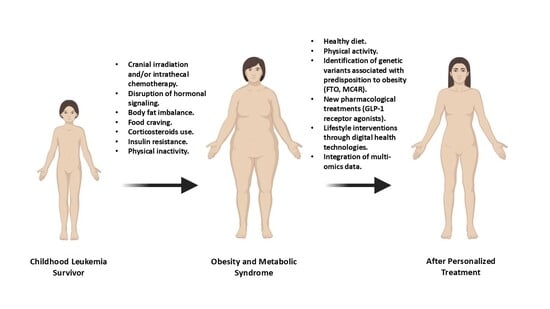
Obesity and Metabolic Syndrome in Childhood Leukemia and in Long-Term Survivors: Causes and Personalized Treatments
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Obesity and MS in Leukemia Survivors
Obesity in Leukemia Survivors
| Reference | Methods and Patients | Main Findings |
|---|---|---|
| [13] | Data from the North American Childhood Cancer Survivor Study. 1765 ALL survivors and 2167 siblings. | Higher rates of obesity among ALL survivors were associated with increasing age. ALL survivors who were treated with CRT had a significantly greater increase in BMI and younger age at CRT exposure significantly modified risk. |
| [16] | Data from Children’s Oncology Group and the Children’s Cancer Group: 1635 Children with ALL. | Prevalence of obesity is significantly lower at diagnosis than at the end of therapy (14 vs. 23%). |
| [17] | 183 children with ALL. | Prevalence of obesity is significantly lower at diagnosis (19%) than at the end of therapy (23%). |
| [18] | 165 children with ALL. | 11% of obese patients at diagnosis compared with 21% at the end of therapy. |
| [23] | 768 AML children from the Children’s Cancer Group-2961. | Overweight AML children were significantly less likely to survive and more likely to experience treatment-related mortality than middle-weight patients. |
| [24] | 748 participants treated with CRT from St Jude Lifetime Cohort study | ALL patients with CRT doses > 20 Gy presented anterior hypopituitarism with significant deficiencies in GH and LH/FSH. |
| [25] | 114 young adult survivors of childhood ALL | Increased total body fat, abdominal fat, visceral fat, and serum leptin levels among survivors treated with CRT when compared to those not treated with CRT |
| [26] | ALL survivors who received CRT (n = 82) compared to those who did not (n = 116) | Increase in leptin levels and reduction in soluble leptin receptor in plasma overweight patients compared to the non-overweight patients. |
| [27] | Systematic review with 863 ALL children and adolescents | Levels of ghrelin in children with ALL were lower than in controls. Higher leptin serum levels were associated with body fatness in ALL survivors. |
| [30] | Childhood Cancer Survivor Study; n = 600 ALL patients. | LEPR Gln223Arg polymorphism may influence obesity in female survivors of childhood ALL, particularly those exposed to CRT. |
| [31] | 191 ALL patients receiving CRT. | 9939609T allele of the FTO gene could be a protecting factor against obesity as a negative association was found between this allele and overweight in ALL survivors who received CRT. |
| [32] | GWAS among 1996 adult CCS from the St. Jude Lifetime Cohort (median age at diagnosis, 7.2 years; median age at follow-up, 32.4 years). | Potential genetic predictors of obesity were found on chromosomes 13 (FAM155A), 2 (SOX11), 4 (GLRA3), and 5 (CDH18 and BASP1) among patients exposed to CRT. Most of them were associated with neuronal growth, repair, and connectivity. |
| [33] | 1458 adult survivors of childhood ALL (median time from diagnosis, 20 years) from Childhood Cancer Survivor Study and St. Jude Lifetime Cohort Study. | Although adult survivors of childhood ALL have a similar genetic heritability for BMI to that observed in the general population, treatment with CRT could modify the effect of genetic variants on adult BMI. |
