
- Madersbacher, S.; Schmidt, J.; Eberle, J.M.; Thoeny, H.C.; Burkhard, F.; Hochreiter, W.; Studer, U.E. Long-term outcome of ileal conduit diversion. J. Urol. 2003, 169, 985–990. [Google Scholar] [CrossRef]
- Yang, W.J.; Cho, K.S.; Rha, K.H.; Lee, H.Y.; Chung, B.H.; Hong, S.J.; Yang, S.C.; Choi, Y.D. Long-term effects of ileal conduit urinary diversion on upper urinary tract in bladder cancer. Urology 2006, 68, 324–327. [Google Scholar] [CrossRef]
- Carter, M.F.; Dalton, D.P.; Garnett, J.E. Simultaneous diversion of the urinary and fecal streams utilizing a single abdominal stoma: The double-barreled wet colostomy. J. Urol. 1989, 141, 1189–1191. [Google Scholar] [CrossRef]
- Carter, M.F.; Dalton, D.P.; Garnett, J.E. The double-barreled wet colostomy: Long-term experience with the first 11 patients. J. Urol. 1994, 152 Pt 2, 2312–2315. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Traeger, L.; Vather, R.; Overall, B.; Cho, J.; Sammour, T. Double barrelled uro-colostomy versus Ileal conduit for urinary diversion following pelvic exenteration: A single centre experience. ANZ J. Surg. 2023, 93, 2450–2456. [Google Scholar] [CrossRef]
- Backes, F.J.; Tierney, B.J.; Eisenhauer, E.L.; Bahnson, R.R.; Cohn, D.E.; Fowler, J.M. Complications after double-barreled wet colostomy compared to separate urinary and fecal diversion during pelvic exenteration: Time to change back? Gynecol. Oncol. 2013, 128, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Limmer, A.M.; Lendzion, R.J.; Leung, C.; Wong, E.; Gilmore, A.J. A single centre experience on the formation of double barrelled uro-colostomy in pelvic exenteration surgery: A cohort study. ANZ J. Surg. 2024, 94, 1161–1166. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 151, 264–269. [Google Scholar]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Lago, V.; Pradillo Aramendi, T.; Segarra-Vidal, B.; Padilla-Iserte, P.; Matute, L.; Gurrea, M.; Pontones, J.L.; Delgado, F.; Domingo, S. Comparation between the Bricker ileal conduit vs double-barrelled wet colostomy after pelvic exenteration for gynaecological malignancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 282, 140–145. [Google Scholar] [CrossRef]
- Chokshi, R.J.; Kuhrt, M.P.; Arrese, D.; Parks, L.; Johnson, M.; Martin, E.W. Single-institution experience comparing double-barreled wet colostomy to ileal conduit for urinary and fecal diversion. J. Clin. Oncol. 2011, 29, 542. [Google Scholar] [CrossRef]
