Comparative Effectiveness of Interventions to Treat Cancer Treatment-Related Cognitive Impairment in Adult Cancer Survivors Following Systemic Therapy: A Systematic Review with Network Meta-Analyses
Simple Summary
Abstract
1. Introduction
Review Question
2. Methods
2.1. Protocol, Registration, and Reporting
2.2. Patient and Public Involvement
2.3. Study Eligibility Criteria
2.4. Data Sources and Search Strategy
2.5. Study Selection Process
2.6. Data Extraction
Cognitive Function Assessment Tools of Interest
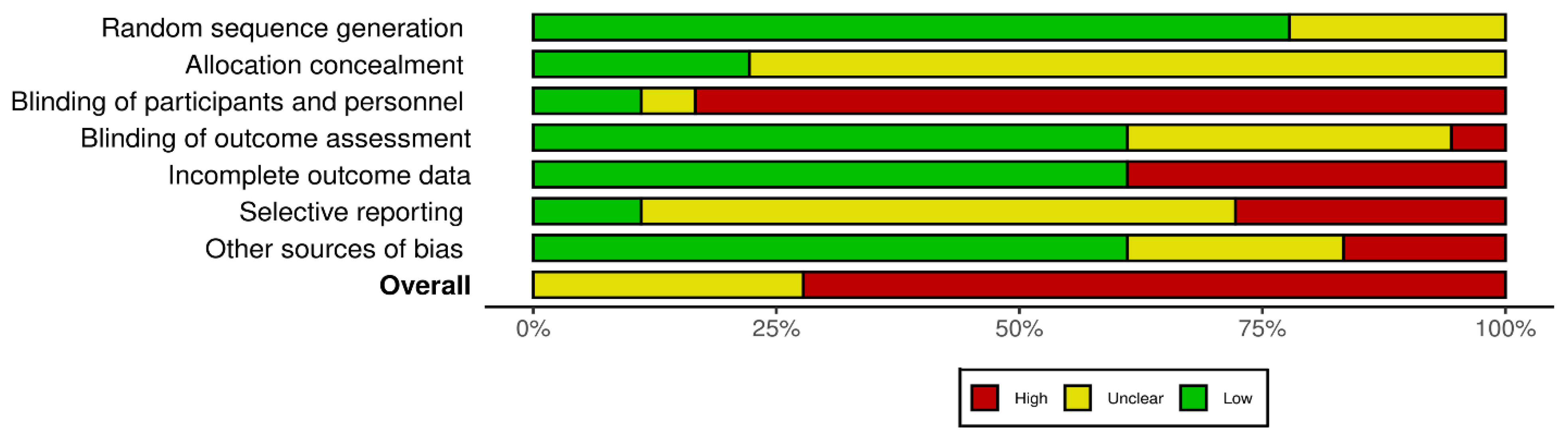
2.7. Risk of Bias and Certainty of Evidence Assessments
2.8. Data Synthesis and Statistical Methods
2.8.1. Network Meta-Analysis Feasibility Assessment
2.8.2. Data Optimization Strategies
2.8.3. Direct Intervention Comparisons
2.8.4. Approach to NMA
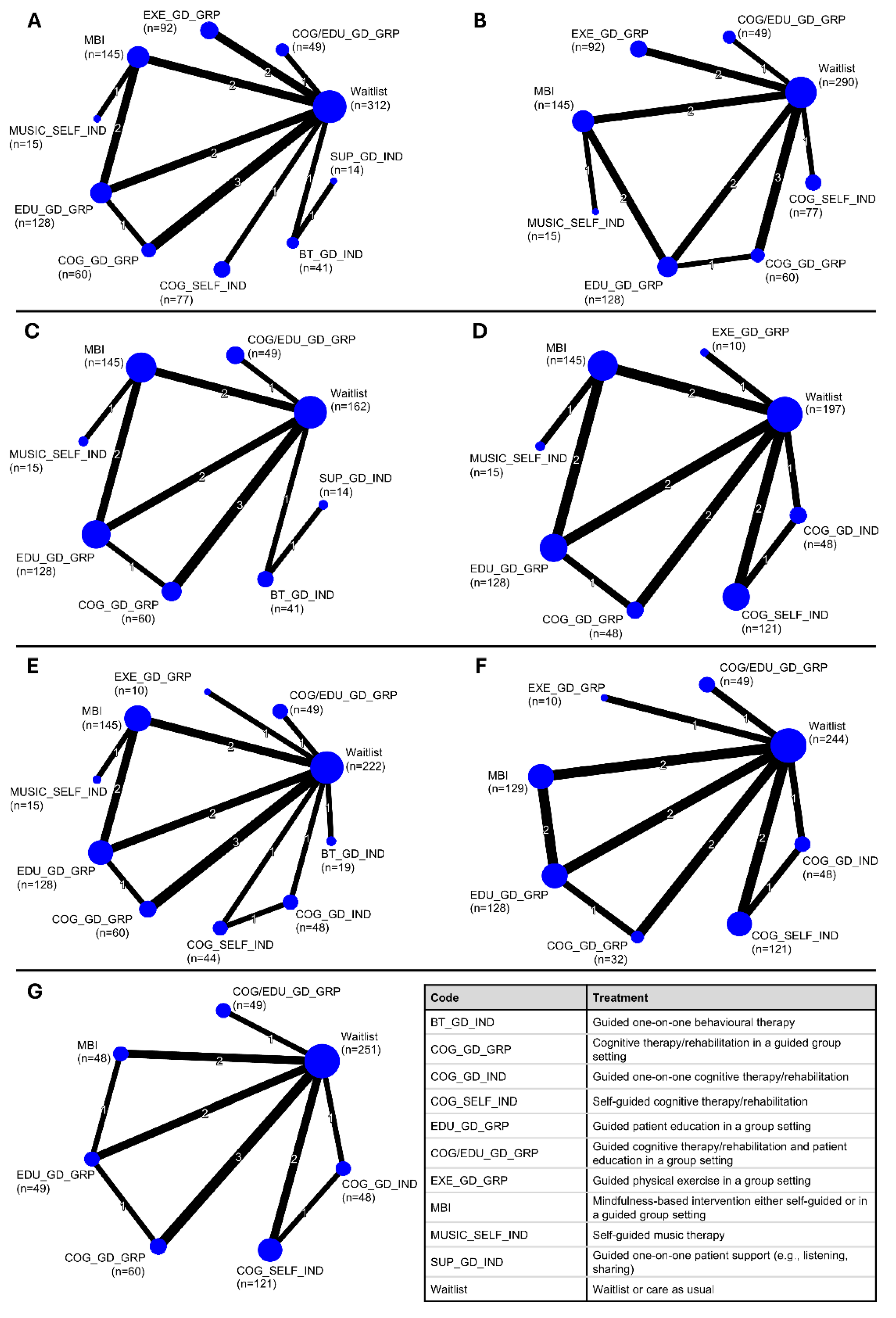
3. Results
3.1. Study Characteristics
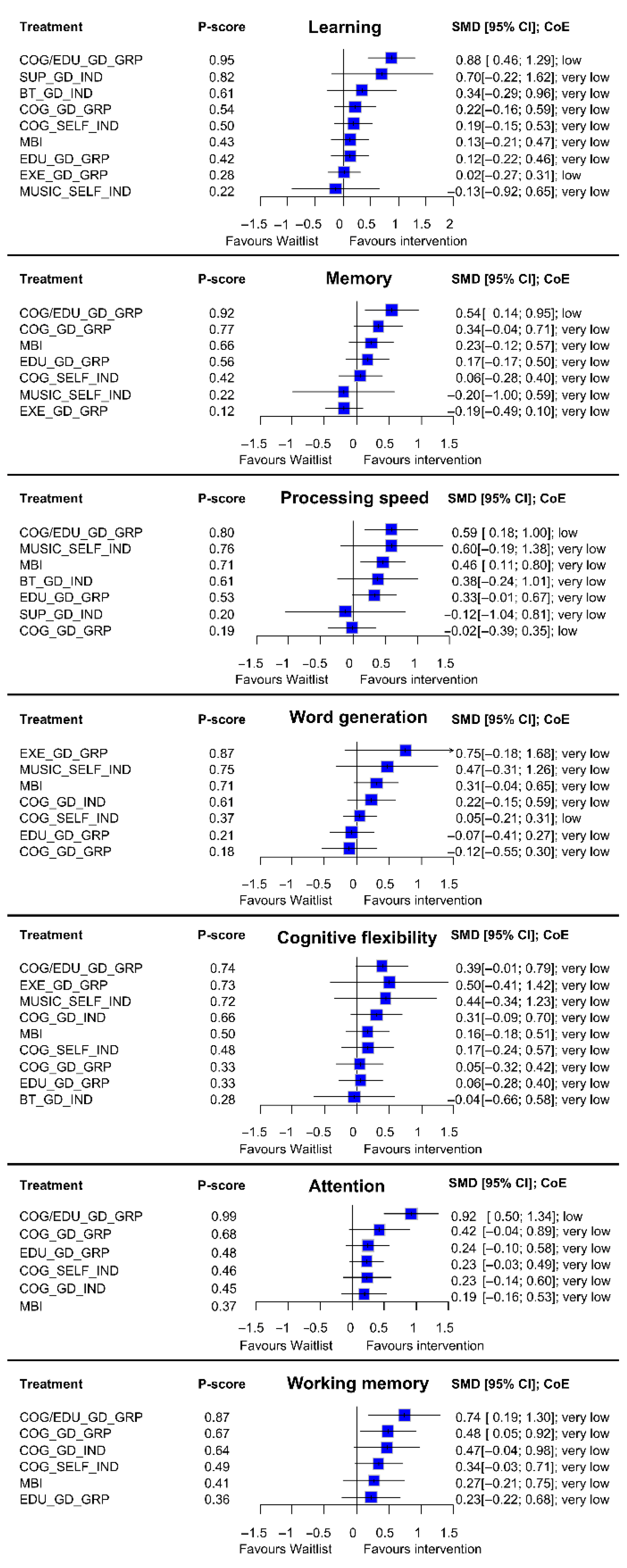
3.2. Results from NMAs at Immediate Post-Intervention
3.2.1. Learning
3.2.2. Memory
3.2.3. Processing Speed
3.2.4. Word Generation
3.2.5. Cognitive Flexibility
3.2.6. Attention
3.2.7. Working Memory
3.3. Results from NMAs at Long-Term Follow-Up
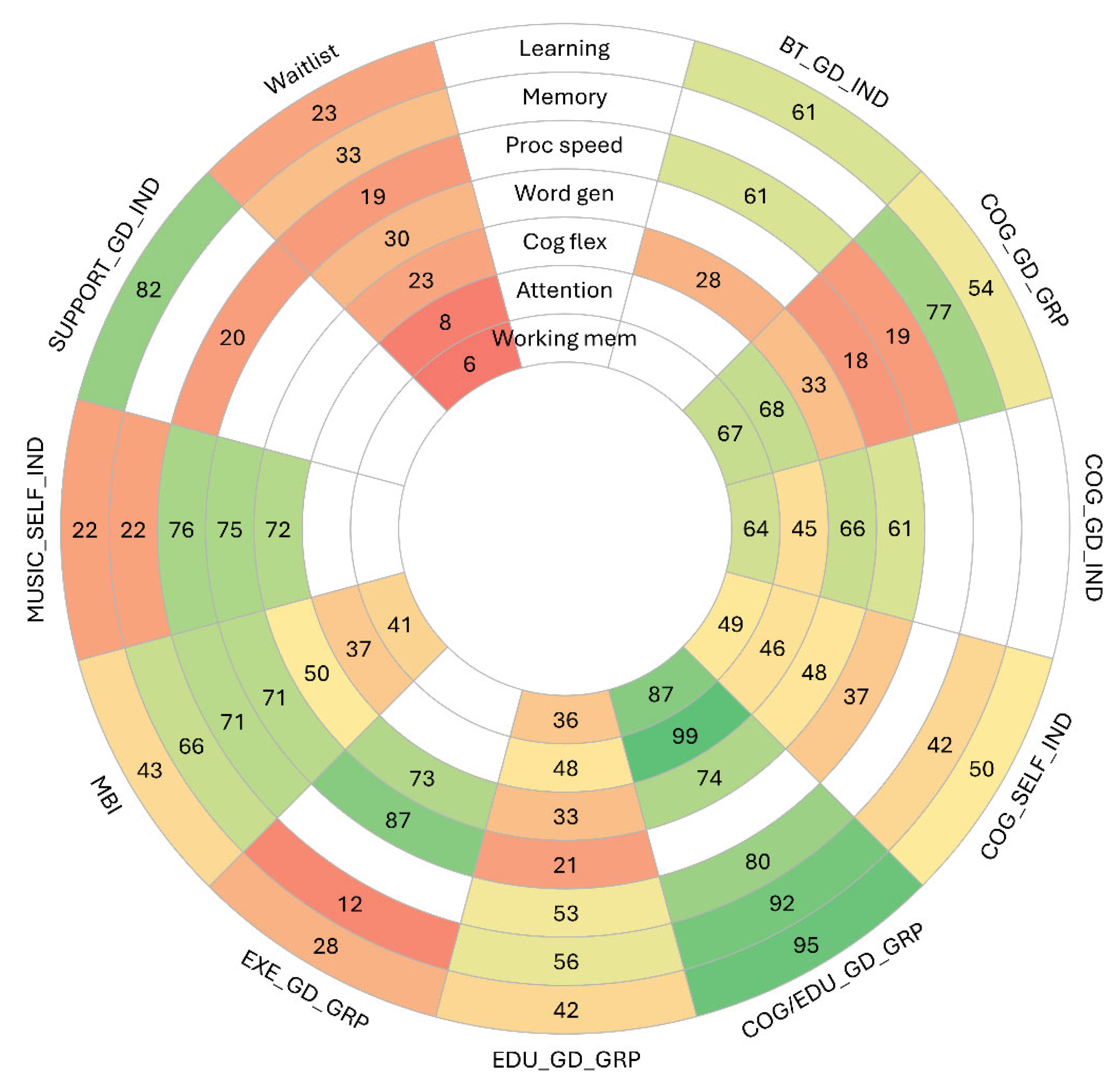
3.4. Summary of Findings Across Outcomes
3.5. Syntheses of Additional Evidence
3.5.1. Abstraction Domain
3.5.2. Pharmacological Therapies
3.5.3. Other Non-Pharmacological Therapies
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rick, O.; Gerhardt, A.; Schilling, G. Cancer-Related Cognitive Dysfunction: A Narrative Review for Clinical Practice. Oncol. Res. Treat. 2024, 47, 218–223. [Google Scholar] [CrossRef]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; De Ruiter, M.B.; Castel, H. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef]
- Olson, B.; Marks, D.L. Pretreatment Cancer-Related Cognitive Impairment-Mechanisms and Outlook. Cancers 2019, 11, 687. [Google Scholar] [CrossRef]
- Oliva, G.; Giustiniani, A.; Danesin, L.; Burgio, F.; Arcara, G.; Conte, P. Cognitive impairment following breast cancer treatments: An umbrella review. Oncologist 2024, 29, e848–e863. [Google Scholar] [CrossRef] [PubMed]
- Turrina, S.; Gibelli, F.; De Leo, D. Chemotherapy-induced cognitive impairment from the forensic medicine perspective: A review of the updated literature. J. Forensic Leg. Med. 2020, 76, 102070. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M. Chemotherapy-related cognitive impairment: What we need to know and what we can do. Asia Pac. J. Oncol. Nurs. 2024, 11, 100334. [Google Scholar] [CrossRef]
- Mulholland, M.M.; Prinsloo, S.; Kvale, E.; Dula, A.N.; Palesh, O.; Kesler, S.R. Behavioral and biologic characteristics of cancer-related cognitive impairment biotypes. Brain Imaging Behav. 2023, 17, 320–328. [Google Scholar] [CrossRef]
- Onyedibe, M.C.; Schmidt, M.E.; Bizer, P.; Zimmer, P.; Steindorf, K. Subjective and Objective Cancer-Related Cognitive Impairments Among Systemic and Radiation Therapy-Naive Female Cancer Patients. Cancer Med. 2025, 14, e70908. [Google Scholar] [CrossRef] [PubMed]
- Fleming, B.; Edison, P.; Kenny, L. Cognitive impairment after cancer treatment: Mechanisms, clinical characterization, and management. BMJ 2023, 380, e071726. [Google Scholar] [CrossRef]