| [35] | Retrospective cohort of 183 pediatric ALL patients | During ALL therapy, patients are at risk for early development of elevated BMI and blood pressure, which places them at potentially increased risk for future adverse health conditions. |
| [36] | 893 CCS with a mean follow-up of 14.9 years from Emma Children’s Hospital/Academic Medical Center | Increased prevalence of obesity in CCS. Risk factors for developing a high BMI at follow-up were a younger age, a high BMI at diagnosis and treatment with cranial radiotherapy. |
| [37] | 3467 participating in the Childhood Cancer Survivor Study | Survivors treated with CRT or SRT exhibited a two- to threefold increased risk of adult short stature, hypothyroidism, and infertility. CRT was associated with an increased risk of being overweight/obese. |
| [38] | 36 children and adolescents between 10–21 years old newly diagnosed ALL treated with intravenous methotrexate | Delayed elimination at 48 h of plasma methotrexate was associated with approximately 2-fold higher risk for larger size and greater obesity. |
4. Metabolic Syndrome in Leukemia Survivors
5. Dietary Patterns in Childhood Cancer Survivors
6. Weight Gain and Effect of Therapy in Childhood Leukemia
7. Personalized and Precision Interventions to Prevent Obesity and MS
8. Limitations
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| AML | Acute myeloid leukemia |
| BMI | Body mass index |
| CCS | childhood cancer survivors |
| CNS | Central nervous system |
| CRT | cranial radiation therapy |
| DRI | Dietary Reference Intake |
| EFS | Event-free survival |
| FTO | Fat mass and obesity-associated gene |
| GWAS | Genome wide association study |
| LEPR | Leptin receptor gene |
| MC4R | Melanocortin-4 Receptor gene |
| MS | Metabolic syndrome |
| OS | Overall survival |
| RDI | Recommended daily intake |
| TARDBP | Trans-activation response DNA binding protein |
References
- Mohammadian-Hafshejani, A.; Farber, I.M.; Kheiri, S. Global incidence and mortality of childhood leukemia and its relationship with the Human Development Index. PLoS ONE 2024, 19, e0304354. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, G.; Cai, X.; Liu, Y.; Qian, B.; Li, D. Global, regional, and national burden of acute myeloid leukemia, 1990–2021: A systematic analysis for the global burden of disease study 2021. Biomark. Res. 2024, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, F.; Frederiksen, L.E.; Bonaventure, A.; Mader, L.; Hasle, H.; Robison, L.L.; Winther, J.F. Childhood cancer: Survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. 2021, 71, 101733. [Google Scholar] [CrossRef]
- Lee, S.H.R.; Yang, W.; Gocho, Y.; John, A.; Rowland, L.; Smart, B.; Williams, H.; Maxwell, D.; Hunt, J.; Yang, W.; et al. Pharmacotypes across the genomic landscape of pediatric acute lymphoblastic leukemia and impact on treatment response. Nat. Med. 2023, 29, 170–179. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2023. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html (accessed on 25 March 2025).
- Kakaje, A.; Alhalabi, M.M.; Ghareeb, A.; Karam, B.; Mansour, B.; Zahra, B.; Hamdan, O. Rates and trends of childhood acute lymphoblastic leukaemia: An epidemiology study. Sci. Rep. 2020, 10, 6756. [Google Scholar] [CrossRef]
- Pui, C.-H.; Yang, J.J.; Bhakta, N.; Rodriguez-Galindo, C. Global efforts toward the cure of childhood acute lymphoblastic leukaemia. Lancet Child Adolesc. Health 2018, 2, 440–454. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Xu, L.; Găman, M.-A.; Zou, Z. The genesis and evolution of acute myeloid leukemia stem cells in the microenvironment: From biology to therapeutic targeting. Cell Death Discov. 2022, 8, 397. [Google Scholar] [CrossRef]