- Fahy, M.R.; Hayes, C.; Kelly, M.E.; Winter, D.C. Updated systematic review of the approach to pelvic exenteration for locally advanced primary rectal cancer. Eur. J. Surg. Oncol. 2022, 48, 2284–2291. [Google Scholar] [CrossRef]
- Pellino, G.; Biondo, S.; Codina Cazador, A.; Enríquez-Navascues, J.M.; Espín-Basany, E.; Roig-Vila, J.V.; García-Granero, E. Pelvic exenterations for primary rectal cancer: Analysis from a 10-year national prospective database. World J. Gastroenterol. 2018, 24, 5144–5153. [Google Scholar] [CrossRef]
- Yang, J.; Luo, Y.; Tian, T.; Dong, P.; Fu, Z. Effects of Neoadjuvant Radiotherapy on Postoperative Complications in Rectal Cancer: A Meta-Analysis. J. Oncol. 2022, 2022, 8197701. [Google Scholar] [CrossRef] [PubMed]
- Alemozaffar, M.; Nam, C.S.; Said, M.A.; Patil, D.; Carney, K.J.; David, S.; Master, V.A. Avoiding the Need for Bowel Anastomosis during Pelvic Exenteration—Urinary Sigmoid or Descending Colon Conduit—Short and Long Term Complications. Urology 2019, 129, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, M.J.; Ceranic, M.S.; Nale, D.P.; Latincic, S.M.; Kecmanovic, D.M. Double-Barreled Wet Colostomy versus Ileal Conduit and Terminal Colostomy for Urinary and Fecal Diversion: A Single Institution Experience. Scand. J. Surg. 2014, 103, 189–194. [Google Scholar] [CrossRef]
- Gan, J.; Hamid, R. Literature Review: Double-Barrelled Wet Colostomy (One Stoma) versus Ileal Conduit with Colostomy (Two Stomas). Urol. Int. 2017, 98, 249–254. [Google Scholar] [CrossRef]
- Bertherat, W.; Pelette, R.; Beaujouan, F.; Chicaud, M.; Ducos, V.; Rousseau, S.; Lescure, V.; Plainard, X.; Descazeaud, A. How do patients manage their urostomy in everyday life? A questionnaire survey. Prog. Urol. 2022, 32, 32–39. [Google Scholar] [CrossRef]
- Van der Aa, F.; Joniau, S.; Van Den Branden, M.; Van Poppel, H. Metabolic changes after urinary diversion. Adv. Urol. 2011, 2011, 764325. [Google Scholar] [CrossRef]
- Cano Megías, M.; Muñoz Delgado, E.G. Bone and metabolic complications of urinary diversions. Endocrinol. Nutr. 2015, 62, 100–105. [Google Scholar] [CrossRef]
