Parallel Alterations in Gut and Tumor Microbiota in Pediatric Oncology: Potential Impacts on Disease Progression and Treatment Response
Simple Summary
Abstract
1. Introduction
Development of Gut Microbiome
2. Microbiome and Cancer
2.1. Parallel Alterations Between the Gut and Tumor Microbiota in Cancer
2.2. Therapeutic Response
2.3. Tumor Microbiome
2.3.1. Gastrointestinal Tumor Microbiome
2.3.2. Respiratory Tract Tumor Microbiome
2.3.3. Central Nervous System Tumor Microbiome
2.3.4. Future Implications
3. Pediatric Specificities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Ryan, C.A.; Boyaval, P.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Maternal Vertical Transmission Affecting Early-life Microbiota Development. Trends Microbiol. 2020, 28, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.M.; Levy, E.I.; Vandenplas, Y. The impact of Cesarean section on the infant gut microbiome. Acta Paediatr. 2021, 110, 60–67. [Google Scholar] [CrossRef]
- Jiménez, E.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Is meconium from healthy newborns sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef]
- Kramer, M.S.; Kakuma, R. Optimal duration of exclusive breastfeeding. In Cochrane Database of Systematic Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Ip, S.; Chung, M.; Raman, G.; Chew, P.; Magula, N.; Devine, D.; Trikalinos, T.; Lau, J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid. Rep. Technol. Assess. 2007, 153, 1–186. [Google Scholar] [PubMed] [PubMed Central]
- Bridgman, S.L.; Azad, M.B.; Field, C.J.; Haqq, A.M.; Becker, A.B.; Mandhane, P.J.; Subbarao, P.; Turvey, S.E.; Sears, M.R.; Scott, J.A.; et al. Fecal Short-Chain Fatty Acid Variations by Breastfeeding Status in Infants at 4 Months: Differences in Relative versus Absolute Concentrations. Front. Nutr. 2017, 4, 11. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A.; et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laursen, M.F.; Zachariassen, G.; Bahl, M.I.; Bergström, A.; Høst, A.; Michaelsen, K.F.; Licht, T.R. Having older siblings is associated with gut microbiota development during early childhood. BMC Microbiol. 2015, 15, 154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.-S.; He, K.-X.; Li, J.-J.; Zhong, Z.-F.; Wang, F.-X.; Pan, L.-L.; Lin, J.-J. Comparison of Gut Microbiome in Human Colorectal Cancer in Paired Tumor and Adjacent Normal Tissues. OncoTargets Ther. 2020, 13, 635–646. [Google Scholar] [CrossRef]
- Liao, K.; Wen, J.; Liu, Z.; Zhang, B.; Zhang, X.; Fu, Y.; Zhang, W.; Hu, H.; Ai, K.; Zhu, W.; et al. The role of intratumoral microbiome in the occurrence, proliferation, metastasis of colorectal cancer and its underlying therapeutic strategies. Ageing Res. Rev. 2025, 111, 102820. [Google Scholar] [CrossRef]