- Hao, T.K.; Van Ha, C.; Son, N.H.; Hiep, P.N. Long-Term Outcome of Childhood Acute Myeloid Leukemia: A 10-Year Retrospective Cohort Study. Pediatr. Rep. 2020, 12, 22–25. [Google Scholar] [CrossRef]
- Bélanger, V.; Morel, S.; Napartuk, M.; Bouchard, I.; Meloche, C.; Curnier, D.; Sultan, S.; Laverdière, C.; Sinnett, D.; Marcil, V. Abnormal HDL lipid and protein composition following pediatric cancer treatment: An associative study. Lipids Health Dis. 2023, 22, 72. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Vallianou, N.G.; Spyrou, N.; Kounatidis, D.; Christodoulatos, G.S.; Karampela, I.; Dalamaga, M. Obesity and Leukemia: Biological Mechanisms, Perspectives, and Challenges. Curr. Obes. Rep. 2023, 13, 1–34. [Google Scholar] [CrossRef]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in children and adolescents: Epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef]
- Jung, A.; Kay, S.S.; Robinson, J.L.; Sheppard, B.B.; Mayer, D.K. Large-scale North American cancer survivorship surveys: 2011–2019 update. J. Cancer Surviv. 2021, 16, 1236–1267. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Alsalhe, T.A.; Chalghaf, N.; Riccò, M.; Bragazzi, N.L.; Wu, J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: An analysis of the Global Burden of Disease Study. PLoS Med. 2020, 17, e1003198. [Google Scholar] [CrossRef] [PubMed]
- Pourhassan, H.; Murphy, L.; Aldoss, I. Glucocorticoid Therapy in Acute Lymphoblastic Leukemia: Navigating Short-Term and Long-Term Effects and Optimal Regimen Selection. Curr. Hematol. Malign. Rep. 2024, 19, 175–185. [Google Scholar] [CrossRef]
- Canner, J.; Alonzo, T.A.; Franklin, J.; Freyer, D.R.; Gamis, A.; Gerbing, R.B.; Lange, B.J.; Meshinchi, S.; Woods, W.G.; Perentesis, J.; et al. Differences in outcomes of newly diagnosed acute myeloid leukemia for adolescent/young adult and younger patients. Cancer 2013, 119, 4162–4169. [Google Scholar] [CrossRef]
- Chow, E.J.; Pihoker, C.; Hunt, K.; Wilkinson, K.; Friedman, D.L. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer 2007, 110, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Esbenshade, A.J.; Simmons, J.H.; Koyama, T.; Lindell, R.B.; Friedman, D.L. Obesity and insulin resistance in pediatric acute lymphoblastic leukemia worsens during maintenance therapy. Pediatr. Blood Cancer 2013, 60, 1287–1291. [Google Scholar] [CrossRef]
- Nakayama, H.; Noguchi, M.; Fukano, R.; Ueda, T.; Taguchi, S.; Yoshimaru, K.; Namie, M.; Shimokawa, M.; Okamura, J. Sarcopenia and obesity in long-term survivors of childhood leukemia/lymphoma: A report from a single institution. Ultrasound Med. Biol. 2021, 51, 1100–1106. [Google Scholar] [CrossRef]
- Podpeskar, A.; Crazzolara, R.; Kropshofer, G.; Hetzer, B.; Rabensteiner, E.; Meister, B.; Obexer, P.; Salvador, C. Recommendations for Nutritional Supplementation in Pediatric Oncology: A Compilation of the Facts. Nutrients 2023, 15, 3239. [Google Scholar] [CrossRef]
- Kurkure, P.; Prasad, M.; Arora, B.; Chinnaswamy, G.; Vora, T.; Narula, G.; Banavali, S. Nutritional status in survivors of childhood cancer: Experience from Tata Memorial Hospital, Mumbai. Indian J. Cancer 2015, 52, 219–223. [Google Scholar] [CrossRef]