- Pergolotti, M.; Battisti, N.M.L.; Padgett, L.; Sleight, A.G.; Abdallah, M.; Newman, R.; Van Dyk, K.; Covington, K.R.; Williams, G.R.; van den Bos, F.; et al. Embracing the complexity: Older adults with cancer-related cognitive decline-A Young International Society of Geriatric Oncology position paper. J. Geriatr. Oncol. 2020, 11, 237–243. [Google Scholar] [CrossRef]
- Dijkshoorn, A.B.C.; van Stralen, H.E.; Sloots, M.; Schagen, S.B.; Visser-Meily, J.M.A.; Schepers, V.P.M. Prevalence of cognitive impairment and change in patients with breast cancer: A systematic review of longitudinal studies. Psychooncology 2021, 30, 635–648. [Google Scholar] [CrossRef]
- Amani, O.; Mazaheri, M.A.; Moghani, M.M.; Zarani, F.; Choolabi, R.H. Chemotherapy-induced cognitive impairment in breast cancer survivors: A systematic review of studies from 2000 to 2021. Cancer Rep. 2024, 7, e1989. [Google Scholar] [CrossRef]
- Ho, M.H.; Cheung, D.S.T.; Wang, T.; Wang, L.; Wong, J.W.H.; Lin, C.C. Cancer-related cognitive impairment in patients with hematologic malignancies after CAR T cell therapy: A systematic review and meta-analysis of prevalence. Support. Care Cancer 2025, 33, 312. [Google Scholar] [CrossRef]
- Ho, M.H.; So, T.W.; Fan, C.L.; Chung, Y.T.; Lin, C.C. Prevalence and assessment tools of cancer-related cognitive impairment in lung cancer survivors: A systematic review and proportional meta-analysis. Support. Care Cancer 2024, 32, 209. [Google Scholar] [CrossRef]
- Treanor, C.J.; Li, J.; Donnelly, M. Cognitive impairment among prostate cancer patients: An overview of reviews. Eur. J. Cancer Care 2017, 26, e12642. [Google Scholar] [CrossRef]
- Whittaker, A.L.; George, R.P.; O’Malley, L. Prevalence of cognitive impairment following chemotherapy treatment for breast cancer: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 2135. [Google Scholar] [CrossRef]
- Koppelmans, V.; Breteler, M.M.; Boogerd, W.; Seynaeve, C.; Gundy, C.; Schagen, S.B. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J. Clin. Oncol. 2012, 30, 1080–1086. [Google Scholar] [CrossRef]
- Wefel, J.S.; Vardy, J.; Ahles, T.; Schagen, S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011, 12, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Treanor, C.J.; McMenamin, U.C.; O’Neill, R.F.; Cardwell, C.R.; Clarke, M.J.; Cantwell, M.; Donnelly, M. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Syst. Rev. 2016, 2016, CD011325. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Hu, Q.; Zhang, L.; Shen, A.; Zhang, Z.; Wang, Q.; Lu, Q. Effects of non-pharmacological interventions on cancer-related cognitive impairment in patients with breast cancer: A systematic review and network meta-analysis. Eur. J. Oncol. Nurs. 2025, 75, 102804. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, C.; Yu, T.; Zhang, H. Comparative Effects of Exercise Interventions and Mindfulness-Based Interventions for Cognitive Impairment and Quality of Life in Breast Cancer Survivors During or After Cancer Treatment: A Systematic Review and Bayesian Network Meta-analysis. Am. J. Phys. Med. Rehabil. 2024, 103, 777–788. [Google Scholar] [CrossRef]
