Genetic, Epidemiological, Clinical, and Therapeutic Trajectories in Colon and Rectal Cancers
Simple Summary
Abstract
1. Introduction
2. Most Typical Genetic Alterations in Colon Cancer: APC, p53, KRAS, SMAD4
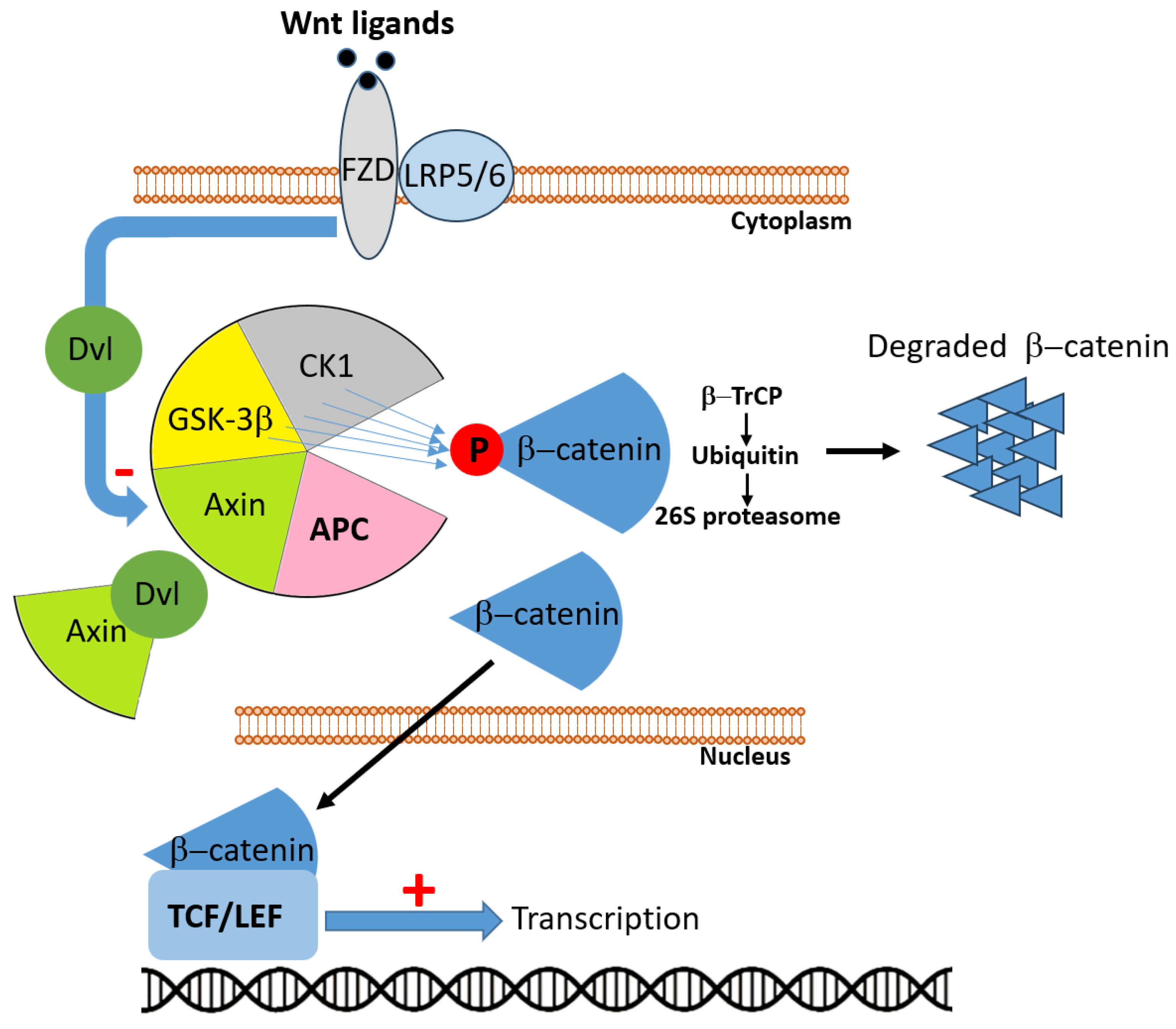
2.1. APC
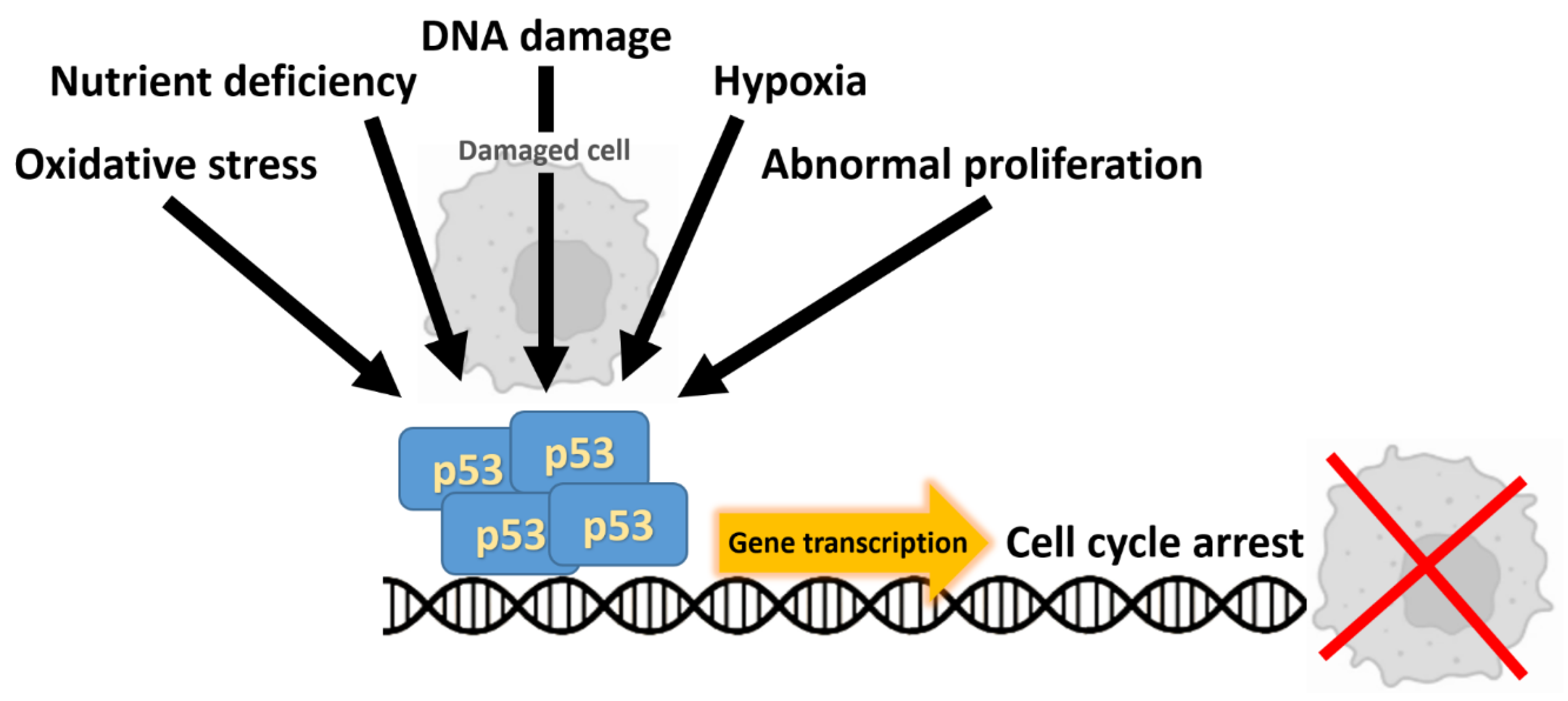
2.2. TP53
2.3. RAS
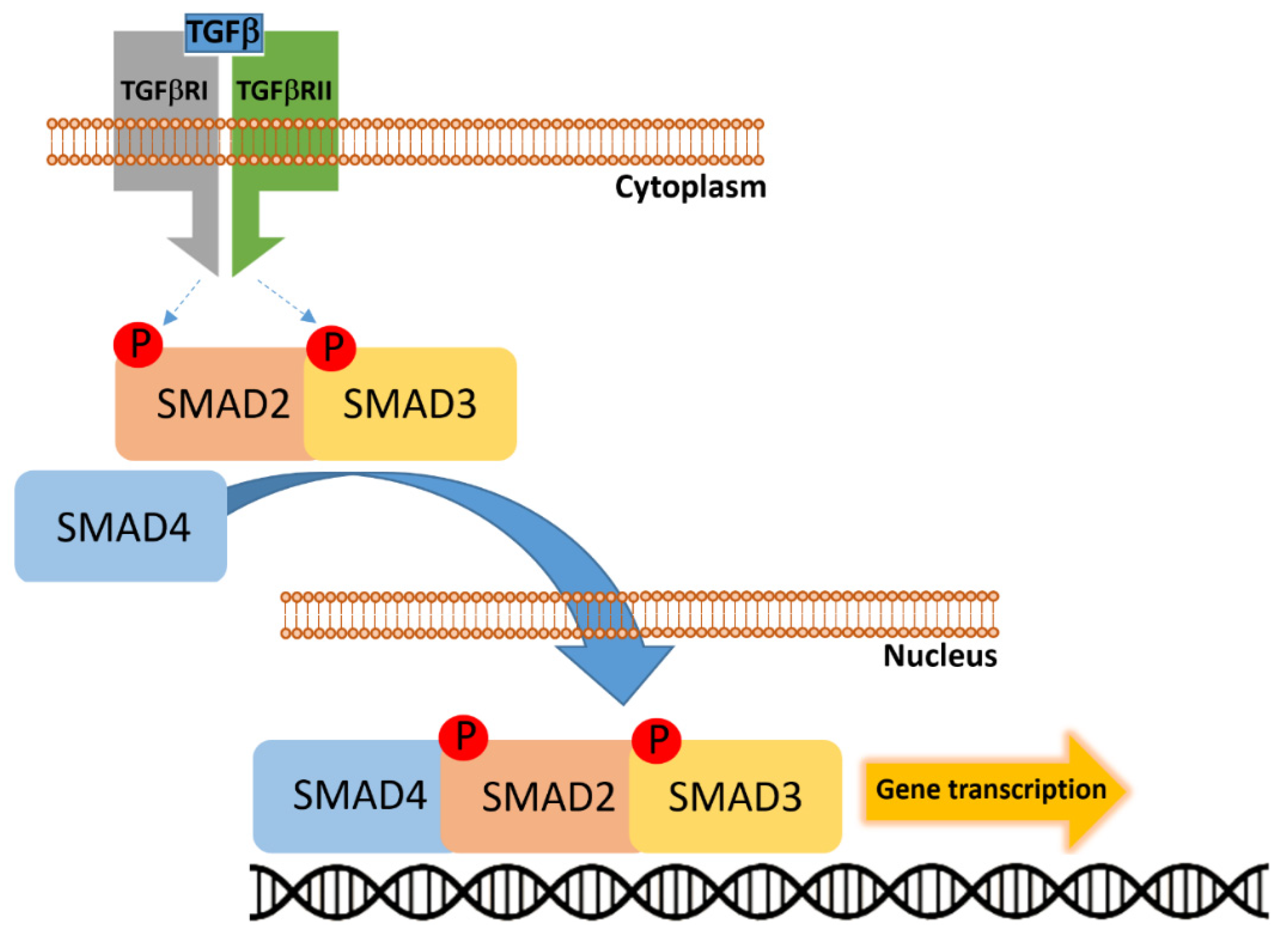
2.4. SMAD4
3. Additional Actionable Alterations in CRC: BRAF and HER2
3.1. BRAF
3.2. HER2
4. Distinct Genomic and Microenvironmental Trajectories in Colon Versus Rectal Cancer
| Feature | Right-Sided Colon Cancer | Left-Sided Colon Cancer | Rectal Cancer |
|---|---|---|---|
| Embryologic origin | Midgut (cecum, ascending, proximal 2/3 transverse colon) | Hindgut (distal 1/3 transverse, descending, sigmoid) | Hindgut |
| Molecular phenotype | Enriched for MSI-H, CIMP, higher mutational burden | Predominantly CIN, frequent somatic copy-number alterations | Higher TP53 and FBXW7 mutation frequencies |
| Key mutations/alterations | BRAF p.V600E, certain PIK3CA mutations | High TP53 mutation rate, recurrent CNV-driven oncogenic events | TP53, FBXW7 mutations; higher TOPO1 expression and ERBB2 alterations |
| Transcriptomic CMS subtypes | More frequent CMS1 (immune-high) | More frequent CMS2 (“canonical” epithelial); subset CMS4 (mesenchymal/stromal, desmoplastic) | More frequent CMS2; enrichment for CMS4 in post-neoadjuvant resection specimens |
| Tumor microenvironment | Immune-infiltrated (particularly in MSI-H) | Variable immune composition; subset enriched in stromal/desmoplastic signatures (CMS4) | Stromal/CAF enrichment |
| Microbiome | Fusobacterium dominance | Bacteroides/Desulfovibrio shift | Bacteroides/Desulfovibrio shift |
5. Epidemiology of Colon and Rectal Cancers
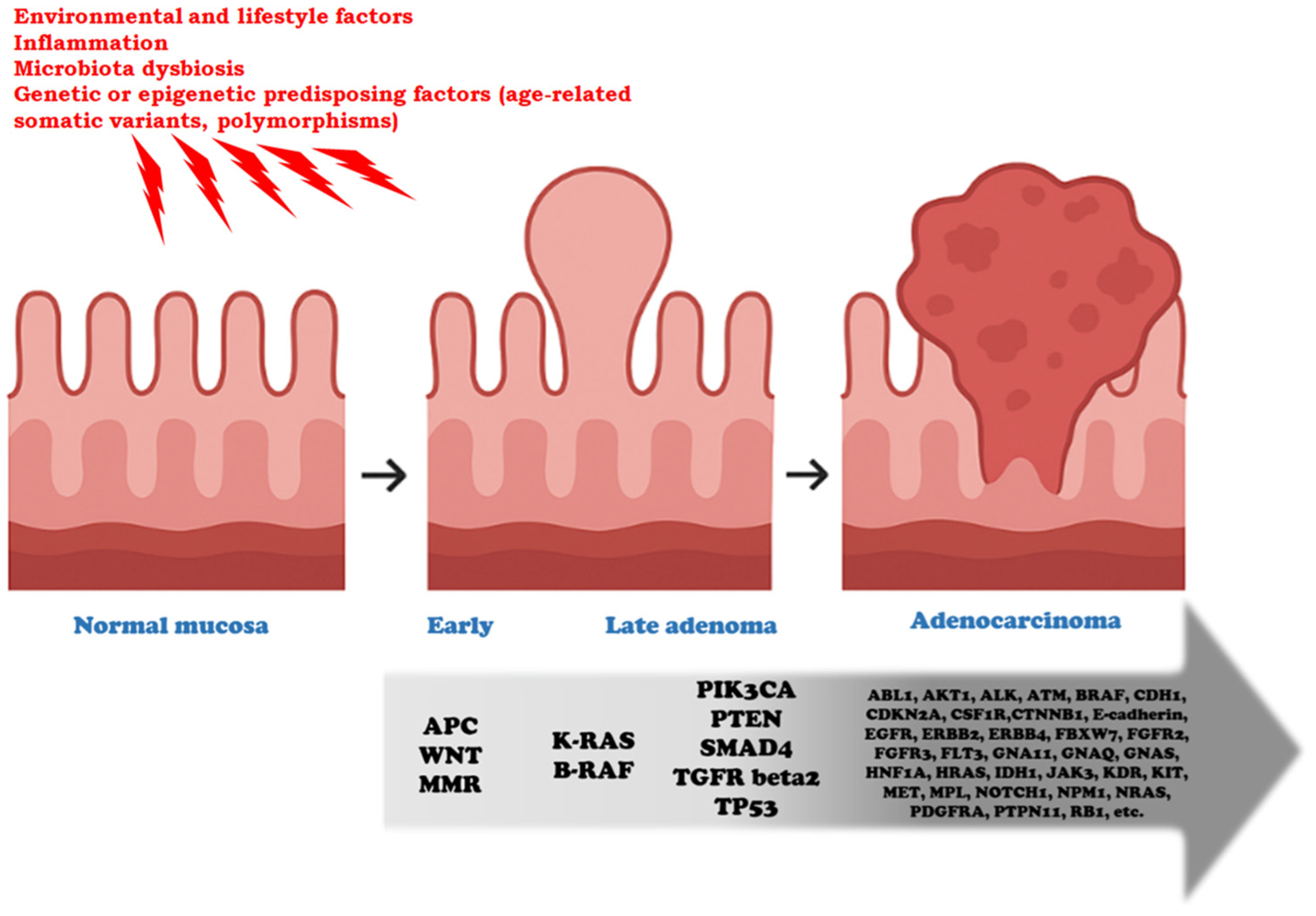
6. Clinical Manifestations of Colon Cancer: From Polyps to Adenocarcinoma and Stages I–IV
6.1. Polyps and Progression to Adenocarcinoma
6.2. Clinical Manifestations of Polyps and Non-Metastatic Disease
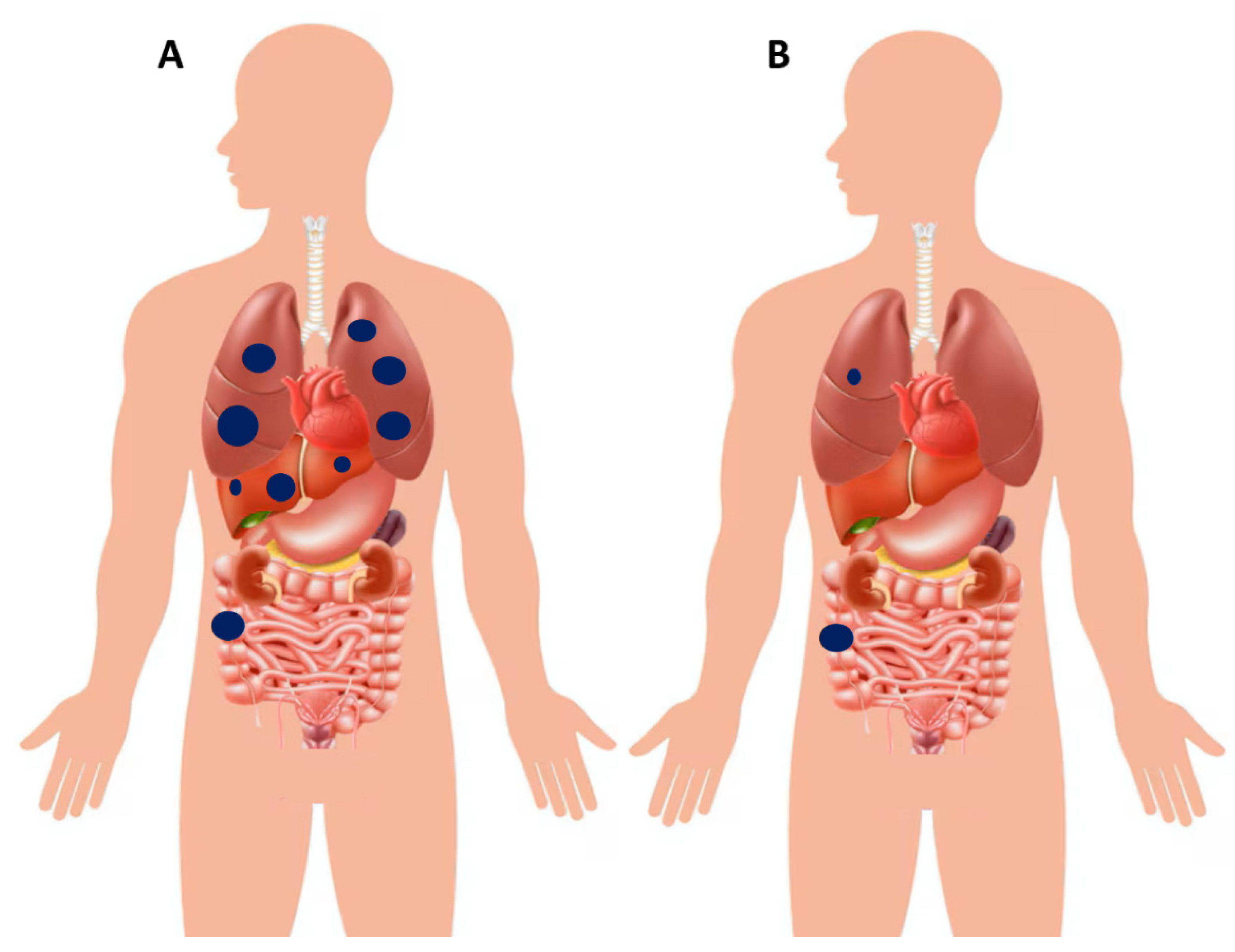
6.3. Clinical Manifestations of Metastatic Colorectal Cancer (Stage IV Disease)
7. Poly-Metastatic and Oligo-Metastatic Colon Cancer: Need for Different Therapeutic Approaches
Oligo-Metastatic Disease
8. Systemic Treatments and the “Continuum of Care” Concept
8.1. General Considerations in Stages I–III and Liver-Limited Disease
8.2. The “Continuum of Care” Paradigm
8.3. First-Line Strategies in Poly-Metastatic Colorectal Cancer
8.4. Second-Line and Beyond in Poly-Metastatic Colorectal Cancer
8.5. Breaking the Therapeutic Line Paradigm: Rechallenge and Treatment Recycling Strategies in Metastatic Colorectal Cancer
9. Methodological Challenges in Colorectal Cancer Research
9.1. Studying Intermittent Therapy: An Urgent Need but Also a Formidable Methodological Challenge
9.1.1. Studying Intermittent Therapy: Rationale and Definitions
9.1.2. Methodological Challenges and Biological Considerations
9.1.3. Endpoints and Future Directions in Precision Intermittent Therapy
9.2. The Role of Circulating Tumor DNA and Variant Allele Frequency in Precision Oncology of Colorectal Cancer
9.2.1. Clinical Utility of ctDNA Across CRC Stages
9.2.2. Variant Allele Frequency as a Surrogate of Clonal Dominance
9.2.3. Technical and Methodological Considerations in ctDNA Analysis
9.3. Refining Patient Selection: Challenges and Opportunities
10. Other Unresolved Challenges and Future Directions
10.1. Early Detection and Prevention
10.2. Overcoming Therapeutic Resistance
10.3. Expanding the Role of Immunotherapy
10.4. Bridging Disparities in Colorectal Cancer Outcomes
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Ottaiano, A.; Santorsola, M.; Caraglia, M.; Circelli, L.; Gigantino, V.; Botti, G.; Nasti, G. Genetic regressive trajectories in colorectalcancer: A new hallmark of oligo-metastaticdisease? Transl. Oncol. 2021, 14, 101131. [Google Scholar] [CrossRef]
- Mizutani, T.; Boretto, M.; Lim, S.; Drost, J.; González, D.M.; Oka, R.; Geurts, M.H.; Begthel, H.; Korving, J.; van Es, J.H.; et al. Recapitulating the adenoma-carcinoma sequence by selection of four spontaneous oncogenic mutations in mismatch-repair-deficient human colon organoids. Nat. Cancer 2024, 5, 1852–1867. [Google Scholar] [CrossRef]
- Joo, J.E.; Viana-Errasti, J.; Buchanan, D.D.; Valle, L. Genetics, genomics and clinical features of adenomatous polyposis. Fam. Cancer 2025, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Molina, R.; Martínez, R.; Suárez, M.; Peña-Cabia, A.; Calderón, M.C.; Mateo, J. Lynch syndrome and colorectal cancer: A review of current perspectives in molecular genetics and clinical strategies. Oncol. Res. 2025, 33, 1531–1545. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.Q.; Luo, Q.; Wang, L.; Song, G.B.; Sheng, J.P.; Xu, B. Signaling pathways involved in colorectal cancer: Pathogenesis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Santorsola, M. Revolutionizing KRAS p.G12C therapy in metastatic colorectal cancer: The triumph of dual inhibition. Med 2023, 4, 857–859. [Google Scholar] [CrossRef]
- Fakih, M.G.; Salvatore, L.; Esaki, T.; Modest, D.P.; Lopez-Bravo, D.P.; Taieb, J.; Karamouzis, M.V.; Ruiz-Garcia, E.; Kim, T.W.; Kuboki, Y.; et al. Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 389, 2125–2139. [Google Scholar] [CrossRef]
- Maiorano, B.A.; Parisi, A.; Maiello, E.; Ciardiello, D. The Interplay between Anti-Angiogenics and Immunotherapy in Colorectal Cancer. Life 2022, 12, 1552. [Google Scholar] [CrossRef]
- Sambo, M.; Bailoni, A.; Mariani, F.; Granai, M.; Calomino, N.; Mancini, V.; D’Antiga, A.; Montagnani, F.; Tumbarello, M.; Lazzi, S.; et al. Prevalence, Incidence and Predictors of Anal HPV Infection and HPV-Related Squamous Intraepithelial Lesions in a Cohort of People Living with HIV. Diagnostics 2025, 15, 198. [Google Scholar] [CrossRef]
- Bardelčíková, A.; Šoltys, J.; Mojžiš, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Shahgoli, V.K.; Noorolyai, S.; Ahmadpour Youshanlui, M.; Saeidi, H.; Nasiri, H.; Mansoori, B.; Holmskov, U.; Baradaran, B. Inflammatory bowel disease, colitis, and cancer: Unmasking the chronic inflammation link. Int. J. Color. Dis. 2024, 39, 173. [Google Scholar] [CrossRef]
- Burgos-Molina, A.M.; Téllez Santana, T.; Redondo, M.; Bravo Romero, M.J. The Crucial Role of Inflammation and the Immune System in Colorectal Cancer Carcinogenesis: A Comprehensive Perspective. Int. J. Mol. Sci. 2024, 25, 6188. [Google Scholar] [CrossRef]
- Wang, T.; Fu, J.; Huang, Y.; Fu, C. Mechanism of APC truncation involved in colorectal cancer tumorigenesis (Review). Oncol. Lett. 2024, 29, 2. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Xicola, R.M.; Nguyen, V.; Lim, J.; Thorne, C.; Salhia, B.; Llor, X.; Ellis, N.; Padi, M. Molecular drivers of tumor progression in microsatellite stable APC mutation-negative colorectal cancers. Sci. Rep. 2021, 11, 23507. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Furth, E.E.; Rustgi, A.K.; Klein, P.S. When You Come to a Fork in the Road, Take It: WntSignaling Activates Multiple Pathways through the APC/Axin/GSK-3 Complex. Cells 2023, 12, 2256. [Google Scholar] [CrossRef]
- He, K.; Gan, W.J. Wnt/β-Catenin Signaling Pathway in the Development and Progression of Colorectal Cancer. Cancer Manag. Res. 2023, 15, 435–448. [Google Scholar] [CrossRef]