- Botta, L.; Capocaccia, R.; Vener, C.; Bernasconi, A.; Trama, A.; Didoné, F.; Demuru, E.; De Angelis, R.; Rossi, S.; Mousavi, S.M.; et al. Estimating cure and risk of death from other causes of adolescent and young adult cancer patients in Europe. Eur. J. Cancer 2025, 222, 115443. [Google Scholar] [CrossRef]
- Lange, B.J.; Gerbing, R.B.; Feusner, J.; Skolnik, J.; Sacks, N.; Smith, F.O.; Alonzo, T.A. Mortality in Overweight and Underweight Children with Acute Myeloid Leukemia. JAMA 2005, 293, 203–211. [Google Scholar] [CrossRef]
- Chemaitilly, W.; Li, Z.; Huang, S.; Ness, K.K.; Clark, K.L.; Green, D.M.; Barnes, N.; Armstrong, G.T.; Krasin, M.J.; Srivastava, D.K.; et al. Anterior Hypopituitarism in Adult Survivors of Childhood Cancers Treated with Cranial Radiotherapy: A Report From the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2015, 33, 492–500. [Google Scholar] [CrossRef]
- Janiszewski, P.M.; Oeffinger, K.C.; Church, T.S.; Dunn, A.L.; Eshelman, D.A.; Victor, R.G.; Brooks, S.; Turoff, A.J.; Sinclair, E.; Murray, J.C.; et al. Abdominal Obesity, Liver Fat, and Muscle Composition in Survivors of Childhood Acute Lymphoblastic Leukemia. J. Clin. Endocrinol. Metab. 2007, 92, 3816–3821. [Google Scholar] [CrossRef] [PubMed]
- Skoczen, S.; Tomasik, P.J.; Bik-Multanowski, M.; Surmiak, M.; Balwierz, W.; Pietrzyk, J.J.; Sztefko, K.; Gozdzik, J.; Galicka-Latała, D.; Strojny, W. Plasma levels of leptin and soluble leptin receptor and polymorphisms of leptin gene -18G > A and leptin receptor genes K109R and Q223R, in survivors of childhood acute lymphoblastic leukemia. J. Exp. Clin. Cancer Res. 2011, 30, 64. [Google Scholar] [CrossRef] [PubMed]
- Fayh, A.P.T.; Bezerra, A.D.d.L.; Friedman, R. Appetite hormones in children and adolescents with cancer: A systematic review of observational studies. Nutr. Hosp. 2020, 35, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Lövgren, I.; Abravan, A.; Bryce-Atkinson, A.; van Herk, M. The late effects of cranial irradiation in childhood on the hypothalamic–pituitary axis: A radiotherapist’s perspective. Endocr. Connect. 2022, 11, e220298. [Google Scholar] [CrossRef]
- Bhandari, R.; Chen, Y.; Chow, E.J.; Howell, R.M.; Kenney, L.B.; Krull, K.R.; Leisenring, W.; Nathan, P.C.; Neglia, J.P.; Ness, K.K.; et al. Health Outcomes Beyond Age 50 Years in Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2025, JCO2500385. [Google Scholar] [CrossRef]
- Ross, J.A.; Oeffinger, K.C.; Davies, S.M.; Mertens, A.C.; Langer, E.K.; Kiffmeyer, W.R.; Sklar, C.A.; Stovall, M.; Yasui, Y.; Robison, L.L. Genetic Variation in the Leptin Receptor Gene and Obesity in Survivors of Childhood Acute Lymphoblastic Leukemia: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2004, 22, 3558–3562. [Google Scholar] [CrossRef]
- Szymon, S.; Bik-Multanowski, M.; Balwierz, W.; Pietrzyk, J.J.; Surmiak, M.; Strojny, W.; Galicka-Latala, D.; Gozdzik, J. Homozygosity for the rs9939609T allele of the FTO gene may have protective effect on becoming overweight in survivors of childhood acute lymphoblastic leukaemia. J. Genet. 2011, 90, 365–368. [Google Scholar] [CrossRef]
- Wilson, C.L.; Liu, W.; Yang, J.J.; Kang, G.; Ojha, R.P.; Neale, G.A.; Srivastava, D.K.; Gurney, J.G.; Hudson, M.M.; Robison, L.L.; et al. Genetic and clinical factors associated with obesity among adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort. Cancer 2015, 121, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.A.; Brown, A.L.; Belmont, J.W.; Scheurer, M.E.; Arroyo, V.M.; Foster, K.L.; Kern, K.D.; Hudson, M.M.; Leisenring, W.M.; Okcu, M.F.; et al. Genetic variation in the body mass index of adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study and the St. Jude Lifetime Cohort. Cancer 2020, 127, 310–318. [Google Scholar] [CrossRef]