- Cheng, A.S.K.; Wang, X.; Niu, N.; Liang, M.; Zeng, Y. Neuropsychological Interventions for Cancer-Related Cognitive Impairment: A Network Meta-Analysis of Randomized Controlled Trials. Neuropsychol. Rev. 2022, 32, 893–905. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.E.; Chen, S.; Zhao, F.; Chen, L.; Li, R. Effectiveness of Nonpharmacologic Interventions for Chemotherapy-Related Cognitive Impairment in Breast Cancer Patients: A Systematic Review and Network Meta-analysis. Cancer Nurs. 2022, 46, E305–E319. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Dong, J.; Huang, M.; Zhang, J.E.; Zhang, X.; Xie, M.; Wefel, J.S. Nonpharmacological interventions for cancer-related cognitive impairment in adult cancer patients: A network meta-analysis. Int. J. Nurs. Stud. 2020, 104, 103514. [Google Scholar] [CrossRef]
- Mackenzie, L.; Marshall, K. Effective non-pharmacological interventions for cancer related cognitive impairment in adults (excluding central nervous system or head and neck cancer): Systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2022, 58, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jung, S.J.; Lee, L.J.; Rhu, J.; Bae, S.H. Impact of nonpharmacological interventions on cognitive impairment in women with breast cancer: A systematic review and meta-analysis. Asia-Pac. J. Oncol. Nurs. 2023, 10, 100212. [Google Scholar] [CrossRef]
- Pinheiro, V.H.G.; Pinto, S.S.; Cardozo, P.S.; Andrade, L.S.; Alberton, C.L. The effect of exercise on cognitive function in breast cancer survivors: Systematic review and meta-analysis of randomized controlled trials. Support. Care Cancer 2025, 33, 577. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, X.; Sun, J.; Hui, Z.; Lei, S.; Wang, C.; Wang, M. Effects of physical exercise on cognitive function of breast cancer survivors receiving chemotherapy: A systematic review of randomized controlled trials. Breast 2022, 63, 113–122. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, X.; Liu, S.; Yu, L.; Zhu, J.; Qiu, S. Therapies for cognitive impairment in breast cancer survivors treated with chemotherapy: A protocol for systematic review. Medicine 2020, 99, e20092. [Google Scholar] [CrossRef]
- Alwi, S.M.S.; Narayanan, V.; Din, N.C.; Taib, N.A.M. Cognitive Rehabilitation Programs for Survivors of Breast Cancer Treated With Chemotherapy: A Systematic Review. Rehabil. Oncol. 2021, 39, 155–167. [Google Scholar] [CrossRef]
- Baydoun, M.; Oberoi, D.; Flynn, M.; Moran, C.; McLennan, A.; Piedalue, K.L.; Carlson, L.E. Effects of Yoga-Based Interventions on Cancer-Associated Cognitive Decline: A Systematic Review. Curr. Oncol. Rep. 2020, 22, 100. [Google Scholar] [CrossRef]
- Binarelli, G.; Joly, F.; Tron, L.; Lefevre Arbogast, S.; Lange, M. Management of Cancer-Related Cognitive Impairment: A Systematic Review of Computerized Cognitive Stimulation and Computerized Physical Activity. Cancers 2021, 13, 5161. [Google Scholar] [CrossRef]
- Floyd, R.; Dyer, A.H.; Kennelly, S.P. Non-pharmacological interventions for cognitive impairment in women with breast cancer post-chemotherapy: A systematic review. J. Geriatr. Oncol. 2021, 12, 173–181. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A.e. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022), GEN. Cochrane. Updated February 2022. 2022. Available online: www.training.cochrane.org/handbook (accessed on 27 April 2023).