- Zhang, L.; Shay, J.W. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109, djw332. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Liu, J.; Pan, S.; Hsieh, M.H.; Ng, N.; Sun, F.; Wang, T.; Kasibhatla, S.; Schuller, A.G.; Li, A.G.; Cheng, D.; et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. USA 2013, 110, 20224–20229. [Google Scholar] [CrossRef]
- Jang, M.K.; Mashima, T.; Seimiya, H. Tankyrase Inhibitors Target Colorectal Cancer Stem Cells via AXIN-Dependent Downregulation of c-KIT Tyrosine Kinase. Mol. Cancer Ther. 2020, 19, 765–776. [Google Scholar] [CrossRef]
- Huang, J.L.; Chang, Y.T.; Hong, Z.Y.; Lin, C.S. Targeting DNA Damage Response and Immune Checkpoint for Anticancer Therapy. Int. J. Mol. Sci. 2022, 23, 3238. [Google Scholar] [CrossRef]
- Capuozzo, M.; Santorsola, M.; Bocchetti, M.; Perri, F.; Cascella, M.; Granata, V.; Celotto, V.; Gualillo, O.; Cossu, A.M.; Nasti, G.; et al. p53: From FundamentalBiology to Clinical Applications in Cancer. Biology 2022, 11, 1325. [Google Scholar] [CrossRef]
- Liebl, M.C.; Hofmann, T.G. The Role of p53 Signaling in Colorectal Cancer. Cancers 2021, 13, 2125. [Google Scholar] [CrossRef]
- Ottaiano, A.; Santorsola, M.; Capuozzo, M.; Perri, F.; Circelli, L.; Cascella, M.; Ianniello, M.; Sabbatino, F.; Granata, V.; Izzo, F.; et al. The prognosticrole of p53 mutations in metastatic colorectal cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2023, 186, 104018. [Google Scholar] [CrossRef]
- Punekar, S.R.; Velcheti, V.; Neel, B.G.; Wong, K.K. The current state of the art and future trends in RAS-targeted cancer therapies. Nat. Rev. Clin. Oncol. 2022, 19, 637–655. [Google Scholar] [CrossRef]
- Fernández Montes, A.; Alonso Orduña, V.; Asensio Martínez, E.; Rodríguez Salas, N.; Torres, E.; Cacho Lavín, D.; Rodríguez Alonso, R.M.; Falcó, E.; Oliva, J.C.; Cirera, L.; et al. The Frequency of Specific KRAS Mutations, and Their Impact on Treatment Choice and Survival, in Patients with Metastatic Colorectal Cancer. Oncologist 2023, 28, e902–e909. [Google Scholar] [CrossRef] [PubMed]
- Meriggi, F.; Vermi, W.; Bertocchi, P.; Zaniboni, A. The Emerging Role of NRAS Mutations in Colorectal Cancer Patients Selected for Anti-EGFR Therapies. Rev. Recent. Clin. Trials 2014, 9, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ji, Q.; Li, Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: Underlying mechanisms and reversal strategies. J. Exp. Clin. Cancer Res. 2021, 40, 328. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Normanno, N.; Facchini, S.; Cassata, A.; Nappi, A.; Romano, C.; Silvestro, L.; De Stefano, A.; Rachiglio, A.M.; Roma, C.; et al. Study of Ras Mutations’ Prognostic Value in Metastatic Colorectal Cancer: STORIA Analysis. Cancers 2020, 12, 1919. [Google Scholar] [CrossRef]
- Ottaiano, A.; Sabbatino, F.; Perri, F.; Cascella, M.; Sirica, R.; Patrone, R.; Capuozzo, M.; Savarese, G.; Ianniello, M.; Petrillo, N.; et al. KRAS p.G12C Mutation in Metastatic Colorectal Cancer: Prognostic Implications and Advancements in Targeted Therapies. Cancers 2023, 15, 3579. [Google Scholar] [CrossRef]
- Ríos-Hoyo, A.; Monzonís, X.; Vidal, J.; Linares, J.; Montagut, C. Unveiling acquired resistance to anti-EGFR therapies in colorectal cancer: A long and winding road. Front. Pharmacol. 2024, 15, 1398419. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Tian, G.; Zhang, Y.; Qiu, Z. Exploring the molecular mechanisms and therapeutic potential of SMAD4 in colorectal cancer. Cancer Biol. Ther. 2024, 25, 2392341. [Google Scholar] [CrossRef] [PubMed]
- Fleming, N.I.; Jorissen, R.N.; Mouradov, D.; Christie, M.; Sakthianandeswaren, A.; Palmieri, M.; Day, F.; Li, S.; Tsui, C.; Lipton, L.; et al. SMAD2, SMAD3 and SMAD4 mutations in colorectal cancer. Cancer Res. 2013, 73, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.H.; Basta, D.W.; Hornick, J.L.; Dong, F. Loss of function SMAD4 nonstop mutations in human cancer. Histopathology 2023, 82, 1098–1104. [Google Scholar] [CrossRef]
- Wasserman, I.; Lee, L.H.; Ogino, S.; Marco, M.R.; Wu, C.; Chen, X.; Datta, J.; Sadot, E.; Szeglin, B.; Guillem, J.G.; et al. SMAD4 Loss in Colorectal Cancer Patients Correlates with Recurrence, Loss of Immune Infiltrate, and Chemoresistance. Clin. Cancer Res. 2019, 25, 1948–1956. [Google Scholar] [CrossRef]
- Itatani, Y.; Kawada, K.; Fujishita, T.; Kakizaki, F.; Hirai, H.; Matsumoto, T.; Iwamoto, M.; Inamoto, S.; Hatano, E.; Hasegawa, S.; et al. Loss of SMAD4 from colorectal cancer cells promotes CCL15 expression to recruit CCR1+ myeloid cells and facilitate liver metastasis. Gastroenterology 2013, 145, 1064–1075.e11. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawada, K.; Itatani, Y.; Inamoto, S.; Okamura, R.; Iwamoto, M.; Miyamoto, E.; Chen-Yoshikawa, T.F.; Hirai, H.; Hasegawa, S.; et al. Loss of SMAD4 Promotes Lung Metastasis of Colorectal Cancer by Accumulation of CCR1+ Tumor-Associated Neutrophils through CCL15-CCR1 Axis. Clin. Cancer Res. 2017, 23, 833–844. [Google Scholar] [CrossRef]
- Voorneveld, P.W.; Kodach, L.L.; Jacobs, R.J.; Liv, N.; Zonnevylle, A.C.; Hoogenboom, J.P.; Biemond, I.; Verspaget, H.W.; Hommes, D.W.; de Rooij, K.; et al. Loss of SMAD4 alters BMP signaling to promote colorectal cancer cell metastasis via activation of Rho and ROCK. Gastroenterology 2014, 147, 196–208.e13. [Google Scholar] [CrossRef]
- Loevenich, L.P.; Tschurtschenthaler, M.; Rokavec, M.; Silva, M.G.; Jesinghaus, M.; Kirchner, T.; Klauschen, F.; Saur, D.; Neumann, J.; Hermeking, H.; et al. SMAD4 Loss Induces c-MYC-Mediated NLE1 Upregulation to Support Protein Biosynthesis, Colorectal Cancer Growth, and Metastasis. Cancer Res. 2022, 82, 4604–4623. [Google Scholar] [CrossRef]
- Katz, L.H.; Li, Y.; Chen, J.S.; Muñoz, N.M.; Majumdar, A.; Chen, J.; Mishra, L. Targeting TGF-βsignaling in cancer. Expert Opin. Ther. Targets 2013, 17, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Lambert, A.W.; Ozturk, S.; Papageorgis, P.; Lopez, D.; Shen, N.; Sen, Z.; Abdolmaleky, H.M.; Győrffy, B.; Feng, H.; et al. Targeting RICTOR Sensitizes SMAD4-Negative Colon Cancer to Irinotecan. Mol. Cancer Res. 2020, 18, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, I.; Hirota, T.; Shinozaki, E. BRAF Mutation in Colorectal Cancers: From Prognostic Marker to Targetable Mutation. Cancers 2020, 12, 3236. [Google Scholar] [CrossRef] [PubMed]
- Ugai, T.; Akimoto, N.; Haruki, K.; Harrison, T.A.; Cao, Y.; Qu, C.; Chan, A.T.; Campbell, P.T.; Berndt, S.I.; Buchanan, D.D.; et al. Prognostic role of detailed colorectal location and tumor molecular features: Analyses of 13,101 colorectal cancer patients including 2994 early-onset cases. J. Gastroenterol. 2023, 58, 229–245. [Google Scholar] [CrossRef]
- Poulikakos, P.I.; Sullivan, R.J.; Yaeger, R. Molecular Pathways and Mechanisms of BRAF in Cancer Therapy. Clin. Cancer Res. 2022, 28, 4618–4628. [Google Scholar] [CrossRef]
- Tan, E.; Whiting, J.; Xie, H.; Imanirad, I.; Carballido, E.; Felder, S.; Frakes, J.; Mo, Q.; Walko, C.; Permuth, J.B.; et al. BRAF Mutations Are Associated with Poor Survival Outcomes in Advanced-stage Mismatch Repair-deficient/Microsatellite High Colorectal Cancer. Oncologist 2022, 27, 191–197. [Google Scholar] [CrossRef]