- Ji, H.; Jiang, Z.; Wei, C.; Ma, Y.; Zhao, J.; Wang, F.; Zhao, B.; Wang, D.; Tang, D. Intratumoural microbiota: From theory to clinical application. Cell Commun. Signal. 2023, 21, 164. [Google Scholar] [CrossRef]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; DeStefano Shields, C.E.; Hechenbleikner, E.M.; Huso, D.L.; et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 2018, 359, 592–597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Zou, S.; Yang, C.; Zhang, J.; Zhong, D.; Meng, M.; Zhang, L.; Chen, H.; Fang, L. Multi-omic profiling reveals associations between the gut microbiome, host genome, and transcriptome in patients with colorectal cancer. J. Transl. Med. 2024, 22, 175. [Google Scholar] [CrossRef]
- Shimogama, T.; Tahara, T.; Shijimaya, T.; Yamazaki, J.; Kobayashi, S.; Nakamura, N.; Takahashi, Y.; Tomiyama, T.; Honzawa, Y.; Fukui, T.; et al. Gastric microbiome in gastric cancer sequence depicts diverse microbial structures associated with cancer risk and prognosis. J. Transl. Med. 2025, 23, 1039. [Google Scholar] [CrossRef]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Hitaka, Y.; Hirata, H.; Yamamoto, Y.; Kobayashi, K.; Isoyama, N.; Matsubara, T.; Watanabe, K.; Mizukami, Y.; Nakagawa, S.; et al. Exploration of predictive factors based on oral and intestinal bacterial flora for treating patients with urothelial carcinoma. PLoS ONE 2025, 20, e0324814. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 2022, 28, 315–324. [Google Scholar] [CrossRef]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium blunts colitis-associated tumourigenesis by modulation of CD8+ T cells in mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, Z.W.; Xia, K.; Liu, Y.W.; Liu, J.H.; Rao, S.S.; Hu, X.K.; Chen, C.Y.; Xu, R.; Wang, Z.X.; Xie, H. Extracellular Vesicles from Akkermansia muciniphila Elicit Antitumor Immunity Against Prostate Cancer via Modulation of CD8+ T Cells and Macrophages. Int. J. Nanomed. 2021, 16, 2949–2963. [Google Scholar] [CrossRef]
- Yang, L.; Wu, B.; Sun, T.; Sun, H.; Ma, J.; Liu, X.; Zhou, D.; Yang, S. Excess Cholesterol Biosynthesis by Up-Regulated HMGCS1 in Colorectal Cancer Cells Induced M2-Like Tumor-Associated Macrophage Polarization Via Extracellular Vesicles. Cancer Res. Treat. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.F.; Fan, Z.Y.; Zhou, B.; Zhan, H.X. Role of the intratumoral microbiome in tumor progression and therapeutic implications. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 189014. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.; Hand, C.K.; Shanahan, F.; Murphy, T.; O’Toole, P.W. Mutagenesis by Microbe: The Role of the Microbiota in Shaping the Cancer Genome. Trends Cancer 2020, 6, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Pongpanich, M.; Porntaveetus, T. Unraveling metagenomics through long-read sequencing: A comprehensive review. J. Transl. Med. 2024, 22, 111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tyagi, S.; Katara, P. Metatranscriptomics: A Tool for Clinical Metagenomics. OMICS J. Integr. Biol. 2024, 28, 394–407. [Google Scholar] [CrossRef]