- Wolfe, D.M.; Hamel, C.; Rice, D.; Veroniki, A.A.; Skidmore, B.; Kanji, S.; Rabheru, K.; McGee, S.F.; Forbes, L.; Liu, M.; et al. Comparative effectiveness of interventions for cancer treatment-related cognitive impairment in adult cancer survivors: Protocol for a systematic review. Syst. Rev. 2024, 13, 207. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Healthcare Interventions: Checklist and Explanations. Ann. Intern. Med. 2015; in press. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Canada’s Drug Agency. Grey Matters: A Tool for Searching Health-related Grey Literature. Available online: https://greymatters.cda-amc.ca (accessed on 18 March 2025).
- EndNote. Version EndNote 20; Clarivate: Philadelphia, PA, USA, 2013. [Google Scholar]
- Hamel, C.; Hersi, M.; Kelly, S.E.; Tricco, A.C.; Straus, S.; Wells, G.; Pham, B.; Hutton, B. Guidance for using artificial intelligence for title and abstract screening while conducting knowledge syntheses. BMC Med Res. Methodol. 2021, 21, 285. [Google Scholar] [CrossRef] [PubMed]
- Connors, E.J.; Hauson, A.O.; Barlet, B.D.; Sarkissians, S.; Stelmach, N.P.; Walker, A.D.; Nemanim, N.M.; Greenwood, K.L.; Chesher, N.J.; Wollman, S.C.; et al. Neuropsychological Assessment and Screening in Heart Failure: A Meta-Analysis and Systematic Review. Neuropsychol. Rev. 2021, 31, 312–330. [Google Scholar] [CrossRef]
- Grant, I.; Gonzalez, R.; Carey, C.L.; Natarajan, L.; Wolfson, T. Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. J. Int. Neuropsychol. Soc. 2003, 9, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.G.; Hauson, A.O.; Wollman, S.C.; Allen, K.E.; Connors, E.J.; Stern, M.J.; Kimmel, C.L.; Stephan, R.A.; Sarkissians, S.; Barlet, B.D.; et al. Neuropsychological comparisons of cocaine versus methamphetamine users: A research synthesis and meta-analysis. Am. J. Drug Alcohol Abus. 2018, 44, 277–293. [Google Scholar] [CrossRef]
- Wollman, S.C.; Hauson, A.O.; Hall, M.G.; Connors, E.J.; Allen, K.E.; Stern, M.J.; Stephan, R.A.; Kimmel, C.L.; Sarkissians, S.; Barlet, B.D.; et al. Neuropsychological functioning in opioid use disorder: A research synthesis and meta-analysis. Am. J. Drug Alcohol Abus. 2019, 45, 11–25. [Google Scholar] [CrossRef]
- Zakzanis, K.K.; Campbell, Z.; Polsinelli, A. Quantitative evidence for distinct cognitive impairment in anorexia nervosa and bulimia nervosa. J. Neuropsychol. 2010, 4 Pt 1, 89–106. [Google Scholar] [CrossRef]
- Sherman, E.M.S.; Tan, J.E.; Hrabok, M. A Compendium of Neuropsychological Tests: Fundamentals of Neuropsychological Assessment and Test Reviews for Clinical Practice, 4th ed.; Oxford University Press: New York, NY, USA, 2022. [Google Scholar]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, A.; Higgins, J.P.T.; Papakonstantinou, T.; Chaimani, A.; Del Giovane, C.; Egger, M.; Salanti, G. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020, 17, e1003082. [Google Scholar] [CrossRef]
- Papakonstantinou, T.; Nikolakopoulou, A.; Higgins, J.P.T.; Egger, M.; Salanti, G. CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst. Rev. 2020, 16, e1080. [Google Scholar] [CrossRef]
- Spineli, L.M.; Papadimitropoulou, K.; Kalyvas, C. Exploring the Transitivity Assumption in Network Meta-Analysis: A Novel Approach and Its Implications. Stat. Med. 2025, 44, e70068. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Fitzpatrick, R.; Haines, A.; Kinmonth, A.L.; Sandercock, P.; Spiegelhalter, D.; Tyrer, P. Framework for design and evaluation of complex interventions to improve health. BMJ 2000, 321, 694–696. [Google Scholar] [CrossRef] [PubMed]
- Lokker, C.; McKibbon, K.A.; Colquhoun, H.; Hempel, S. A scoping review of classification schemes of interventions to promote and integrate evidence into practice in healthcare. Implement Sci. 2015, 10, 27. [Google Scholar] [CrossRef]
- Michie, S.; Johnston, M.; Abraham, C.; Lawton, R.; Parker., D.; Walker, A. Making psychological theory useful for implementing evidence based practice: A consensus approach. Qual. Saf. Health Care 2005, 14, 26–33. [Google Scholar] [CrossRef]
- Michie, S.; van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement Sci. 2011, 6, 42. [Google Scholar] [CrossRef]
- Van Stan, J.H.; Dijkers, M.P.; Whyte, J.; Hart, T.; Turkstra, L.S.; Zanca, J.M.; Chen, C. The Rehabilitation Treatment Specification System: Implications for Improvements in Research Design, Reporting, Replication, and Synthesis. Arch. Phys. Med. Rehabil. 2019, 100, 146–155. [Google Scholar] [CrossRef]
- Lopez-Lopez, J.A.; Page, M.J.; Lipsey, M.W.; Higgins, J.P.T. Dealing with effect size multiplicity in systematic reviews and meta-analyses. Res. Synth. Methods 2018, 9, 336–351. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Chapter 24: Multiple Outcomes or Time-Points within a Study. In Introduction to Meta-Analysis; John Wiley and Sons, Ltd.: Chichester, UK, 2009; pp. 225–238, Chapter 24. [Google Scholar]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D. Doing Meta-Analysis with R: A Hands-On Guide; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Murad, M.H.; Wang, Z.; Chu, H.; Lin, L. When continuous outcomes are measured using different scales: Guide for meta-analysis and interpretation. BMJ 2019, 364, k4817. [Google Scholar] [CrossRef]
- da Costa, B.R.; Nuesch, E.; Rutjes, A.W.; Johnston, B.C.; Reichenbach, S.; Trelle, S.; Guyatt, G.H.; Jüni, P. Combining follow-up and change data is valid in meta-analyses of continuous outcomes: A meta-epidemiological study. J. Clin. Epidemiol. 2013, 66, 847–855. [Google Scholar] [CrossRef]
- Ostinelli, E.G.; Efthimiou, O.; Luo, Y.; Miguel, C.; Karyotaki, E.; Cuijpers, P.; Furukawa, T.A.; Salanti, G.; Cipriani, A. Combining endpoint and change data did not affect the summary standardised mean difference in pairwise and network meta-analyses: An empirical study in depression. Res. Synth. Methods 2024, 15, 758–768. [Google Scholar] [CrossRef]