- Janku, F. Advances on the BRAF Front in Colorectal Cancer. Cancer Discov. 2018, 8, 389–391. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- Elez, E.; Yoshino, T.; Shen, L.; Lonardi, S.; Van Cutsem, E.; Eng, C.; Kim, T.W.; Wasan, H.S.; Desai, J.; Ciardiello, F.; et al. Encorafenib, Cetuximab, and mFOLFOX6 in BRAF-Mutated Colorectal Cancer. N. Engl. J. Med. 2025, 392, 2425–2437. [Google Scholar] [CrossRef]
- Siena, S.; Sartore-Bianchi, A.; Marsoni, S.; Hurwitz, H.I.; McCall, S.J.; Penault-Llorca, F.; Srock, S.; Bardelli, A.; Trusolino, L. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann. Oncol. 2018, 29, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, Y.; Sun, Y.; Wang, W.; Qian, X.; Yu, X.; Pan, Y. Prevalence, prognosis and predictive status of HER2 amplification in anti-EGFR-resistant metastatic colorectal cancer. Clin. Transl. Oncol. 2020, 22, 813–822. [Google Scholar] [CrossRef]
- Cheng, S.; Gomez, C.G.; Ferrell, M.; Giza, R.; Syed, M.P.; Magge, T.; Gorantla, V.; Hsieh, R.W.; Bao, R.; Singhi, A.; et al. Investigating incidence of RAS/RAF and PIK3CA alterations in HER2-amplified colorectal cancer: A comprehensive analysis. Oncologist 2025, 30, oyaf158. [Google Scholar] [CrossRef]
- Strickler, J.H.; Cercek, A.; Siena, S.; André, T.; Ng, K.; Van Cutsem, E.; Wu, C.; Paulson, A.S.; Hubbard, J.M.; Coveler, A.L.; et al. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): A multicentre, open-label, phase 2 study. Lancet Oncol. 2023, 24, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Cho, M.S.; Kim, N.K. Difference between right-sided and left-sided colorectal cancers: From embryology to molecular subtype. Expert Rev. Anticancer Ther. 2018, 18, 351–358. [Google Scholar] [CrossRef]
- Imperial, R.; Ahmed, Z.; Toor, O.M.; Erdoğan, C.; Khaliq, A.; Case, P.; Case, J.; Kennedy, K.; Cummings, L.S.; Melton, N.; et al. Comparative proteogenomic analysis of right-sided colon cancer, left-sided colon cancer and rectal cancer reveals distinct mutational profiles. Mol. Cancer 2018, 17, 177. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectalcancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.E.; Weinberg, B.A.; Xiu, J.; El-Deiry, W.S.; Hwang, J.J.; Gatalica, Z.; Philip, P.A.; Shields, A.F.; Lenz, H.J.; Marshall, J.L. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget 2017, 8, 86356–86368. [Google Scholar] [CrossRef]
- Sanz-Pamplona, R.; Cordero, D.; Berenguer, A.; Lejbkowicz, F.; Rennert, H.; Salazar, R.; Biondo, S.; Sanjuan, X.; Pujana, M.A.; Rozek, L.; et al. Gene expression differences between colon and rectum tumors. Clin. Cancer Res. 2011, 17, 7303–7312. [Google Scholar] [CrossRef]
- Ciepiela, I.; Szczepaniak, M.; Ciepiela, P.; Hińcza-Nowak, K.; Kopczyński, J.; Macek, P.; Kubicka, K.; Chrapek, M.; Tyka, M.; Góźdź, S.; et al. Tumor location matters, next generation sequencing mutation profiling of left-sided, rectal, and right-sided colorectal tumors in 552 patients. Sci. Rep. 2024, 14, 4619. [Google Scholar] [CrossRef]
- Kim, K.; Castro, E.J.T.; Shim, H.; Advincula, J.V.G.; Kim, Y.W. Differences Regarding the Molecular Features and Gut Microbiota Between Right and Left Colon Cancer. Ann. Coloproctol. 2018, 34, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.D.; Ju, J.K. Immunological Differences Between Right-Sided and Left-Sided Colorectal Cancers: A Comparison of Embryologic Midgut and Hindgut. Ann. Coloproctol. 2019, 35, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Paschke, S.; Jafarov, S.; Staib, L.; Kreuser, E.D.; Maulbecker-Armstrong, C.; Roitman, M.; Holm, T.; Harris, C.C.; Link, K.H.; Kornmann, M. Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer. Int. J. Mol. Sci. 2018, 19, 2577. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; de la Chapelle, A. Hereditary colorectal cancer. N. Engl. J. Med. 2003, 348, 919–932. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Bocchetti, M.; Ferraro, M.G.; Ricciardiello, F.; Ottaiano, A.; Luce, A.; Cossu, A.M.; Scrima, M.; Leung, W.Y.; Abate, M.; Stiuso, P.; et al. The Role of microRNAs in Development of Colitis-Associated Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 3967. [Google Scholar] [CrossRef]
- Capuozzo, M.; Celotto, V.; Landi, L.; Ferrara, F.; Sabbatino, F.; Perri, F.; Cascella, M.; Granata, V.; Santorsola, M.; Ottaiano, A. Beyond Body Size: Adiponectinas a Key Player in Obesity-Driven Cancers. Nutr. Cancer 2023, 75, 1848–1862. [Google Scholar] [CrossRef]
- Ottaiano, A.; Santorsola, M.; Circelli, L.; Perri, F.; Cascella, M.; Sabbatino, F.; Capuozzo, M.; Granata, V.; Zappavigna, S.; Lombardi, A.; et al. Hypertension, type 2 diabetes, obesity, and p53 mutations negatively correlate with metastatic colorectal cancer patients’ survival. Front. Med. 2023, 10, 1091634. [Google Scholar] [CrossRef]
- Ottaiano, A.; De Divitiis, C.; Capozzi, M.; Avallone, A.; Pisano, C.; Pignata, S.; Tafuto, S. Obesity and Cancer: Biological Links and Treatment Implications. Curr. Cancer Drug Targets 2018, 18, 231–238. [Google Scholar] [CrossRef]
- Botteri, E.; Borroni, E.; Sloan, E.K.; Bagnardi, V.; Bosetti, C.; Peveri, G.; Santucci, C.; Specchia, C.; van den Brandt, P.; Gallus, S.; et al. Smoking and Colorectal Cancer Risk, Overall and by Molecular Subtypes: A Meta-Analysis. Am. J. Gastroenterol. 2020, 115, 1940–1949. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Obesity and colon and rectal cancer risk: A meta-analysis of prospective studies. Am. J. Clin. Nutr. 2007, 86, 556–565. [Google Scholar] [CrossRef]
- Fedirko, V.; Tramacere, I.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Negri, E.; Straif, K.; Romieu, I.; La Vecchia, C.; et al. Alcohol drinking and colorectalcancer risk: An overall and dose-response meta-analysis of published studies. Ann. Oncol. 2011, 22, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Qi, L.; Sun, M.; Liu, W.; Zhang, X.; Yu, Y.; Tian, Z.; Ni, Z.; Zheng, R.; Li, Y. Global esophageal cancer epidemiology in 2022 and predictions for 2050: A comprehensive analysis and projections based on GLOBOCAN data. Chin. Med. J. 2024, 137, 3108–3116. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Joo, J.E.; Chu, Y.L.; Georgeson, P.; Walker, R.; Mahmood, K.; Clendenning, M.; Meyers, A.L.; Como, J.; Joseland, S.; Preston, S.G.; et al. Intratumoral presence of the genotoxic gut bacteria pks+ E. coli, Enterotoxigenic Bacteroides fragilis, and Fusobacterium nucleatum and their association with clinicopathological and molecular features of colorectal cancer. Br. J. Cancer 2024, 130, 728–740. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Diaconu, C.C.; Gheorghe, G.; Mihai, M.M.; Diaconu, C.C.; Bostan, M.; Bleotu, C. Gut Microbiota and Colorectal Cancer: A Balance Between Risk and Protection. Int. J. Mol. Sci. 2025, 26, 3733. [Google Scholar] [CrossRef]
- Godos, J. Decreasing adherence to the Mediterranean diet: Health and environmental foe. Int. J. Food Sci. Nutr. 2023, 74, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef]