- Gregoriou, K.; Craigie, I.; Gibson, B.; Mason, A.; Shaikh, M.G. Risk factors and management of corticosteroid-induced hyperglycaemia in paediatric acute lymphoblastic leukaemia. Pediatr. Blood Cancer 2019, 67, e28085. [Google Scholar] [CrossRef]
- Esbenshade, A.J.; Simmons, J.H.; Koyama, T.; Koehler, E.; Whitlock, J.A.; Friedman, D.L. Body mass index and blood pressure changes over the course of treatment of pediatric acute lymphoblastic leukemia. Pediatr. Blood Cancer 2010, 56, 372–378. [Google Scholar] [CrossRef]
- van Santen, H.M.; Geskus, R.B.; Raemaekers, S.; van Trotsenburg, A.S.P.; Vulsma, T.; van der Pal, H.J.H.; Caron, H.N.; Kremer, L.C.M. Changes in body mass index in long-term childhood cancer survivors. Cancer 2015, 121, 4197–4204. [Google Scholar] [CrossRef]
- Chow, E.J.; Liu, W.; Srivastava, K.; Leisenring, W.M.; Hayashi, R.J.; Sklar, C.A.; Stovall, M.; Robison, L.L.; Baker, K.S. Differential effects of radiotherapy on growth and endocrine function among acute leukemia survivors: A childhood cancer survivor study report. Pediatr. Blood Cancer 2012, 60, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Orgel, E.; Nabais, T.; Douglas, C.; Mittelman, S.D.; Neely, M. Effect of Body Fat on Population Pharmacokinetics of High-Dose Methotrexate in Pediatric Patients with Acute Lymphoblastic Leukemia. J. Clin. Pharmacol. 2020, 61, 755–762. [Google Scholar] [CrossRef]
- Cho, Y.K.; Jung, C.H.; Kang, Y.M.; Hwang, J.Y.; Kim, E.H.; Yang, D.H.; Kang, J.; Park, J.; Kim, H.; Lee, W.J. 2013 ACC/AHA Cholesterol Guideline Versus 2004 NCEP ATP III Guideline in the Prediction of Coronary Artery Calcification Progression in a Korean Population. J. Am. Hear. Assoc. 2016, 5, e003410. [Google Scholar] [CrossRef]
- Bielorai, B.; Pinhas-Hamiel, O. Type 2 Diabetes Mellitus, the Metabolic Syndrome, and Its Components in Adult Survivors of Acute Lymphoblastic Leukemia and Hematopoietic Stem Cell Transplantations. Curr. Diabetes Rep. 2018, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Buchanan, G.R.; Eshelman, D.A.; Denke, M.A.; Andrews, T.C.; Germak, J.A.; Tomlinson, G.E.; Snell, L.E.; Foster, B.M. Cardiovascular Risk Factors in Young Adult Survivors of Childhood Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. 2001, 23, 424–430. [Google Scholar] [CrossRef]
- Link, K.; Moëll, C.; Garwicz, S.; Cavallin-Ståhl, E.; Björk, J.; Thilén, U.; Ahrén, B.; Erfurth, E.M. Growth Hormone Deficiency Predicts Cardiovascular Risk in Young Adults Treated for Acute Lymphoblastic Leukemia in Childhood. J. Clin. Endocrinol. Metab. 2004, 89, 5003–5012. [Google Scholar] [CrossRef]
- Oudin, C.; Simeoni, M.-C.; Sirvent, N.; Contet, A.; Coroller, A.B.-L.; Bordigoni, P.; Curtillet, C.; Poirée, M.; Thuret, I.; Play, B.; et al. Prevalence and risk factors of the metabolic syndrome in adult survivors of childhood leukemia. Blood 2011, 117, 4442–4448. [Google Scholar] [CrossRef][Green Version]
- van Waas, M.; Neggers, S.J.C.M.M.; Pieters, R.; Heuvel-Eibrink, M.M.v.D. Components of the metabolic syndrome in 500 adult long-term survivors of childhood cancer. Ann. Oncol. 2009, 21, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Siviero-Miachon, A.A.; Spinola-Castro, A.M.; Lee, M.L.d.M.; Monteiro, C.M.d.C.; Carvalho, A.C.d.C.; Geloneze, B.; Guerra-Junior, G. Subcutaneous adipose tissue plays a beneficial effect on subclinical atherosclerosis in young survivors of acute lymphocytic leukemia. Vasc. Health Risk Manag. 2015, 11, 479–488. [Google Scholar] [CrossRef]