- Vickers, A.J.; Altman, D.G. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ 2001, 323, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Jackson, D.; Viechtbauer, W.; Bender, R.; Bowden, J.; Knapp, G.; Kuss, O.; Higgins, J.P.; Langan, D.; Salanti, G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods 2016, 7, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Jackson, D.; Bender, R.; Kuss, O.; Langan, D.; Higgins, J.P.T.; Knapp, G.; Salanti, G. Methods to calculate uncertainty in the estimated overall effect size from a random-effects meta-analysis. Res. Synth. Methods 2019, 10, 23–43. [Google Scholar] [CrossRef]
- Dias, S.; Sutton, A.J.; Ades, A.E.; Welton, N.J. Evidence synthesis for decision making 2: A generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med. Decis. Mak. 2013, 33, 607–617. [Google Scholar] [CrossRef]
- Dias, S.; Sutton, A.J.; Welton, N.J.; Ades, A.E. Evidence synthesis for decision making 3: Heterogeneity-subgroups, meta-regression, bias, and bias-adjustment. Med. Decis. Mak. 2013, 33, 618–640. [Google Scholar] [CrossRef]
- Dias, S.; Welton, N.; Sutton, A.; Caldwell, D.; Lu, G.; Ades, A. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. Available online: https://www.ncbi.nlm.nih.gov/books/n/nicedsutsd4/pdf/ (accessed on 22 October 2025).
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.3.3; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 22 October 2025).
- Veroniki, A.A.; Vasiliadis, H.S.; Higgins, J.P.; Salanti, G. Evaluation of inconsistency in networks of interventions. Int. J. Epidemiol. 2013, 42, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Jackson, D.; Barrett, J.K.; Lu, G.; Ades, A.E.; White, I.R. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth. Methods 2012, 3, 98–110. [Google Scholar] [CrossRef]
- Rhodes, K.M.; Turner, R.M.; Higgins, J.P. Predictive distributions were developed for the extent of heterogeneity in meta-analyses of continuous outcome data. J. Clin. Epidemiol. 2015, 68, 52–60. [Google Scholar] [CrossRef]
- Veroniki, A.A.; Straus, S.E.; Fyraridis, A.; Tricco, A.C. The rank-heat plot is a novel way to present the results from a network meta-analysis including multiple outcomes. J. Clin. Epidemiol. 2016, 76, 193–199. [Google Scholar] [CrossRef]
- Campbell, K.L.; Kam, J.W.Y.; Neil-Sztramko, S.E.; Liu Ambrose, T.; Handy, T.C.; Lim, H.J.; Hayden, S.; Hsu, L.; Kirkham, A.A.; Gotay, C.C.; et al. Effect of aerobic exercise on cancer-associated cognitive impairment: A proof-of-concept RCT. Psychooncology 2018, 27, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Cherrier, M.M.; Anderson, K.; David, D.; Higano, C.S.; Gray, H.; Church, A.; Willis, S.L. A randomized trial of cognitive rehabilitation in cancer survivors. Life Sci. 2013, 93, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Cherrier, M.M.; Higano, C.S.; Gray, H.J. Cognitive skill training improves memory, function, and use of cognitive strategies in cancer survivors. Support. Care Cancer 2022, 30, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Damholdt, M.F.; Mehlsen, M.; O’Toole, M.S.; Andreasen, R.K.; Pedersen, A.D.; Zachariae, R. Web-based cognitive training for breast cancer survivors with cognitive complaints-a randomized controlled trial. Psychooncology 2016, 25, 1293–1300. [Google Scholar] [CrossRef]
- Dos Santos, M.; Hardy-Leger, I.; Rigal, O.; Licaj, I.; Dauchy, S.; Levy, C.; Noal, S.; Segura, C.; Delcambre, C.; Allouache, D.; et al. Cognitive rehabilitation program to improve cognition of cancer patients treated with chemotherapy: A 3-arm randomized trial. Cancer 2020, 126, 5328–5336. [Google Scholar] [CrossRef]
- Ercoli, L.M.; Petersen, L.; Hunter, A.M.; Castellon, S.A.; Kwan, L.; Kahn-Mills, B.A.; Embree, L.M.; Cernin, P.A.; Leuchter, A.F.; Ganz, P.A. Cognitive rehabilitation group intervention for breast cancer survivors: Results of a randomized clinical trial. Psychooncology 2015, 24, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.J.; McDonald, B.C.; Rocque, M.A.; Furstenberg, C.T.; Horrigan, S.; Ahles, T.A.; Saykin, A.J. Development of CBT for chemotherapy-related cognitive change: Results of a waitlist control trial. Psychooncology 2012, 21, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.J.; Sigmon, S.T.; Pritchard, A.J.; LaBrie, S.L.; Goetze, R.E.; Fink, C.M.; Garrett, A.M. A randomized trial of videoconference-delivered cognitive behavioral therapy for survivors of breast cancer with self-reported cognitive dysfunction. Cancer 2016, 122, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Henneghan, A.M.; Becker, H.; Harrison, M.L.; Inselmann, K.; Fico, B.; Schafer, H.; King, E.; Patt, D.; Kesler, S. A randomized control trial of meditation compared to music listening to improve cognitive function for breast cancer survivors: Feasibility and acceptability. Complement. Ther. Clin. Pract. 2020, 41, 101228. [Google Scholar] [CrossRef]
- Henneghan, A.M.; Becker, H.; Phillips, C.; Kesler, S. Sustained effects of mantra meditation compared to music listening on neurocognitive outcomes of breast cancer survivors: A brief report of a randomized control trial. J. Psychosom. Res. 2021, 150, 110628. [Google Scholar] [CrossRef]
- Koevoets, E.W.; Schagen, S.B.; de Ruiter, M.B.; Geerlings, M.I.; Witlox, L.; van der Wall, E.; Stuiver, M.M.; Sonke, G.S.; Velthuis, M.J.; Jobsen, J.J.; et al. Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: A randomized controlled trial (PAM study). Breast Cancer Res. 2022, 24, 36. [Google Scholar] [CrossRef]
- Lawrence, J.A.; Griffin, L.; Balcueva, E.P.; Groteluschen, D.L.; Samuel, T.A.; Lesser, G.J.; Naughton, M.J.; Case, L.D.; Shaw, E.G.; Rapp, S.R. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J. Cancer Surviv. 2016, 10, 176–184. [Google Scholar] [CrossRef]
- Lengacher, C.A.; Reich, R.R.; Rodriguez, C.S.; Nguyen, A.T.; Park, J.Y.; Meng, H.; Tinsley, S.; Hueluer, G.; Donovan, K.A.; Moscoso, M.S.; et al. Efficacy of Mindfulness-Based Stress Reduction for Breast Cancer (MBSR(BC)) a Treatment for Cancer-related Cognitive Impairment (CRCI): A Randomized Controlled Trial. J. Integr. Complement. Med. 2024, 31, 75–91. [Google Scholar] [CrossRef]