- Brusnic, O.; Onisor, D.; Boicean, A.; Hasegan, A.; Ichim, C.; Guzun, A.; Chicea, R.; Todor, S.B.; Vintila, B.I.; Anderco, P.; et al. Fecal Microbiota Transplantation: Insights into Colon Carcinogenesis and Immune Regulation. J. Clin. Med. 2024, 13, 6578. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Meng, Y.; Tan, Z.; Zhen, J.; Xiao, D.; Cai, L.; Dong, W.; Chen, C. Global, regional, and national burden of early-onset colorectal cancer from 1990 to 2021: A systematic analysis based on the Global Burden of Disease Study 2021. BMC Med. 2025, 23, 34. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Gomez de Agüero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Al Nabhani, Z.; Eberl, G. Imprinting of the immune system by the microbiota early in life. Mucosal Immunol. 2020, 13, 183–189. [Google Scholar] [CrossRef]
- Gharib, E.; Robichaud, G.A. From Crypts to Cancer: A Holistic Perspective on Colorectal Carcinogenesis and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 9463. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Leslie, A.; Carey, F.A.; Pratt, N.R.; Steele, R.J. The colorectal adenoma-carcinoma sequence. Br. J. Surg. 2002, 89, 845–860. [Google Scholar] [CrossRef]
- Armaghany, T.; Wilson, J.D.; Chu, Q.; Mills, G. Genetic alterations in colorectal cancer. Gastrointest. Cancer Res. 2012, 5, 19–27. [Google Scholar]
- Powell, S.M.; Zilz, N.; Beazer-Barclay, Y.; Bryan, T.; Hamilton, S.R.; Thibodeau, S.N.; Vogelstein, B.; Kinzler, K.W. APC mutations occur early during colorectal tumorigenesis. Nature 1992, 359, 235–237. [Google Scholar] [CrossRef]
- Rajagopalan, H.; Bardelli, A.; Lengauer, C.; Kinzler, K.W.; Vogelstein, B.; Velculescu, V.E. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature 2002, 418, 934. [Google Scholar] [CrossRef]
- Baker, S.J.; Preisinger, A.C.; Jessup, J.M.; Paraskeva, C.; Markowitz, S.; Willson, J.K.; Hamilton, S.R.; Vogelstein, B. p53 gene mutations occur in combination with 17p allelic deletions as late events in colorectal tumorigenesis. Cancer Res. 1990, 50, 7717–7722. [Google Scholar] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Sottoriva, A.; Kang, H.; Ma, Z.; Graham, T.A.; Salomon, M.P.; Zhao, J.; Marjoram, P.; Siegmund, K.; Press, M.F.; Shibata, D.; et al. A Big Bang model of human colorectal tumor growth. Nat. Genet. 2015, 47, 209–216. [Google Scholar] [CrossRef]
- Toh, T.B.; Lim, J.J.; Chow, E.K.H. Epigenetics in cancer stem cells. Mol. Cancer 2017, 16, 29. [Google Scholar] [CrossRef]
- Half, E.; Bercovich, D.; Rozen, P. Familial adenomatous polyposis. Orphanet J. Rare Dis. 2009, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef] [PubMed]
- Avery, A.; Alden, J.; Kramish, C.; Caballero, C.; Wright-Void, C.; Bruner, E.T. The pathologic diagnosis of carcinoma in various tissues. Adv. Cancer Res. 2022, 154, 1–14. [Google Scholar] [CrossRef]
- Winawer, S.J.; Zauber, A.G.; Ho, M.N.; O’Brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Waye, J.D.; Gottlieb, S.; Adelstein, S.; Levin, B.; et al. Prevention of colorectal cancer by colonoscopic polypectomy. N. Engl. J. Med. 1993, 329, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Robertson, D.J.; Riddle, M.S.; Sawhney, M.S.; Shaukat, A.; et al. Colorectal cancer screening: Recommendations for physicians and patients from the US Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2017, 112, 1016–1030. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R. Genetic alterations underlying colorectal tumorigenesis. Cancer Surv. 1992, 12, 119–136. [Google Scholar] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Lieberman, D.A.; Rex, D.K.; Winawer, S.J.; Giardiello, F.M.; Johnson, D.A.; Levin, T.R. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012, 143, 844–857. [Google Scholar] [CrossRef]
- Compton, C.; Fenoglio-Preiser, C.M.; Pettigrew, N.; Fielding, L.P. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer 2000, 88, 1739–1757. [Google Scholar] [CrossRef]
- Mikalonis, M.; Avlund, T.H.; Løve, U.S. Danish guidelines for treating acute colonic obstruction caused by colorectal cancer-a review. Front. Surg. 2024, 11, 1400814. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H.; Levin, T.R.; Lavin, P.; Lidgard, G.P.; Ahlquist, D.A.; Berger, B.M. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 2014, 370, 1287–1297. [Google Scholar] [CrossRef]
- Lin, J.S.; Piper, M.A.; Perdue, L.A.; Rutter, C.M.; Webber, E.M.; O’Connor, E.; Smith, N.; Whitlock, E.P. Screening for colorectal cancer: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016, 315, 2576–2594. [Google Scholar] [CrossRef]
- Colquhoun, P.; Chen, H.C.; Kim, J.I.; Efron, J.; Weiss, E.G.; Nogueras, J.J.; Vernava, A.M.; Wexner, S.D. High compliance rates observed for follow up colonoscopy post polypectomy are achievableoutside of clinical trials: Efficacy of polypectomyisnotreduced by low compliance for follow up. Colorectal Dis. 2004, 6, 158–161. [Google Scholar] [CrossRef]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef]
- Yang, Q.; Qu, R.; Lu, S.; Zhang, Y.; Zhang, Z.; Fu, W. Biological and Clinical Characteristics of Proximal Colon Cancer: Far from Its Anatomical Subsite. Int. J. Med. Sci. 2024, 21, 1824–1839. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef]
- Manfredi, S.; Lepage, C.; Hatem, C.; Coatmeur, O.; Faivre, J.; Bouvier, A.M. Epidemiology and management of liver metastases from colorectal cancer. Ann. Surg. 2006, 244, 254–259. [Google Scholar] [CrossRef]
- He, K.; Wang, Z.; Luo, M.; Li, B.; Ding, N.; Li, L.; He, B.; Wang, H.; Cao, J.; Huang, C.; et al. Metastasis organotropism in colorectal cancer: Advancing toward innovative therapies. J. Transl. Med. 2023, 21, 612. [Google Scholar] [CrossRef]
- Niibe, Y.; Chang, J.Y.; Onishi, H.; Salama, J.; Hiraki, T.; Yamashita, H. Oligometastases/Oligo-recurrence of lung cancer. Pulm. Med. 2013, 2013, 438236. [Google Scholar] [CrossRef] [PubMed]
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I.; Méndez Romero, A.; Nevens, D.; Palma, D.; Park, C.; et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother. Oncol. 2020, 148, 157–166. [Google Scholar] [CrossRef]
- Withers, H.R.; Lee, S.P. Modeling growth kinetics and statistical distribution of oligometastases. Semin. Radiat. Oncol. 2006, 16, 111–119. [Google Scholar] [CrossRef]
- Niibe, Y.; Jingu, K.; Onishi, H. Oligometastases: History and future vision of breast cancer. Transl. Cancer Res. 2020, 9, 5028–5031. [Google Scholar] [CrossRef]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; Nandita, M.D.; Dingemans, A.M.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef] [PubMed]
- Tree, A.C.; Khoo, V.S.; Eeles, R.A.; Ahmed, M.; Dearnaley, D.P.; Hawkins, M.A.; Huddart, R.A.; Nutting, C.M.; Ostler, P.; van As, N.J.; et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013, 14, e28–e37. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef]
- Adam, R.; de Gramont, A.; Figueras, J.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; Sobrero, A.; et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat. Rev. 2015, 41, 729–741. [Google Scholar] [CrossRef]
- Gonzalez, M.; Poncet, A.; Combescure, C.; Robert, J.; Ris, H.B.; Gervaz, P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: A systematic review and meta-analysis. Ann. Surg. Oncol. 2013, 20, 572–579. [Google Scholar] [CrossRef]