- Duarte, V.D.S.; Porcellato, D. Host DNA depletion methods and genome-centric metagenomics of bovine hindmilk microbiome. mSphere 2024, 9, e0047023. [Google Scholar] [CrossRef]
- Saarenpää, S.; Shalev, O.; Ashkenazy, H.; Carlos, V.; Lundberg, D.S.; Weigel, D.; Giacomello, S. Spatial metatranscriptomics resolves host–bacteria–fungi interactomes. Nat. Biotechnol. 2024, 42, 1384–1393. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260.e16. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The Intestinal Microbiota in Colorectal Cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Dienstmann, R.; Salazar, R.; Tabernero, J. Molecular Subtypes and the Evolution of Treatment Decisions in Metastatic Colorectal Cancer. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 231–238. [Google Scholar] [CrossRef]
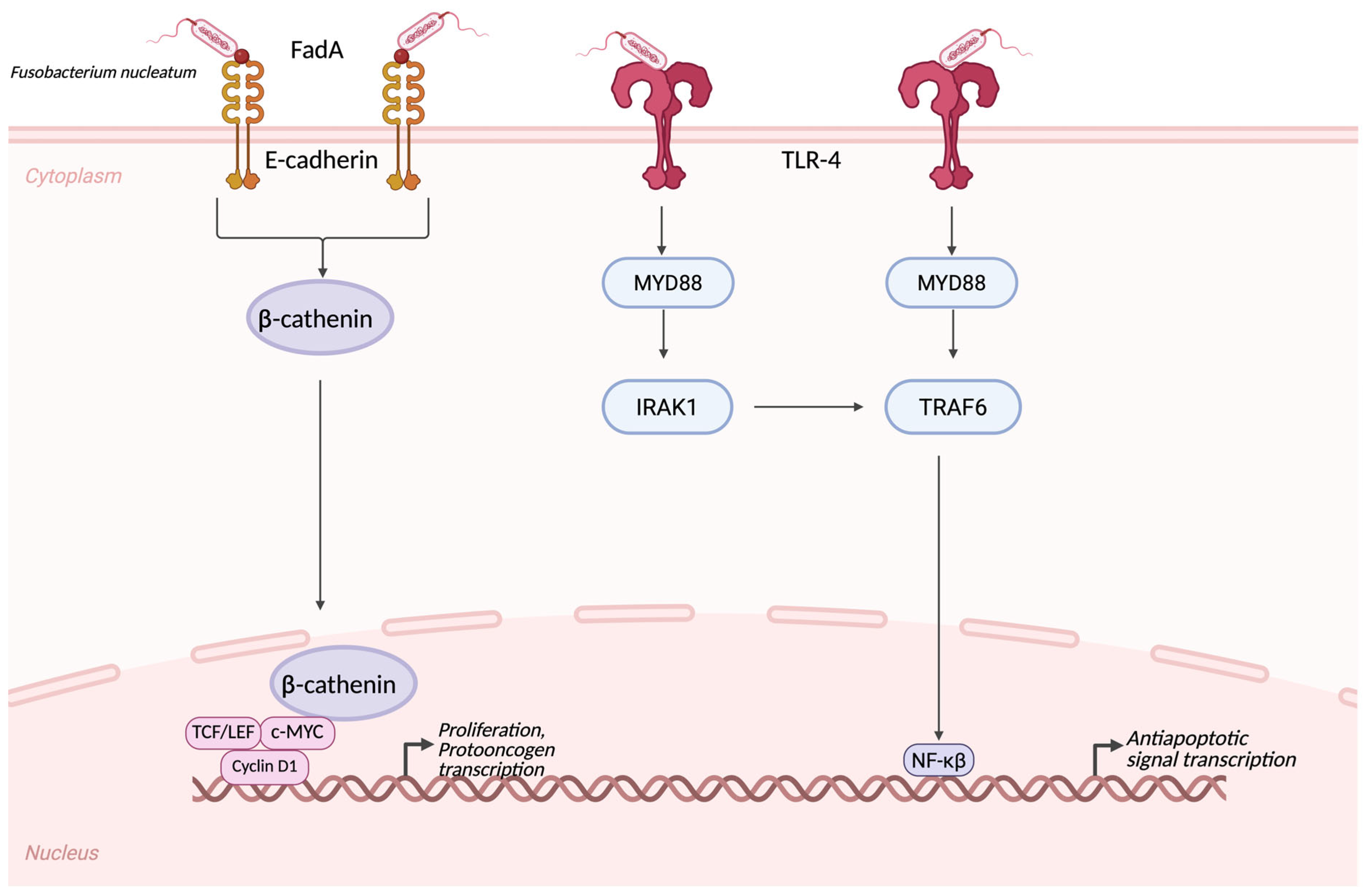
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Stanietsky, N.; Rovis, T.L.; Glasner, A.; Seidel, E.; Tsukerman, P.; Yamin, R.; Enk, J.; Jonjic, S.; Mandelboim, O. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur. J. Immunol. 2013, 43, 2138–2150. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Li, M.; Hu, D.; Li, C.; Ge, B.; Jin, B.; Fan, Z. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ. 2013, 20, 456–464. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting the NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Balaji, S.; Ahmed, M.; Lorence, E.; Yan, F.; Nomie, K.; Wang, M. NF-κB signaling and its relevance to the treatment of mantle cell lymphoma. J. Hematol. Oncol. 2018, 11, 83. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y. Intratumor microbiome in cancer progression: Current developments, challenges and future trends. Biomark. Res. 2022, 10, 37. [Google Scholar] [CrossRef]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bellotti, R.; Speth, C.; Adolph, T.E.; Lass-Flörl, C.; Effenberger, M.; Öfner, D.; Maglione, M. Micro- and Mycobiota Dysbiosis in Pancreatic Ductal Adenocarcinoma Development. Cancers 2021, 13, 3431. [Google Scholar] [CrossRef]
- Guan, S.W.; Lin, Q.; Yu, H.B. Intratumour microbiome of pancreatic cancer. World J. Gastrointest. Oncol. 2023, 15, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liao, H.; Chen, Y.; Ye, C.; Huang, L.; Xu, M.; Du, J.; Zhang, J.; Huang, D.; Cai, S.; et al. Airway microbiota-associated D-phenylalanine promotes non-small cell lung cancer metastasis through epithelial-mesenchymal transition. J. Transl. Med. 2025, 23, 673. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The Microbiome and the Respiratory Tract. Annu. Rev. Physiol. 2016, 78, 481–504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akinosoglou, K.S.; Karkoulias, K.; Marangos, M. Infectious complications in patients with lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 8–18. [Google Scholar] [PubMed]