- Melis, M.; Schroyen, G.; Leenaerts, N.; Smeets, A.; Sunaert, S.; Van der Gucht, K.; Deprez, S. The impact of mindfulness on cancer-related cognitive impairment in breast cancer survivors with cognitive complaints. Cancer 2023, 129, 1105–1116. [Google Scholar] [CrossRef]
- Milbury, K.; Chaoul, A.; Biegler, K.; Wangyal, T.; Spelman, A.; Meyers, C.A.; Arun, B.; Palmer, J.L.; Taylor, J.; Cohen, L. Tibetan sound meditation for cognitive dysfunction: Results of a randomized controlled pilot trial. Psychooncology 2013, 22, 2354–2363. [Google Scholar] [CrossRef]
- Rapp, S.R.; Dressler, E.V.; Brown, W.M.; Wade, J.L., 3rd; Le-Lindqwister, N.; King, D.; Rowland, K.M.; Weaver, K.E.; Klepin, H.D.; Shaw, E.G.; et al. Phase III Randomized, Placebo-Controlled Clinical Trial of Donepezil for Treatment of Cognitive Impairment in Breast Cancer Survivors After Adjuvant Chemotherapy (WF-97116). J. Clin. Oncol. 2024, 42, 2546–2557. [Google Scholar] [CrossRef]
- Van der Gucht, K.; Ahmadoun, S.; Melis, M.; de Cloe, E.; Sleurs, C.; Radwan, A.; Blommaert, J.; Takano, K.; Vandenbulcke, M.; Wildiers, H.; et al. Effects of a mindfulness-based intervention on cancer-related cognitive impairment: Results of a randomized controlled functional magnetic resonance imaging pilot study. Cancer 2020, 126, 4246–4255. [Google Scholar] [CrossRef]
- Vardy, J.L.; Pond, G.R.; Bell, M.L.; Renton, C.; Dixon, A.; Dhillon, H.M. A randomised controlled trial evaluating two cognitive rehabilitation approaches for cancer survivors with perceived cognitive impairment. J. Cancer Surviv. 2023, 17, 1583–1595. [Google Scholar] [CrossRef]
- Wu, L.M.; Valdimarsdottir, H.B.; Amidi, A.; Reid, K.J.; Ancoli-Israel, S.; Bovbjerg, K.; Fox, R.S.; Walker, L.; Matharu, A.; Kaseda, E.T.; et al. Examining the Efficacy of Bright Light Therapy on Cognitive Function in Hematopoietic Stem Cell Transplant Survivors. J. Biol. Rhythm. 2022, 37, 471–483. [Google Scholar] [CrossRef]
- Haywood, D.; Henneghan, A.M.; Chan, A.; Chan, R.J.; Dhillon, H.M.; Lustberg, M.B.; Vardy, J.L.; O’Connor, M.; Elvidge, N.; Dauer, E.; et al. The effect of non-pharmacological interventions on cognitive function in cancer: An overview of systematic reviews. Support. Care Cancer 2025, 33, 151. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Bellens, A.; Roelant, E.; Sabbe, B.; Peeters, M.; van Dam, P.A. A video-game based cognitive training for breast cancer survivors with cognitive impairment: A prospective randomized pilot trial. Breast 2020, 53, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bray, V.J.; Dhillon, H.M.; Bell, M.L.; Kabourakis, M.; Fiero, M.H.; Yip, D.; Boyle, F.; Price, M.A.; Vardy, J.L. Evaluation of a Web-Based Cognitive Rehabilitation Program in Cancer Survivors Reporting Cognitive Symptoms After Chemotherapy. J. Clin. Oncol. 2017, 35, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.; Louis, C.C.; Moser, J.; Grunfeld, E.A.; Derakshan, N. Benefits of adaptive cognitive training on cognitive abilities in women treated for primary breast cancer: Findings from a 1-year randomised control trial intervention. Psychooncology 2023, 32, 1848–1857. [Google Scholar] [CrossRef]
- Hartman, S.J.; Nelson, S.H.; Myers, E.; Natarajan, L.; Sears, D.D.; Palmer, B.W.; Weiner, L.S.; Parker, B.A.; Patterson, R.E. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: The memory & motion study. Cancer 2018, 124, 192–202. [Google Scholar] [CrossRef]
- Maeir, T.; Makranz, C.; Peretz, T.; Odem, E.; Tsabari, S.; Nahum, M.; Gilboa, Y. Cognitive Retraining and Functional Treatment (CRAFT) for adults with cancer related cognitive impairment: A preliminary efficacy study. Support. Care Cancer 2023, 31, 152. [Google Scholar] [CrossRef]
- Mihuta, M.E.; Green, H.J.; Shum, D.H.K. Web-based cognitive rehabilitation for survivors of adult cancer: A randomised controlled trial. Psychooncology 2018, 27, 1172–1179. [Google Scholar] [CrossRef]
- Vega, J.N.; Albert, K.M.; Mayer, I.A.; Taylor, W.D.; Newhouse, P.A. Nicotinic treatment of post-chemotherapy subjective cognitive impairment: A pilot study. J. Cancer Surviv. 2019, 13, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Von Ah, D.; Carpenter, J.S.; Saykin, A.; Monahan, P.; Wu, J.; Yu, M.; Rebok, G.; Ball, K.; Schneider, B.; Weaver, M.; et al. Advanced cognitive training for breast cancer survivors: A randomized controlled trial. Breast Cancer Res. Treat. 2012, 135, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.M.; Amidi, A.; Tanenbaum, M.L.; Winkel, G.; Gordon, W.A.; Hall, S.J.; Bovbjerg, K.; Diefenbach, M.A. Computerized cognitive training in prostate cancer patients on androgen deprivation therapy: A pilot study. Support. Care Cancer 2018, 26, 1917–1926. [Google Scholar] [CrossRef]
- Sarvghadi, P.; Ghaffari, A.; Rostami, H.R. The effects of neurofeedback training on short-term memory and quality of life in women with breast cancer. Int. J. Ther. Rehabil. 2019, 26, 1–8. [Google Scholar] [CrossRef]
- Jung, S.O.; Kim, J.E.E.; Kim, H.J. Assessing objective cognitive impairments in cancer survivors: Features and validity of measures for research and clinical applications. Asia Pac. J. Oncol. Nurs. 2023, 10, 100309. [Google Scholar] [CrossRef]
- Saita, K.; Amano, S.; Kaneko, F.; Okamura, H. A scoping review of cognitive assessment tools and domains for chemotherapy-induced cognitive impairments in cancer survivors. Front. Hum. Neurosci. 2023, 17, 1063674. [Google Scholar] [CrossRef]
- Clapp, J.D.; Luta, G.; Small, B.J.; Ahles, T.A.; Root, J.C.; Graham, D.; Hurria, A.; Jacobsen, P.B.; Jim, H.; McDonald, B.C.; et al. The Impact of Using Different Reference Populations on Measurement of Breast Cancer-Related Cognitive Impairment Rates. Arch. Clin. Neuropsychol. 2018, 33, 956–963. [Google Scholar] [CrossRef]