- Pfannschmidt, J.; Dienemann, H.; Hoffmann, H. Surgical resection of pulmonary metastases from colorectal cancer: A systematic review of published series. Ann. Thorac. Surg. 2007, 84, 324–338. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Sloothen, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- Quenet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomized, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Batra, A.; Rigo, R.; Sheka, D.; Cheung, W.Y. Real-world evidence on adjuvant chemotherapy in older adults with stage II/III colon cancer. World J. Gastrointest. Oncol. 2020, 12, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Yamanaka, T.; Shiozawa, M.; Manaka, D.; Kotaka, M.; Gamoh, M.; Shiomi, A.; Makiyama, A.; Munemoto, Y.; Rikiyama, T.; et al. Oxaliplatin-basedadjuvantchemotherapy duration (3 versus 6 months) for high-risk stage II colon cancer: The randomizedphase III ACHIEVE-2 trial. Ann. Oncol. 2021, 32, 77–84. [Google Scholar] [CrossRef]
- Wörmann, B.; Bokemeyer, C.; Burmeister, T.; Köhne, C.H.; Schwab, M.; Arnold, D.; Blohmer, J.U.; Borner, M.; Brucker, S.; Cascorbi, I.; et al. Dihydropyrimidine Dehydrogenase Testing prior to Treatment with 5-Fluorouracil, Capecitabine, and Tegafur: A Consensus Paper. Oncol. Res. Treat. 2020, 43, 628–636. [Google Scholar] [CrossRef]
- Chang, C.L.; Yuan, K.S.; Wu, A.T.H.; Wu, S.Y. Adjuvant Therapy for High-Risk Stage II or III Colon Adenocarcinoma: A Propensity Score-Matched, Nationwide, Population-Based Cohort Study. Cancers 2019, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Wang, Y.; Lo, S.N.; Lahouel, K.; Cohen, J.D.; Wong, R.; Shapiro, J.D.; Harris, S.J.; Khattak, A.; Burge, M.E.; et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer: 5-year outcomes of the randomized DYNAMIC trial. Nat. Med. 2025, 31, 1509–1518. [Google Scholar] [CrossRef]
- Martín-Arana, J.; Gimeno-Valiente, F.; Henriksen, T.V.; García-Micó, B.; Martínez-Castedo, B.; Gambardella, V.; Martínez-Ciarpaglini, C.; Palomar, B.; Huerta, M.; Camblor, D.G.; et al. Whole-exome tumor-agnostic ctDNA analysis enhances minimal residual disease detection and reveals relapse mechanisms in localized colon cancer. Nat. Cancer 2025, 6, 1000–1016. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, M.; Roselló, S.; Tarazona, N.; Huerta, M.; Pérez-Santiago, L.; Fleitas, T.; Pla-Martí, V.; Puccini, A.; Roda, D.; Cervantes, A. Advances in the management of locally advanced rectal cancer: A shift toward a patient-centred approach to balance outcomes and quality of life. Cancer Treat. Rev. 2025, 140, 103015. [Google Scholar] [CrossRef]
- Kasi, A.; Abbasi, S.; Handa, S.; Al-Rajabi, R.; Saeed, A.; Baranda, J.; Sun, W. Total Neoadjuvant Therapy vs. Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2030097. [Google Scholar] [CrossRef]
- Khoo, E.; O’Neill, S.; Brown, E.; Wigmore, S.J.; Harrison, E.M. Systematic review of systemic adjuvant, neoadjuvant and perioperative chemotherapy for resectable colorectal-liver metastases. HPB 2016, 18, 485–493. [Google Scholar] [CrossRef]
- Leonard, G.D.; Brenner, B.; Kemeny, N.E. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J. Clin. Oncol. 2005, 23, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Salihu, F.; Corro, C.; Ris, F.; Meurette, G.; Durham, A.; Genoud, V.; Bornand, A.; Meyer, J.; Koessler, T. Impact of Neoadjuvant Immunotherapy in Localized Rectal Cancer. A Systematic Review. Clin Color. Cancer, 2025; in press. [Google Scholar]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lonardi, S.; Masi, G.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Spadi, R.; Zaniboni, A.; et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: Results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann. Oncol. 2015, 26, 1188–1194. [Google Scholar] [CrossRef]
- Heinemann, V.; Stintzing, S.; Modest, D.P.; Giessen-Jung, C.; Michl, M.; Mansmann, U.R. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur. J. Cancer 2015, 51, 1927–1936. [Google Scholar] [CrossRef]
- Fadlallah, H.; El Masri, J.; Fakhereddine, H.; Youssef, J.; Chemaly, C.; Doughan, S.; Abou-Kheir, W. Colorectal cancer: Recent advances in management and treatment. World J. Clin. Oncol. 2024, 15, 1136–1156. [Google Scholar] [CrossRef]
- Loupakis, F.; Cremolini, C.; Masi, G.; Lonardi, S.; Zagonel, V.; Salvatore, L.; Cortesi, E.; Tomasello, G.; Ronzoni, M.; Spadi, R.; et al. Initial therapy with FOLFOXIRI and bevacizumab for metastaticcolorectalcancer. N. Engl. J. Med. 2014, 371, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Antoniotti, C.; Rossini, D.; Lonardi, S.; Loupakis, F.; Pietrantonio, F.; Bordonaro, R.; Latiano, T.P.; Tamburini, E.; Santini, D.; et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastaticcolorectalcancer (TRIBE2): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Bennouna, J.; Sastre, J.; Arnold, D.; Österlund, P.; Greil, R.; Van Cutsem, E.; von Moos, R.; Viéitez, J.M.; Bouché, O.; Borg, C.; et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 29–37. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined with Cetuximab or Bevacizumab on Overall Survival in Patients with KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef]
- Rossini, D.; Boccaccino, A.; Carullo, M.; Antoniotti, C.; Dima, G.; Ciracì, P.; Marmorino, F.; Moretto, R.; Masi, G.; Cremolini, C. Primarytumour side as a driver for treatment choice in RAS wild-type metastatic colorectal cancer patients: A systematic review and pooled analysis of randomised trials. Eur. J. Cancer 2023, 184, 106–116. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.H.; Hitre, E.; Zaluski, J.; Chang Chien, C.R.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef]
- Abdel Hamid, M.; Pammer, L.M.; Oberparleiter, S.; Günther, M.; Amann, A.; Gruber, R.A.; Mair, A.; Nocera, F.I.; Ormanns, S.; Zimmer, K.; et al. Multidimensional differences of right- and left-sided colorectal cancer and their impact on targeted therapies. NPJ Precis. Oncol. 2025, 9, 116. [Google Scholar] [CrossRef]
- Kennel, K.B.; Greten, F.R. The immune microenvironment of colorectal cancer. Nat. Rev. Cancer 2025, 1–20. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastaticcolorectalcancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Colle, R.; Pudlarz, T.; Heran, M.; Duval, A.; Svrcek, M.; André, T. Immune Checkpoint Inhibition in Metastatic Colorectal Cancer Harboring Microsatellite Instability or Mismatch Repair Deficiency. Cancers 2021, 13, 1149. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomized, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [CrossRef]
- Parseghian, C.M.; Loree, J.M.; Morris, V.K.; Liu, X.; Clifton, K.K.; Napolitano, S.; Henry, J.T.; Pereira, A.A.; Vilar, E.; Johnson, B.; et al. Anti-EGFR-resistant clones decay exponentially after progression: Implications for anti-EGFR re-challenge. Ann. Oncol. 2019, 30, 243–249. [Google Scholar] [CrossRef]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouche, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomized, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef]