- Zareifar, S.; Haghpanah, S.; Shorafa, E.; Shakibazad, N.; Karamizadeh, Z. Evaluation of Metabolic Syndrome and Related Factors in Children Affected by Acute Lymphoblastic Leukemia. Indian J. Med. Paediatr. Oncol. 2017, 38, 97–102. [Google Scholar] [PubMed]
- Shams-White, M.; Kelly, M.J.; Gilhooly, C.; Liu, S.; Must, A.; Parsons, S.K.; Saltzman, E.; Zhang, F.F. Food craving and obesity in survivors of pediatric ALL and lymphoma. Appetite 2016, 96, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ladas, E.J.; Orjuela, M.; Stevenson, K.; Cole, P.D.; Lin, M.; Athale, U.H.; Clavell, L.A.; Leclerc, J.-M.; Michon, B.; Schorin, M.A.; et al. Dietary intake and childhood leukemia: The Diet and Acute Lymphoblastic Leukemia Treatment (DALLT) cohort study. Nutrition 2016, 32, 1103–1109.e1. [Google Scholar] [CrossRef]
- Zhang, F.F.; Saltzman, E.; Kelly, M.J.; Liu, S.; Must, A.; Parsons, S.K.; Roberts, S.B. Comparison of childhood cancer survivors’ nutritional intake with US dietary guidelines. Pediatr. Blood Cancer 2015, 62, 1461–1467. [Google Scholar] [CrossRef]
- Delvin, E.; Marcil, V.; Alos, N.; Laverdière, C.; Sinnett, D.; Krajinovic, M.; Bélanger, V.; Drouin, S.; Nyalendo, C.; Levy, E. Is there a relationship between vitamin D nutritional status and metabolic syndrome in childhood acute lymphoblastic leukemia survivors? A PETALE study. Clin. Nutr. ESPEN 2019, 31, 28–32. [Google Scholar] [CrossRef]
- Tandon, S.; Moulik, N.R.; Kumar, A.; Mahdi, A.A.; Kumar, A. Effect of pre-treatment nutritional status, folate and vitamin B12 levels on induction chemotherapy in children with acute lymphoblastic leukemia. Indian Pediatr. 2015, 52, 385–389. [Google Scholar] [CrossRef]
- Kijima, T.; Kato, T.; Goto, Y.; Kuribayashi, K.; Mikami, K.; Negi, Y.; Murakami, S.; Yoshida, T.; Homma, M.; Wakana, A.; et al. KEYNOTE-A17: First-Line Pembrolizumab Plus Cisplatin–Pemetrexed in Japanese Participants with Advanced Pleural Mesothelioma. Cancer Sci. 2025, 116, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, X.; Wang, C.; Wang, Y.; Chen, Y.; Li, J.; Niu, C.; Gao, P. Impact of SLCO1B1 521T > C variant on leucovorin rescue and risk of relapse in childhood acute lymphoblastic leukemia treated with high-dose methotrexate. Pediatr. Blood Cancer 2014, 61, 2203–2207. [Google Scholar] [CrossRef] [PubMed]
- Sterba, J.; Dusek, L.; Demlova, R.; Valik, D. Pretreatment Plasma Folate Modulates the Pharmacodynamic Effect of High-Dose Methotrexate in Children with Acute Lymphoblastic Leukemia and Non-Hodgkin Lymphoma: “Folate Overrescue” Concept Revisited. Clin. Chem. 2006, 52, 692–700. [Google Scholar] [CrossRef]
- Nasra, S.; Bhatia, D.; Kumar, A. Targeted Macrophage Re-Programming: Synergistic Therapy with Methotrexate and RELA siRNA Folate-Liposome in RAW264.7 Cells and Arthritic Rats. Adv. Health Mater. 2024, 13, e2400679. [Google Scholar] [CrossRef]
- van Vliet, M.M.; Schoenmakers, S.; Willemsen, S.P.; Sinclair, K.D.; Steegers-Theunissen, R.P.M. First-trimester maternal folate and vitamin B12 concentrations and their associations with first-trimester placental growth: The Rotterdam Periconception Cohort. Hum. Reprod. 2025, 40, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Grigorescu, A.; Riza, A.-L.; Streata, I.; Netea, M.G. Metabolic dysregulation of trained immunity in immune aging and the impact of dietary patterns. Am. J. Physiol. Physiol. 2025, 329, C456–C470. [Google Scholar] [CrossRef]