- Collins, K.; Yocum, B.P.; Idrees, M.T.; Saeed, O. Carcinoma arising in ileal conduit or orthotopic ileal neobladder reconstruction: A 20-year single institute experience. Histopathology 2024, 85, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Ali-El-Dein, B.; El-Tabey, N.; Abdel-Latif, M.; Abdel-Rahim, M.; El-Bahnasawy, M.S. Late uro-ileal cancer after incorporation of ileum into the urinary tract. J. Urol. 2002, 167, 84–88. [Google Scholar] [CrossRef] [PubMed]
- van Kesteren, L.J.; Moolenaar, L.R.; Nieuwenhuijzen, J.A.; de Bruijn, V.; Moldovan, O.C.; Vlug, M.S.; Lameris, W.; Hompes, R.; Tuynman, J.B. Double-Barrel Urocolostomy After Pelvic Exenteration: Short-Term Morbidity and Patient-Reported Quality of Life. Ann. Surg. Oncol. 2025, 32, 4534–4541. [Google Scholar] [CrossRef] [PubMed]
- Lopes de Queiroz, F.; Barbosa-Silva, T.; Pyramo Costa, L.M.; Werneck Côrtes, B.J.; Figueiredo, J.A.; Guerra, F.; Campos, M.H.; Salgado, F.A.; Silva Lemos, G.; Rosa da Silva, P. Double-barrelled wet colostomy with simultaneous urinary and faecal diversion: Results in 9 patients and review of the literature. Color. Dis. 2006, 8, 353–359. [Google Scholar] [CrossRef]
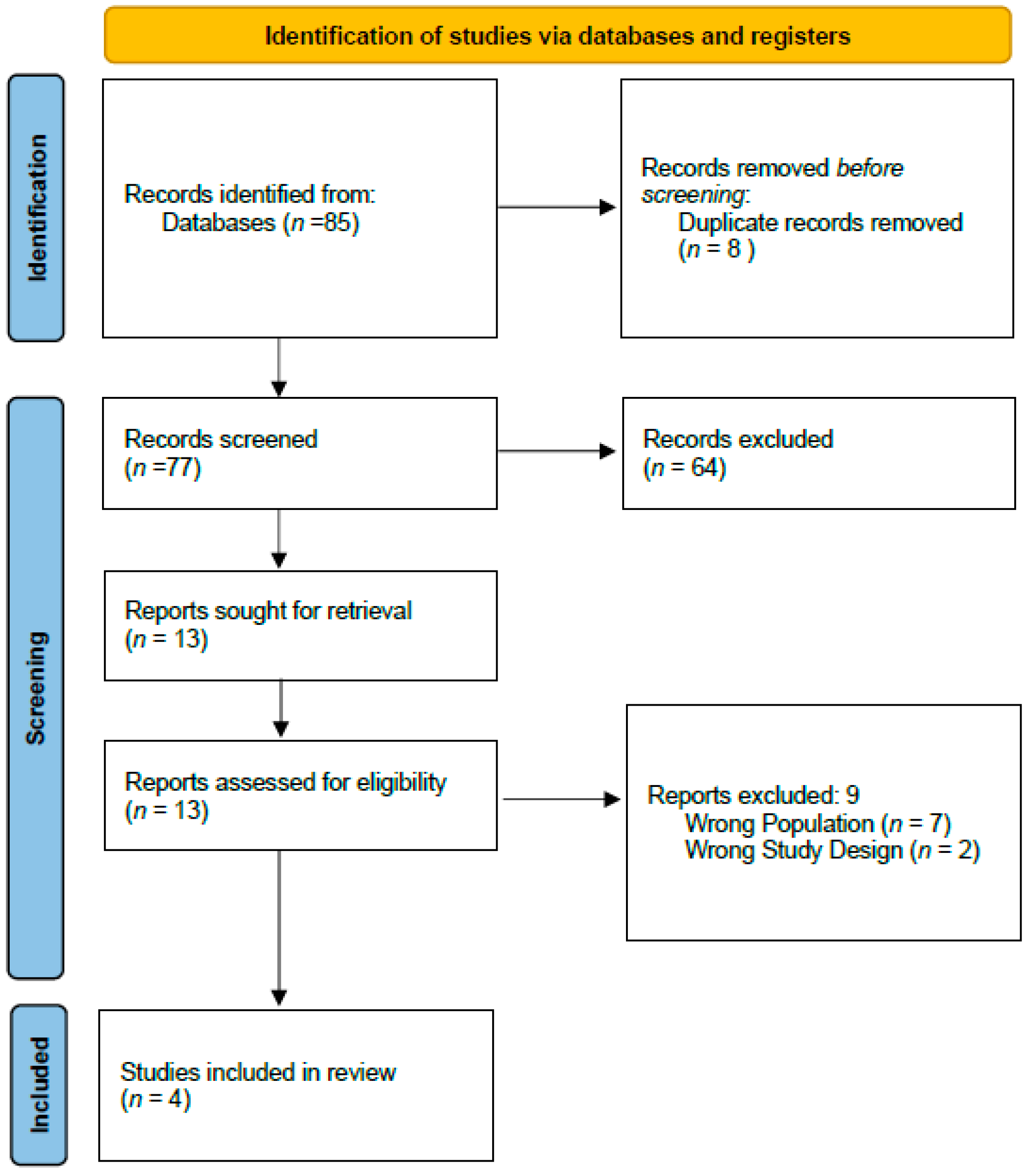

| Study | Group | n | Age (Mean ± SD or Median [Range]) | BMI (Mean or Median) | Pre-op RT (%) | Type of Exenteration (%) | Operative Time (min) | Length of Stay (Days) Median ([Range]) | Flap Use (% and Type) |
|---|---|---|---|---|---|---|---|---|---|
| Lago et al. [24] | DBUC | 20 | 62 ± 11 | 26 ± 6.5 (not group-specific) | 84 (not group-specific) | Total 65, Anterior 20, Posterior 15 (not group-specific) | 412 ± 124 (not group-specific) | 16 (3–38) | e-VRAM 30%, Omental 44%, Gracilis 3%, V-Y 7% (not group-specific) |
| IC | 29 | 57 ± 11 | - | - | - | - | 22 (7–72) | - | |
| Chokshi et al. [25] | DBUC | 43 | 56 [38–79] | - | 77 | Total 100 | 547 [240–864] | 14 [6–57] | - |
| IC | 10 | 62 [46–80] | - | 60 | Total 100 | 549 [279–720] | 16.5 [8–37] | - | |
| Backes et al. [20] | DBUC | 12 | 54 [42–72] | 29 | 100 | - | 610 [406–853] | 14.5 [10–57] | - |
| IC | 11 | 57 [28–76] | 25 | 100 | - | 720 [635–1003] | 26 [14–37] | - | |
| Nguyen et al. [19] | DBUC | 16 | 56.6 ± 18.1 | 26.5 [17–36] | 93.8 | Total 100 | 566.3 ± 204.6 | 18.5 [8–73] | None 6.3%, VRAM + other 37.5%, Omental 31.3%, VRAM 25%, |
| IC | 23 | 66.5 ± 11.5 | 26 [22–45] | 65.2 | Total 52.2, Modified Total 8.7, Anterior 30.4, Other 8.7 | 414.6 ± 167.9 | 16 [9–57] | None 54.5%, VRAM 18.2%, Omental 13.6%, VRAM + other 4.5%, Gracilis 9.1% |