- Ottaiano, A.; Santorsola, M.; Perri, F.; Granata, V.; Cascella, M.; Sabbatino, F.; Nasti, G. Survival and Toxicities of Metastatic Colorectal Cancer Patients Treated with Regorafenib before TAS-102 or Vice Versa: A Mono-Institutional Real-Practice Study. J. Clin. Med. 2023, 12, 596. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Yilmaz, M.; Möller, S.; Zitnjak, D.; Krogh, M.; Petersen, L.N.; Poulsen, L.Ø.; Winther, S.B.; Thomsen, K.G.; Qvortrup, C. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: An investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastaticcolorectalcancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Overman, M.J.; Hartman, L.; Khoukaz, T.; Brutcher, E.; Lenz, H.J.; Atasoy, A.; Shangguan, T.; Zhao, H.; El-Rayes, B. Safety of Nivolumab plus Low-Dose Ipilimumab in Previously Treated Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer. Oncologist 2019, 24, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Ellebæk, S.B.; Graversen, M.; Detlefsen, S.; Lundell, L.; Fristrup, C.W.; Pfeiffer, P.; Mortensen, M.B. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC)-directed treatment of peritoneal metastasis in end-stage colo-rectal cancer patients. Pleura Peritoneum 2020, 5, 20200109. [Google Scholar] [CrossRef]
- Raoof, M.; Whelan, R.L.; Sullivan, K.M.; Ruel, C.; Frankel, P.H.; Cole, S.E.; Tinsley, R.; Eng, M.; Fakih, M.; Chao, J.; et al. Safety and Efficacy of Oxaliplatin Pressurized Intraperitoneal Aerosolized Chemotherapy (PIPAC) in Colorectal and Appendiceal Cancer with Peritoneal Metastases: Results of a Multicenter Phase I Trial in the USA. Ann. Surg. Oncol. 2023, 30, 7814–7824. [Google Scholar] [CrossRef]
- Constantinides, A.; Lansu, N.; Mosen, P.; Rauwerdink, P.; Strating, E.; Völlmy, F.; Nederend, M.; Leusen, J.H.W.; Rovers, K.; Wassenaar, E.; et al. Treatment of colorectal peritoneal metastases with oxaliplatin induces biomarkers predicting response to immune checkpoint blockade. Transl. Oncol. 2025, 59, 102464. [Google Scholar] [CrossRef]
- Ciappina, G.; Toscano, E.; Ottaiano, A.; Capuozzo, M.; Consolo, P.; Maiorana, E.; Carroccio, P.; Franchina, T.; Ieni, A.; Di Mauro, A.; et al. Negative Hyperselection in MetastaticColorectal Cancer for First-Line Anti-EGFR Therapy: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 2216. [Google Scholar] [CrossRef]
- Siravegna, G.; Mussolin, B.; Buscarino, M.; Corti, G.; Cassingena, A.; Crisafulli, G.; Ponzetti, A.; Cremolini, C.; Ahronian, L.G.; Lazzari, L.; et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 2015, 21, 795–801. [Google Scholar] [CrossRef]
- Ciardiello, D.; Martinelli, E.; Troiani, T.; Mauri, G.; Rossini, D.; Martini, G.; Napolitano, S.; Famiglietti, V.; Del Tufo, S.; Masi, G.; et al. Anti-EGFR Rechallenge in Patients with Refractoryct DNA RAS/BRAF wt Metastatic Colorectal Cancer: A Nonrandomized Controlled Trial. JAMA Netw. Open 2024, 7, e245635. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Pietrantonio, F.; Lonardi, S.; Mussolin, B.; Rua, F.; Crisafulli, G.; Bartolini, A.; Fenocchio, E.; Amatu, A.; Manca, P.; et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: The phase 2 CHRONOS trial. Nat. Med. 2022, 28, 1612–1618. [Google Scholar] [CrossRef]
- Tournigand, C.; André, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J. Clin. Oncol. 2004, 22, 229–237. [Google Scholar] [CrossRef]
- Cassidy, J.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F.; et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 2006–2012. [Google Scholar] [CrossRef]
- Tosi, F.; Sartore-Bianchi, A.; Lonardi, S.; Amatu, A.; Leone, F.; Ghezzi, S.; Martino, C.; Bencardino, K.; Bonazzina, E.; Bergamo, F.; et al. Long-term Clinical Outcome of Trastuzumab and Lapatinib for HER2-positive Metastatic Colorectal Cancer. Clin. Color. Cancer 2020, 19, 256–262.e2. [Google Scholar] [CrossRef]
- De Cuyper, A.; Van DenEynde, M.; Machiels, J.P. HER2 as a Predictive Biomarker and Treatment Target in Colorectal Cancer. Clin. Color. Cancer 2020, 19, 65–72. [Google Scholar] [CrossRef]
- Haas, S.; Mikkelsen, A.H.; Kronborg, C.J.S.; Oggesen, B.T.; Møller, P.F.; Fassov, J.; Frederiksen, N.A.; Krogsgaard, M.; Graugaard-Jensen, C.; Ventzel, L.; et al. Management of treatment-related sequelae following colorectal cancer. Color. Dis. 2023, 25, 458–488. [Google Scholar] [CrossRef]
- Eng, C. Toxic effects and their management: Daily clinical challenges in the treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2009, 6, 207–218. [Google Scholar] [CrossRef]
- Di Maio, M.; Basch, E.; Bryce, J.; Perrone, F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 319–325. [Google Scholar] [CrossRef]
- Chiang, T.Y.; Hsu, H.C.; Jane, S.W.; Chen, S.C. EGFRI-associated health-related quality of life by severity of skin toxicity in metastatic colorectal cancer patients receiving epidermal growth factor receptor inhibitor target therapy. Support. Care Cancer 2020, 28, 4771–4779. [Google Scholar] [CrossRef]
- Joshi, S.S.; Ortiz, S.; Witherspoon, J.N.; Rademaker, A.; West, D.P.; Anderson, R.; Rosenbaum, S.E.; Lacouture, M.E. Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer 2010, 116, 3916–3923. [Google Scholar] [CrossRef]
- Hertz, D.L.; Dockter, T.J.; Satele, D.V.; Loprinzi, C.L.; Le-Rademacher, J. Neuropathy severity at the time of oxaliplatin treatment alteration in patients with colon cancer (Alliance A151912). Support. Care Cancer 2021, 29, 7855–7863. [Google Scholar] [CrossRef]
- Wasan, H.; Meade, A.M.; Adams, R.; Wilson, R.; Pugh, C.; Fisher, D.; Sydes, B.; Madi, A.; Sizer, B.; Lowdell, C.; et al. Intermittent chemotherapy plus either intermittent or continuous cetuximab for first-line treatment of patients with KRAS wild-type advanced colorectal cancer (COIN-B): A randomised phase 2 trial. Lancet Oncol. 2014, 15, 631–639. [Google Scholar] [CrossRef]
- Boige, V.; Blons, H.; François, E.; Ben Abdelghani, M.; Phelip, J.M.; Le Brun-Ly, V.; Mineur, L.; Galais, M.P.; Villing, A.L.; Hautefeuille, V.; et al. Maintenance Therapy with Cetuximab After FOLFIRI Plus Cetuximab for RAS Wild-Type Metastatic Colorectal Cancer: A Phase 2 Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2333533. [Google Scholar] [CrossRef]
- Avallone, A.; Giuliani, F.; De Stefano, A.; Santabarbara, G.; Nasti, G.; Montesarchio, V.; Rosati, G.; Cassata, A.; Leo, S.; Romano, C.; et al. Intermittent or Continuous Panitumumab Plus Fluorouracil, Leucovorin, and Irinotecan for First-Line Treatment of RAS and BRAF Wild-Type Metastatic Colorectal Cancer: The IMPROVE Trial. J. Clin. Oncol. 2025, 43, 829–839. [Google Scholar] [CrossRef]
- Seymour, M.T.; Maughan, T.S.; Ledermann, J.A.; Topham, C.; James, R.; Gwyther, S.J.; Smith, D.B.; Shepherd, S.; Maraveyas, A.; Ferry, D.R.; et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): A randomised controlled trial. Lancet 2007, 370, 143–152. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Lenz, H.J.; Köhne, C.H.; Heinemann, V.; Tejpar, S.; Melezínek, I.; Beier, F.; Stroh, C.; Rougier, P.; van Krieken, J.H.; et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J. Clin. Oncol. 2015, 33, 692–700. [Google Scholar] [CrossRef]
- Folprecht, G.; Gruenberger, T.; Bechstein, W.; Raab, H.R.; Weitz, J.; Lordick, F.; Hartmann, J.T.; Stoehlmacher-Williams, J.; Lang, H.; Trarbach, T.; et al. Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann. Oncol. 2014, 25, 1018–1025. [Google Scholar] [CrossRef]
- Tao, X.Y.; Li, Q.Q.; Zeng, Y. Clinical application of liquid biopsy in colorectal cancer: Detection, prediction, and treatment monitoring. Mol. Cancer 2024, 23, 145. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef]