- Singer, A.W.; Selvin, S.; Block, G.; Golden, C.; Carmichael, S.L.; Metayer, C. Maternal prenatal intake of one-carbon metabolism nutrients and risk of childhood leukemia. Cancer Causes Control. 2016, 27, 929–940. [Google Scholar] [CrossRef]
- Bailey, H.D.; Miller, M.; Langridge, A.; de Klerk, N.H.; van Bockxmeer, F.M.; Attia, J.; Scott, R.J.; Armstrong, B.K.; Milne, E. Maternal Dietary Intake of Folate and Vitamins B6 and B12 During Pregnancy and the Risk of Childhood Acute Lymphoblastic Leukemia. Nutr. Cancer 2012, 64, 1122–1130. [Google Scholar] [CrossRef]
- Bruzzi, P.; Bigi, E.; Predieri, B.; Bonvicini, F.; Cenciarelli, V.; Felici, F.; Iughetti, L. Long-term effects on growth, development, and metabolism of ALL treatment in childhood. Expert Rev. Endocrinol. Metab. 2018, 14, 49–61. [Google Scholar] [CrossRef]
- Egnell, C.; Heyman, M.; Jónsson, Ó.G.; Raja, R.A.; Niinimäki, R.; Albertsen, B.K.; Schmiegelow, K.; Stabell, N.; Vaitkeviciene, G.; Lepik, K.; et al. Obesity as a predictor of treatment-related toxicity in children with acute lymphoblastic leukaemia. Br. J. Haematol. 2021, 196, 1239–1247. [Google Scholar] [CrossRef]
- Shimony, S.; Flamand, Y.; Valtis, Y.K.; Place, A.E.; Silverman, L.B.; Vrooman, L.M.; Brunner, A.M.; Sallan, S.E.; Stone, R.M.; Wadleigh, M.; et al. Effect of BMI on toxicities and survival among adolescents and young adults treated on DFCI Consortium ALL trials. Blood Adv. 2023, 7, 5234–5245. [Google Scholar] [CrossRef] [PubMed]
- Orgel, E.; Tucci, J.; Alhushki, W.; Malvar, J.; Sposto, R.; Fu, C.H.; Freyer, D.R.; Abdel-Azim, H.; Mittelman, S.D. Obesity is associated with residual leukemia following induction therapy for childhood B-precursor acute lymphoblastic leukemia. Blood 2014, 124, 3932–3938. [Google Scholar] [CrossRef] [PubMed]
- Birenboim, S.B.; Rabinowicz, R. Acute therapy-related toxicities in pediatric acute lymphoblastic leukemia. Haematologica 2025, 110, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Mosaad, Y.M.; Morzak, M.; Chennawi, F.A.E.A.E.; Elsharkawy, A.A.; Abdelsalam, M. Evaluation of the role of FTO (rs9939609) and MC4R (rs17782313) gene polymorphisms in type 1 diabetes and their relation to obesity. J. Pediatr. Endocrinol. Metab. 2023, 37, 110–122. [Google Scholar] [CrossRef]
- Oever, S.R.v.D.; Mulder, R.L.; Oeffinger, K.C.; A Gietema, J.; Skinner, R.; Constine, L.S.; Wallace, W.H.; Armenian, S.; Barnea, D.; Bardi, E.; et al. Metabolic syndrome in childhood, adolescent, and young adult cancer survivors: Recommendations for surveillance from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Eur. J. Endocrinol. 2025, 192, S27–S40. [Google Scholar] [CrossRef]
- Mai, Y.; Jing, Z.; Sun, P.; Wang, Y.; Dong, P.; Liu, J. TARDBP drives T-cell acute lymphoblastic leukemia progression by binding MDM2 mRNA, involving β-catenin pathway. FASEB J. 2024, 38, e70110. [Google Scholar] [CrossRef]
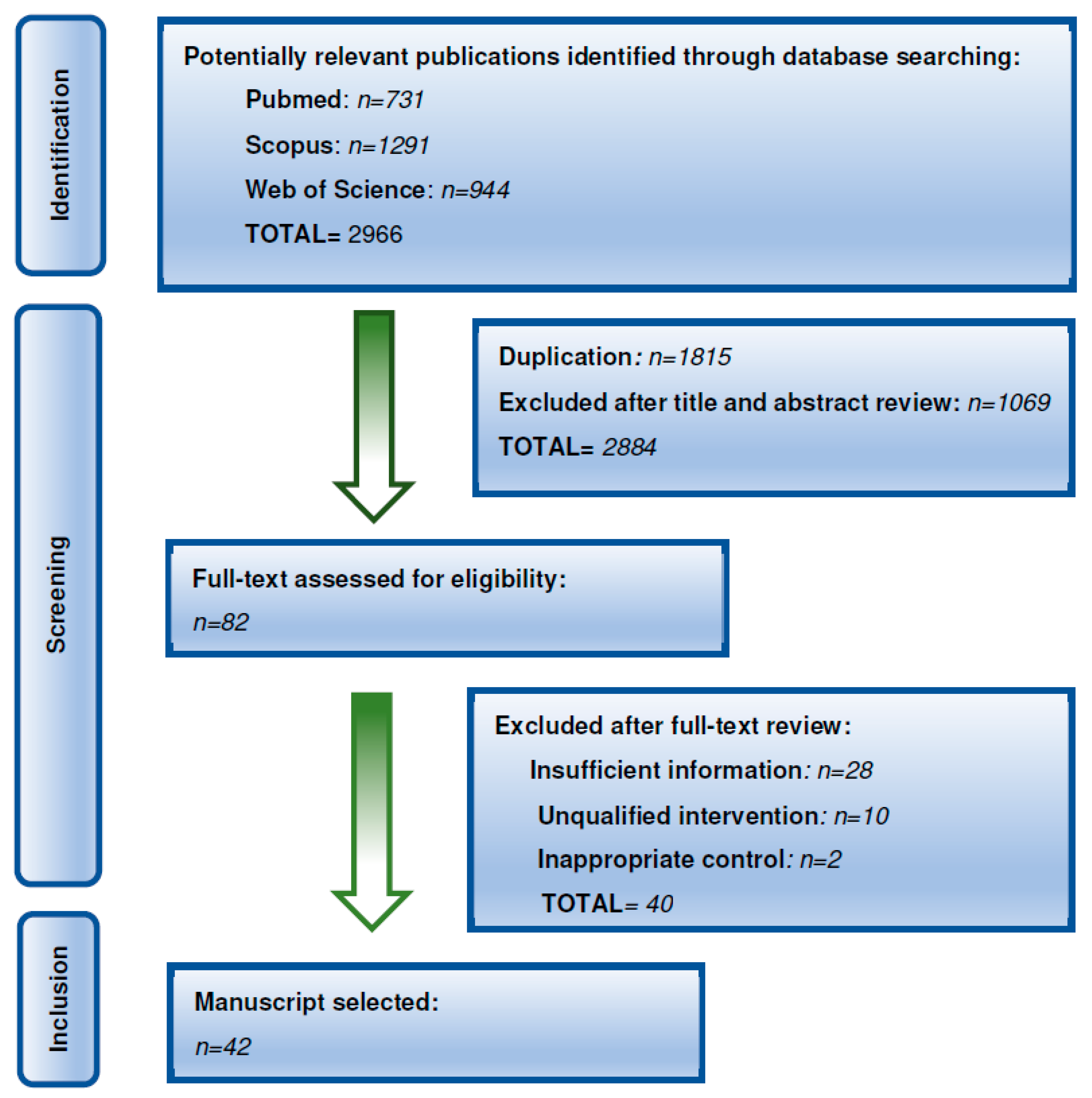
| Reference | Methods and Patients | Main Findings |
|---|---|---|
| [41] | 26 survivors of childhood ALL. | 62% had at least one CVRF related to their treatment, and 30% had more than two. Subjects who were treated with CRT had an increased BMI (p = 0.039), triglyceride (p = 0.027) and VLDL levels (p = 0.022) when compared with those who received only chemotherapy. Survivors of childhood ALL treated with CRT were at risk for MS. |