| Study Country Randomized Controlled Trial (RCT) or Feasibility Trial Funding | Study Objective | Study Dates | Interventions (Sample Size Per Arm in Network Meta-Analyses) | Intervention Duration; Follow-up Time(s) Since Intervention | Cognitive Impairment Eligibility Criteria | Mean (SD) Months Since End of Chemotherapy; Mean (SD) Age (Years); % with Breast Cancer | Systemic Cancer Therapies: Chemo, Radiation, Hormone, Targeted | Education | Baseline Comorbidities: Cognitive Impairment; Depression; Anxiety; Fatigue |
|---|---|---|---|---|---|---|---|---|---|
| Lengacher et al., 2024 [88] USA RCT Non-industry | “…to evaluate if BCS [breast cancer survivors] assigned to either the MBSR(BC) [Mindfulness-Based Stress Reduction for breast cancer survivors], Breast Cancer Education Support (BCES), or Usual Care (UC) regimens experienced greater improvements at 6, 12, and 26 weeks on objective and subjective cognitive performance” | October 2015 to July 2020 † | MBI (n = 81) EDU_GD_GRP (n = 79) | 6 weeks; 0 and 5 months | A positive response to at least one of two scaled questions from the European Organization for Research and Treatment of Cancer Quality of Life questionnaire (EORTC-QLQ): (1) “Please rate on a scale from 0 to 10, the difficulty level you have in concentrating on things, like reading a newspaper or watching television?”, (2) “Please rate on a scale from 0 to 10, the difficulty level you have in remembering things.” (0 = no difficulty, 10 = very difficult) | ~24.1 (NR); 56.4 (10.6); 100% | 100% 77% Not reported Not reported | Less than a college degree: 21.7%, Some college or AA degree: 28.8%, College degree: 25.0%, Graduate/ professional school: 23.6% | Severe; Mild; Severe; Mild |
| Melis et al., 2023 [89] Belgium RCT Non-industry | …to evaluate “the potential of MBI to reduce CTRCI [cancer-related cognitive impairment]” | 1 Oct 2018 ‡ | Waitlist (n = 32) MBI (n = 36) EDU_GD_GRP (n = 31) | 8 weeks; 0 and 3 months | Significant cognitive complaints (Cognitive Failures Questionnaire [CFQ] total score > 42.9 [mean + 1 SD] or at least two of the four extra CFQ questions >mean 1 + SD | 25 (14.4); 48.4 (8.7); 100% | 100% 73% 71% Not reported | Secondary school: 26.5%, Higher education: 73.5% | Severe; Not reported; Not reported; Not reported |
| Vardy et al., 2023 [93] Australia RCT Non-industry | “…to evaluate two cognitive rehabilitation programs used in non-cancer populations against standard-of-care control in solid tumour survivors self-reporting cognitive impairment after chemotherapy” | September 2015 to November 2020 § | Waitlist (n = 16) COG_GD_GRP (n = 20) EDU_GD_GRP (n = 18) | 6 weeks; 0 and 6 months | Self-reported cognitive change on the 2-item EORTC-QLQ-C30 Cognitive Functioning scale as “quite a bit” or greater in one or both domains | 26.3 (11.9); 54.5 (8.8); 92.3% | 100% 71% 72% Not reported | mean (SD) years: 14.4 (3.3) | Severe; None; None; Mild |
| Cherrier et al., 2022 [78] USA RCT Non-industry | …to examine “the effectiveness of a behavioral skills training intervention to improve objectively measured cognitive performance as well as individual participant defined cognitive symptoms in cancer survivors” | Not reported | Waitlist (n = 47) COG/EDU_GD_GRP (n = 49) | 7 weeks; 0 months | Subjective concern about declines in cognitive functioning related to a diagnosis of cancer and/or cancer-related treatment (obtained through phone screening by requiring a Yes response to the question: “Do you have concerns about your memory or other thinking abilities related to your cancer or cancer treatment?” | 52.8 (63.6); 59.2 (10.4); Not reported | 89% 54% Not reported Not reported | mean (SD) years: 16.8 (2.6) | Severe; Mild; None; Moderate |
| Koevoets et al., 2022 [86] Netherlands RCT Non-industry | …to investigate “whether exercise training improves cognition in chemotherapy-exposed breast cancer patients 2–4 years after diagnosis” | December 2016 and September 2020 ¶ | Waitlist (n = 82) EXE_GD_GRP (n = 82) | 26 weeks; 0 months | Needed to self-report cognitive problems after cancer diagnosis, which was confirmed by lower than expected performance on neuropsychological testing | 31.2 (7.8); 52.3 (8.6); 100% | 100% 75% 61% 21% | High: 43.1%, Middle: 55.8%, Low: 0, Missing: 1.1% | Moderate; None; Mild; None |
| Dos Santos et al., 2020 [80] France RCT Non-industry | “…to evaluate the impact of computer-assisted CR [cognitive rehabilitation] on subjective and objective cognition, QOL [quality of life], anxiety, and depression among cancer patients treated with chemotherapy and reporting cognitive complaints” | September 2012 to July 2017 † | Waitlist (n = 51) COG_GD_IND (n = 48) COG_SELF_IND (n = 44) | 12 weeks; 0 months | Reported cognitive complaint (during or within five years of chemotherapy completion) | 11.4 (2.3); 51.3 (10.3); 83.8% | 100% 79% 59% Not reported | Primary school: 5.4%, Middle school: 16.2%, High school: 16.8%, University: 50.3%, Unknown: 11.4% | Severe; Mild; Moderate; None |
| Henneghan et al., 2020 [84,85] USA Feasibility/pilot Non-industry | “...to determine the feasibility of delivering an eight-week daily KK [Kirtan Kriya] intervention program remotely to BCS” and “...to evaluate the preliminary effects of the program on cognitive function (performance and perceived), psychological functioning (anxiety, depression, stress), fatigue, and self-efficacy in BCS compared to classical music listening (ML)” | October 2018 to July 2019 † | MUSIC_SELF_IND (n = 15) MBI (n = 16) | 8 weeks; 0 and 2 months | Reported cognitive deficits (a minimum of five cognitive symptoms occurring “sometimes” or more, per the Perceived Deficits Questionnaire | 31.4 (19.4); 49.5 (9.9); 100% | 100% 84% Previous: 65% Current: 67% 42% | Bachelors or higher: 71.0% | Severe; None; None; Mild |