- Horgan, D.; Van denBulcke, M.; Malapelle, U.; Troncone, G.; Normanno, N.; Capoluongo, E.D.; Prelaj, A.; Rizzari, C.; Trapani, D.; Singh, J.; et al. Tackling the implementation gap for the uptake of NGS and advanced molecular diagnostics into healthcare systems. Heliyon 2023, 10, e23914. [Google Scholar] [CrossRef]
- Grivicich, I.; Mans, D.R.; Peters, G.J.; Schwartsmann, G. Irinotecan and oxaliplatin: An overview of the novel chemotherapeutic options for the treatment of advanced colorectal cancer. Braz. J. Med. Biol. Res. 2001, 34, 1087–1103. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Chai, Y.; Liu, J.L.; Zhang, S.; Li, N.; Xu, D.Q.; Liu, W.J.; Fu, R.J.; Tang, Y.P. The effective combination therapies with irinotecan for colorectal cancer. Front. Pharmacol. 2024, 15, 1356708. [Google Scholar] [CrossRef]
- Wang, T.L.; Diaz, L.A., Jr.; Romans, K.; Bardelli, A.; Saha, S.; Galizia, G.; Choti, M.; Donehower, R.; Parmigiani, G.; Shih, I.E.M.; et al. Digital karyotyping identifies thymidylate synthase amplification as a mechanism of resistance to 5-fluorouracil in metastatic colorectal cancer patients. Proc. Natl. Acad. Sci. USA 2004, 101, 3089–3094. [Google Scholar] [CrossRef]
- Gongora, C.; Vezzio-Vie, N.; Tuduri, S.; Denis, V.; Causse, A.; Auzanneau, C.; Collod-Beroud, G.; Coquelle, A.; Pasero, P.; Pourquier, P.; et al. New Topoisomerase I mutations are associated with resistance to camptothecin. Mol. Cancer 2011, 10, 64. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Q.; Huang, R.; Gao, Z.; Yuan, Z.; Tang, Q.; Gao, F.; Wang, M.; Zhang, W.; Ma, T.; et al. Genomic Analysis Reveals Heterogeneity Between Lesions in Synchronous Primary Right-Sided and Left-Sided Colon Cancer. Front. Mol. Biosci. 2021, 8, 689466. [Google Scholar] [CrossRef]
- Liang, L.; Zeng, J.H.; Qin, X.G.; Chen, J.Q.; Luo, D.Z.; Chen, G. Distinguishable Prognostic Signatures of Left- and Right-Sided Colon Cancer: A Study Based on Sequencing Data. Cell Physiol. Biochem. 2018, 48, 475–490. [Google Scholar] [CrossRef]
- Capuozzo, M.; Ferrara, F.; Santorsola, M.; Zovi, A.; Ottaiano, A. Circulating Tumor Cells as Predictive and Prognostic Biomarkers in Solid Tumors. Cells 2023, 12, 2590. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef]
- Taïeb, J.; Sullo, F.G.; Lecanu, A.; Bourreau, C.; Barbier, E.; Gandini, A.; Bez, J.; Mulot, C.; Di Fiore, F.; Elhajbi, F.; et al. Early ctDNA and Survival in Metastatic Colorectal Cancer Treated with Immune Checkpoint Inhibitors: A Secondary Analysis of the SAMCO-PRODIGE 54 Randomized Clinical Trial. JAMA Oncol. 2025, 11, 874–882. [Google Scholar] [CrossRef]
- Holz, A.; Paul, B.; Zapf, A.; Pantel, K.; Joosse, S.A. Circulating tumor DNA as prognostic marker in patients with metastatic colorectal cancer undergoing systemic therapy: A systematic review and meta-analysis. Cancer Treat. Rev. 2025, 139, 102999. [Google Scholar] [CrossRef]
- Botticelli, A.; Cremolini, C.; Scagnoli, S.; Biffoni, M.; Lonardi, S.; Fornaro, L.; Guarneri, V.; De Giorgi, U.; Ascierto, P.; Blandino, G.; et al. The Impact of ConcordanceBetween Liquid and Tissue Biopsy for Actionable Mutations: Insights from the Rome Trial. Clin. Cancer Res. 2025; ahead of print. [Google Scholar]
- Deveson, I.W.; Gong, B.; Lai, K.; LoCoco, J.S.; Richmond, T.A.; Schageman, J.; Zhang, Z.; Novoradovskaya, N.; Willey, J.C.; Jones, W.; et al. SEQC2 Oncopanel Sequencing Working Group. Evaluating the analytical validity of circulating tumor DNA sequencing assays for precision oncology. Nat. Biotechnol. 2021, 39, 1115–1128. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, J.; Wu, S.; Si, H.; Gao, C.; Xu, W.; Abdullah, S.E.; Higgs, B.W.; Dennis, P.A.; van der Heijden, M.S.; et al. Prognostic and Predictive Impact of Circulating Tumor DNA in Patients with Advanced Cancers Treated with Immune Checkpoint Blockade. Cancer Discov. 2020, 10, 1842–1853. [Google Scholar] [CrossRef]
- Salichos, L.; Meyerson, W.; Warrell, J.; Gerstein, M. Estimating growth patterns and driver effects in tumor evolution from individual samples. Nat. Commun. 2020, 11, 732. [Google Scholar] [CrossRef]
- Parackal, S.; Zou, D.; Day, R.; Black, M.; Guilford, P. Comparison of Roche Cell-Free DNA collection Tubes ® to Streck Cell-Free DNA BCT ® s for sample stability using healthy volunteers. Pract. Lab. Med. 2019, 16, e00125. [Google Scholar] [CrossRef]
- Lee, J.S.; Cho, E.H.; Kim, B.; Hong, J.; Kim, Y.G.; Kim, Y.; Jang, J.H.; Lee, S.T.; Kong, S.Y.; Lee, W.; et al. Clinical Practice Guideline for Blood-based Circulating Tumor DNA Assays. Ann. Lab. Med. 2024, 44, 195–209. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, X.; Xun, G.; Li, Z.; Chong, Y.; Yang, L.; Wang, H.; Zhang, F.; Luo, S.; Cui, L.; et al. Argonaute integrated single-tube PCR system enables supersensitive detection of rare mutations. Nucleic Acids Res. 2021, 49, e75. [Google Scholar] [CrossRef]
- Pedrosa, L.; Esposito, F.; Thomson, T.M.; Maurel, J. The Tumor Microenvironment in Colorectal Cancer Therapy. Cancers 2019, 11, 1172. [Google Scholar] [CrossRef]
- Ottaiano, A.; Ianniello, M.; Santorsola, M.; Ruggiero, R.; Sirica, R.; Sabbatino, F.; Perri, F.; Cascella, M.; Di Marzo, M.; Berretta, M.; et al. From Chaos to Opportunity: Decoding Cancer Heterogeneity for Enhanced Treatment Strategies. Biology 2023, 12, 1183. [Google Scholar] [CrossRef]
- Trotta, A.M.; Ottaiano, A.; Romano, C.; Nasti, G.; Nappi, A.; De Divitiis, C.; Napolitano, M.; Zanotta, S.; Casaretti, R.; D’Alterio, C.; et al. Prospective Evaluation of Cetuximab-Mediated Antibody-Dependent Cell Cytotoxicity in Metastatic Colorectal Cancer Patients Predicts Treatment Efficacy. Cancer Immunol. Res. 2016, 4, 366–374. [Google Scholar] [CrossRef]
- Calemma, R.; Ottaiano, A.; Trotta, A.M.; Nasti, G.; Romano, C.; Napolitano, M.; Galati, D.; Borrelli, P.; Zanotta, S.; Cassata, A.; et al. Fc gamma receptor IIIa polymorphisms in advanced colorectal cancer patients correlated with response to anti-EGFR antibodies and clinical outcome. J. Transl. Med. 2012, 10, 232. [Google Scholar] [CrossRef]
- Ottaiano, A.; Scala, S.; Normanno, N.; Napolitano, M.; Capozzi, M.; Rachiglio, A.M.; Roma, C.; Trotta, A.M.; D’Alterio, C.; Portella, L.; et al. Cetuximab, irinotecan and fluorouracile in fiRst-line treatment of immunologically-selected advanced colorectal cancer patients: The CIFRA study protocol. BMC Cancer 2019, 19, 899. [Google Scholar] [CrossRef]
- Sahu, P.; Mitra, A.; Ganguly, A. Targeting KRAS and SHP2 signaling pathways for immunomodulation and improving treatment outcomes in solid tumors. Int. Rev. Cell Mol. Biol. 2024, 386, 167–222. [Google Scholar] [CrossRef]
- D’Andrea, E.; Ahnen, D.J.; Sussman, D.A.; Najafzadeh, M. Quantifying the impact of adherence to screening strategies on colorectal cancer incidence and mortality. Cancer Med. 2020, 9, 824–836. [Google Scholar] [CrossRef]
- Hines, J.B.; Kacew, A.J.; Sweis, R.F. The Development of STING Agonists and Emerging Results as a Cancer Immunotherapy. Curr. Oncol. Rep. 2023, 25, 189–199. [Google Scholar] [CrossRef]
- Overwijk, W.W.; Tagliaferri, M.A.; Zalevsky, J. Engineering IL-2 to Give New Life to T Cell Immunotherapy. Annu. Rev. Med. 2021, 72, 281–311. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Chong, A.; Hong, Y.; Min, J.J. Bioengineering of bacteria for cancer immunotherapy. Nat. Commun. 2023, 14, 3553. [Google Scholar] [CrossRef]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib Plus Nivolumab in Patients with Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef]