- Yu, G.; Gail, M.H.; Consonni, D.; Carugno, M.; Humphrys, M.; Pesatori, A.C.; Caporaso, N.E.; Goedert, J.J.; Ravel, J.; Landi, M.T. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol. 2016, 17, 163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, X.; Yang, M.; Liu, J.; Gao, R.; Hu, J.; Li, J.; Zhang, L.; Shi, Y.; Guo, H.; Cheng, J.; et al. Discovery and validation of potential bacterial biomarkers for lung cancer. Am. J. Cancer Res. 2015, 5, 3111–3122. [Google Scholar] [PubMed] [PubMed Central]
- Lee, S.H.; Sung, J.Y.; Yong, D.; Chun, J.; Kim, S.Y.; Song, J.H.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; et al. Characterization of microbiome in bronchoalveolar lavage fluid of patients with lung cancer compared with benign mass-like lesions. Lung Cancer 2016, 102, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.J.; Wu, B.G.; Sulaiman, I.; Gershner, K.; Schluger, R.; Li, Y.; Yie, T.A.; Meyn, P.; Olsen, E.; Perez, L.; et al. Lower Airway Dysbiosis Affects Lung Cancer Progression. Cancer Discov. 2021, 11, 293–307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teruya, H.; Higa, F.; Akamine, M.; Ishikawa, C.; Okudaira, T.; Tomimori, K.; Mukaida, N.; Tateyama, M.; Heuner, K.; Fujita, J.; et al. Mechanisms of Legionella pneumophila-induced interleukin-8 expression in human lung epithelial cells. BMC Microbiol. 2007, 7, 102. [Google Scholar] [CrossRef]
- Sims, G.P.; Rowe, D.C.; Rietdijk, S.T.; Herbst, R.; Coyle, A.J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 2010, 28, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zhang, Q.; Zeh HJ3rd Lotze, M.T.; Tang, D. HMGB1 in cancer: Good, bad, or both? Clin. Cancer Res. 2013, 19, 4046–4057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Lange, E.C. The physiological characteristics and transcytosis mechanisms of the blood-brain barrier (BBB). Curr. Pharm. Biotechnol. 2012, 13, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Henrik Heiland, D.; Ravi, V.M.; Behringer, S.P.; Frenking, J.H.; Wurm, J.; Joseph, K.; Garrelfs, N.W.C.; Strähle, J.; Heynckes, S.; Grauvogel, J.; et al. Tumor-associated reactive astrocytes aid the evolution of an immunosuppressive environment in glioblastoma. Nat. Commun. 2019, 10, 2541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zeng, W.; Zhang, X.; Pei, Y.; Zhang, H.; Li, Y. The role of gut microbiota in patients with benign and malignant brain tumors: A pilot study. Bioengineered 2022, 13, 7846–7858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, H.; Wang, Y.; Han, M.; Wang, L.; Li, X.; Kuang, X.; Du, J.; Peng, F. Multi-omics-based investigation of Bifidobacterium’s inhibitory effect on glioma: Regulation of tumor and gut microbiota, and MEK/ERK cascade. Front. Microbiol. 