| Study | Group | n | Urinary Leaks | Pyelonephritis | Electrolyte Disturbances | Urinary Fistula | Enteric Fistula |
|---|---|---|---|---|---|---|---|
| Lago et al. (2023) [24] | DBUC | 17 | 0 | 9 (53%) | 3 (18%) | 2 (12%) | 2 (12%) |
| IC | 29 | 1 (3.4%) | 17 (59%) | 7 (24%) | 0 (0%) | 8 (28%) | |
| Chokshi et al. (2011) [25] | DBUC | 43 | 8 (18.6%) | 1 (2.3%) | - | 6 (14%) | 13 (30.2%) |
| IC | 10 | 3 (30%) | 1 (10%) | - | 6 (60%) | 1 (10%) | |
| Backes et al. (2012) [20] | DBUC | 12 | 0 | 1 (8%) | 0 (0%) | - | - |
| IC | 11 | 5 (45%) | 3 (27%) | 1 (9%) | - | - | |
| Nguyen et al. (2023) [19] | DBUC | 16 | 1 (6.25%) | - | 4 (25.00%) | - | 0 (0%) |
| IC | 23 | 2 (8.70%) | - | 5 (21.74%) | - | 1 (4.35%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, A.; Calpin, G.; Salama, M.; Creavin, B.; Maguire, P.J.; Lonergan, P.; Cho, J.; Abu Saadeh, F.; McLoughlin, L.; Sammour, T.; et al. Double-Barrel Uro-Colostomy Versus Ileal Conduit for Urinary Diversion After Pelvic Exenteration: A Systematic Review and Meta-Analysis of Comparative Outcomes. Cancers 2025, 17, 3479. https://doi.org/10.3390/cancers17213479
Salama A, Calpin G, Salama M, Creavin B, Maguire PJ, Lonergan P, Cho J, Abu Saadeh F, McLoughlin L, Sammour T, et al. Double-Barrel Uro-Colostomy Versus Ileal Conduit for Urinary Diversion After Pelvic Exenteration: A Systematic Review and Meta-Analysis of Comparative Outcomes. Cancers. 2025; 17(21):3479. https://doi.org/10.3390/cancers17213479
Chicago/Turabian StyleSalama, Ahmed, Gavin Calpin, Mahmoud Salama, Ben Creavin, Patrick J. Maguire, Peter Lonergan, Jonathan Cho, Feras Abu Saadeh, Louise McLoughlin, Tarik Sammour, and et al. 2025. "Double-Barrel Uro-Colostomy Versus Ileal Conduit for Urinary Diversion After Pelvic Exenteration: A Systematic Review and Meta-Analysis of Comparative Outcomes" Cancers 17, no. 21: 3479. https://doi.org/10.3390/cancers17213479
APA StyleSalama, A., Calpin, G., Salama, M., Creavin, B., Maguire, P. J., Lonergan, P., Cho, J., Abu Saadeh, F., McLoughlin, L., Sammour, T., & Kelly, M. E. (2025). Double-Barrel Uro-Colostomy Versus Ileal Conduit for Urinary Diversion After Pelvic Exenteration: A Systematic Review and Meta-Analysis of Comparative Outcomes. Cancers, 17(21), 3479. https://doi.org/10.3390/cancers17213479