| [42] | Matched case–control study with 44 ALL survivors treated with CRT or chemotherapy at a median of 17 years after treatment. | Significantly higher levels of insulin (p = 0.002), blood glucose (p = 0.01), LDL (p < 0.05), apolipoprotein B (p < 0.05), triglycerides (p < 0.05), and leptin (p < 0.05) were found among ALL patients compared with controls. ALL patients had higher BMI, waist to hip ratio, higher fat mass and lower lean mass (p < 0.001). Impaired indicators of cardiac function (ejection fraction; p < 0.001, shortening fraction; p = 0.01) were also observed among survivors. More than 90% of patients were GH deficient. |
| [43] | Prospective multicentric study (n = 184), evaluating the prevalence of MS in young adults surviving childhood leukemia with a mean follow-up of 15.4 years | Overall prevalence of MS was 9.2% with a significant higher risk observed in the group treated with TBI in the univariate and in the multivariate analysis. TBI was also associated with higher blood levels of triglycerides (OR = 4.5; p = 0.004), low levels of HDL (OR = 2.5; p = 0.02), and elevated fasting glucose (OR = 6.1; p = 0.04). |
| [44] | 500 adult CCS, with a median follow-up time of 19 years | ALL survivors treated with cranial irradiation had an increased risk of developing MS compared with ALL survivors not treated with cranial irradiation (23% vs. 7%, p = 0.011) |
| [45] | Cross-sectional study in 55 young ALL survivors (25 irradiated and 30 non-irradiated) and 24 leukemia-free controls | Treatment with CRT had a significant effect on visceral adipose tissue and subcutaneous adipose tissue accumulation. cIMT positively correlated with exposure to CRT (p = 0.029), diastolic blood pressure (p = 0.016), and leptin-to-adiponectin ratio (p = 0.048). CRT modified the distribution of fat and could play a critical role in atherosclerosis. Leptin-to-adiponectin ratio and diastolic blood pressure, both associated with MS, also influenced cIMT. |
| [46] | 53 ALL children | An increase of 20% of overweight patients was observed at the end of treatment (p = 0.04). Mean blood leptin level was also higher. BMI Z-score significantly increased over the study period (p = 0.001). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corominas-Herrero, F.J.; Navas-Carrillo, D.; Ortega-García, J.A.; Martínez-Romera, I.; Orenes-Piñero, E. Obesity and Metabolic Syndrome in Childhood Leukemia and in Long-Term Survivors: Causes and Personalized Treatments. Cancers 2025, 17, 3446. https://doi.org/10.3390/cancers17213446
Corominas-Herrero FJ, Navas-Carrillo D, Ortega-García JA, Martínez-Romera I, Orenes-Piñero E. Obesity and Metabolic Syndrome in Childhood Leukemia and in Long-Term Survivors: Causes and Personalized Treatments. Cancers. 2025; 17(21):3446. https://doi.org/10.3390/cancers17213446
Chicago/Turabian StyleCorominas-Herrero, Francisco José, Diana Navas-Carrillo, Juan Antonio Ortega-García, Isabel Martínez-Romera, and Esteban Orenes-Piñero. 2025. "Obesity and Metabolic Syndrome in Childhood Leukemia and in Long-Term Survivors: Causes and Personalized Treatments" Cancers 17, no. 21: 3446. https://doi.org/10.3390/cancers17213446
APA StyleCorominas-Herrero, F. J., Navas-Carrillo, D., Ortega-García, J. A., Martínez-Romera, I., & Orenes-Piñero, E. (2025). Obesity and Metabolic Syndrome in Childhood Leukemia and in Long-Term Survivors: Causes and Personalized Treatments. Cancers, 17(21), 3446. https://doi.org/10.3390/cancers17213446