2024, 15, 1344284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdugheni, R.; Wang, W.Z.; Wang, Y.J.; Du, M.X.; Liu, F.L.; Zhou, N.; Jiang, C.Y.; Wang, C.Y.; Wu, L.; Ma, J.; et al. Metabolite profiling of human-originated Lachnospiraceae at the strain level. iMeta 2022, 1, e58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mattes, J.; Hulett, M.; Xie, W.; Hogan, S.; Rothenberg, M.E.; Foster, P.; Parish, C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: An eotaxin and STAT6-dependent process. J. Exp. Med. 2003, 197, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patrizz, A.; Dono, A.; Zorofchian, S.; Hines, G.; Takayasu, T.; Husein, N.; Otani, Y.; Arevalo, O.; Choi, H.A.; Savarraj, J.; et al. Glioma and temozolomide-induced alterations in gut microbiome. Sci. Rep. 2020, 10, 21002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, J.; Shin, T.-S.; Kim, J.S.; Jee, Y.-K.; Kim, Y.-K. A new horizon of precision medicine: Combination of the microbiome and extracellular vesicles. Exp. Mol. Med. 2022, 54, 466–482. [Google Scholar] [CrossRef]
- Cheung, S.Y.A.; Hay, J.L.; Lin, Y.W.; de Greef, R.; Bullock, J. Pediatric oncology drug development and dosage optimization. Front. Oncol. 2024, 13, 1235947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vyas, C.; Moshyk, A.; Fusaro, G.; Zacharoulis, S.; Fazeli, M.S.; Gaind, N.; Behyan, S.; Thakkar, P. Immune checkpoint inhibitors in pediatric patients with melanoma: A systematic literature review. Melanoma Manag. 2024, 11, 2382075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sprokkerieft, J.; van der Beek, J.N.; Spreafico, F.; Selle, B.; Thebaud, E.; Chowdhury, T.; Brok, J.; Ottóffy, G.; Sun, X.; Ramírez Villar, G.L.; et al. Targeted therapies in children with renal cell carcinoma (RCC): An International Society of Pediatric Oncology-Renal Tumor Study Group (SIOP-RTSG)-related retrospective descriptive study. Cancer Med. 2024, 13, e6782. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Xue, J.; Zhou, X.; You, M.; Du, Q.; Yang, X.; He, J.; Zou, J.; Cheng, L.; Li, M.; et al. Oral microbiota distinguishes acute lymphoblastic leukemia pediatric hosts from healthy populations. PLoS ONE 2014, 9, e102116, Correction in PLoS ONE 2014, 9, e110449. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, M.; Rüchel, N.; Janssen, S.; Borkhardt, A.; Gössling, K.L. The Microbiome in Childhood Acute Lymphoblastic Leukemia. Cancers 2021, 13, 4947. [Google Scholar] [CrossRef]
- Rajagopala, S.V.; Yooseph, S.; Harkins, D.M.; Moncera, K.J.; Zabokrtsky, K.B.; Torralba, M.G.; Tovchigrechko, A.; Highlander, S.K.; Pieper, R.; Sender, L.; et al. Gastrointestinal microbial populations can distinguish pediatric and adolescent Acute Lymphoblastic Leukemia (ALL) at the time of disease diagnosis. BMC Genom. 2016, 17, 635. [Google Scholar] [CrossRef]