- Fakih, M.; Raghav, K.P.S.; Chang, D.Z.; Larson, T.; Cohn, A.L.; Huyck, T.K.; Cosgrove, D.; Fiorillo, J.A.; Tam, R.; D’Adamo, D.; et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: A single-arm, open-label, multicentre phase 2 study. eClinicalMedicien 2023, 58, 101917. [Google Scholar] [CrossRef]
- Saeed, A.; Park, R.; Pathak, H.; Al-Bzour, A.N.; Dai, J.; Phadnis, M.; Al-Rajabi, R.; Kasi, A.; Baranda, J.; Sun, W.; et al. Clinical and biomarker results from a phase II trial of combined cabozantinib and durvalumab in patients with chemotherapy-refractory colorectal cancer (CRC): CAMILLA CRC cohort. Nat. Commun. 2024, 15, 1533. [Google Scholar] [CrossRef]
- Antoniotti, C.; Rossini, D.; Pietrantonio, F.; Salvatore, L.; Lonardi, S.; Tamberi, S.; Marmorino, F.; Moretto, R.; Prisciandaro, M.; Tamburini, E.; et al. Upfront Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan Plus Bevacizumab with or Without Atezolizumab for Patients with Metastatic Colorectal Cancer: Updated and Overall Survival Results of the ATEZOTRIBE Study. J. Clin. Oncol. 2024, 42, 2637–2644. [Google Scholar] [CrossRef]
- Kawazoe, A.; Xu, R.H.; García-Alfonso, P.; Passhak, M.; Teng, H.W.; Shergill, A.; Gumus, M.; Qvortrup, C.; Stintzing, S.; Towns, K.; et al. Lenvatinib Plus Pembrolizumab Versus Standard of Care for Previously Treated Metastatic Colorectal Cancer: Final Analysis of the Randomized, Open-Label, Phase III LEAP-017 Study. J. Clin. Oncol. 2024, 42, 2918–2927. [Google Scholar] [CrossRef]
- Cao, D.; Zhou, A. Tumor Immune Microenvironment and Current Status of Immune Checkpoint Inhibitor Therapy in Colorectal Cancer Liver Metastasis. Curr. Oncol. 2025, 32, 493. [Google Scholar] [CrossRef]
- Yousef, M.; Yousef, A.; Chowdhury, S.; Fanaeian, M.M.; Knafl, M.; Peterson, J.; Zeineddine, M.; Alfaro, K.; Zeineddine, F.; Goldstein, D.; et al. Molecular, Socioeconomic, and Clinical Factors Affecting Racial and Ethnic Disparities in Colorectal Cancer Survival. JAMA Oncol. 2024, 10, 1519–1529. [Google Scholar] [CrossRef]

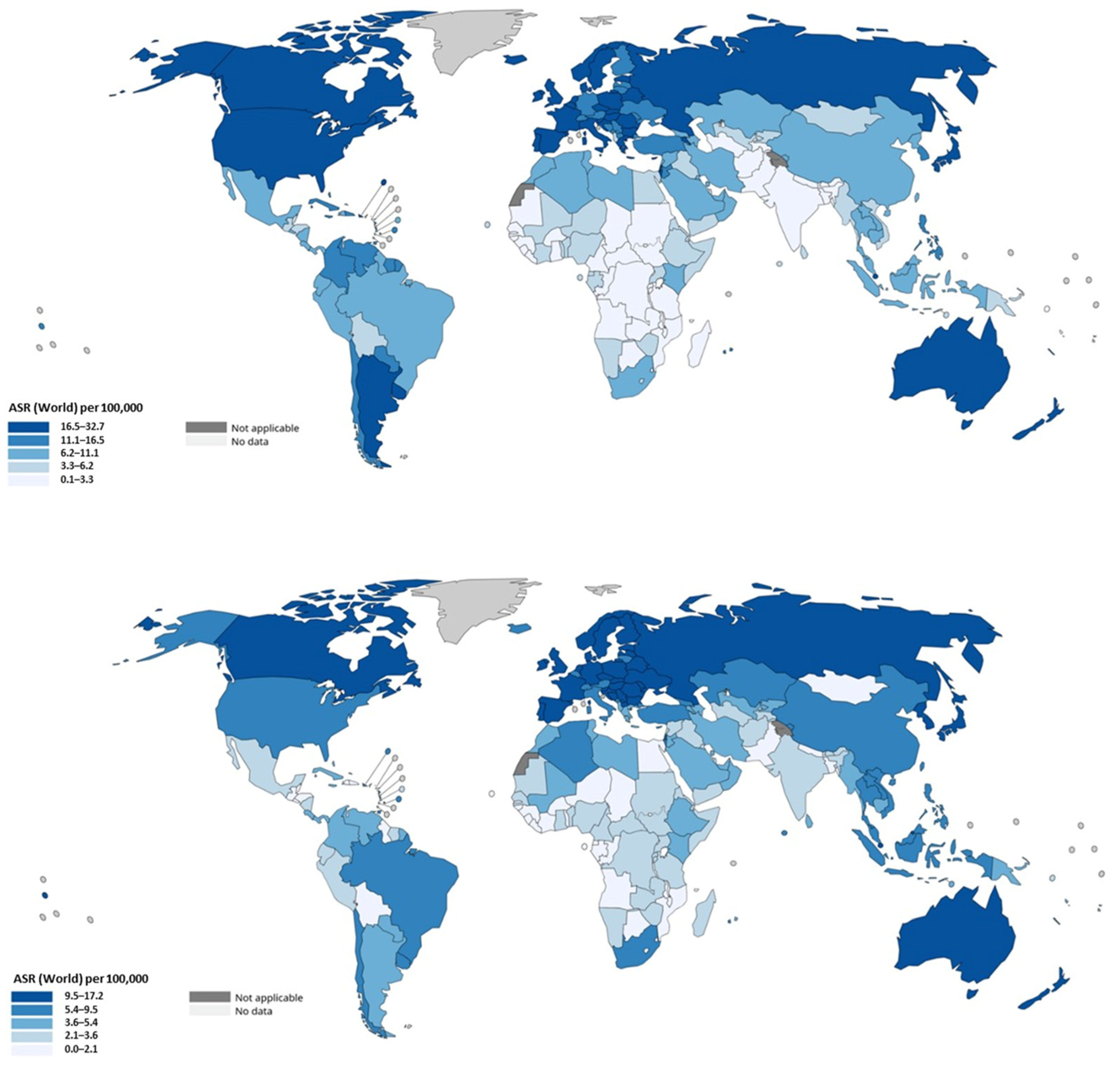

| Region/Continent | CRC Incidence (Colon + Rectal) ASR/100,000 (Both Sexes) | Colon/Rectal Ratio | Key Notes and Trends |
|---|---|---|---|
| Worldwide | ~18.4 | 2.0 | Global colon cancer incidence is approximately double rectal; overall CRC incidence rising modestly worldwide. EOCRC (<50 years) increasing in high-income countries and emerging in low/middle-income countries. |
| Australia/New Zealand | ~35 | 1.6 | Among the highest CRC ASIR globally. Screening and early detection are established; colon vs. rectal cancer both high; some decline in older age incidence; rise in younger. |
| Europe (Western/Northern) | ~30–45 in many high-rate countries (e.g., Denmark ~48.1, Norway ~45.3, Netherlands ~42.8) | 2.0 | High rates for colon cancer; rectal cancer also high. Some Western European countries show stable or declining colon cancer incidence in older age groups; rectal incidence among younger increasing. |
| North America (U.S., Canada) | ~37.1 | 2.3 | U.S., Canada show declines in older adults but strong increases among younger, especially for rectal cancer. Distinctions in colon vs. rectal trends visible. |
| East Asia | China ~20.1 Japan ~36.6 | 2.5 | East Asia has among highest absolute numbers due to large population; both colon and rectal cancer incidence rising. EOCRC burden increasing. |
| Latin America/Caribbean | ~18–25 | 2.5 | Lower ASIR, but increasing trends; colon cancer rising as lifestyle changes; rectal cancer also contributes significantly. |
| South/South-Central Asia | ~4.9 | 2.5 | Underreporting may be an issue; less screening; incidence rising slowly but mortality high because of late diagnosis. |
| Sub-Saharan Africa | <10 | 3.0 | Countries with increasing obesity and diet shifts have rising CRC (colon + rectum); projections expect increases. Some nations show divergence where rectal cancer rises faster in younger cohorts. |
| Study | Sample Size | Induction Regimen | Study Arms | Primary Endpoint | Statistical Hypothesis | Biomarker-Guided Stop? | Study Conclusions |
|---|---|---|---|---|---|---|---|
| COIN-B | 169 | 12 weeks FOLFOX + cetuximab | Randomization after induction: intermittent FOLFOX/cetuximab vs. intermittent FOLFOX + cetuximab maintenance (reintroduction of FOLFOX at progression) | FFS | A’Hern’s design to detect a 10-month FFS of 50% versus 35%, based on prior MRC FOCUS and phase II trial; 80% power and 5% one-sided α | No | The trial did not meet its primary endpoint; intermittent chemotherapy with cetuximab maintenance was feasible but failed to demonstrate superiority in FFS |
| PRODIGE-28 | 139 | 8 cycles FOLFIRI + cetuximab | Randomization after induction: intermittent FOLFIRI/cetuximab vs. intermittent FOLFIRI + cetuximab maintenance (reintroduction of FOLFIRI at progression) | PFR | Fleming one-step design with a 6-month PFS rate of 40% as null hypothesis; 80% power and 5% one-sided α | No | The study showed activity of induction plus cetuximab but did not achieve the predefined efficacy threshold; intermittent treatment did not improve PFR compared to continuous therapy |
| IMPROVE | 137 | 8 cycles FOLFIRI + panitumumab | Randomization after induction: continuous FOLFIRI + panitumumab until progression vs. intermittent strategy with planned treatment-free intervals | PFSot | Hypothesis based on lower 95% CI of CRYSTAL study, median PFSot time ≤7 months as null hypothesis; 80% power and 10% one-sided α | No | The trial met its statistical design but provided no evidence that intermittent strategies with panitumumab were superior; clinical benefit remained comparable to continuous therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capuozzo, M.; Picone, C.; Sabbatino, F.; Santorsola, M.; Caraglia, F.; Iervolino, D.; Sirica, R.; Gualillo, O.; Di Mauro, G.; Castiello, R.; et al. Genetic, Epidemiological, Clinical, and Therapeutic Trajectories in Colon and Rectal Cancers. Cancers 2025, 17, 3438. https://doi.org/10.3390/cancers17213438
Capuozzo M, Picone C, Sabbatino F, Santorsola M, Caraglia F, Iervolino D, Sirica R, Gualillo O, Di Mauro G, Castiello R, et al. Genetic, Epidemiological, Clinical, and Therapeutic Trajectories in Colon and Rectal Cancers. Cancers. 2025; 17(21):3438. https://doi.org/10.3390/cancers17213438
Chicago/Turabian StyleCapuozzo, Maurizio, Carmine Picone, Francesco Sabbatino, Mariachiara Santorsola, Francesco Caraglia, Domenico Iervolino, Roberto Sirica, Oreste Gualillo, Giordana Di Mauro, Rosa Castiello, and et al. 2025. "Genetic, Epidemiological, Clinical, and Therapeutic Trajectories in Colon and Rectal Cancers" Cancers 17, no. 21: 3438. https://doi.org/10.3390/cancers17213438
APA StyleCapuozzo, M., Picone, C., Sabbatino, F., Santorsola, M., Caraglia, F., Iervolino, D., Sirica, R., Gualillo, O., Di Mauro, G., Castiello, R., Ianniello, M., Cossu, A. M., Nebbioso, A., Altucci, L., Izzo, F., Patrone, R., Belli, A., Berretta, M., Cascella, M., ... Ottaiano, A. (2025). Genetic, Epidemiological, Clinical, and Therapeutic Trajectories in Colon and Rectal Cancers. Cancers, 17(21), 3438. https://doi.org/10.3390/cancers17213438