| Van der Gucht et al., 2020 [92] Belgium Feasibility/pilot Non-industry | “…to investigate the effect of a blended-care mindfulness-based intervention (MBI) on chemotherapy-related cognitive impairment and functional brain changes” | Not reported | Waitlist (n = 14) MBI (n = 12) | 8 weeks; 0 and 3 months | Had significant cognitive complaints, as measured by the CFQ: a CFQ total score > 42.9 or a score greater than the accepted mean score ± 1 SD on ≥2 CFQ extra questions. | 19 (Not reported); 45.5 (6); 100% | 100% 36% 79% Not reported | Secondary school: 45%; Higher education degree: 45%; Never finished secondary school: 10% | Severe; Moderate; Not reported; Mild |
| Campbell et al., 2018 [76] Canada Feasibility/pilot Non-industry | …to test “the effect of a 24-week aerobic exercise intervention compared to usual lifestyle control on measures of cancer-associated cognitive impairment in early stage BCS reporting persistent cognitive concerns” | January 2011 to June 2013 † | Waitlist (n = 9) EXE_GD_GRP (n = 10) | 24 weeks; 0 and 6 months | Self-report cognitive impairment | 11.2 (6.7); 52.4 (6.2); 100% | 100% 90% Not reported Not reported | Vocational training/some college or less: 31.6%, College graduate: 43.4%, Some postgraduate or more: 21.1% | Severe; None; None; Mild |
| Damholdt et al., 2016 [79] Denmark RCT Non-industry | …to examine “effects of web-based cognitive training in a large sample of breast cancer survivors, applying a broad cognitive training program with baseline and short- and long-term telephone-based neuropsychological assessments” | March 2013 to December 2014 † | Waitlist (n = 59) COG_SELF_IND (n = 77) | 6 weeks; 0 and 5 months | Subjective complaints of cognitive impairment and scoring above the sample median (≥27) on the CFQ | Months since diagnosis: 55.4 (21.9); 54.8 (8.6); 100% | 83% 85% 69% Not reported | Municipal primary and lower secondary school, incl. apprenticeships: 24.2%, Short (<3 years): 21.7%, Medium (3–4 years): 33.1%, Long (+5 years): 19.7% | Severe; None; None; Not reported |
| Ferguson et al., 2016 [83] USA RCT Non-industry | …to evaluate Memory and Attention Adaptation Training (MAAT), a cognitive behavioral therapy, compared with a supportive therapy control | Not reported | SUP_GD_IND (n = 14) BT_GD_IND (n = 22) | 8 weeks; 0 and 2 months | Reported cognitive problems attributed to chemotherapy; A score of ≤10 on the FACT-Cog Impact of Quality of Life Scale | 52.6 (46.3); 54.6 (12.12); 100% | 100% Not reported Not reported Not reported | mean (SD) years: 15.6 (2.8) | Severe; None; None; Moderate |
| Ercoli et al., 2015 [81] USA RCT Non-industry | …to evaluate “the efficacy of a cognitive rehabilitation (CR) intervention compared with a waitlist (WL) control condition on cognitive complaints, neuropsychological and brain functioning in breast cancer survivors (BCS)” | January 2012 and April 2013 † | Waitlist (n = 16) COG_GD_GRP (n = 28) | 5 weeks; 0 and 2 months | Self-reported cognitive difficulties interfering with everyday activities | Months since diagnosis: 33.6 (13.2); 53.8 (8.2); 100% | 77% 75% Currently: 71% 26% | Less than college: 25%, College graduate: 20.8%, Post-college graduate: 54.2% | Mild; None; Not reported; Not reported |
| Cherrier et al., 2013 [77] USA RCT Non-industry | …to examine “the effectiveness of a group-based cognitive rehabilitation intervention in cancer survivors” | Not reported | Waitlist (n = 16) COG_GD_GRP (n = 12) | 7 weeks; 0.25 and 0.5 months | Subjective concern about declines in cognitive functioning related to a diagnosis of cancer and/or cancer related treatment, which was assessed by a Yes response to the question “Do you have concerns about your memory or other thinking abilities following cancer treatment?” | 58.1 (12); 58.9 (2.4); Not reported | 89% 46% Not reported Not reported | mean (SD) years: 17.1 (0.5) | Severe; Mild; None; Severe |
| Ferguson et al., 2012 [82] USA RCT Non-industry | “To evaluate the efficacy of a brief cognitive-behavioral therapy (CBT) that is being developed for management of cognitive dysfunction following chemotherapy among breast cancer survivors” | Not reported | Waitlist (n = 21) BT_GD_IND (n = 19) | 8 weeks; 0 and 2 months | Complaint of memory and attention problems following chemotherapy | >18; 50.3 (6.4); 100% | 100% Not reported Not reported Not reported | mean (SD) years: 16.4 (2.4) | Mild; Not reported; Not reported; Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolfe, D.M.; Hamel, C.; Berard, J.; Veroniki, A.A.; Skidmore, B.; Kanji, S.; Rabheru, K.; McGee, S.F.; Forbes, L.; de Lima Machado, I.; et al. Comparative Effectiveness of Interventions to Treat Cancer Treatment-Related Cognitive Impairment in Adult Cancer Survivors Following Systemic Therapy: A Systematic Review with Network Meta-Analyses. Cancers 2025, 17, 3430. https://doi.org/10.3390/cancers17213430
Wolfe DM, Hamel C, Berard J, Veroniki AA, Skidmore B, Kanji S, Rabheru K, McGee SF, Forbes L, de Lima Machado I, et al. Comparative Effectiveness of Interventions to Treat Cancer Treatment-Related Cognitive Impairment in Adult Cancer Survivors Following Systemic Therapy: A Systematic Review with Network Meta-Analyses. Cancers. 2025; 17(21):3430. https://doi.org/10.3390/cancers17213430
Chicago/Turabian StyleWolfe, Dianna M., Candyce Hamel, Jason Berard, Areti Angeliki Veroniki, Becky Skidmore, Salmaan Kanji, Kiran Rabheru, Sharon F. McGee, Leta Forbes, Igor de Lima Machado, and et al. 2025. "Comparative Effectiveness of Interventions to Treat Cancer Treatment-Related Cognitive Impairment in Adult Cancer Survivors Following Systemic Therapy: A Systematic Review with Network Meta-Analyses" Cancers 17, no. 21: 3430. https://doi.org/10.3390/cancers17213430
APA StyleWolfe, D. M., Hamel, C., Berard, J., Veroniki, A. A., Skidmore, B., Kanji, S., Rabheru, K., McGee, S. F., Forbes, L., de Lima Machado, I., Liu, M., Saunders, D., Vandermeer, L., Clemons, M., & Hutton, B. (2025). Comparative Effectiveness of Interventions to Treat Cancer Treatment-Related Cognitive Impairment in Adult Cancer Survivors Following Systemic Therapy: A Systematic Review with Network Meta-Analyses. Cancers, 17(21), 3430. https://doi.org/10.3390/cancers17213430