- Hakim, H.; Dallas, R.; Wolf, J.; Tang, L.; Schultz-Cherry, S.; Darling, V.; Johnson, C.; Karlsson, E.A.; Chang, T.-C.; Jeha, S.; et al. Gut Microbiome Composition Predicts Infection Risk During Chemotherapy in Children with Acute Lymphoblastic Leukemia. Clin. Infect. Dis. 2018, 67, 541–548. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, X.; Yang, X.; Que, J.; Du, Q.; Zhang, Q.; Zou, J. Oral Health, Caries Risk Profiles, and Oral Microbiome of Pediatric Patients with Leukemia Submitted to Chemotherapy. BioMed Res. Int. 2021, 2021, 6637503. [Google Scholar] [CrossRef]
- De Pietri, S.; Ingham, A.C.; Frandsen, T.L.; Rathe, M.; Krych, L.; Castro-Mejía, J.L.; Nielsen, D.S.; Nersting, J.; Wehner, P.S.; Schmiegelow, K.; et al. Gastrointestinal toxicity during induction treatment for childhood acute lymphoblastic leukemia: The impact of the gut microbiota. Int. J. Cancer 2020, 147, 1953–1962. [Google Scholar] [CrossRef]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Chiu, H.T.; Alshawsh, M.A. Faecalibacterium prausnitzii Ameliorates Colorectal Tumorigenesis and Suppresses Proliferation of HCT116 Colorectal Cancer Cells. Biomedicines 2022, 10, 1128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soltani, S.; Tabibzadeh, A.; Yousefi, P.; Zandi, M.; Zakeri, A.; Akhavan Rezayat, S.; Ramezani, A.; Esghaei, M.; Farahani, A. HPV infections in retinoblastoma: A systematic review. J. Clin. Lab. Anal. 2021, 35, e23981. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polychronopoulou, S.; Kostaridou, S.; Panagiotou, J.P.; Stefanaki, K.; Papadakis, V.; Florentin, L.; Houlakis, M.; Christopoulos, G.; Haidas, S. Nasopharyngeal Carcinoma in Childhood and Adolescence: A Single Institution’s Experience with Treatment Modalities During the Last 15 Years. Pediatr. Hematol. Oncol. 2004, 21, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, E.P.; Krischer, J.P.; Smith, B.E.; Hawkins, H.K.; Finegold, M.J. Nasopharyngeal carcinoma in children: Retrospective review and demonstration of Epstein-Barr viral genomes in tumor cell cytoplasm: A report of the Pediatric Oncology Group. Hum. Pathol. 1990, 21, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Du, X.; Cui, X.; Fan, R.; Pan, J.; Wang, Z. Oral microbiome characteristics in patients with pediatric solid tumor. Front. Microbiol. 2024, 14, 1286522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, C.; Chen, Y.; Yao, W.; Zhang, P.; Wang, X.; Qu, G.; Ren, Z.; Wang, J. Gut microbiome and serum metabolome alterations in osteosarcoma patients. Front. Microbiol. 2025, 16, 1616603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
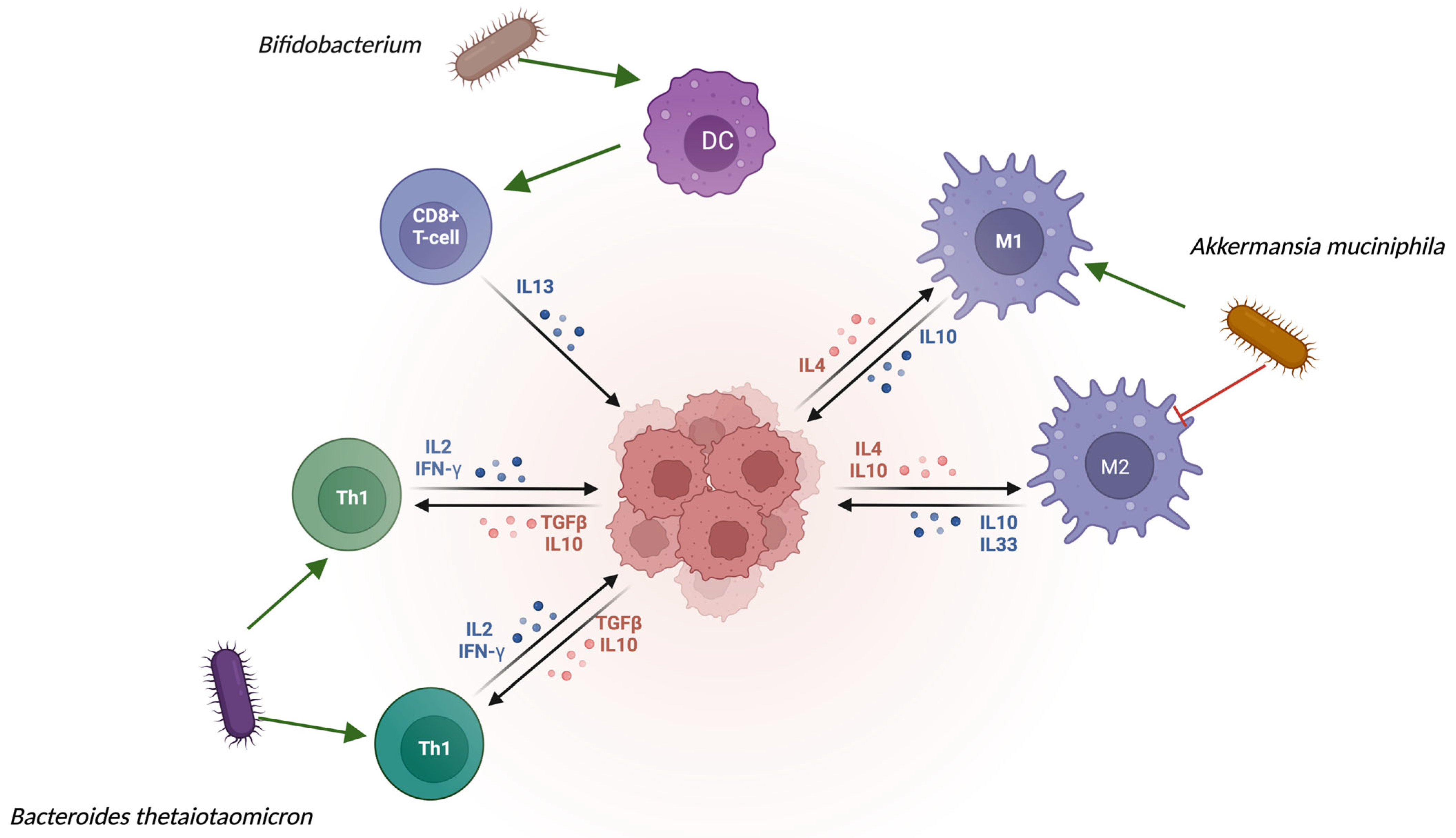
| Microorganism | Change in the Gut Microbiota | Alteration in the Tumor Microbiota | Biological Role |
|---|---|---|---|
| Fusobacterium nucleatum | Increased in fecal samples of CRC patients | Strong overrepresentation in tumorous tissue, local invasion to the tumor stroma | Induces local inflammation via TLR-4-MYD88-NFκB pathway; induces β-catenin activation [13]. |
| Escherichia coli | Elevated in patients of CRC and HCC | It can be found within the tumor epithelium | Colibactin can cause a dsDNA break, and therefore genotoxic stress, mutagenesis, and chromosomal instability [15]. |
| Bacteroides fragilis | Overrepresented among patients with CRC | Present in tumorous tissue and in the peritumoral mucosa | Induces IL-17 inflammation, disrupts E-cadherin [16]. |
| Akkermansia muciniphila | Decreased among cancer patients, especially in ICI non-responders | Rarely found in the tumorous tissue, but it can alter T-cell infiltration | It can enhance the anti-tumoral efficacy [17]. |
| Prevotella intermedia | Increased in feces of GI cancer patients | Could be found in the tumorous tissue occasionally | It is common to be found with TP53 mutation [18]. |
| Helicobacter pylori | Can be present in the gastric mucosa as part of a chronic infection | Dominant species in gastric tumors | Induces DNA-methylation and TP53 mutation, and causes a chronic smoldering inflammation [19]. |
| Malessezia spp. | It can be found in feces of PDAC patients | Can also be found in tumorous tissue | Activates the complement cascade via the mannose-MBL binding. It can cause a tumor-promoting inflammation [20]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, P.J.; Sági, V.; Kassai, L.K.; Kiss-Miki, R.M.; Makra, N.; Szabó, D.; Garami, M. Parallel Alterations in Gut and Tumor Microbiota in Pediatric Oncology: Potential Impacts on Disease Progression and Treatment Response. Cancers 2025, 17, 3426. https://doi.org/10.3390/cancers17213426
Szabó PJ, Sági V, Kassai LK, Kiss-Miki RM, Makra N, Szabó D, Garami M. Parallel Alterations in Gut and Tumor Microbiota in Pediatric Oncology: Potential Impacts on Disease Progression and Treatment Response. Cancers. 2025; 17(21):3426. https://doi.org/10.3390/cancers17213426
Chicago/Turabian StyleSzabó, Patrik József, Viktória Sági, Levente Károly Kassai, Renáta Mária Kiss-Miki, Nóra Makra, Dóra Szabó, and Miklós Garami. 2025. "Parallel Alterations in Gut and Tumor Microbiota in Pediatric Oncology: Potential Impacts on Disease Progression and Treatment Response" Cancers 17, no. 21: 3426. https://doi.org/10.3390/cancers17213426
APA StyleSzabó, P. J., Sági, V., Kassai, L. K., Kiss-Miki, R. M., Makra, N., Szabó, D., & Garami, M. (2025). Parallel Alterations in Gut and Tumor Microbiota in Pediatric Oncology: Potential Impacts on Disease Progression and Treatment Response. Cancers, 17(21), 3426. https://doi.org/10.3390/cancers17213426








